Abstract
Recent studies have demonstrated the presence of estrogen receptor (ER)β in the mitochondria in various cell types and tissues, but the exact function of this localization remains unclear. In this study, we have examined the function of mitochondrial ERβ in non-small-cell lung cancer (NSCLC) cells. Down-regulation of ERβ by short hairpin RNA constructs sensitized NSCLC cells to various apoptosis-inducing agents such as cisplatin, taxol, and etoposide. The increased growth inhibition and induction of apoptosis in ERβ-knockdown cells was observed irrespective of estrogen treatment, suggesting a ligand-independent role of ERβ in regulating the intrinsic apoptotic pathway. Further, ERβ from the mitochondrial fraction physically interacted with the proapoptotic protein Bad, in a ligand-independent manner. Glutathione-S-transferase pull-down assays and molecular modeling studies revealed that the DNA-binding domain and hinge region of ERβ, and the BH3 domain of Bad were involved in these interactions. Further investigations revealed that ERβ inhibited Bad function by disrupting Bad-Bcl-XL and Bad-Bcl-2 interactions. Reintroduction of ERβ in the mitochondria of ERβ knockdown cells reversed their sensitivity to cisplatin. Overall, our results demonstrate a ligand-independent role of ERβ in regulating apoptosis, revealing a novel function for ERβ in the mitochondria.
Mitochondrial ERβ in lung cancer cells interacts with pro-apoptotic protein Bad in a ligand-independent manner and inhibits apoptosis.
Estrogens are key signaling molecules that regulate various physiological processes such as cell growth, development, and differentiation and also play a major role in many pathological processes of hormone-dependent diseases (for review, see Ref. 1). Estrogens exert their biological effect through two estrogen receptor (ER) subtypes, ERα and ERβ. In the classical genomic mode of action, these receptors regulate gene expression by binding to estrogen-response elements in the promoter region of their target genes. In some cases, estrogens can also activate signaling through nongenomic mechanisms by binding to membrane-associated ERs, resulting in rapid cellular responses such as activation of kinase pathways (2,3).
Although discovered in the mid-1990s, the molecular mechanisms of ERβ have been poorly understood when compared with ERα. Like ERα, ERβ possesses transactivation properties, with a strong activation function (AF)-2 domain but a weak AF-1 function, when tested under similar conditions (4,5). In addition, ERβ also demonstrates nongenomic signaling properties in various cell lines (6,7,8). Interestingly, in certain model systems, endogenous ERβ appears to be completely extranuclear and does not translocate into the nucleus after estrogen treatment. We and several others have reported the presence of ERβ in the mitochondria in a number of cell types and tissues, including human non-small-cell lung cancer (NSCLC) cells (7), human breast cancer MCF-7 cells (9), human lens epithelial cells (10), human osteosarcoma SaOS-2 cells, and liver cancer HepG2 cells (11), murine hippocampal HT-22 cells, and rat primary neurons and cardiomyocytes (12). The presence of a mitochondrial localization signal in the hinge region of ERβ has been suggested (9). Further, estrogens have been shown to up-regulate the mitochondrial DNA genes that encode the mitochondrial respiratory chain complex (for review, see Ref. 13). However, it remains unclear whether ERβ regulates mitochondrial DNA transcription directly or whether the nuclear target genes of ERs, in turn, regulate mitochondrial DNA transcription. Two recent studies demonstrated that down-regulation of ERβ by small interfering RNA (SiRNA) constructs resulted in loss of mitochondrial membrane potential, an indicator of apoptotic progression, suggesting a role for ERβ role in regulating apoptosis (14,15). However, the precise mechanism underlying the antiapoptotic function of ERβ in the mitochondria is unknown.
We previously demonstrated that ERβ was present in the cytoplasm in lung cancer cells and failed to translocate to the nucleus in the presence of estrogen (7). We also showed that ERβ was localized to the mitochondria in NSCLC cells. In this study, we have examined in detail the function of mitochondrial ERβ in NSCLC cells. We demonstrate that down-regulation of ERβ sensitizes the lung cancer cells to various apoptosis-inducing agents. Further, we show that mitochondrial ERβ physically interacts with Bad in a ligand-independent manner and sequesters Bad from interacting with Bcl-XL and Bcl-2.
Results
Loss of ERβ sensitizes lung cancer cells to various apoptosis-inducing agents
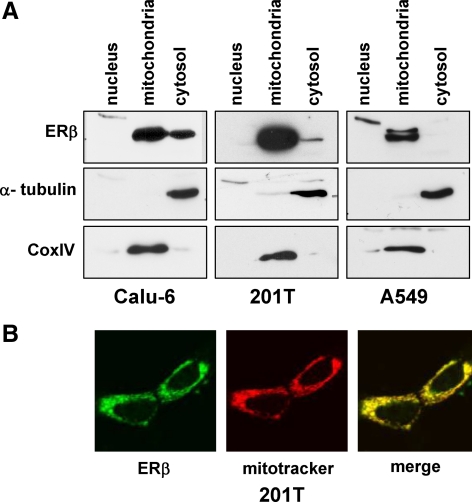
We previously reported that NSCLC cells express ERβ but not ERα, and ERβ was localized in an extranuclear fashion in all cell lines, including localization at the mitochondria (7). We performed subcellular fractionation studies to further confirm the presence of ERβ in the mitochondria of NSCLC cells. Western blotting analysis demonstrated that ERβ was present predominantly in the mitochondrial fraction in Calu-6, 201T, and A549 NSCLC cells (Fig. 1A). Cox IV and α-tubulin were used as markers for mitochondrial and cytoplasmic fractions, respectively (Fig. 1A). Further, confocal microscopy studies also demonstrated that endogenous ERβ colocalized with the mitochondrial marker Mitotracker Red in 201T lung cancer cells (Fig. 1B).
Figure 1.
ERβ is localized to mitochondria in NSCLC cells. A, Nuclear, mitochondrial, and cytosolic fractions were prepared from NSCLC cells and analyzed for ERβ by Western blotting. Cox IV and α-tubulin served as controls for mitochondrial and cytosolic fractions, respectively. B, Confocal microscopy of 201T cells stained with antibodies against ERβ (green) and Mitotracker Red (red). Overlap is shown in yellow.
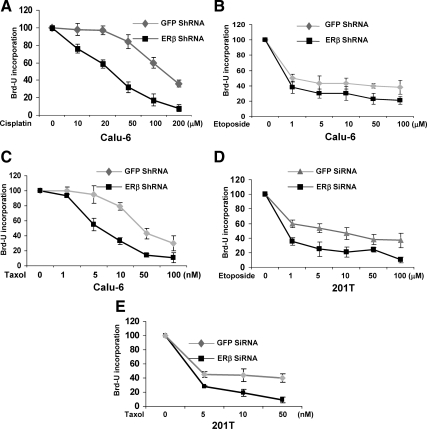
During the course of our experiments, we observed that ERβ short hairpin RNA (ShRNA) cells displayed reduced proliferation rates when compared with control green fluorescent protein (GFP) ShRNA cells (Supplemental Fig. 1 published on The Endocrine Society’s Journals web site at http://mend.endojournals.org) and exhibited apoptotic-like characteristics. Because many mitochondrial proteins are involved in the intrinsic apoptotic pathway, we investigated the role of ERβ in regulating apoptosis. We examined the effect of various apoptosis-inducing agents such as cisplatin, taxol, and etoposide on Calu-6 cells stably expressing ERβ ShRNA constructs (7) (Supplemental Fig. 2). As shown in Fig. 2, A–C, the growth inhibition was enhanced in response to chemotherapeutic agents when ERβ was down-regulated in Calu-6 lung cancer cells. This increased growth inhibition was observed irrespective of estrogen treatment, suggesting a ligand-independent growth-inhibitory function of ERβ. To rule out cell-specific effects, we carried out similar experiments in 201T cells transfected with GFP and ERβ SiRNA oligos (Supplemental Fig. 2). As expected, ERβ-down-regulated 201T cells were more sensitive to etoposide and taxol-induced growth inhibition, when compared with control cells (Fig. 2, D and E). The IC50 values for each of these agents are described in Table 1. In addition, ERβ knockdown cells were also sensitive to UV radiation, another apoptosis-inducing agent, when compared with control cells (Supplemental Fig. 3). Of note, the SiRNA oligos used in Calu-6 cells and SiRNA oligos used in 201T cells targeted different sites of ERβ mRNA and potentially rules out off-target effects that might contribute toward increased sensitivity to chemotherapy agents.
Figure 2.
ERβ ShRNA cells exhibit increased growth inhibition to chemotherapeutic agents. A–C, Calu-6 cells (stably expressing GFP or ERβ ShRNA constructs) and (D and E) 201T cells (transfected with GFP or ERβ SiRNA oligos) were grown in phenol red-free media under estrogen-free conditions and treated with various concentrations of cisplatin, taxol, or etoposide for 2–4 d. Cell growth was measured by Brd-U incorporation. Values are represented as mean (±sd) from five identical wells. Statistical analysis was performed by one-way ANOVA (P < 0.05). All the experiments were performed more than three times, and a representative experiment is shown.
Table 1.
IC50 values of chemotherapeutic agents in control and ERβ down-regulated NSCLC cells
| IC50 values
|
|||
|---|---|---|---|
| Cells | Drug | GFP ShRNA | ERβ ShRNA |
| Calu-6 | Cisplatin | 135 μm | 26 μm |
| Calu-6 | Etoposide | 6.1 μm | 0.62 μm |
| Calu-6 | Taxol | 40 nm | 6.0 nm |
| 201T | Etoposide | 6.3 μm | 88 nm |
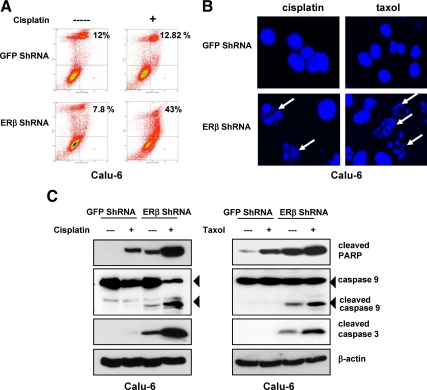
We next examined whether the increased growth inhibition in ERβ down-regulated cells was due to an increase in apoptotic activity. Cisplatin-treated Calu-6 GFP ShRNA and ERβ ShRNA cells were stained with Annexin V/propidium iodide, followed by flow cytometric analysis. Cisplatin treatment increased Annexin V staining 5-fold in ERβ ShRNA cells, when compared with control cells (Fig. 3A). Nuclear staining by Hoechst revealed fragmented and condensed nuclei, characteristic of apoptotic cells, in cisplatin- or taxol-treated Calu-6 ERβ ShRNA cells (Fig. 3B). Further evidence of increased apoptosis in ERβ ShRNA cells was demonstrated by Western blotting with antibodies against cleaved poly(ADP-ribose) polymerase (PARP), caspase-9, and cleaved caspase-3 (Fig. 3C). Taken together, these results suggest that loss of ERβ sensitizes lung cancer cells to apoptosis induction.
Figure 3.
ERβ ShRNA cells exhibit increased apoptosis. A, Calu-6 GFP and ERβ ShRNA cells grown under estrogen-free conditions were treated with cisplatin (100 μm) for 24 h. Cells were stained with Annexin V and propidium iodide and analyzed by flow cytometry. The percentage of Annexin V-positive cells in the two right quadrants is indicated. B, After the indicated treatments, cells were stained with Hoechst, and fragmented nuclei were visualized by confocal microscopy. The fragmented nuclei are denoted by arrows. C, Calu-6 cells were treated with cisplatin (100 μm) or taxol (20 nm) for 24 h, and the presence of cleaved caspase-3/9 and PARP was analyzed by Western blotting.
ERβ interacts with proapoptotic protein Bad
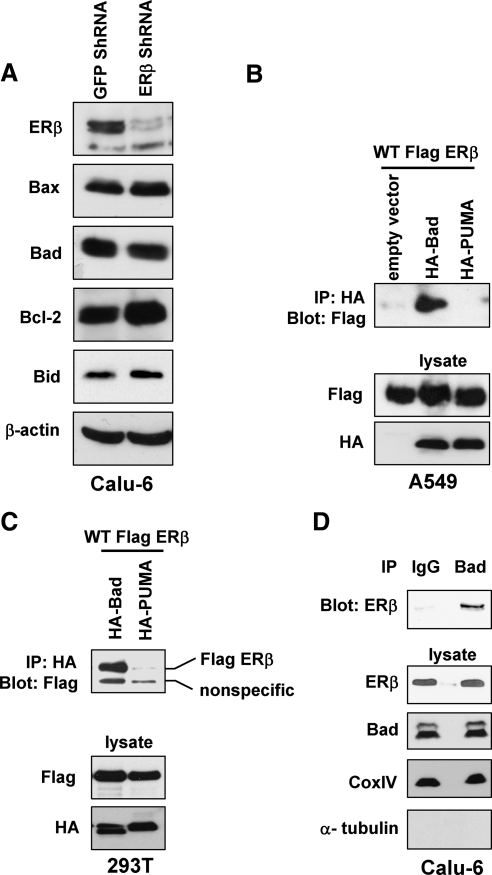
Because ERβ-down-regulated cells exhibited increased apoptosis, we examined whether the expression of various Bcl-2 family members were altered in ERβ ShRNA cells, when compared with control cells. Western blotting analysis revealed no difference in expression levels between Calu-6 GFP ShRNA and ERβ ShRNA cells, including the levels of estrogen-regulated Bcl-2 (Fig. 4A). Next, we hypothesized that ERβ inhibited apoptosis by interacting with proapoptotic proteins that are the regulators of the intrinsic apoptotic pathway. To test this hypothesis, we performed coimmunoprecipitations in A549 cells by transfecting Flag-ERβ along with hemagglutinin (HA)-Bad or HA-PUMA constructs. Antibodies against the HA-epitope efficiently coimmunoprecipitated Flag-ERβ in cells cotransfected with Bad, but not PUMA (Fig. 4B). Similar physical interactions were observed in human embryonic kidney (HEK)293T cells transfected with Flag-ERβ and HA-Bad (Fig. 4C). We next determined whether endogenous ERβ and Bad formed complexes in NSCLC cells. As shown in Fig. 4D, endogenous ERβ was coimmunoprecipitated from the mitochondrial fraction by antibodies against Bad, but not by control IgG.
Figure 4.
ERβ coimmunoprecipitates with Bad. A, Western blotting analysis of various Bcl-2 family members in Calu-6 GFP and ERβ ShRNA cells. B, A549 cells were transfected with Flag-ERβ along with HA-Bad or HA-PUMA constructs. Immunoprecipitations were performed with anti-HA antibodies followed by Western blotting with anti-Flag antibodies. C, Coimmunoprecipitation assays in 293T cells transfected with Flag-ERβ, HA-Bad, and HA-PUMA constructs. D, Endogenous Bad from mitochondrial fraction was immunoprecipitated with control IgG or anti-Bad antibodies and Western blotted with anti-ERβ antibodies. Cox IV and α-tubulin served as controls for mitochondrial and cytosolic fractions, respectively. IP, Immunoprecipitation; WT, wild type.
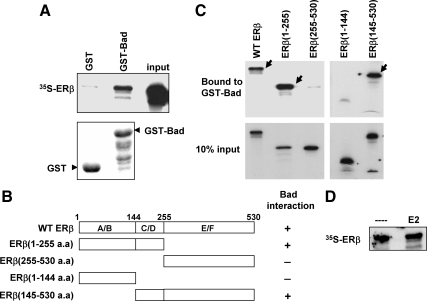
To demonstrate direct interaction between ERβ and Bad, we performed GST pull-down assays. In vitro translated [35S]ERβ interacted with immobilized GST-Bad but not with GST (Fig. 5A). To identify the domain on ERβ responsible for interaction with Bad, we generated several ERβ mutants (Fig. 5B). GST pull-down assays revealed that the C/D domain [145-255 amino acids (a.a.)], which encompasses the DNA-binding domain (DBD) and hinge region of ERβ, was crucial for interaction with GST-Bad (Fig. 5C). Because the DBD is highly conserved between ERα and ERβ, we examined whether Bad also interacted with ERα. As expected, GST pull-down experiments demonstrated a requirement of ERα DBD and hinge region for interactions with Bad (data not shown). Thus, our observations demonstrate the involvement of DBD in protein interactions and are similar to the previously reported studies wherein p53 was shown to make physical contacts with another apoptotic protein, Bcl-XL, via its DBD (16).
Figure 5.
GST pull-down assays demonstrate ERβ and Bad interactions. A (top panel), Immobilized GST or GST-Bad was incubated with 35S-labeled ERβ, and bound proteins were separated by SDS-PAGE and visualized by autoradiography. Bottom panel, Coomassie-stained gel of recombinant GST and GST-Bad proteins. B, Schematic diagram of various ERβ deletion mutants. C, GST pull-down assays between GST-Bad and 35S-labeled ERβ mutants. GST-Bad-bound ERβ proteins are denoted by solid arrows in the upper panel. D, GST pull-down assays between Bad and [35S]ERβ in the presence or absence of 17β-estradiol (E2) (100 nm). WT, Wild type.
We also examined whether the ligand would alter the interactions between ERβ and Bad using in vitro assays. 17β-Estradiol did not affect the binding of ERβ to GST-Bad (Fig. 5D) and is in agreement with our previous results that showed ligand-independent effects of ERβ in regulating apoptotic cell death (Fig. 3). Together, these studies identify a novel interaction between ERβ and proapoptotic protein Bad.
Identification of the ERβ-interacting domain on Bad
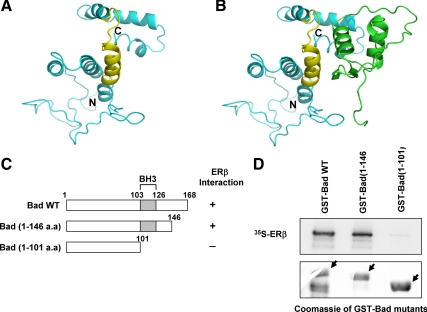
To identify potential ERβ-interacting surfaces on Bad, we first developed structural models for these two proteins (Fig. 6A), followed by protein-protein docking using ClusPro software (17,18). The top-ranked models were analyzed with respect to their binding energy and to predict the residues located at the interface formed by the two proteins in the docked complex. In seven of the top 10 most highly ranked conformations based on energy and number of structures in the cluster (corresponding to ranks 2, 4, 5, 6, 7, 9, 10, and representing 261 of 395 docked conformations), a single binding area on the Bad protein showing contacts with the ERβ DBD was observed. The other three structures were located at the N terminus of Bad, with little contact area at the interface. Given the lack of defined secondary structure area in the N terminus, we did not consider these three models further. The preferred binding pocket is shown in Fig. 6B. The set of Bad residues that contributed to the stabilizing electrostatic and desolvation free energy interactions in this conformation (ranked second) were from the BH3 domain residues (110-Tyr, 112-Arg, 113-Glu, 114-Leu, 115-Arg, 117-Met, 118-Ser, 119-Asp, 120-Glu, 121-Phe, 123-Asp) and the C-terminal helix residues (147-Trp, 150-Val, 159-Leu, 163-Ser, and 164-Ser). The BH3 contribution to the interaction was the largest, with a total of approximately 85% of electrostatic interactions and about 70% of free energy contacts (Supplemental Table 1). This result suggests that the BH3 domain on Bad plays a major role in its interaction with ERβ, whereas a further stabilization may be achieved through interaction with the C-terminal helix. The corresponding residues on the ERβ DBD were 141-Gly, 143-Lys, 144-Arg, 161-Tyr, 170-Lys, 174-Lys, 175-Arg, 178-Gln, and 216-Lys. Interestingly, when these residues were compared with the homologous residues in the ERα crystal structure (PDB identification no. 1HCQ), most of these residues were within 5Å from the DNA, indicating that there is significant overlap between the DNA-binding and Bad-binding interfaces on ER.
Figure 6.
Molecular modeling studies of ERβ-Bad complex. A, Predicted three-dimensional structure of human Bad with the BH3 domain shown in yellow. B, Computer modeling of ERβ DBD-Bad complex with ERβ and Bad shown in green and cyan, respectively. C, Schematic representation of Bad deletion mutants. D (top panel), GST pull-down assays between Bad mutants and 35S-labeled ERβ. Bottom panel, Coomassie-stained gel of recombinant GST-Bad proteins. WT, Wild type.
Based on these predicted interacting domains, three Bad deletion mutants were generated (Fig. 6C) and expressed in bacteria as GST fusion proteins. We then performed GST pull-down assays to identify the region of Bad involved in heterodimerization with ERβ. ERβ interacted with wild-type Bad and Bad (1-146), but not with Bad (1-101), suggesting that the BH3 domain is required for this interaction whereas the C-terminal helix plays a less critical role (Fig. 6D).
ERβ sequesters Bad and inhibits Bad-Bcl-XL and Bad-Bcl-2 interactions
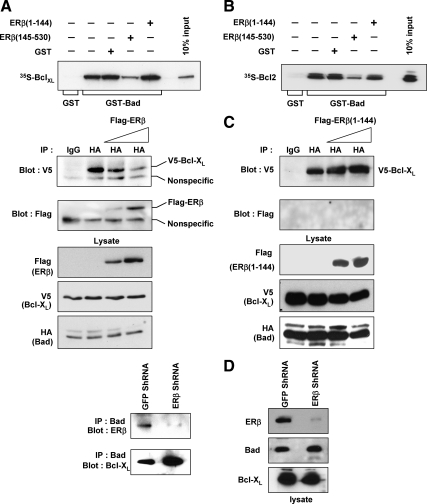
We next investigated the mechanism by which ERβ inhibited Bad function and contributed to inhibition of apoptosis. Bad, like other BH3-only proteins, binds to the antiapoptotic proteins Bcl-2 and Bcl-XL, causing displacement of the proapoptotic molecules Bax and Bak, thus freeing them from the inhibitory complex to initiate caspase activation (for review, see Ref. 19). Because the BH3 domain of Bad was involved in the interaction with ERβ, we hypothesized that ERβ might sequester Bad and inhibit its interaction with Bcl-XL. To examine this, we performed GST pull-down assays by incubating in vitro translated 35S-Bcl-XL with immobilized GST-Bad. As expected, Bcl-XL interacted with GST-Bad but not with GST (Fig. 7A, lanes 2 and 1, respectively). However, when the assay was performed in the presence of recombinant ERβ (145-530 a.a.), the interaction between GST-Bad and Bcl-XL diminished (Fig. 7A, compare lanes 2 and 4). By contrast, addition of recombinant GST protein or the N-terminal ERβ mutant (ERβ 1-144 a.a.) did not alter GST-Bad-Bcl-XL interactions (Fig. 7A, lanes 3 and 5). Similar results were obtained when experiments were performed with GST-Bad and [35S]Bcl-2 (Fig. 7B).
Figure 7.
ERβ inhibits Bad-Bcl-XL and Bad-Bcl-2 interactions. A, 35S-labeled Bcl-XL was incubated with immobilized GST or GST-Bad. Where indicated, these assays were performed in the presence of equal amounts of recombinant proteins GST (lane 3), GST-ERβ (a.a. 145-530) (lane 4), or GST-ERβ (1-144) (lane 5), and bound complexes were resolved on SDS-PAGE and visualized by autoradiography. B, Similar GST pull-down experiments were performed using GST-Bad and [35S]Bcl-2. C (left panel), 293T cells were transfected with Flag-ERβ, HA-Bad, and V5-Bcl-XL constructs. Lysates were immunoprecipitated with anti-HA antibodies and Western blotted with anti-V5 antibodies. The blots were stripped and reprobed with anti-Flag antibodies. C (right panel), Similar immunoprecipitations were performed in 293T cells transfected with Flag-ERβ (1-144), HA-Bad, and V5-Bcl-XL constructs. D (left panel), Endogenous Bad from cytosolic fractions of Calu-6 GFP ShRNA and ERβ ShRNA cells were immunoprecipitated with anti-Bad antibodies, and immune complexes were Western blotted with antibodies against ERβ or Bcl-XL. D (right panel), Western blots of ERβ, Bad, and Bcl-XL using cytosolic lysates. IP, Immunoprecipitation.
We further examined whether overexpression of ERβ inhibited Bad-Bcl-XL interactions in cells. 293T cells were transfected with HA-Bad and V5-Bcl-XL constructs along with increasing amounts of Flag-ERβ, and immunoprecipitation assays were performed. Antibodies against HA-epitope coimmunoprecipitated V5-Bcl-XL efficiently but was significantly reduced in lysates from cells transfected with Flag-ERβ (Fig. 7C, left panel). When the blot was stripped and reprobed with antibodies against Flag, we observed reciprocal increase in Flag-ERβ levels, indicating that ERβ competed with Bcl-XL for binding to Bad. Under similar conditions, mutant Flag-ERβ (1-144 a.a.) failed to dissociate Bad-Bcl-XL interactions (Fig. 7C, right panel). In agreement, endogenous Bad was found predominantly associated with ERβ in GFP ShRNA cells, whereas it was complexed with Bcl-XL in ERβ ShRNA cells (Fig. 7D). Together, these studies demonstrate that ERβ competes with Bcl-XL and Bcl-2 for binding to Bad.
Targeting ERβ to the mitochondria increases cell survival in lung cancer cells
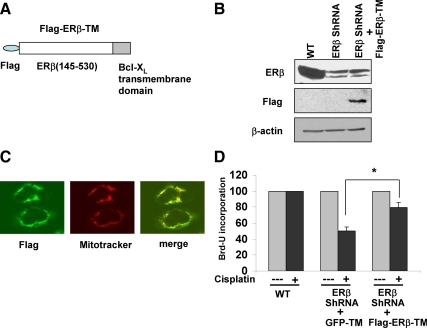
To further demonstrate the cell survival role of ERβ in the mitochondria, we transiently overexpressed mutant ERβ (145-530 a.a.) in the mitochondria as a Flag-tagged protein in ERβ knockdown cells. To target the protein to mitochondria, we fused the 30 amino acid transmembrane (TM) domain of Bcl-XL to the C terminus of Flag-ERβ (145-530 a.a.) and refer to this protein as Flag-ERβ-TM (Fig. 8A). This strategy has previously been employed by other groups to direct exogenous proteins to the mitochondria (16). Because the ERβ ShRNA sequences used are targeted toward N terminus of ERβ, we expected that the expression of Flag-ERβ-TM would not be altered, and thus would serve as a strategy to rescue ERβ ShRNA cell sensitivity toward cisplatin. The expression of Flag-ERβ-TM protein in Calu-6 ERβ knockdown cells was analyzed by Western blotting. As expected, the wild-type cells expressed endogenous ERβ protein whereas the ERβ knockdown cells expressed mutant Flag-ERβ-TM protein (Fig. 8B). The antibodies against ERβ recognized only the endogenous protein but not Flag-ERβ-TM, because the epitope lies in the N-terminal region. Further, the mitochondrial localization of Flag-ERβ-TM was confirmed by confocal microscopy (Fig. 8C).
Figure 8.
ERβ targeted to mitochondria promotes resistance to cisplatin-induced apoptosis. A, Schematic diagram of Flag-ERβ-TM construct. B, Western blotting of Calu-6 wild-type (WT) and ERβ ShRNA cells transfected with Flag-ERβ-TM construct. Lysates were probed with antibodies against ERβ, Flag, or β-actin. C, Confocal microscopy of ERβ ShRNA cells transfected with Flag-ERβ-TM. Cells were stained with antibodies against Flag (green) and with Mitotracker Red. Overlap is seen in yellow. D, Calu-6 wild-type (WT) and ERβ ShRNA cells transiently transfected with GFP-TM or Flag-ERβ-TM constructs were grown in phenol red-free media under estrogen-free conditions and treated with cisplatin (20 μm) for 3 d. Cell growth was measured by Brd-U incorporation. Values are represented as mean (±sd) from five identical wells. Statistical analysis was performed by one-way ANOVA (*, P < 0.05).
We examined whether expression of Flag-ERβ-TM would render the cells resistant to cisplatin treatment, as assessed by bromodeoxyuridine (Brd-U) incorporation. As shown in Fig. 8D, cisplatin-induced growth inhibition was greater in ERβ ShRNA cells transfected with GFP-TM (cells expressing GFP protein targeted to mitochondria and serves as control) when compared with cells expressing Flag-ERβ-TM. Taken together, these results suggest that mitochondrial ERβ promotes cell survival and contributes to resistance to chemotherapy agents in NSCLC cells.
Discussion
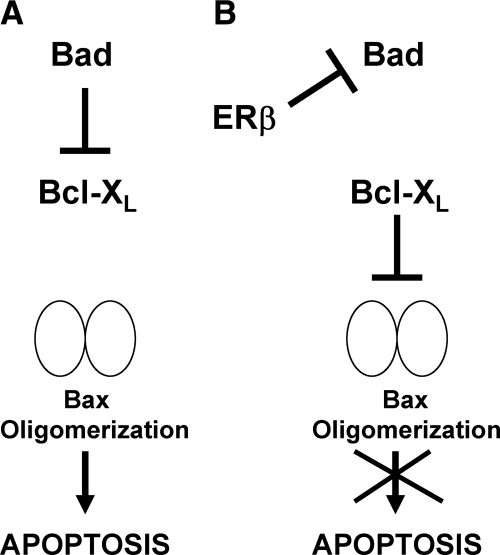
A delicate balance exists between signaling pathways that promote cell growth and cell death to maintain tissue homeostasis. The present study reveals a new cell-survival function of mitochondrial ERβ in lung cancer cells. As shown in the schematic diagram (Fig. 9), we propose that in the absence of ERβ, Bad is able to interact with Bcl-XL (and Bcl-2) to free Bax to oligomerize and initiate apoptosis. However, in the presence of ERβ, Bad is sequestered and prevented from interaction with Bcl-XL, thus inhibiting apoptosis. Thus, loss of ERβ in lung cancer cells results in tilting the balance toward induction of apoptosis, and this is further enhanced by apoptosis-inducing chemotherapy agents. The mitochondrial action of ERβ might contribute toward chemotherapy resistance in NSCLC cells.
Figure 9.
Schematic representation depicting the effects of ERβ knockdown. A, In the absence of ERβ, Bad interacts with Bcl-XL to free Bax to oligomerize and initiate apoptosis. B, In the presence of ERβ, Bad is sequestered and prevented from interaction with Bcl-XL, thus inhibiting apoptosis.
The current work parallels with studies that describe the transcriptional-independent apoptosis-regulatory function of several nuclear proteins in the mitochondria. For example, mitochondrial p53 has been shown to interact with Bcl-XL via its DBD and promote apoptosis (16). Binding of p53 to Bcl-XL induced structural changes in the BH3 domain and enhanced Bcl-XL interaction with BH3-only proteins in an allosteric manner (20). p53 can also directly interact with another proapoptotic protein Bak at the outer mitochondrial membrane and cause oligomerization of Bak and release of cytochrome c from the mitochondria (21). Similarly, the nuclear receptor Nur77 has been demonstrated to interact with Bcl-2 and regulate apoptosis. This interaction altered Bcl-2 conformation and converted it from an antiapoptotic protein to a proapoptotic protein and induced cell death (22).
Our work contrasts with studies done by Ellis Levin’s group (23) who have demonstrated estrogen-dependent function of ERs in the mitochondria of breast cancer cells. By using cell-free mitochondrial studies they have shown that estrogen, probably through ERα and ERβ, up-regulates mitochondrial manganese superoxide dismutase enzyme activity, which in turn reduces formation of reactive oxygen species leading to inhibition of apoptosis. We did not examine the regulation of manganese superoxide dismutase activity in NSCLC cells in this study, and this requires further investigation. Alternatively, the ligand-independent mechanisms of apoptotic regulation also exist in breast cancer cells and might not be unique to lung cancer cells. For example, a similar paradigm exists in breast cancer cells wherein ERα interacts and inhibits proapoptotic protein p53 in a ligand-independent manner (24). Similar ligand-independent functions of ERα and ERβ have been demonstrated in regulating MAPK pathways in osteoblasts (25). Further, knockdown of ERβ in HT22 murine hippocampal cells resulted in a decrease in mitochondrial membrane potential and an increase in cytochrome c oxidase activity, in the absence of estrogen treatment (14). Collapse of mitochondrial membrane potential is one of early indicators of apoptosis, and these observations support a role for ERβ in regulating apoptosis independent of the ligand. Thus, it is possible that extranuclear ERs regulate apoptosis by both ligand-dependent and -independent mechanisms.
Apart from our report, many groups have studied the role of ERs in modulating mitochondrial structure and function, albeit in the context of ligand-dependent function. 17β-Estradiol increased the transcript levels of many mitochondrial respiratory chain complex proteins, which are encoded by both nuclear and mitochondrial DNA (13). Chen et al. (26) have demonstrated binding of both ERα and ERβ to estrogen response element-like sequences present in mitochondrial DNA by gel shift assays. However, it is not established whether mitochondrial genes are regulated directly by transcriptional activation of ERs. Several recent studies have shown the role of nuclear respiratory factor-1 (NRF-1) in mediating this process. Estrogens up-regulated NRF-1 gene through ERα and ERβ, which in turn induced the nuclear encoded mitochondrial transcription factor A (TFAM) (27,28). TFAM then shuttled to the mitochondria to modulate the transcription of mitochondrial DNA.
Our present study is confined only to ERβ, and not to ERα, in lung cancer cells. The presence of ERα in lung cancer cells is confounded by conflicting reports. Although we and others have reported only ERβ expression in lung cancer cells (7,29,30), some studies have reported the expression of both ERα and ERβ (31,32,33). Two groups have demonstrated the presence of truncated ERα transcripts generated due to deletion of exons 3, 4, or 7, by RT-PCR analysis (33,34). The physiological functions of these truncated ERα isoforms is not clear because these proteins lack the essential DNA binding or the C-terminal AF-2 domains (35). In contrast, using real-time PCR analysis, we detected very low ERα mRNA levels in lung cancer cells that were comparable to what is seen in ERα-negative COS-1 cells (7). We conclude that lung cancer cells do not express detectable levels of full-length ERα.
Although the localization of ERβ in the mitochondria has been demonstrated by various groups in different systems, the exact mechanism of this mitochondrial import is not known because a localizing signal sequence has yet to be identified. Chen et al. (9) have predicted a putative mitochondrial targeting sequence in the hinge region of ERβ by computer analysis, but this remains to be validated by biochemical experiments. ERβ belongs to an increasing number of nuclear proteins that are imported into the mitochondria such as CREB, RXRα, PPARγ, GR, AP1, NF-kB, c-Myc, p53, and Nurr 77 (for reviews, see Refs. 36 and 37). However, many of these proteins lack the cleavable N-terminal presequences or the internal signal sequences that are required for recognition by the mitochondrial translocase of outer membrane (TOM) protein complex (38). A consensus motif in these signal sequences that is recognized by Tom20, a subunit of TOM complex, was identified by nuclear magnetic resonance and peptide- library analysis (39,40) and is represented by φXXφφ where φ is a hydrophobic amino acid (such as leucine, valine, phenylalanine, tryptophan, isoleucine, and tyrosine), and X represents any amino acid. Interestingly, ERβ contains three LXXLL motifs, two VXXLL motifs, and one FXXLL motif in the ligand-binding domain. The involvement of these motifs in mitochondrial targeting remains to be investigated. Further, ERβ is shown to colocalize with Tom20 at the mitochondria in HT22 murine hippocampal cells (41). In some proteins, internal cryptic mitochondrial targeting signals are revealed by endoprotease processing. A recent study has identified a cytosolic serine endoprotease involved in mitochondrial targeting of proteins such as CYP1A1, GR, RXRα, and p53 (42). Further studies are warranted to investigate the molecular mechanisms of mitochondrial import of ERβ.
The BH3 domain of proapoptotic proteins is critically involved in making contacts with the antiapoptotic proteins, Bcl-XL and Bcl-2. The three-dimensional structural studies have shown that BH3 sequences in proapoptotic proteins adopt an amphipathic α-helix conformation while making contact with the hydrophobic binding pocket of Bcl-2 family members (43,44). Our studies reveal that the BH3 domain of Bad is involved in interaction with ERβ. Interestingly, the binding interface on ERβ DBD is not hydrophobic, but basic in nature. Further, three of the four conserved hydrophobic residues that constitute the BH3 domain (Leu-114, Met-117, Phe-121) appear to make contact with ERβ. Our modeling studies also predict that Asp-123 of Bad is involved in strong electrostatic interactions with ERβ. In contrast, the same residue was shown to have a destabilizing effect in Bcl-XL-Bad interactions (45). It is possible that the structural determinants for ERβ-Bad are quite different from those of Bcl-XL-Bad interactions and that the interaction of acidic Asp-123 of Bad with the basic interface of ERβ DBD might be critical in stabilizing this complex. We also would like to point out that in our preliminary studies, the DBD and hinge region of ERβ by itself failed to interact with Bad, in GST pull-down assays (data not shown). However, the binding was efficient when expressed along with either the N-terminal A/B domain or the ligand-binding domain (Fig. 5C). Thus, it is not clear whether these regions are required for stabilizing the interactions or whether the GST-ERβ DBD recombinant protein used in our studies was in a suboptimal conformation. Three-dimensional structural studies of the complex would throw more light on these interactions. Together, our work has identified a novel interaction between ERβ and proapoptotic protein Bad. It is tempting to speculate that ERβ regulates apoptosis in mitochondria by binding to multiple BH3-family proteins, given the requirement of the BH3 domain for the interaction. These interactions might serve as a therapeutic target to increase the efficiency of chemotherapy in lung cancer cells.
Materials and Methods
Reagents
We purchased 17β-estradiol, and antibodies against β-actin, α-tubulin, and FLAG epitope from Sigma-Aldrich (St. Louis, MO); polyclonal antibodies against ERβ were purchased from Millipore Corp. (Bedford, MA; catalog no. 05-824); polyclonal antibodies against Bad, cleaved PARP, and cleaved caspase-3 were purchased from Cell Signaling Technology (Danvers, MA); antibodies against V5 epitope and Cox IV, Hoechst, Mitotracker Red CMXRos, and secondary antibody conjugated to Alexa-488 were purchased from Invitrogen Life Technologies (Carlsbad, CA); antibodies against ERβ, Bax, and GFP were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); cisplatin, taxol, and etoposide were purchased from Enzo Life Sciences, Inc. (Farmingdale, NY); antibodies against Bcl-2 was purchased from DAKO (Carpinteria, CA); and antibodies against caspase-9 was purchased from Assay Designs, Inc., (Ann Arbor, MI).
Plasmid constructs
Human ERβ [1-530 amino acids (a.a.)] cDNA with a Flag tag was cloned into pcDNA vector (Invitrogen), and subsequent deletion mutants were generated by PCR. Human Bad and PUMA cDNA constructs with HA tag were cloned into pCEP4 vector (Invitrogen). Human Bcl-XL cDNA was cloned into pcDNA-V5/HisA vector (Invitrogen). Wild-type and mutant GST-Bad cDNA constructs were generated by PCR and cloned into pGEX4T1 vector (Amersham/GE). Flag-ERβ (145-530)-TM was constructed by cloning the ERβ (a.a. 145-530) fragment into pcDNA 3.1 vector (Invitrogen). The Bcl-XL TM domain of 30 amino acids (46) was generated by PCR and cloned downstream of Flag-ERβ (a.a. 145-530).
Cell lines
Calu-6 and 201T are lung adenocarcinoma cell lines (7). A549 is a bronchoalveolar cell line. NSCLC cells were maintained in RPMI containing 10% fetal bovine serum. 293T cells were maintained in DMEM containing 10% fetal bovine serum.
SiRNA oligos
GFP ShRNA and ERβ ShRNA constructs have been described before (7). ERβ was down-regulated in 201T cells by transfecting the following SiRNA oligo combination that targets two different regions 5′-AAGCCCAAATGTGTTGTGG; 5′-CCTTACCTGTAAACAGAGA. Cells were transfected with the above oligos using Oligofectamine (Invitrogen) followed by a second round of transfection after 72 h (15). Cells were treated with chemotherapeutic agents 48 h after the second transfection, and Brd-U incorporation assay was performed.
Coimmunoprecipitations
Mitochondria were isolated using the Mitochondria Isolation Kit from Pierce Chemical Co. (Rockford, IL). Mitochondrial lysates (400 μg) or whole-cell lysates (600 μg) were incubated with control IgG or antibodies against Bad or HA overnight at 4 C. Immune complexes were washed with a buffer containing 50 mm Tris (pH 7.4), 100 mm NaCl, 0.1% Nonidet P40 or 1% CHAPS four times and subjected to Western blotting. Where mentioned, the blot was stripped and reprobed for normalization and loading control.
GST pull-down assays
GST and GST-Bad mutants (5 μg) were immobilized on glutathione agarose beads and incubated with in vitro translated 35S-labeled Bcl-XL, Bcl-2, or ERβ for 30 min on ice in a binding buffer containing 50 mm Tris-HCl (pH 7.4), 100 mm NaCl, and 0.1% Nonidet P40. Protein complexes were washed four times with the same buffer, and bound materials were resolved by SDS-PAGE and visualized by autoradiography. In some experiments, these assays were carried out in the presence of 17β-estradiol (100 nm) or recombinant GST-ERβ (a.a. 1-144) and GST-ERβ (a.a. 145-530) proteins (7.5 μg each).
Cell proliferation assays
NSCLC cells grown in phenol red-free media containing charcoal-stripped serum were seeded onto 96-well plates (5 × 103 cells per well) and treated with various chemotherapeutic agents (cisplatin, taxol, etoposide) for 2–4 d with different concentrations. Cells were incubated 2 h before harvest with Brd-U. Brd-U incorporation was measured colorimetrically using Cell Proliferation ELISA kit from Roche (Indianapolis, IN). Each experiment was performed three times, and data were expressed as the means and standard deviations from five identical wells.
Apoptosis assays
For flow cytometry, Calu-6 cells grown in phenol red-free media were treated with cisplatin (100 μm) for 24 h. Cells were stained with Annexin V-FITC (BD Biosciences, Palo Alto, CA) and propidium iodide, and apoptosis was analyzed by flow cytometry. For nuclear fragmentation, cells grown on coverslips were treated with cisplatin or taxol for 24 h. Cells were fixed in methanol/acetone, stained with Hoechst, and visualized by confocal microscopy. For Western blotting, whole-cell lysates were probed with antibodies against cleaved PARP, cleaved caspase-3, and caspase-9.
Molecular modeling studies of ERβ-Bad complex
ERβ and Bad structural models were generated using homology modeling. The DBDs of ERα and ERβ exhibit 98% sequence homology, and an alignment between both DBDs was generated using ClustalW (47). The Bad structure was modeled based on the crystal structure of the human Bid protein (48) using the alignment procedure described (49). The protein sequences corresponding to human Bad (Q92934) and ERβ (Q92731) were extracted from the Swissprot sequence database. Using the respective sequence alignments and crystal structures, the three-dimensional structural models of the ERβ DBD domain (residues 141-254) and full-length human Bad were built by homology modeling using the MODELLER software (50). The resulting structural models were evaluated using MOLPROBITY (51).
Protein-protein docking was performed on structural models of ERβ and Bad by application of the ClusPro docking software (17,18). The DOT algorithm along with the available default parameters was used in the docking procedure, and conformations with minimum desolvation energy and electrostatic energy were selected. The resulting top 10 docked models were submitted to the FastContact program (52) to analyze the binding energy of the respective conformations and to identify the residues located at the interface between the two proteins in the complex.
Supplementary Material
Footnotes
This work was supported by the Career Development Award from the University of Pittsburgh Specialized Program of Research Excellence (SPORE) in Lung Cancer and by the Core Grant for Vision Research EY08098.
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 21, 2010
Abbreviations: AF, Activation function; a.a., Amino acids; Brd-U, bromodeoxyuridine; DBD, DNA-binding domain; ER, estrogen receptor; GFP, green fluorescent protein; GST, glutathione-S-transferase; HA, hemagglutinin; NSCLC, non-small-cell lung cancer; PARP, poly(ADP-ribose) polymerase; ShRNA, short hairpin RNA; SiRNA, small interfering RNA; 293T, human embryonic kidney (HEK) cells; TM, transmembrane; TOM, translocase of outer membrane.
References
- Ascenzi P, Bocedi A, Marino M 2006 Structure-function relationship of estrogen receptor α and β: impact on human health. Mol Aspects Med 27:299–402 [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA 2003 Estrogen signaling: a subtle balance between ER α and ER β. Mol Interv 3:281–292 [DOI] [PubMed] [Google Scholar]
- Levin ER 2005 Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol 19:1951–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP 1999 The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 140:5566–5578 [DOI] [PubMed] [Google Scholar]
- Cowley SM, Parker MG 1999 A comparison of transcriptional activation by ERα and ERβ. J Steroid Biochem Mol Biol 69:165–175 [DOI] [PubMed] [Google Scholar]
- Pandini G, Genua M, Frasca F, Squatrito S, Vigneri R, Belfiore A 2007 17β-Estradiol up-regulates the insulin-like growth factor receptor through a nongenotropic pathway in prostate cancer cells. Cancer Res 67:8932–8941 [DOI] [PubMed] [Google Scholar]
- Zhang G, Liu X, Farkas AM, Parwani AV, Lathrop KL, Lenzner D, Land SR, Srinivas H 2009 Estrogen receptor β functions through nongenomic mechanisms in lung cancer cells. Mol Endocrinol 23:146–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majidi M, Al-Wadei HA, Takahashi T, Schuller HM 2007 Nongenomic β estrogen receptors enhance β1 adrenergic signaling induced by the nicotine-derived carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in human small airway epithelial cells. Cancer Res 67:6863–6871 [DOI] [PubMed] [Google Scholar]
- Chen JQ, Delannoy M, Cooke C, Yager JD 2004 Mitochondrial localization of ERα and ERβ in human MCF7 cells. Am J Physiol Endocrinol Metab 286:E1011–E1022 [DOI] [PubMed] [Google Scholar]
- Cammarata PR, Chu S, Moor A, Wang Z, Yang SH, Simpkins JW 2004 Subcellular distribution of native estrogen receptor α and β subtypes in cultured human lens epithelial cells. Exp Eye Res 78:861–871 [DOI] [PubMed] [Google Scholar]
- Solakidi S, Psarra AM, Sekeris CE 2005 Differential subcellular distribution of estrogen receptor isoforms: localization of ERα in the nucleoli and ERβ in the mitochondria of human osteosarcoma SaOS-2 and hepatocarcinoma HepG2 cell lines. Biochim Biophys Acta 1745:382–392 [DOI] [PubMed] [Google Scholar]
- Yang SH, Liu R, Perez EJ, Wen Y, Stevens Jr SM, Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW 2004 Mitochondrial localization of estrogen receptor β. Proc Natl Acad Sci USA 101:4130–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JQ, Cammarata PR, Baines CP, Yager JD 2009 Regulation of mitochondrial respiratory chain biogenesis by estrogens/estrogen receptors and physiological, pathological and pharmacological implications. Biochim Biophys Acta 1793:1540–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Sarkar SN, Liu R, Perez EJ, Wang X, Wen Y, Yan LJ, Simpkins JW 2009 Estrogen receptor β as a mitochondrial vulnerability factor. J Biol Chem 284:9540–9548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JM, Dimitrijevich SD, Younes M, Skliris G, Murphy LC, Cammarata PR 2008 Role of wild-type estrogen receptor-β in mitochondrial cytoprotection of cultured normal male and female human lens epithelial cells. Am J Physiol Endocrinol Metab 295:E637–E647 [DOI] [PubMed] [Google Scholar]
- Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM 2003 p53 has a direct apoptogenic role at the mitochondria. Mol Cell 11:577–590 [DOI] [PubMed] [Google Scholar]
- Comeau SR, Gatchell DW, Vajda S, Camacho CJ 2004 ClusPro: an automated docking and discrimination method for the prediction of protein complexes. Bioinformatics 20:45–50 [DOI] [PubMed] [Google Scholar]
- Comeau SR, Gatchell DW, Vajda S, Camacho CJ 2004 ClusPro: a fully automated algorithm for protein-protein docking. Nucleic Acids Res 32:W96–W99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Strasser A 2008 The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9:47–59 [DOI] [PubMed] [Google Scholar]
- Hagn F, Klein C, Demmer O, Marchenko N, Vaseva A, Moll UM, Kessler H 2010 BclxL changes conformation upon binding to wild-type but not mutant p53 DNA binding domain. J Biol Chem 285:3439–3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu JI, Dumont P, Hafey M, Murphy ME, George DL 2004 Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol 6:443–450 [DOI] [PubMed] [Google Scholar]
- Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, Dawson MI, Reed JC, Zhang XK 2004 Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell 116:527–540 [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Wallace DC, Levin ER 2006 Functional estrogen receptors in the mitochondria of breast cancer cells. Mol Biol Cell 17:2125–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Konduri SD, Bansal S, Nayak BK, Rajasekaran SA, Karuppayil SM, Rajasekaran AK, Das GM 2006 Estrogen receptor-α binds p53 tumor suppressor protein directly and represses its function. J Biol Chem 281:9837–9840 [DOI] [PubMed] [Google Scholar]
- Aguirre JI, Plotkin LI, Gortazar AR, Millan MM, O'Brien CA, Manolagas SC, Bellido T 2007 A novel ligand-independent function of the estrogen receptor is essential for osteocyte and osteoblast mechanotransduction. J Biol Chem 282:25501–25508 [DOI] [PubMed] [Google Scholar]
- Chen JQ, Eshete M, Alworth WL, Yager JD 2004 Binding of MCF-7 cell mitochondrial proteins and recombinant human estrogen receptors α and β to human mitochondrial DNA estrogen response elements. J Cell Biochem 93:358–373 [DOI] [PubMed] [Google Scholar]
- Mattingly KA, Ivanova MM, Riggs KA, Wickramasinghe NS, Barch MJ, Klinge CM 2008 Estradiol stimulates transcription of nuclear respiratory factor-1 and increases mitochondrial biogenesis. Mol Endocrinol 22:609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virbasius JV, Scarpulla RC 1994 Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci USA 91:1309–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omoto Y, Kobayashi Y, Nishida K, Tsuchiya E, Eguchi H, Nakagawa K, Ishikawa Y, Yamori T, Iwase H, Fujii Y, Warner M, Gustafsson JA, Hayashi SI 2001 Expression, function, and clinical implications of the estrogen receptor β in human lung cancers. Biochem Biophys Res Commun 285:340–347 [DOI] [PubMed] [Google Scholar]
- Hershberger PA, Vasquez AC, Kanterewicz B, Land S, Siegfried JM, Nichols M 2005 Regulation of endogenous gene expression in human non-small cell lung cancer cells by estrogen receptor ligands. Cancer Res 65:1598–1605 [DOI] [PubMed] [Google Scholar]
- Márquez-Garbán DC, Chen HW, Fishbein MC, Goodglick L, Pietras RJ 2007 Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids 72:135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollerup S, Jørgensen K, Berge G, Haugen A 2002 Expression of estrogen receptors α and β in human lung tissue and cell lines. Lung Cancer 37:153–159 [DOI] [PubMed] [Google Scholar]
- Stabile LP, Davis AL, Gubish CT, Hopkins TM, Luketich JD, Christie N, Finkelstein S, Siegfried JM 2002 Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor α and β and show biological responses to estrogen. Cancer Res 62:2141–2150 [PubMed] [Google Scholar]
- Ivanova MM, Mazhawidza W, Dougherty SM, Klinge CM 2010 Sex differences in estrogen receptor subcellular location and activity in lung adenocarcinoma cells. Am J Respir Cell Mol Biol 42:320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herynk MH, Fuqua SA 2004 Estrogen receptor mutations in human disease. Endocr Rev 25:869–898 [DOI] [PubMed] [Google Scholar]
- Psarra AM, Sekeris CE 2008 Nuclear receptors and other nuclear transcription factors in mitochondria: regulatory molecules in a new environment. Biochim Biophys Acta 1783:1–11 [DOI] [PubMed] [Google Scholar]
- Lee J, Sharma S, Kim J, Ferrante RJ, Ryu H 2008 Mitochondrial nuclear receptors and transcription factors: who’s minding the cell? J Neurosci Res 86:961–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N, Wiedemann N, Meisinger C, Lithgow T 2004 Assembling the mitochondrial outer membrane. Nat Struct Mol Biol 11:1044–1048 [DOI] [PubMed] [Google Scholar]
- Obita T, Muto T, Endo T, Kohda D 2003 Peptide library approach with a disulfide tether to refine the Tom20 recognition motif in mitochondrial presequences. J Mol Biol 328:495–504 [DOI] [PubMed] [Google Scholar]
- Muto T, Obita T, Abe Y, Shodai T, Endo T, Kohda D 2001 NMR identification of the Tom20 binding segment in mitochondrial presequences. J Mol Biol 306:137–143 [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Yang SH, Sarkar SN, Pearce V 2008 Estrogen actions on mitochondria—physiological and pathological implications. Mol Cell Endocrinol 290:51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boopathi E, Srinivasan S, Fang JK, Avadhani NG 2008 Bimodal protein targeting through activation of cryptic mitochondrial targeting signals by an inducible cytosolic endoprotease. Mol Cell 32:32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong SL, Ng SL, Fesik SW 1996 X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature 381:335–341 [DOI] [PubMed] [Google Scholar]
- Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW 1997 Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science 275:983–986 [DOI] [PubMed] [Google Scholar]
- Petros AM, Nettesheim DG, Wang Y, Olejniczak ET, Meadows RP, Mack J, Swift K, Matayoshi ED, Zhang H, Thompson CB, Fesik SW 2000 Rationale for Bcl-xL/Bad peptide complex formation from structure, mutagenesis, and biophysical studies. Protein Sci 9:2528–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talos F, Petrenko O, Mena P, Moll UM 2005 Mitochondrially targeted p53 has tumor suppressor activities in vivo. Cancer Res 65:9971–9981 [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ 1994 CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou JJ, Li H, Salvesen GS, Yuan J, Wagner G 1999 Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell 96:615–624 [DOI] [PubMed] [Google Scholar]
- Yang J 2009 Molecular modeling of human BAD and its interaction with PKAc or PP1c. J Theoret Biol 257:159–169 [DOI] [PubMed] [Google Scholar]
- Fiser A, Sali A 2003 Modeller: generation and refinement of homology-based protein structure models. Methods Enzymol 374:461–491 [DOI] [PubMed] [Google Scholar]
- Davis IW, Leaver-Fay A, Chen VB, Block JN, Kapral GJ, Wang X, Murray LW, Arendall III WB, Snoeyink J, Richardson JS, Richardson DC 2007 MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res 35:W375–W383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champ PC, Camacho CJ 2007 FastContact: a free energy scoring tool for protein-protein complex structures. Nucleic Acids Res 35:W556–W560 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.