Abstract
β-Cell mass expansion is one mechanism by which obese animals compensate for insulin resistance and prevent diabetes. FoxM1 is a transcription factor that can regulate the expression of multiple cell cycle genes and is necessary for the maintenance of adult β-cell mass, β-cell proliferation, and glucose homeostasis. We hypothesized that FoxM1 is up-regulated by nondiabetic obesity and initiates a transcriptional program leading to β-cell proliferation. We performed gene expression analysis on islets from the nondiabetic C57BL/6 Leptinob/ob mouse, the diabetic BTBR Leptinob/ob mouse, and an F2 Leptinob/ob population derived from these strains. We identified obesity-driven coordinated up-regulation of islet Foxm1 and its target genes in the nondiabetic strain, correlating with β-cell mass expansion and proliferation. This up-regulation was absent in the diabetic strain. In the F2 Leptinob/ob population, increased expression of Foxm1 and its target genes segregated with higher insulin and lower glucose levels. We next studied the effects of FOXM1b overexpression on isolated mouse and human islets. We found that FoxM1 stimulated mouse and human β-cell proliferation by activating many cell cycle phases. We asked whether FOXM1 expression is also responsive to obesity in human islets by collecting RNA from human islet donors (body mass index range: 24–51). We found that the expression of FOXM1 and its target genes is positively correlated with body mass index. Our data suggest that β-cell proliferation occurs in adult obese humans in an attempt to expand β-cell mass to compensate for insulin resistance, and that the FoxM1 transcriptional program plays a key role in this process.
FoxM1 plays a central role in transcriptional regulation of β-cell proliferation in response to obesity in mouse and human islets.
Both type 1 and type 2 diabetes result from reduced functional pancreatic β-cell mass. In type 1 diabetes, β-cell mass is lost due to autoimmune destruction. In type 2 diabetes, there is an increased requirement for β-cells due to peripheral insulin resistance, and this demand cannot be met. Patients with type 2 diabetes, or even impaired fasting glucose, have reduced β-cell mass compared with nondiabetic controls (1,2). In nondiabetics, there is a compensatory increase in β-cell mass with obesity (1,3,4). This response is even more robust in rodents, in which β-cell replication is important for increasing β-cell mass (5,6). However, obesity-driven human β-cell proliferation is controversial in nondiabetic patients (1,3). Understanding the mechanisms of β-cell proliferation in response to obesity may enable us to harness these physiological pathways to expand β-cell mass in the setting of diabetes.
We have previously shown that numerous cell cycle genes are coordinately up-regulated in pancreatic islets in response to obesity in nondiabetic C57BL/6 (B6) Leptinob/ob mice (7). Among these genes is Foxm1, a transcription factor that regulates cell cycle progression through transactivation of many critical cell cycle genes (reviewed in Ref. 8).
FoxM1 appears to be a key transcriptional regulator of cell cycle progression in the β-cell. Using a pancreas-specific knockout of Foxm1, Gannon and colleagues (9) previously demonstrated that FoxM1 is necessary for adult β-cell proliferation to maintain β-cell mass and to increase β-cell mass in response to partial pancreatectomy (10) and during pregnancy (11).
FoxM1 regulates all phases of the cell cycle. FoxM1 can regulate the G1/S transition by activating the cyclin-dependent kinases, Cdk4, Cdk6, and Cdk2, by several mechanisms. FoxM1 can stimulate transcriptional up-regulation of Cyclin D (12,13), which binds to and activates Cdk4 and Cdk6. FoxM1 can trigger transcription of Cyclin A (14,15) and Cyclin E (16), which bind to and activate Cdk2. Cdc25a is another FoxM1 target gene, which dephosphorylates and activates Cdk2 (17).
FoxM1 also activates the G2/M transition through effects on Cdk1, the gatekeeper for M-phase entry. It directly induces the expression of Cdk1 (13,14) and its binding partner, Cyclin B (14,18,19). In addition, Cdk1 is activated by the phosphatases Cdc25b (14,15,17) and Cdc25c (13), which are also FoxM1 target genes.
FoxM1 is also necessary for proper mitotic progression. Loss of FoxM1 expression leads to mitotic spindle defects, chromosome missegregation, mitotic delay, and failure of cytokinesis (15,18). FoxM1 stimulates the transcription of many mitotic genes to ensure proper mitosis, including centromere proteins A (Cenpa) (17), B (Cenpb) (17), and F (Cenpf) (18), and the chromosome passenger complex (CPC) genes Survivin (Birc5) (17), Aurora Kinase B (Aurkb) (17), and Polo-like kinase 1 (Plk1) (18,20). Finally, Cdc20, a key component of the anaphase-promoting complex, is FoxM1 dependent (14) and necessary to degrade proteins like Cyclin B to promote mitotic exit.
We set out to investigate the transcriptional regulation of islet proliferation in response to obesity. Our pre vious microarray results identified Foxm1 as a transcription factor that was coordinately regulated with obesity and β-cell proliferation in a mouse model (7). Additionally, Gannon and colleagues (9,10,11) demonstrated that FoxM1 is necessary for β-cell proliferation in response to other physiological stimuli. We first asked: does Foxm1 expression segregate with diabetes phenotypes in a genetically diverse Leptinob/ob population? Then, we determined whether overexpression of FOXM1b is sufficient to drive β-cell replication in mouse and human islets. Finally, we asked whether FOXM1 and its target genes are up-regulated in human islets in response to obesity.
Results
FoxM1 is induced by obesity in diabetes-resistant mice
We previously reported the clinical and gene expression phenotype of the B6 and the BTBR mouse strains as a function of strain, age, and leptin-deficient obesity (via introduction of the Leptinob mutation) (7). Leptin deficiency in the B6 mouse evokes a robust β-cell proliferative response, leading to very high plasma insulin concentrations and protection from diabetes. In contrast, β-cell proliferation is unresponsive to obesity in the BTBR Leptinob/ob mouse, leading to the rapid onset of diabetes (7). In islets, we identified a gene set consisting of 217 highly correlated mRNA transcripts that enriched for cell cycle function. The expression of the islet cell cycle module correlated with islet cell proliferation and showed the same strain-specific response to obesity as direct measurements of islet cell proliferation: increasing in obese B6, but not in obese BTBR mice. Embedded within this module were Foxm1 and many of its transcriptional targets (7).
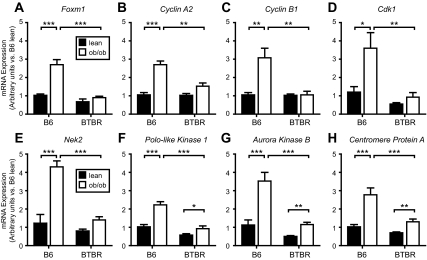
To confirm these microarray results, we performed quantitative RT-PCR analysis in islets from 10-wk-old lean and obese B6 and BTBR mice. Foxm1 mRNA increased 2.6-fold in response to obesity in B6, but not in BTBR mice (Fig. 1A). Additionally, Foxm1 mRNA was 3.0-fold more abundant in B6 Leptinob/ob islets than in BTBR Leptinob/ob islets (Fig. 1A).
Figure 1.
Foxm1 and its target genes are up-regulated in obese nondiabetic mouse islets but not obese diabetic islets. Quantitative RT-PCR analysis of lean (solid bars) and obese (open bars), C57BL/6 (B6) (nondiabetic) and BTBR (diabetic), 10 wk-old islets. Cycle threshold (Ct) values were normalized to β-actin to yield Delta Ct (ΔCt) values. B6 lean ΔCt values were averaged for each gene, and fold changes were calculated vs. average B6 lean ΔCt values. Comparisons were made by one-way ANOVA followed by Bonferroni-corrected t tests. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (n = 5–7 for each group).
The FoxM1 transcriptional targets, Cyclin A2 (14,15), Cyclin B1 (14,19), Cdk1 (14), and Nek2 (15,18,21), demonstrated a strain-specific response to obesity. All four mRNAs increased more than 2.6-fold in islets from B6 Leptinob/ob vs. islets from lean mice (Fig. 1, B–E), but failed to be induced by obesity in the BTBR strain. Similarly, the FoxM1 target gene Survivin (Birc5) (17) displayed an identical expression pattern in the same cohort of mice (22).
Three additional FoxM1 transcriptional targets, Plk1, Aurkb, and Cenpa, showed a similar, but not identical, response to obesity (17). These three genes increased in B6 Leptinob/ob vs. B6 lean islets, respectively (Fig. 1, F–H), but in the BTBR islets, the up-regulation in response to obesity was blunted by 25–35%. All three mRNAs were expressed at more than 2.0-fold higher levels in islets from nondiabetic B6 Leptinob/ob vs. islets from diabetic BTBR Leptinob/ob mice (Fig. 1, F–H).
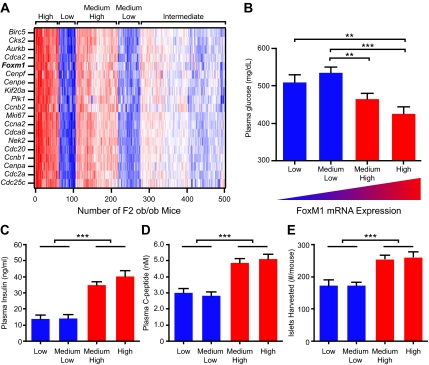
To investigate the relationship between islet Foxm1 expression and plasma glucose and insulin, we surveyed a population of 499 F2 B6:BTBR Leptinob/ob mice (at 10 wk of age). Islet gene expression in these animals was examined using microarray. These mice represent a genetically diverse mixture of alleles from the two founder strains; essentially, we are shuffling the two genomes and asking whether the relationship between Foxm1 expression and diabetes-related traits remain correlated across this population. We performed hierarchical unsupervised clustering of the 499 F2 animals based upon the islet expression of Foxm1, its known target genes, and several additional cell cycle transcripts, the expression of which is highly correlated with that of Foxm1. We identified four groups of mice with tightly correlated expression of Foxm1 and its targets (Fig. 2A). The animals were categorized into four groups based on their Foxm1 transcript expression level: high (n = 62), medium high (n = 112), medium low (n = 59), and low (n = 44). The remaining 222 mice demonstrated an intermediate expression of Foxm1 and its target genes. There was an average 2.78-fold difference in expression levels between the high and low expression groups for all genes. Raw expression data are available as Supplemental Data published on The Endocrine Society’s Journals web site at http://mend.endojournals.org. We next compared the clinical phenotypes of the F2 mice that were clustered according to Foxm1 expression.
Figure 2.
The expression of Foxm1 and its target genes can predict metabolic phenotypes in F2 Leptinob/ob mice. A, Unsupervised clustering of 499 F2 Leptinob/ob mice based upon the transcript levels of Foxm1 and its target genes. Red indicates increased transcript levels, and blue indicates decreased mRNA levels. Four groups were identified from left to right. High expressers, low expressers, medium-high expressers, and medium-low expressers. Plasma glucose (panel B), plasma insulin (panel C), and plasma C-peptide levels (panel D) of the four identified groups at 10 wk of age after a 4-h fast. E, Total number of islets isolated at 10 wk of age. Comparisons were made by one-way ANOVA followed by Bonferroni-corrected t tests. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The high Foxm1 expression groups demonstrated an improved metabolic phenotype when compared with the low expression groups (Fig. 2, B–D). The expression of Foxm1 across the groups was negatively correlated with fasting plasma glucose levels; the high and low Foxm1 expression groups differed in fasting plasma glucose by 73 mg/dl (Fig. 2B). Fasting plasma insulin was positively correlated with Foxm1 across the groups. The low and high expression groups had a nearly 3-fold difference in fasting plasma insulin (Fig. 2C). Similarly, fasting plasma C-peptide measurements were more than 1.5-fold higher in the high Foxm1 expression groups than in the low expression groups (Fig. 2D). Because all islets were used for gene expression analysis, we were unable to directly measure β-cell mass and instead used the number of islets isolated from each individual as a rough estimate of islet mass. The low Foxm1 expression groups each yielded an average of 174 islets per mouse, whereas the medium high and high Foxm1 expression groups yielded 255 and 261 islets, respectively (Fig. 2E). These groups of mice demonstrated an intermediate diabetogenic phenotype compared with the parental strains (complete data available in Ref. 7), which is expected for a segregating F2 population. Overall, the expression level of Foxm1 and its target genes segregated with improved glucose levels, increased insulin production, and increased islet number in our F2 Leptinob/ob mice.
FoxM1 is sufficient to stimulate β-cell proliferation in isolated mouse and human islets
Because Foxm1 expression is only up-regulated in islets from the relatively diabetes-resistant B6 obese mice and is necessary for β-cell proliferation (9,10), we hypothesized that FoxM1 is sufficient to drive β-cell replication. We tested this hypothesis by overexpressing FoxM1 in mouse and human islets. We used an adenovirus that drives human FOXM1b expression via the cytomegalovirus (CMV) promoter (AdCMV-FOXM1b) (14). As expected, AdCMV-FOXM1b increased FOXM1b mRNA and protein in mouse and human islets (Supplemental Fig. 1).
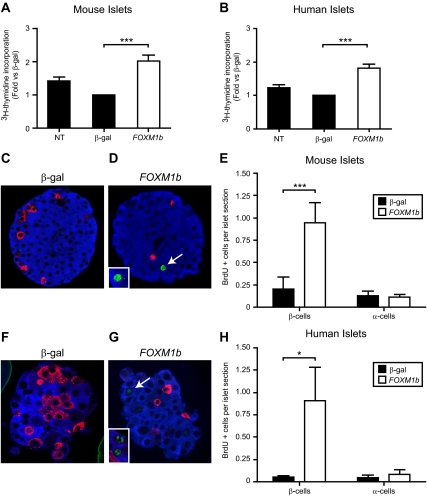
We tested the effects of FOXM1b overexpression on mouse and human islet cell proliferation by measuring [3H]thymidine incorporation into DNA. In BTBR islets, AdCMV-FOXM1b increased [3H]thymidine incorporation into islet DNA 2.0-fold vs. AdCMV-β-gal control islets (Fig. 3A). In human islets, FOXM1b overexpression triggered a 1.8-fold increase in [3H]thymidine incorporation into islet cell DNA (Fig. 3B).
Figure 3.
Overexpression of FOXM1b in mouse and human islets predominantly stimulates β-cell proliferation. [3H]thymidine incorporation assays in mouse (panel A, n = 12) and human (panel B, n = 10) islets. NT, No treatment. Comparisons were made by repeated-measures ANOVA followed by Bonferroni-corrected paired t tests. C–H, BrdU incorporation assays in mouse (C–E) and human (F–H) islets. Representative images of AdCMV-β-gal (solid bars) and AdCMV-FOXM1b (open bars) treated mouse (C and D) and human (F and G) islets are shown. A close up image of other representative BrdU-positive cells is shown within the white box in D and G. Insulin is blue, glucagon is red, and BrdU is green. Quantitation of BrdU positive β-cells and α-cells in mouse (panel E, n = 5) and human (panel H, n = 4) islets. Comparisons were made by repeated-measures ANOVA followed by Bonferroni-corrected paired t tests. *, P < 0.05; **, P < 0.01; ***, P < 0.001. White arrows indicate BrdU-positive β-cells.
To determine which cell types were induced to divide by FOXM1b overexpression, we measured 5-bromo-2′-deoxyuridine (BrdU) incorporation into α- and β-cell nuclei. Overexpression of FOXM1b in isolated mouse and human islets elicited a 4.7-fold increase in BrdU incorporation into DNA in mouse β-cells (Fig. 3E) and 16.3-fold increase in human β-cells (Fig. 3H). Despite the high expression levels driven by the CMV promoter and the high proportion of α-cells present in human islets, AdCMV-FOXM1b did not increase BrdU incorporation into mouse or human α-cell DNA (Fig. 3, E and H). In short, FOXM1b overexpression in mouse and human islets predominantly stimulates β-cell replication.
FoxM1-stimulated β-cell proliferation does not diminish insulin secretion
Stimulation of β-cell proliferation in culture tends to diminish β-cell function (23). We tested whether FoxM1-stimulated β-cell proliferation affects β-cell function by measuring glucose-stimulated insulin secretion. In mouse islets, FOXM1b overexpression had no effect on insulin secretion in response to low or high glucose concentrations when compared with AdCMV-β-gal-treated islets (Supplemental Fig. 2, A and B). Both adenoviral treatments, however, reduced stimulated insulin secretion when compared with untreated islets (Supplemental Fig. 2B, P < 0.05).
As in mouse islets, the insulin secretion in response to glucose was equal between AdCMV-β-gal and AdCMV-FOXM1b-treated human islets (Supplemental Fig. 2D). However, any adenoviral treatment led to diminished total insulin secretion at both basal and stimulated glucose conditions (Supplemental Fig. 2C). In summary, no significant differences were detected in any parameter of glucose-stimulated insulin secretion when comparing FoxM1 to β-gal overexpression.
FoxM1 activates multiple phases of the cell cycle
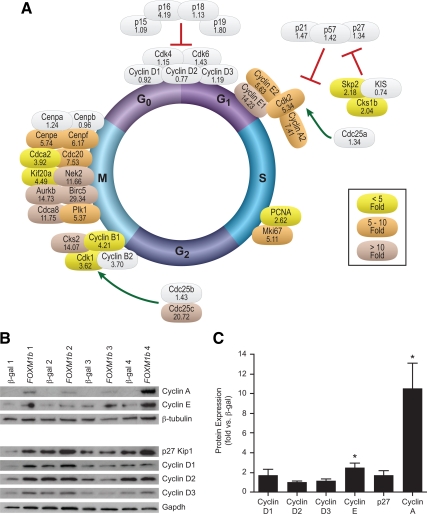
Overexpression of FoxM1 activates the G1/S transition of the cell cycle. Despite the many studies demonstrating a role for Cyclin D in β-cell proliferation (reviewed in Ref. 24), the mRNA and protein expression of D-type Cyclins were not induced by FOXM1b overexpression in mouse or human islets (Fig. 4 and Fig. 5).
Figure 4.
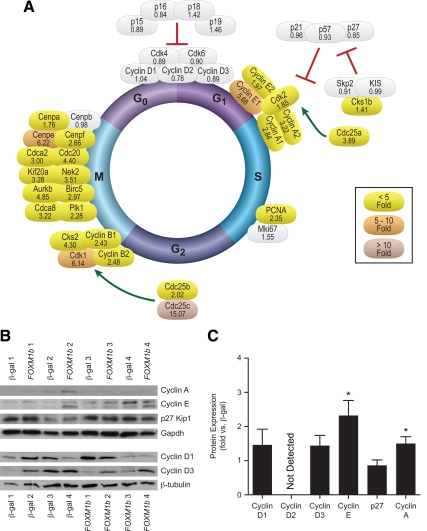
Overexpression of FOXM1b stimulates all phases of the cell cycle in mouse islets. Quantitative RT-PCR analysis of Cyclin, Cdk, and other cell cycle molecule mRNA abundance. Ct values were normalized to β-actin to yield ΔCt values. Fold changes for each mouse experiment were determined and averaged (n = 4). Gray shading indicates nonsignificant changes. Colored shading indicates at least P < 0.05. Fold changes are given beneath the gene name. Mean se and exact P values can be found in Supplemental Table 5. B and C, Western blot analyis for G1/S proteins in mouse islets. B, Representative Western blots. C, Quantitation of Western blots. Densitometry values were normalized to β-tubulin or Gapdh. Fold changes were determined for each experiment and averaged (n = 4–6). Comparisons were made by Student’s paired t tests. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Figure 5.
Overexpression of FOXM1b stimulates many phases of the cell cycle in human islets. Quantitative RT-PCR analysis of CYCLIN, CDK, and other cell cycle molecule mRNA abundance. Ct values were normalized to β-actin to yield ΔCt values. Fold changes for each human preparation were determined and averaged (n = 4–5). Gray shading indicates nonsignificant changes. Colored shading indicates at least P < 0.05. Fold changes are given under the gene name. Mean se and exact P values can be found in Supplemental Table 6. B and C, Western blot analyis for G1/S proteins in human islets. B, Representative western blots. C, Quantitation of Western blots. Densitometry values were normalized to β-tubulin or Gapdh. Fold changes were determined for each experiment and averaged (n = 6–11). Comparisons were made by Student’s paired t-tests. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Instead, FoxM1 stimulated the G1/S transition by activating Cdk2. FOXM1b overexpression increased the mRNA abundance of Cyclin E by more than 5-fold in mouse (Fig. 4A) and 2-fold in human islets (Fig. 5A). At the protein level, we confirmed a more than 2-fold up-regulation of Cyclin E in mouse (Fig. 4C) and human islets (Fig. 5C) by Western blot analysis. FOXM1b overexpression also increased Cyclin A mRNA 7.4-fold in mouse islets (Fig. 4A) and more than 2-fold in human islets (Fig. 5A). Similarly, we detected a more than 10-fold and 1.5-fold increase in Cyclin A protein in mouse and human islets, respectively (Figs. 4C and 5C). Cdk2 mRNA abundance was also elevated in mouse and human islets (Figs. 4A and 5A). Additionally, we found increased mRNA expression of CDC25A in human islets (Fig. 4A). Therefore, in islets, FoxM1 up-regulates Cdk2, its binding partners Cyclin E and Cyclin A, and its activator Cdc25a to progress through the G1/S transition of the cell cycle.
FOXM1b overexpression also activates the G2/M transition. Overexpression of FOXM1b triggers up-regulation of Cyclin B mRNA in mouse and human islets (Figs. 4A and 5A). AdCMV-FOXM1b treatment additionally increased Cdk1 mRNA abundance (Figs. 4A and 5A). FOXM1b overexpression also up-regulated the mRNA levels of the Cdk1 phosphatases CDC25B (human islets only, Fig. 5A) and Cdc25c more than 15-fold in mouse and human islets (Figs. 4A and 5A). Therefore, similar to the G1/S transition, FOXM1b overexpression activated the G2/M transition by increasing Cdk1 expression, the expression of its Cyclin-binding partners, and its Cdc25 activators.
AdCMV-FOXM1b stimulated the expression of many transcripts encoding the mitotic machinery. This includes the chromosome passenger complex (CPC), which is necessary for proper centrosome function in mitosis and contains many known FoxM1 target genes. FOXM1b overexpression increased the mRNA abundance of CPC genes Survivin (Birc5), Aurkb, Plk1, and Cdca8 in mouse and human islets (Figs. 4A and 5A). Taken together, our results demonstrate that FoxM1 stimulates S-phase entry (independent of Cyclin D/Cdk4/Cdk6 complex up-regulation), transition through G2 to M-phase, and an increase in the M-phase machinery to ensure proper and complete islet cell division.
FoxM1 is up-regulated in obese nondiabetic human islet donors
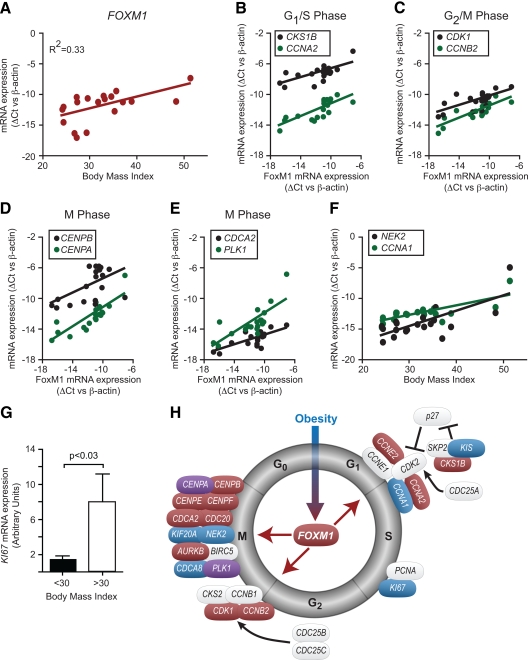
To determine whether FOXM1 and its targets are responsive to obesity in human islets as they are in mouse islets, we obtained a panel of 19 human islet RNA samples. The human islet donors did not have diabetes, spanned a body mass index (BMI) range of 24–51, were aged 27–60 yr, and comprised eight males and 11 females. We performed quantitative RT-PCR analysis to measure the expression of FOXM1. Using a multiple regression model that tests for BMI, age, and sex as covariate adjustments, we found a significant positive correlation between BMI and FOXM1 transcript levels (Fig. 6A; P = 0.01). The coefficient of determination was 0.33, suggesting that one third of the FOXM1 variance can be explained by BMI alone (Fig. 6A). When simply comparing islets from obese (BMI > 30) to nonobese (BMI < 30) human donors, we also found an average 7.4-fold (P = 0.01) increase in FOXM1 mRNA expression. We also detected an average 3.8-fold (P = 0.028) increase in KI67 expression in the obese donors (Fig. 6G), suggesting increased β-cell replication.
Figure 6.
FOXM1 and its target genes are up-regulated in obese nondiabetic human islets. Quantitative RT-PCR analysis of 19 human islet preparations over a range of BMI (24–51), age (27–60 yr), and sex (eight male, 11 female). A, Correlation between BMI and FOXM1 mRNA expression (P = 0.01). B–E, Correlation between FOXM1 and various target genes at various cell cycle stages (P < 0.05 for all). F, Correlation of CYCLIN A1 and NEK2 to BMI (P < 0.05). G, KI67 mRNA expression (P = 0.028). Associations were tested using a multiple regression model with BMI, FOXM1, age, and sex as covariate adjustments. P values for all comparisons can be found in Supplemental Table 1. H, Model for the regulation of islet cell cycle genes by BMI. Genes in blue are correlated to BMI or significantly induced by BMI. Genes in red are correlated to FOXM1. Genes in purple are correlated to both BMI and FOXM1. Genes in gray were not correlated to BMI or FOXM1 expression.
The expression of islet Foxm1 and its target genes show coordinate regulation in response to obesity in mice (Figs. 1 and 2). We therefore performed quantitative RT-PCR for FoxM1 transcriptional targets in our human islet panel. We analyzed the data using a multiple regression model as before but additionally tested for associations with FOXM1 itself. We found that G1/S transition regulators CYCLIN A2, CYCLIN E2, and CKS1B were positively correlated with FOXM1 mRNA levels (Fig. 6, B and H; P < 0.05 for all). The G2/M phase regulators CYCLIN B2 (P < 0.001) and CDK1 (P < 0.05) also showed a positive correlation with FOXM1 mRNA abundance (Fig. 6C). Multiple members of the mitotic machinery were positively correlated with FOXM1 expression in human islets. For example, centromere protein mRNAs CENPA (P < 0.001), CENPB (P < 0.05), CENPE (P = 0.01), and CENPF (P < 0.001) displayed positive correlations with FOXM1 transcript levels (Fig. 6, D and H). Interestingly, CENPA (P = 0.01) and PLK1 (P < 0.05) also demonstrated significant correlations with BMI, even after adjusting for FOXM1 levels (Fig. 6H), suggesting multiple layers of transcriptional regulation for these genes. Very few of the genes we examined showed a significant correlation with age or sex (Supplemental Table 1).
A couple of known FOXM1 target genes were positively correlated with BMI but not with FOXM1 in human islets. The S-phase regulator CYCLIN A1 (P < 0.01) and the mitotic kinase NEK2 (P < 0.05) displayed positive correlations with BMI, independent of FOXM1 (Fig. 6F).
We found that the majority of cell cycle regulators that correlated with BMI did so through their correlation with FOXM1 (colored red in Fig. 6H). Because we used a multiple regression model with BMI and FOXM1 as covariates, a statistical correlation with FOXM1 and not BMI suggests a model wherein obesity increases FOXM1, and FoxM1, in turn, stimulates expression of the downstream gene. In contrast, if a gene correlates with BMI independently of FOXM1 (like CYCLIN A1; colored blue in Fig. 6H), then we infer that obesity regulates CYCLIN A1 through a different mechanism than via induction of FOXM1 expression.
Based on these data, we propose the following model of islet cell cycle gene expression in human pancreatic islets (Fig. 6H). Obesity, as an external stimulus, leads to the up-regulation of FOXM1 and multiple cell cycle transcripts, including KI67. Increased FoxM1 then increases the abundance of G1/S (CKS1B, CYCLIN A2, CYCLIN E2), G2/M (CYCLIN B2, CDK1), and M-phase transcripts (Centromere proteins, CDC20, CDCA2, and PLK1). Ultimately, the end result is cell division.
Our results in Figs. 3 and 5 demonstrate that overexpression of FOXM1b triggers human β-cell proliferation by activating multiple phases of the cell cycle. Our data in Fig. 6 demonstrate that FOXM1 expression correlates with obesity. Furthermore, its cadre of target genes from many phases of the cell cycle correlate with obesity through FOXM1. We propose that obesity may stimulate β-cell replication by activating the FoxM1 transcriptional response in human islets as it does in mouse islets.
Discussion
Insufficient insulin production and β-cell mass are key contributors to type 1 and type 2 diabetes (25,26,27). Expansion of β-cell mass is one way that an obese individual may generate an adequate insulin supply to meet the demands imposed by insulin resistance. We now identify FoxM1 as a transcriptional regulator of adaptive proliferation in response to obesity in the mouse. We further demonstrate that overexpression of FOXM1b in isolated islets stimulates mouse and human β-cell proliferation by activating multiple stages of the cell cycle. Lastly, we show, in nondiabetic human pancreatic islets, that obesity induces the expression of FOXM1 and its cell cycle-regulated targets. Harnessing these mechanisms and stimulating β-cell replication, either ex vivo before transplantation or in vivo to compensate for insulin resistance, may provide therapeutic interventions to treat patients with either form of diabetes (23).
The discovery of mitogenic transcriptional regulators in human β-cells has been difficult because the β-cell mitogens and pathways that are effective in rodent islets are often ineffective in human islets. In fact, only three transcription factors have been shown to stimulate human β-cell replication to date. Adenoviral overexpression studies show that hamster Nkx6.1 (28), mouse Pax4 (29), and human p8 (30) are sufficient to drive human islet cell replication in vitro. FoxM1 can now be added to this short list. We show that FOXM1b overexpression increases β-cell proliferation (Fig. 3) by stimulating multiple cell-cycle phases (Fig. 5), suggesting complete cell division rather than DNA damage repair. We also show a preferential effect for β-cells without diminishing insulin-secretory function (Fig. 3 and Supplemental Fig. 2).
FoxM1 overexpression triggers β-cell proliferation by activating multiple phases of the cell cycle
FoxM1 activates islet G1 progression independently of Cyclin D and Cdk4/6 activity. Previous reports on the G1 phase in the β-cell demonstrate that D-type Cyclins and their activation of Cdk4 and Cdk6 are critical for G0 exit and stimulation of β-cell replication. This occurs by Cdk4- and/or Cdk6-dependent phosphorylation of the pocket proteins (Retinoblastoma protein, p107, p130) and derepression of E2F transcription factors (reviewed in Ref. 24). Recent work demonstrates that Cyclin D1 and Cdk6 are sufficient to drive human β-cell proliferation in vitro (31). FoxM1 can increase Cyclin D1 and D2 mRNA expression in liver and in endothelial cells, respectively (12,13). In islets, however, FOXM1b overexpression has no effect upon Cyclin D, Cdk4, or Cdk6 expression (Figs. 4 and 5). Similarly, Gannon and colleagues (10) found no changes in Cyclin D2 mRNA levels in islets after partial pancreatectomy, a model wherein FoxM1 stimulates β-cell proliferation. These data suggest a tissue specificity to the transcriptional program activated by FoxM1 with respect to D-type Cyclins and a lack of reliance for FoxM1 upon increased Cdk4 or Cdk6 activity for islet cell cycle progression.
Instead, FoxM1 activates G1 progression and S-phase entry by increasing Cdk2 activity. Classically, Cdk2 hyperphosphorylates pocket proteins to further prevent them from inhibiting the E2F transcription factors (reviewed in Ref. 24). In the β-cell, it has been previously shown that activation of Cdk2, via overexpression of Cyclin E (28) or Cyclin A (32), is sufficient to drive β-cell proliferation. In our studies, FoxM1 increased Cdk2 activity by up-regulating Cyclin A, Cyclin E, Cdk2, and Cdc25a expression (Figs. 4 and 5). Therefore, FoxM1 activates Cdk2, which is sufficient to phosphorylate the pocket proteins and activate E2F transcription factors to trigger β-cell proliferation in the absence of increased Cdk4 or Cdk6 activity.
FoxM1 stimulates the G2/M transition and mitosis via similar mechanisms as other cell types. FOXM1b overexpression stimulated M-phase entry by enhancing Cdk1 activity via increased expression of Cyclin B, Cdk1, and Cdc25 phosphatases (Figs. 4 and 5). FoxM1 also stimulated proper mitotic progression by up-regulating M-phase machinery like the CPC (Figs. 4 and 5). All of these genes are well documented to be regulated by FoxM1, as previously discussed (8).
In addition to confirming many known FoxM1 transcriptional targets, we have discovered new FoxM1-regulated genes. In the G1/S transition, we found up-regulation of Cdk2 gene expression in islets overexpressing FOXM1b. In the G2/M transition, we found increased expression of Cks2, which was recently reported to be important for Cdk1 activity (33). Finally, we found that Cdca8 (Borealin, part of the CPC), Cenpe, and Cdca2 (an anaphase factor) were up-regulated by FOXM1b to ensure proper mitotic progression (Figs. 4 and 5). Additional evidence for FoxM1 regulation of these transcripts came from our mouse and human in vivo studies. All five of these genes were found to correlate very highly with Foxm1 expression in islets from lean and obese B6 and BTBR mice (data not shown). Cks2, Cdca8, Cenpe, and Cdca2 were also highly correlated with Foxm1 expression in islets from our F2 sample (Fig. 2). In addition, CENPE and CDCA2 gene expression positively correlated with FOXM1 expression in islets from obese nondiabetic human subjects (Fig. 6G). Therefore, we propose these five genes as new FoxM1-regulated transcripts.
A role for FoxM1 in islet cell proliferation in vivo
Whereas the role of FoxM1 and other transcription factors has been elucidated in various models of β-cell proliferation, little is known about the transcriptional regulation of obesity-driven islet cell proliferation (9,10,34,35). Our data suggest that FoxM1 is a key regulator of this process. In diabetes-resistant B6 Leptinob/ob mice, Foxm1 and its targets are up-regulated (Fig. 1) and correlate with β-cell proliferation and mass (7). Islet Foxm1 expression fails to increase with obesity in the BTBR strain (Fig. 1), in which there is reduced β-cell mass, proliferation, and diabetes (7).
There are many genetic and phenotypic differences between any two mouse strains. One way to test for a causal relationship between particular phenotypes is to generate a segregating population from two founder strains and ask whether particular phenotypes cosegregate. When they do, it is often because they share a common cause. To determine whether Foxm1 expression might be causally associated with diabetes-related traits, we screened an F2 sample derived from B6 and BTBR Leptinob/ob mice. This sample has a 10- and 50-fold range of glucose and insulin levels, respectively, giving us a large amount of phenotypic variance within which to test our hypothesis. In this sample, we found that the mRNA abundance of Foxm1 and many of its classic target genes positively correlated with fasting plasma insulin, C-peptide, crude islet mass, and improved fasting plasma glucose (Fig. 2). These data suggest that obesity-stimulated expression of Foxm1 is a protective measure to expand β-cell mass and prevent metabolic decompensation.
To determine whether this obesity-driven FoxM1 expression could independently stimulate β-cell proliferation, we overexpressed FOXM1b in isolated islets. We found that FoxM1 is sufficient to increase β-cell replication (Fig. 3). We even found this effect in BTBR islets, which fail to up-regulate Foxm1 and proliferate in vivo in response to obesity, suggesting that these islets are not inherently resistant to proliferative stimuli. Our data complement those of Gannon and colleagues (9), who demonstrate that increased Foxm1 expression is necessary for adult β-cell proliferation and stimulated β-cell proliferation secondary to partial pancreatectomy (10) and pregnancy (11). We add that increased expression of Foxm1 and its transcriptional program correlate with β-cell proliferation in response to obesity in mice. In contrast, a transgenic mouse ubiquitously overexpressing human FOXM1b did not show a statistically significant increase in β-cell mass in response to partial pancreatectomy, although a trend was apparent, proliferation was not directly measured, and few animals were examined (10).
The role of β-cell proliferation is not as clearly defined in humans as it is in the mouse. Replication of β-cells has been observed in human islets in the setting of new-onset type 1 diabetes and in islets with close proximity to gastrinoma tumors (36,37). Additionally, genome-wide association studies in type 2 diabetes identify single nucleotide polymorphisms near multiple cell cycle-related genes (38,39,40,41), suggesting the importance of β-cell proliferation in type 2 diabetes pathogenesis. Butler and colleagues (1) initially examined the mechanisms of β-cell mass regulation in autopsy specimens derived from lean and obese type 2 diabetics. They presented, as controls, data from lean and obese nondiabetic subjects, in whom no significant difference in Ki67 staining of β-cell nuclei was observed (1). On the basis of these data, it has been widely inferred that in humans, unlike rodents, β-cells do not replicate in response to obesity. Recently, Rosenberg and colleagues (3) performed a similar autopsy study emphasizing new-onset diabetes in their cohort. Increased obesity-driven β-cell proliferation was detected in nondiabetic donors, but not diabetic donors (3). This study not only detects obesity-stimulated human β-cell proliferation, but suggests that lack of proliferation could be causal for reduced β-cell mass and diabetes pathogenesis.
Here, we demonstrate that human islets show increased expression of FOXM1 with obesity (Fig. 6A; P = 0.01). The expression of many FoxM1 target genes is positively correlated with BMI through FOXM1 expression (Fig. 6, B–F). These target genes act at multiple phases of the cell cycle and have been shown to be transcriptionally up-regulated in proliferating cells (42). Accordingly, the proliferative marker KI67 is up-regulated 3.8-fold by obesity in human islets (Fig. 6G). We also demonstrate that FoxM1 is sufficient to preferentially stimulate β-cell proliferation (Fig. 3) and up-regulates these same target genes in isolated human islets (Fig. 4). Our data suggest that the FoxM1 transcriptional program participates in human β-cell proliferation in response to obesity in vivo.
Our study and the previously published work on human β-cell proliferation in response to obesity used different methodologies. We used gene expression analysis from organ donors whereas the studies of Butler and Rosenberg used Ki67 and PCNA immunohistochemistry, respectively, on autopsy pancreata (1,3). Quantitative RT-PCR, which has a greater sensitivity and dynamic range than immunohistochemistry, could explain our ability to detect significant changes, where these were not always detectable with protein staining. Unfortunately, we did not collect islet protein or fix islets for immunostaining from this panel, so a direct mRNA to protein comparison is not possible. Furthermore, we measured many cell cycle transcripts as markers for proliferation rather than relying on a single marker. Unfortunately, no direct measurement of human β-cell proliferation in vivo is yet available. However, our data are consistent with increased β-cell replication in response to obesity.
Although the focus of our analysis was directed to human islet gene expression changes in response to obesity, we also examined the effects of age as a covariate. We did not find a significant negative correlation of proliferative gene expression with age (Supplemental Table 1). Previous work has found reduced basal proliferative rates in organ donor islets with increasing age when measured with immunohistochemistry (43). We think our data differs because our islet donor population was almost entirely between the ages of 40 and 60 yr, causing an age analysis to be underpowered.
Understanding the adaptive response to obesity in human islets leading to expansion of β-cell mass may allow us to utilize these processes to increase regulated insulin production in patients with prediabetes or diabetes. Even in the setting of increased apoptosis and declining β-cell mass in type 2 diabetes, we may be able to restore euglycemia by expanding β-cell mass. By identifying key transcriptional regulators like FoxM1, we can now hope to work to identify mitogens upstream of FoxM1 to develop new type 2 diabetes therapeutics.
Materials and Methods
Animals
Lean and obese B6 and BTBR mice were derived from the same cohort of 10-wk-old male mice described in our previous publication (7). F2 mice were generated by crossing female BTBR.Leptinob/+ and male B6-Leptinob/+ mice to generate F1-Leptinob/ob animals. F1-Leptinob/ob mice were fat transplanted as previously described (44) to restore fertility and were intercrossed to generate F2-Leptinob/ob animals. F2- Leptinob/ob animals were killed at 10 wk of age, as previously described (7). For adenoviral studies, lean BTBR male and female mice aged 8–14 wk were used. Mouse procedures were approved by the Association for Assessment and Accreditation of Laboratory Animal Care to meet acceptable standards of humane animal care.
Plasma measurements
Animals were fasted for 4 h (0530–0930 h), and retroorbital blood samples were taken before animals were euthanized by CO2 asphyxiation. Glucose was measured by the glucose oxidase method (Sigma-Aldrich, St. Louis, MO). Insulin was measured by an ELISA developed in our laboratory using a pair of antiinsulin/proinsulin antibodies (clones D6C4 and D3E7-BT; Research Diagnostics,), as previously described (7).
Islet isolation
Intact pancreatic islets were isolated from mice using a collagenase digestion procedure, as previously described (45), and hand picked under a stereo microscope to remove contaminating acinar tissue. Islets were then either washed in PBS and stored in RLT buffer (QIAGEN, Chatsworth, CA) at −80 C for RNA isolation or used in adenoviral experiments.
Microarray analysis
Gene expression profiling in pancreatic islets was carried out as described previously (7). Briefly, whole-islet RNA was purified from approximately 500 mice using QIAGEN RNeasy Mini Kits according to the manufacturer’s instructions. Total RNA was reverse transcribed (cRNA) and labeled with Cy3. For each mouse, Cy3-labeled cRNA was hybridized against a reference pool of Cy5-labeled cRNA that was constructed from equal aliquots of RNA from 180 randomly selected mice. Gene expression measurements were made using custom-printed microarrays consisting of approximately 40,000 60-mer oligonucleotides (Agilent Technologies, Palo Alto, CA). Expression values are reported as log10 of the ratio of Cy3-cRNA/Cy5-cRNA reference (mlratio) for each mouse. Mice were partitioned into distinct subgroups by unsupervised hierarchical clustering of the normal scores of the expression of FoxM1 and the other genes listed in Fig. 2. This order-preserving procedure forces the expression values to follow a normal distribution, reducing influence of outlier expression values on the clustering algorithm. Mlratio data for all 499 mice for the genes listed in Fig. 2 are included in Supplemental Data.
Adenovirus construction
AdCMV-FOXM1b adenovirus was constructed using human FOXM1b cDNA, as previously described (14) at University of Illinois-Chicago in Dr. Robert Costa’s laboratory. The adenovirus was a generous gift from Dr. Pradip Raychaudhuri (University of Illinois-Chicago). The AdCMV-β-gal virus was a gift from Dr. Chris Newgard (Duke University, Durham, NC) and has been previously described (28). We checked for the contaminant E1A, an adenoviral oncogene, that could confound proliferation results (46). We detected no E1A DNA in AdCMV-FOXM1b adenoviral stocks or E1A mRNA in AdCMV-FOXM1b-treated islets (data not shown). E1A testing was done by quantitative PCR using the following primers: forward, TATGCCAAACCTTGTACCGGAGGT and reverse, CCGGGGTGCTCCACATAATCT.
Islet culture
After handpicking, mouse islets were washed twice in islet media (RPMI 1640 with 8 mm glucose, 10% fetal bovine serum, 1% penicillin/streptomycin) and separated into groups. Islets were then treated with AdCMV-FOXM1b or AdCMV-β-gal with an multiplicity of infection of approximately 120–240 (4.7 × 107 plaque-forming untits/200–400 islets) for 18–20 h.
Human islets were obtained from participating Islet Cell Resource Centers, including the Center at University of Wisconsin. An exemption was granted for all protocols by the Institutional Review Board at the University of Wisconsin-Madison. Islets were treated with AdCMV-FOXM1b or AdCMV-β-gal with a multiplicity of infection of approximately 50–100 (1.26 × 108 plaque-forming units/1000–2000 islet equivalent units) for 18–20 h in CMRL 1066 with 2.5% human serum albumin. After this point, mouse and human islets were treated identically. Islets were then cultured for an additional approximately 54 h with fresh islet media added daily.
[3H]thymidine incorporation into DNA
After 54 h of incubation, an overnight approximately 18-h incubation was performed in the presence of [3H]thymidine (1 mCi/ml, Amersham TRK758). Islets were washed three times with ice-cold PBS. DNA and protein were precipitated by addition of 10% trichloroacetic acid. The precipitate was solubilized in 0.3 n NaOH, and the radioactivity was measured using a liquid scintillation counter. A fraction of the solubilized product was kept to measure total protein by the Bradford assay (47). Sample counts were individually normalized to protein, and an average for each treatment group was determined.
Immunofluorescent staining
After 54 h of incubation, islets were incubated for 18 h with 10 mm BrdU (Sigma B5002). Islets were washed three times with 1 ml of PBS. Islets were fixed in Bouin’s solution for 2 h and maintained in 10% neutral-buffered formalin. After formalin removal, 50 ml of Affi-Gel blue bead slurry (Bio-Rad Laboratories, Hercules, CA) was added to the islets to aid in visualization during sectioning. The islet and bead slurry was embedded in paraffin. (5 μm) Serial sections on glass slides were deparaffinized with xylene and rehydrated in a graded series of ethanol. For mouse islets, BrdU (Calbiochem, La Jolla, CA; NA61 Ab-3, 1:250), glucagon (Santa Cruz Biotechnology, Inc., Santa Cruz, CA; sc-13091, 1:50), and insulin (Sigma; I8510, 1:500) were immunostained. For human islets, slides were boiled for 13 min in Vector H-3300 antigen retrieval solution (Vector Laboratories, Inc., Burlingame, CA) after rehydration. BrdU (Invitrogen, Carlsbad, CA; A21301MP, 1:100), glucagon, and insulin were immunostained. Islet sections were imaged at ×40 via confocal microscopy. Analysis of the photomicrographs was performed in a blinded fashion and scored for BrdU-positive nuclei within cells staining positively for insulin or glucagon. We required the nuclear area containing the BrdU staining to be entirely surrounded by either insulin or glucagon staining to be counted; any poorly stained cells or cells that could not be clearly designated were not counted.
Glucose-stimulated insulin secretion
Three to five islets were washed in Krebs-Ringer bicarbonate secretion buffer (0.5% BSA, 118.41 mm NaCl, 4.69 mm KCl, 1.18 mm MgSO4, 1.18 mm KH2PO4, 25 mm NaHCO3, 5 mm HEPES, and 2.52 mm CaCl2) with 1.67 mm glucose for 45 min. Islets were then either incubated in Krebs-Ringer bicarbonate secretion buffer with 1.67 mm glucose or 16.7 mm glucose for 45 min. Insulin concentrations were measured by our in-house ELISA (mouse insulin) or RIA (human insulin, RI-13K; Linco Research, Inc., St Charles, MO) and normalized to total insulin content.
Quantitative RT-PCR
RNA was isolated with the RNeasy kit (QIAGEN) and cDNA was prepared with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). mRNA measurements were performed by SYBR quantitative RT-PCR, and all values were normalized to β-actin. Primer sequences can be found in Supplemental Tables 2 and 3. For adenoviral studies, changes relative to treatment with a control adenovirus (AdCMV-β-gal) were determined for each independent mouse or human islet preparation and averaged.
Western blotting
Islets were washed in PBS and lysed in 20 mm Tris HCl, 10 mm EDTA, and 1% Triton X-100 containing 10 μg/ml leupeptin, 5 μg/ml aprotinin, 5 μg/ml pepstatin A, 100 mm pefablock, and 1 mm sodium orthovanadate. Whole islet lysate (20–50 μg) was separated on 4–15% Tris HCl gradient gels (Bio-Rad) and transferred to polyvinylidene difluoride membranes. Membranes were blocked in 5% milk Tris-buffered saline with 0.25% Tween 20. Primary antibodies and dilutions can be found in Supplemental Table 4. Blots were quantitated by densitometry with the QuantTL program. Relative changes were determined for each individual mouse or human and averaged.
Statistical methods
Quantitative RT-PCR comparisons were made by one-way ANOVA followed by Bonferroni-corrected t tests (Fig. 1) or Student’s paired t test (Figs. 4 and 5 and Supplemental Tables 5 and 6). Paired analysis was performed because islets from a single mouse or human were separated into two treatment groups. Human islet obesity RT-PCR data were analyzed by both binned obese (BMI > 30) vs. lean (BMI < 30) unpaired Student’s t tests or a multiple regression model using FOXM1, BMI, age, and sex as covariate adjustments (Fig. 6 and Supplemental Table 1). Interactions between BMI, age, and sex were nonsignificant and not reported. F2 islet microarray expression data were clustered with an unsupervised hierarchical approach in R (48). Clinical phenotype comparisons of F2 mice (Fig. 2) were made by one-way ANOVA followed by Bonferroni-corrected t tests. For data derived from measuring [3H]thymidine incorporation into DNA (Fig. 3), comparisons were made by repeated-measures ANOVA followed by Bonferroni-corrected paired t-tests. For the detection of BrdU incorporation into DNA (Fig. 3), comparisons were made by repeated-measures ANOVA followed by Bonferroni-corrected paired t tests. For experiments that measured insulin secretion (Supplemental Fig. 2), comparisons were made by repeated-measures ANOVA followed by Bonferroni-corrected paired t test. Western blot comparisons (Figs. 4 and 5) were made by Student’s paired t tests.
Supplementary Material
Acknowledgments
We thank Kathryn Schueler, Angie Tebon Oler, and Donald Stapleton (University of Wisconsin, Madison, WI) for technical assistance in generation of the F2 Leptinob/ob population and its phenotyping; Dr. Christopher Newgard (Duke University, Durham, NC) and Dr. David Harlan (University of Massachusetts, Worcester, MA) for critical review of this manuscript; Dr. Christopher Newgard for providing the AdCMV-β-gal adenovirus; Dr. Pradip Raychaudhuri (University of Illinois, Chicago, IL) for providing the AdCMV-FOXM1b adenovirus; and Laura Vanderploeg (University of Wisconsin, Madison, WI) for her artistic design of Figs. 4–6. For provision of human islets, we thank the ICR Basic Science Islet Distribution Program, including the following ICR and JDRF centers: University of Wisconsin (Madison, WI), University of Alabama (Birmingham, AL), University of Illinois (Chicago, IL),University of Pennsylvania (Philadelphia, PA), University of Minnesota (Duluth, MN), Northwestern University (Evanston, IL), University of Miami (Coral Gables, FL), Emory University (Atlanta, GA), Invenio Institute (Irvine, CA), Massachusetts General Hospital (Boston, MA), University of Pittsburgh (Pittsburgh, PA), Washington University (St. Louis, MO), and the Southern California Islet Consortium.
Footnotes
This work was supported by the following grants and fellowships: D.B.D. has received support from the Pearl Stetler Foundation, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (Grant 1K08DK083442), Wisconsin Alumni Research Foundation, and the William and Judith Busse Women in Medicine Research Fund. J.A.L. was supported by an National Human Genome Research Institute training grant to the Genomic Sciences Training Program (5T32HG002760). A.D.A. was supported by NIDDK Grants DK58037 and DK66369, Hatch Grant from the University of Wisconsin College of Agriculture and Life Sciences (WIS01069), JDRF (17-2007-1026), and in kind support from Merck Research Laboratories.
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 21, 2010
Abbreviations: B6, C57BL/6; BMI, body mass index; BrdU, 5-bromo-2′-deoxyuridine; cdk, cyclin-dependent kinase; CMV, cytomegalovirus; CPC, chromosome passenger complex; Ct, cycle threshold.
References
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC 2003 β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52:102–110 [DOI] [PubMed] [Google Scholar]
- Ritzel RA, Butler AE, Rizza RA, Veldhuis JD, Butler PC 2006 Relationship between β-cell mass and fasting blood glucose concentration in humans. Diabetes Care 29:717–718 [DOI] [PubMed] [Google Scholar]
- Hanley SC, Austin E, Assouline-Thomas B, Kapeluto J, Blaichman J, Moosavi M, Petropavlovskaia M, Rosenberg L 2010 β-Cell mass dynamics and islet cell plasticity in human type 2 diabetes. Endocrinology 151:1462–1472 [DOI] [PubMed] [Google Scholar]
- Klöppel G, Löhr M, Habich K, Oberholzer M, Heitz PU 1985 Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res 4:110–125 [DOI] [PubMed] [Google Scholar]
- Hull RL, Kodama K, Utzschneider KM, Carr DB, Prigeon RL, Kahn SE 2005 Dietary-fat-induced obesity in mice results in β cell hyperplasia but not increased insulin release: evidence for specificity of impaired β cell adaptation. Diabetologia 48:1350–1358 [DOI] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA 2004 Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429:41–46 [DOI] [PubMed] [Google Scholar]
- Keller MP, Choi Y, Wang P, Davis DB, Rabaglia ME, Oler AT, Stapleton DS, Argmann C, Schueler KL, Edwards S, Steinberg HA, Chaibub Neto E, Kleinhanz R, Turner S, Hellerstein MK, Schadt EE, Yandell BS, Kendziorski C, Attie AD 2008 A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome Res 18:706–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierstra I, Alves J 2007 FOXM1, a typical proliferation-associated transcription factor. Biol Chem 388:1257–1274 [DOI] [PubMed] [Google Scholar]
- Zhang H, Ackermann AM, Gusarova GA, Lowe D, Feng X, Kopsombut UG, Costa RH, Gannon M 2006 The FoxM1 transcription factor is required to maintain pancreatic β-cell mass. Mol Endocrinol 20:1853–1866 [DOI] [PubMed] [Google Scholar]
- Ackermann Misfeldt A, Costa RH, Gannon M 2008 β-Cell proliferation, but not neogenesis, following 60% partial pancreatectomy is impaired in the absence of FoxM1. Diabetes 57:3069–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhang J, Pope CF, Crawford LA, Vasavada RC, Jagasia SM, Gannon M 2010 Gestational diabetes mellitus resulting from impaired β-cell compensation in the absence of FoxM1, a novel downstream effector of placental lactogen. Diabetes 59:143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Yoshida Y, Hughes DE, Costa RH 2006 Increased expression of hepatocyte nuclear factor 6 stimulates hepatocyte proliferation during mouse liver regeneration. Gastroenterology 130:1283–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YY, Gao XP, Zhao YD, Mirza MK, Frey RS, Kalinichenko VV, Wang IC, Costa RH, Malik AB 2006 Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury. J Clin Invest 116:2333–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Krupczak-Hollis K, Tan Y, Dennewitz MB, Adami GR, Costa RH 2002 Increased hepatic forkhead box M1B (FoxM1B) levels in old-aged mice stimulated liver regeneration through diminished p27Kip1 protein levels and increased Cdc25B expression. J Biol Chem 277:44310–44316 [DOI] [PubMed] [Google Scholar]
- Wonsey DR, Follettie MT 2005 Loss of the forkhead transcription factor FoxM1 causes centrosome amplification and mitotic catastrophe. Cancer Res 65:5181–5189 [DOI] [PubMed] [Google Scholar]
- Kalinichenko VV, Gusarova GA, Tan Y, Wang IC, Major ML, Wang X, Yoder HM, Costa RH 2003 Ubiquitous expression of the forkhead box M1B transgene accelerates proliferation of distinct pulmonary cell types following lung injury. J Biol Chem 278:37888–37894 [DOI] [PubMed] [Google Scholar]
- Wang IC, Chen YJ, Hughes D, Petrovic V, Major ML, Park HJ, Tan Y, Ackerson T, Costa RH 2005 Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol Cell Biol 25:10875–10894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laoukili J, Kooistra MR, Brás A, Kauw J, Kerkhoven RM, Morrison A, Clevers H, Medema RH 2005 FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol 7:126–136 [DOI] [PubMed] [Google Scholar]
- Ma RY, Tong TH, Cheung AM, Tsang AC, Leung WY, Yao KM 2005 Raf/MEK/MAPK signaling stimulates the nuclear translocation and transactivating activity of FOXM1c. J Cell Sci 118:795–806 [DOI] [PubMed] [Google Scholar]
- Fu Z, Malureanu L, Huang J, Wang W, Li H, van Deursen JM, Tindall DJ, Chen J 2008 Plk1-dependent phosphorylation of FoxM1 regulates a transcriptional programme required for mitotic progression. Nat Cell Biol 10:1076–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvisi DF, Pinna F, Ladu S, Pellegrino R, Simile MM, Frau M, De Miglio MR, Tomasi ML, Sanna V, Muroni MR, Feo F, Pascale RM 2009 Forkhead box M1B is a determinant of rat susceptibility to hepatocarcinogenesis and sustains ERK activity in human HCC. Gut 58:679–687 [DOI] [PubMed] [Google Scholar]
- Rieck S, White P, Schug J, Fox AJ, Smirnova O, Gao N, Gupta RK, Wang ZV, Scherer PE, Keller MP, Attie AD, Kaestner KH 2009 The transcriptional response of the islet to pregnancy in mice. Mol Endocrinol 23:1702–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmeier HE, Newgard CB 2005 Islets for all? Nat Biotechnol 23:1231–1232 [DOI] [PubMed] [Google Scholar]
- Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, Takane KK, Garcia-Ocaña A, Vasavada R, Stewart AF 2006 Molecular control of cell cycle progression in the pancreatic β-cell. Endocr Rev 27:356–370 [DOI] [PubMed] [Google Scholar]
- Weir GC, Bonner-Weir S 2004 Five stages of evolving β-cell dysfunction during progression to diabetes. Diabetes 53 Suppl 3:S16–S21 [DOI] [PubMed] [Google Scholar]
- Wajchenberg BL 2007 β-Cell failure in diabetes and preservation by clinical treatment. Endocr Rev 28:187–218 [DOI] [PubMed] [Google Scholar]
- Prentki M, Nolan CJ 2006 Islet β-cell failure in type 2 diabetes. J Clin Invest 116:1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisler JC, Fueger PT, Babu DA, Hohmeier HE, Tessem JS, Lu D, Becker TC, Naziruddin B, Levy M, Mirmira RG, Newgard CB 2008 Stimulation of human and rat islet β-cell proliferation with retention of function by the homeodomain transcription factor Nkx6.1. Mol Cell Biol 28:3465–3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun T, He KH, Lupi R, Boehm B, Wojtusciszyn A, Sauter N, Donath M, Marchetti P, Maedler K, Gauthier BR 2008 The diabetes-linked transcription factor Pax4 is expressed in human pancreatic islets and is activated by mitogens and GLP-1. Hum Mol Genet 17:478–489 [DOI] [PubMed] [Google Scholar]
- Päth G, Opel A, Gehlen M, Rothhammer V, Niu X, Limbert C, Romfeld L, Hügl S, Knoll A, Brendel MD, Bretzel RG, Seufert J 2006 Glucose-dependent expansion of pancreatic β-cells by the protein p8 in vitro and in vivo. Am J Physiol Endocrinol Metab 291:E1168–E1176 [DOI] [PubMed] [Google Scholar]
- Fiaschi-Taesch N, Bigatel TA, Sicari B, Takane KK, Salim F, Velazquez-Garcia S, Harb G, Selk K, Cozar-Castellano I, Stewart AF 2009 Survey of the human pancreatic β-cell G1/S proteome reveals a potential therapeutic role for cdk-6 and cyclin D1 in enhancing human β-cell replication and function in vivo. Diabetes 58:882–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WJ, Schreiber WE, Zhong E, Liu FF, Kornfeld BD, Wondisford FE, Hussain MA 2008 Exendin-4 stimulation of cyclin A2 in β-cell proliferation. Diabetes 57:2371–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinsson-Ahlzén HS, Liberal V, Grünenfelder B, Chaves SR, Spruck CH, Reed SI 2008 Cyclin-dependent kinase-associated proteins Cks1 and Cks2 are essential during early embryogenesis and for cell cycle progression in somatic cells. Mol Cell Biol 28:5698–5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Gao N, Gorski RK, White P, Hardy OT, Rafiq K, Brestelli JE, Chen G, Stoeckert Jr CJ, Kaestner KH 2007 Expansion of adult β-cell mass in response to increased metabolic demand is dependent on HNF-4α. Genes Dev 21:756–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo AS, Hay CW, Docherty K 2008 Pancreatic transcription factors and their role in the birth, life and survival of the pancreatic β cell. Mol Cell Endocrinol 294:1–9 [DOI] [PubMed] [Google Scholar]
- Meier JJ, Butler AE, Galasso R, Rizza RA, Butler PC 2006 Increased islet β cell replication adjacent to intrapancreatic gastrinomas in humans. Diabetologia 49:2689–2696 [DOI] [PubMed] [Google Scholar]
- Meier JJ, Lin JC, Butler AE, Galasso R, Martinez DS, Butler PC 2006 Direct evidence of attempted β cell regeneration in an 89-year-old patient with recent-onset type 1 diabetes. Diabetologia 49:1838–1844 [DOI] [PubMed] [Google Scholar]
- Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, et al. 2007 A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316:1341–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeggini E, Scott LJ, Saxena R, Voight BF, Marchini JL, Hu T, de Bakker PI, Abecasis GR, Almgren P, Andersen G, Ardlie K, Boström KB, Bergman RN, Bonnycastle LL, Borch-Johnsen K, Burtt NP, Chen H, Chines PS, Daly MJ, Deodhar P, Ding CJ, Doney AS, Duren WL, Elliott KS, Erdos MR, et al. 2008 Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet 40:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, McCarthy MI, Hattersley AT 2007 Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316:1336–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Boström K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, et al. 2007 Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316:1331–1336 [DOI] [PubMed] [Google Scholar]
- Whitfield ML, Sherlock G, Saldanha AJ, Murray JI, Ball CA, Alexander KE, Matese JC, Perou CM, Hurt MM, Brown PO, Botstein D 2002 Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol Biol Cell 13:1977–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maedler K, Schumann DM, Schulthess F, Oberholzer J, Bosco D, Berney T, Donath MY 2006 Aging correlates with decreased β-cell proliferative capacity and enhanced sensitivity to apoptosis: a potential role for Fas and pancreatic duodenal homeobox-1. Diabetes 55:2455–2462 [DOI] [PubMed] [Google Scholar]
- Barros CC, Almeida SS, Mori MA, Valero VB, Haro AS, Batista EC, Rosa TS, Bacurau RF, Wurtele M, Araújo RC 2009 Efficient method for obtaining Lep(ob)/Lep(ob)-derived animal models using adipose tissue transplantations. Int J Obes (Lond) 33:938–944 [DOI] [PubMed] [Google Scholar]
- Rabaglia ME, Gray-Keller MP, Frey BL, Shortreed MR, Smith LM, Attie AD 2005 α-Ketoisocaproate-induced hypersecretion of insulin by islets from diabetes-susceptible mice. Am J Physiol Endocrinol Metab 289:E218–E224 [DOI] [PubMed] [Google Scholar]
- Lavine JA, Raess PW, Davis DB, Rabaglia ME, Presley BK, Keller MP, Beinfeld MC, Kopin AS, Newgard CB, Attie AD 2010 Contamination with E1A-positive wild-type adenovirus accounts for species-specific stimulation of islet cell proliferation by CCK: a cautionary note. Mol Endocrinol 24:464–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM 1976 A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Team RDC 2009 R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.