Abstract
Glucocorticoids play central roles in the regulation of energy metabolism by shifting it toward catabolism, whereas AMP-activated protein kinase (AMPK) is the master regulator of energy homeostasis, sensing energy depletion and stimulating pathways of increasing fuel uptake and saving on peripheral supplies. We showed here that AMPK regulates glucocorticoid actions on carbohydrate metabolism by targeting the glucocorticoid receptor (GR) and modifying transcription of glucocorticoid-responsive genes in a tissue- and promoter-specific fashion. Activation of AMPK in rats reversed glucocorticoid-induced hepatic steatosis and suppressed glucocorticoid-mediated stimulation of glucose metabolism. Transcriptomic analysis in the liver suggested marked overlaps between the AMPK and glucocorticoid signaling pathways directed mostly from AMPK to glucocorticoid actions. AMPK accomplishes this by phosphorylating serine 211 of the human GR indirectly through phosphorylation and consequent activation of p38 MAPK and by altering attraction of transcriptional coregulators to DNA-bound GR. In human peripheral mononuclear cells, AMPK mRNA expression positively correlated with that of glucocorticoid-responsive glucocorticoid-inducible leucine zipper protein, which correlated also positively with the body mass index of subjects. These results indicate that the AMPK-mediated energy control system modulates glucocorticoid action at target tissues. Because increased action of glucocorticoids is associated with the development of metabolic disorders, activation of AMPK could be a promising target for developing pharmacological interventions to these pathologies.
AMPK, a master regulator of energy homeostasis, phosphorylates human GR at serine 211 through activation of p38 MAPK, and attenuates glucocorticoid-induced hyperglycemia and liver steatosis.
Glucocorticoids, the end-effector hormones of the hypothalamic-pituitary-adrenal axis, play crucial roles in the regulation of basal and stress-related homeostasis (1,2). These hormones influence functions of virtually every organ and tissue and are necessary for the maintenance of many important biological activities, such as the homeostasis of the central nervous system (CNS) and cardiovascular systems, intermediary metabolism, and the immune/inflammatory reaction, influencing mRNA expression of up to 20% of the expressed genome (1,3,4,5).
Glucocorticoids have strong effects on the energy expenditure of the body, shifting energy balance from anabolism to catabolism by stimulating the synthesis/release of glucose in the liver and facilitating lipolysis in adipose tissues and protein degradation in various organs and tissues, including the skeletal muscles, skin, and bones (2,4). Glucocorticoids also stimulate appetite in the CNS, facilitating uptake of nutrients (6). Thus, chronic excess of circulating glucocorticoids or increase of glucocorticoid sensitivity of their target tissues is associated with development of visceral obesity, diabetes mellitus type 2, and hyperlipidemia (4,6,7,8,9).
The glucocorticoid receptor (GR), which belongs to the steroid/sterol/thyroid/retinoid/orphan nuclear receptor superfamily, mediates most of the known actions of glucocorticoids (3,10). The prototype human GR consists of 777 amino acids and three domains: the N-terminal domain; middle, DNA-binding; and C-terminal, ligand-binding domains (10). Upon hormone binding, the GR translocates from the cytoplasm into the nucleus, and binds its specific DNA recognition sequences, the glucocorticoid response elements (GREs), in the regulatory regions of glucocorticoid-responsive genes or interacts with other transcription factors and modifies their actions on their target genes (10,11). DNA-bound GR modulates the transcription of regulated coding sequences by attracting numerous cofactor and coregulator proteins and protein complexes, such as the histone acetyltransferase coactivator complexes, and the mating-type switching/sucrose non-fermenting (SWI/SNF) and vitamin D receptor-interacting protein/thyroid hormone receptor-associated protein chromatin-remodeling complexes, eventually influencing the activity of RNA polymerase II and its ancillary factors (10,12).
Because glucocorticoids are essential for survival and strongly influence the energy balance of the body, several distinct signaling pathways regulate the transcriptional activity of the GR by affecting various activation steps of this receptor (3,10). Such major mechanisms include posttranslational modifications of the GR, such as phosphorylation, acetylation, methylation, nitrosylation, and small ubiquitin-like modifier protein (SUMO)ylation (10). Indeed, several kinases, including the cell cycle-related and mitogen-activated kinases, located downstream of cell surface receptors for growth factors and cytokines, phosphorylate specific serine or threonine residues of the GR and change its transcriptional activity (13,14,15,16,17,18,19,20). In addition, we recently found that the cyclin-dependent kinase 5, a brain-specific serine/threonine kinase essential for fetal brain development and function, phosphorylated the GR at multiple serine residues located in the N-terminal domain and modulated GR-induced transcriptional activity in a promoter-specific fashion (21,22).
AMP-activated protein kinase (AMPK), also an evolutionarily well-conserved serine/threonine kinase, plays central roles as a cellular sensor of metabolic needs and regulator of the body’s energy balance (23). AMPK is composed of three subunits, α, β, and γ, each of which consists of multiple isoforms with different biological activities (23,24). Depletion of intracellular ATP and hence elevation of AMP leads to activation of AMPK by upstream kinases, such as AMPK kinase, liver kinase B1, and calmodulin kinase kinase, through phosphorylation of threonine 172 of the α-subunit of the human AMPK, in cooperation with other activation mechanisms, including allosteric activation and inhibition of dephosphorylation by protein phosphatases (24,25).
This effect of AMP can be mimicked by its synthetic analog 5-aminoimidazole-4-carboxamide-1-β-d-ribonucleoside (AICAR), which is generally used experimentally as a stimulator of AMPK (24,26,27,28). Activation of AMPK by AMP or AICAR phosphorylates downstream substrate molecules, such as the insulin receptor substrate-1, the p38 MAPK, the acetyl-coenzyme A carboxylase, the 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, and the hormone-sensitive lipase (24,25,29,30,31,32,33,34,35).
Activation of these substrates by AMPK then shift the functions of various organs toward increasing energy supply and reducing anabolism (23). Such alterations include an increase of appetite in CNS, stimulation of glucose uptake in the heart and skeletal muscles, inhibition of gluconeogenesis in the liver, inhibition of insulin release from the pancreas, and suppression of fat accumulation/synthesis in adipose tissue (23,25). Interestingly, glucocorticoids also strongly influence all of these activities (2,4).
We hypothesized that these two major signaling pathways crosstalk at numerous points. We demonstrate here that AMPK differentially modulates glucocorticoid action by phosphorylating the human GR at serine 211 indirectly through the activation of p38 MAPK. This regulation is evident in the liver because AMPK suppresses glucocorticoid-induced gene expression of rate-limiting enzymes for gluconeogenesis and glycogenolysis, ultimately attenuating glucocorticoid-induced hepatic steatosis and the elevation of blood glucose levels.
Results
AMPK attenuates dexamethasone-induced elevation of serum glucose and liver steatosis in rats
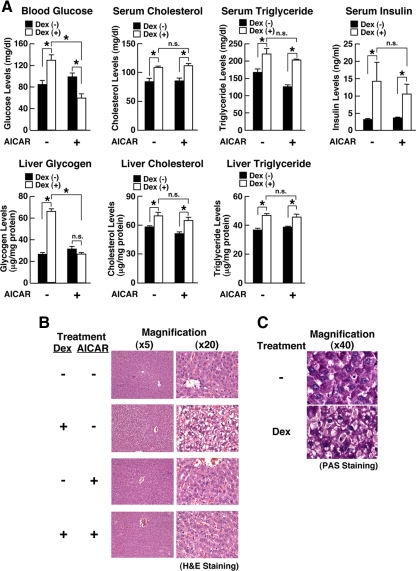
To examine our hypothesis, we first studied the influence of AMPK on the effect of glucocorticoids in vivo by injecting the synthetic glucocorticoid dexamethasone and/or the AMPK activator AICAR into rats (Fig. 1). Animals injected with these compounds appeared grossly normal 24 h after the injections. However, dexamethasone increased blood glucose levels by approximately 30%, whereas added AICAR strongly suppressed this elevation (Fig. 1A).
Figure 1.
AMPK attenuates dexamethasone-induced steatosis in rat liver and suppresses blood glucose levels. Rats were treated with AICAR (0.7 g/kg per animal) and/or dexamethasone (1.5 mg/kg per animal) for 24 h, and their livers, skeletal muscles, sc fat, spleens, and brains were sampled. A, AICAR attenuates dexamethasone-induced elevation of blood glucose and hepatic glycogen levels without affecting serum and hepatic levels of cholesterol and triglycerides in rats. Levels of blood glucose, serum and hepatic cholesterol and triglycerides, and serum insulin were examined in rats treated with the compounds indicated. *, P < 0.01, n.s., not significant, compared with the conditions indicated. B and C, AICAR attenuates dexamethasone-induced steatosis in the liver. The livers of the rats treated with the compounds indicated were stained with hematoxylin and eosin (H&E), and their representative histology images in lower (×5) and higher (×20) magnifications are shown in B, whereas the images of PAS staining for the liver treated with vehicle (−) or dexamethasone (Dex) are shown in C.
Dexamethasone also increased hepatic glycogen and AICAR completely abolished this change. Dexamethasone increased circulating levels of insulin, whereas AICAR did not affect the change induced by dexamethasone. These results indicate that the negative effect of AICAR on dexamethasone-induced elevation of blood glucose appears to be due to reduction of glucose production in the liver.
Dexamethasone also increased serum and hepatic levels of cholesterol and triglycerides, whereas AICAR did not influence such changes by this steroid, suggesting that AMPK mainly regulates glucocorticoid-induced effect on glucose metabolism.
We next examined histology of the liver in these animals (Fig. 1, B and C). Dexamethasone alone caused a severe steatotic change by transforming hepatocytes into foamy cells (Fig. 1B, second top panels). Periodic acid Schiff (PAS) staining revealed that the contents of these foamy cells were carbohydrate macromolecules, such as glycogen, glycoproteins, and proteoglycans, consistent with massive accumulation of glycogen by dexamethasone in the liver (Fig. 1C). Injection of AICAR alone did not influence the histology of the liver (Fig. 1B, third panels), whereas this compound strongly attenuated the steatotic change caused by dexamethasone (Fig. 1B, bottom panels). Taken together these data suggest that AMPK antagonized dexamethasone-induced alteration of glucose metabolism in the liver, explaining in part the AICAR-mediated suppression of dexamethasone-induced elevation of circulating glucose levels.
AMPK regulates GR-induced transcriptional activity in a tissue- and gene-specific fashion
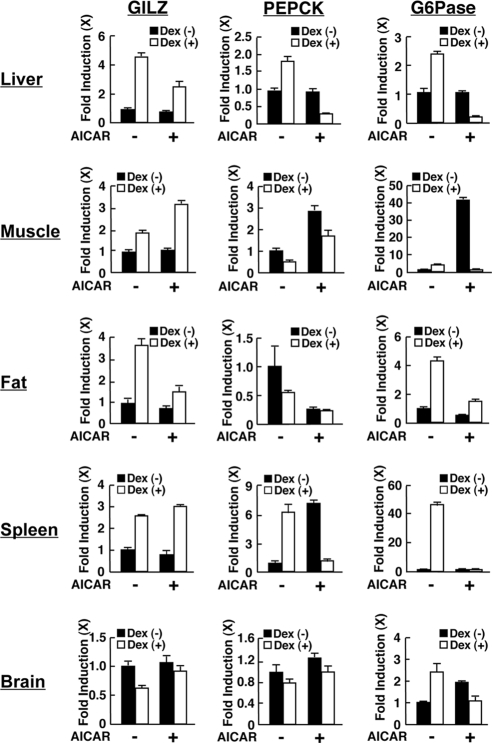
To further examine the interaction between AMPK and GR in glucose metabolism, we examined mRNA expression of the phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) genes along with that of the glucocorticoid-inducible leucine zipper protein (GILZ) in the liver as well as in skeletal muscle, sc fat, spleen, and brain obtained from rats treated with dexamethasone and/or AICAR for 8 h (Fig. 2).
Figure 2.
AICAR differentially regulates the transcriptional activity of glucocorticoid-responsive genes in a tissue- and gene-specific fashion. Rats were treated with AICAR (0.7 g/kg per animal) and/or dexamethasone (1.5 mg/kg per animal) for 8 h, and mRNA expression of GILZ, PEPCK, and G6Pase were examined with SYBR Green-based real-time PCR. the fold induction of mRNA expression of the indicated genes was calculated by dividing values of the mRNA expression with that of the baseline (the value obtained in the absence of AICAR and dexamethasone). Bars represent mean ± se values of the fold induction of mRNA expression in the presence or absence of dexamethasone. See main text for results of statistical analyses.
PEPCK and G6Pase are the rate-limiting enzymes for gluconeogenesis and glycogenolysis in the liver, whereas GILZ is a well-known glucocorticoid-responsive gene (36,37,38,39). Dexamethasone stimulated mRNA expression of the PEPCK (gluconeogenesis) and G6Pase (glycogenolysis) genes in the liver (P < 0.01). AICAR alone showed no influence on the mRNA expression of these genes (P = 0.61 and 0.55, respectively), whereas it strongly suppressed PEPCK and G6Pase mRNA expression in the presence of dexamethasone (P < 0.01).
Dexamethasone suppressed PEPCK mRNA expression in muscle and fat (P < 0.01), whereas AICAR negatively or positively regulated mRNA expression in these tissues, respectively (P < 0.01). Dexamethasone strongly and weakly stimulated G6Pase mRNA expression in fat and the muscle, respectively, whereas AICAR strongly stimulated mRNA expression of this gene in the muscle (P < 0.01). Thus, these results indicate that AICAR mainly suppressed the effect of dexamethasone on PEPCK and G6Pase mRNA expression in these tissues.
It appears that simultaneous treatment with AICAR strongly suppressed dexamethasone-stimulated gluconeogenesis and glycogenolysis in the liver, whereas it stimulated glycogenolysis in the muscle. The integrated effects of such regulation of dexamethasone and AICAR on glucose metabolism in the liver and muscle would have been expected to have caused a dramatic reduction of blood glucose levels and reversal of the liver steatotic changes in animals simultaneously treated with both of these compounds (Fig. 1).
In contrast to PEPCK and G6Pase, dexamethasone stimulated GILZ mRNA expression in the liver, muscle, fat, and spleen and suppressed the mRNA expression of this gene in the brain (P < 0.01), whereas AICAR alone did not affect GILZ mRNA expression in any of these tissues. These results indicate that the GILZ gene is a consistently glucocorticoid-responsive gene in these tissues. AICAR suppressed dexamethasone-induced GILZ mRNA expression in the liver and fat, whereas AICAR enhanced it strongly in the muscle and weakly in the spleen (P < 0.01). AICAR attenuated the suppressive effect of dexamethasone on GILZ mRNA expression in the brain (P = 0.041). Thus, AMPK differentially influences GR-induced transcriptional activity on the glucocorticoid-responsive GILZ gene in an organ/tissue-specific fashion.
AMPK differentially regulates GR-induced transcriptional activity in rat liver in microarray analyses
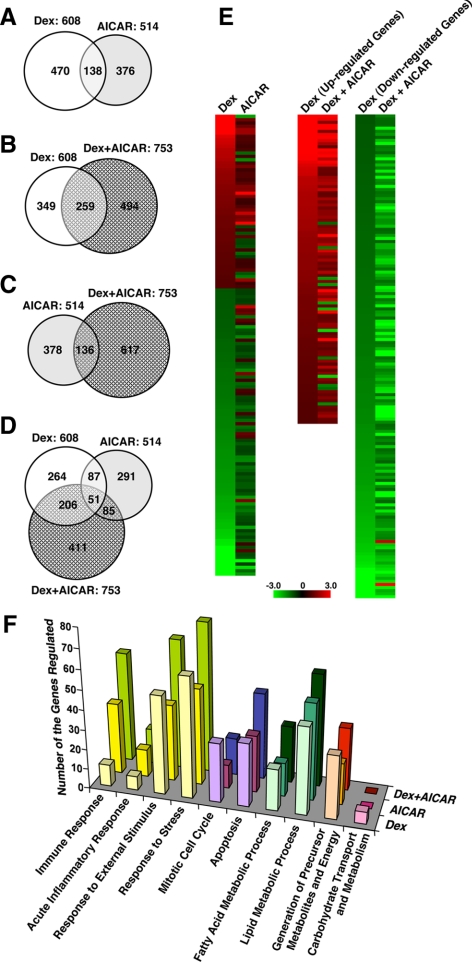
To address further functional interactions between AMPK and GR in global gene expression profiles, we performed microarray analyses using the Rat Genome 230 2.0 tiled array (Affymetrix, Santa Clara, CA) in samples obtained from the liver of the rats treated with dexamethasone and/or AICAR for 8 h (Fig. 3). Among approximately 15,000 genes successfully analyzed, dexamethasone changed mRNA expression of 608 (∼4.1%) genes, whereas AICAR altered that of 514 (∼3.4%) genes (Fig. 3A). Dexamethasone and AICAR shared 138 responsive genes, whereas the direction of their regulation was gene-specific (Fig. 3, A and E, left column). Simultaneous treatment with dexamethasone and AICAR changed mRNA expression of 753 genes, among which 259 genes were also regulated by dexamethasone alone (Fig. 3B). These results indicate that AMPK alters the transcriptional activity of GR in a fraction of glucocorticoid-responsive genes in the liver.
Figure 3.
AICAR alters mRNA expression of dexamethasone-responsive genes in rat liver in microarray analyses. Liver samples obtained in the experiment shown in Fig. 2 were used. A–D, AICAR modulates the transcriptional activity of a fraction of dexamethasone-responsive genes. Venn diagrams demonstrating genes regulated by dexamethasone (Dex) and/or AICAR (A), Dex and/or Dex+AICAR (B), AICAR and/or Dex+ AICAR (C), and Dex, AICAR, and Dex+AICAR (D) are shown. E, AICAR differentially regulates dexamethasone-induced transcriptional activity in a gene-specific fashion in microarray analyses. Cluster analyses of the genes regulated by dexamethasone (Dex) and AICAR (left panel), and those regulated by Dex and Dex+AICAR (right two panels) are shown. F, Dexamethasone and AICAR mutually influence with each other’s activity on the expression of genes with distinct biological activities. The numbers of the genes with the indicated biological activities are shown. Nomenclatures of the demonstrated biologic activities are based on Gene Ontology (http://www.geneontology.org).
The direction of AICAR-mediated regulation on these glucocorticoid-responsive genes was gene-specific (Fig. 3E, right two columns). In contrast to the effect of AMPK on GR-induced transcriptional activity, only 136 genes of 514 AICAR-responsive genes were further affected by dexamethasone (Fig. 3C), suggesting that the effect of GR on AICAR is weak in the liver. A three-way comparison between dexamethasone, AICAR, and dexamethasone+ AICAR also revealed that simultaneous treatment with dexamethasone and AICAR preferentially affected glucocorticoid-regulated genes (Fig. 3D). Lists of the genes regulated by dexamethasone and/or AICAR found in the microarray analysis are shown in Supplemental Tables 1, 2, and 3, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org.
To understand in more detail and categorize the genes regulated by dexamethasone and/or AICAR, we compared numbers of the regulated genes sorted out by their biological activities (Fig. 3F). In this analysis, AICAR completely attenuated the effect of dexamethasone on the genes grouped in carbohydrate transport and metabolism, whereas dexamethasone and AICAR cooperatively altered some genes in the other functional categories. These results further confirm that GR and AMPK mutually influence with each other’s activity in many different biological functions.
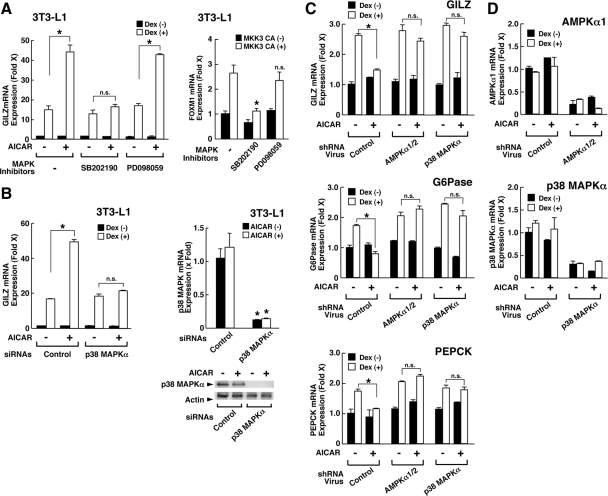
AMPK enhances GR-induced transcriptional activity in preadipocyte 3T3-L1 cells
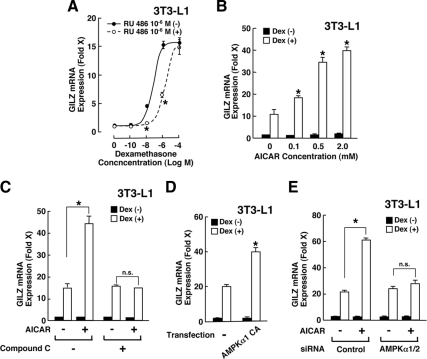
To examine the molecular mechanism(s) underlying AMPK-mediated modulation of glucocorticoid actions, we examined at the cellular level the influence of AICAR on GR-induced transcriptional activity by measuring mRNA expression of the endogenous GILZ gene, whose promoter contains multiple GREs (39,40) (Fig. 4). Indeed, dexamethasone dose-dependently stimulated GILZ mRNA expression greater than 15-fold in 3T3-L1 cells (Fig. 4A). Because the receptor antagonist RU 486 shifted the dexamethasone titration curve rightward, we considered the observed effect of dexamethasone on the GILZ mRNA expression as GR-mediated. AICAR enhanced dexamethasone-stimulated GILZ mRNA expression in a dose-dependent fashion in these cells (Fig. 4B). Addition of compound C, a well-known inhibitor of AMPK, completely abolished AICAR-mediated enhancement of dexamethasone-induced GILZ mRNA expression (Fig. 4C), indicating that AMPK activated by AICAR enhanced GR-induced transcriptional activity.
Figure 4.
AMPK enhances GR-induced transcriptional activity in 3T3-L1 cells. A, Dexamethasone stimulates GILZ mRNA expression though the GR in 3T3-L1 cells. 3T3-L1 cells were incubated with indicated concentrations of dexamethasone in the presence or absence of 10−6 m of RU 486. Circles indicate mean ± se values of fold induction of GILZ mRNA over the baseline (the value obtained in the absence of dexamethasone) in the presence (open circles) or absence (closed circles) of RU 486. *, P < 0.01, compared with GILZ mRNA expression in the presence or absence of RU 486 at the same concentrations of dexamethasone. B, AICAR enhances dexamethasone-induced GILZ mRNA expression in 3T3-L1 cells. 3T3-L1 cells were incubated with indicated concentrations of AICAR in the presence or absence of 10−6 m of dexamethasone (Dex). Fold induction of GILZ mRNA expression was calculated by dividing the values of the mRNA expression with that of the baseline (the value obtained in the absence of AICAR and dexamethasone). Bars represent mean ± se values of the fold induction of GILZ mRNA expression in the presence or absence of dexamethasone. *, P < 0.01, ANOVA followed by Student t test with Bonferroni correction by comparing the results obtained in the presence of increasing concentrations of AICAR and dexamethasone. C, AICAR-induced enhancement of dexamethasone-stimulated GILZ mRNA expression is completely abolished with compound C in 3T3-L1 cells. 3T3-L1 cells were incubated with 2 mm of AICAR and/or 40 μm of compound C in the presence or absence of 10−6 m of dexamethasone. Fold induction of GILZ mRNA expression was calculated by dividing the values of the mRNA expression with that of the baseline (the value obtained in the absence of AICAR, compound C, and dexamethasone). Bars represent mean ± se values of the fold induction of GILZ mRNA expression in the presence or absence of dexamethasone. *, P < 0.01, compared with the conditions indicated. D, The CA form of the α1-subunit of AMPK enhances dexamethasone-stimulated GILZ mRNA expression in 3T3-L1 cells. 3T3-L1 cells were transfected with AMPKα1-CA-expressing plasmid and were incubated in the presence or absence of 10−6 m of dexamethasone. Fold induction of GILZ mRNA expression was calculated by dividing the values of the mRNA expression with that of the baseline (the value obtained in the presence of a control plasmid and in the absence of dexamethasone). Bars represent mean ± se values of the fold induction of GILZ mRNA expression in the presence or absence of dexamethasone. *, P < 0.01, compared with the condition obtained with transfection of the control plasmid, untreated with AICAR and in the presence of dexamethasone. E, Knockdown of the α1- and α2-subunits of AMPK abolishes AICAR-induced enhancement of dexamethasone-stimulated GILZ mRNA expression in 3T3-L1 cells. 3T3-L1 cells were transfected with control or AMPKα1/ AMPKα2 siRNA and were incubated in the presence or absence of 10−6 m of dexamethasone and/or 2 mm of AICAR. The fold induction of GILZ mRNA expression was calculated by dividing the values of the mRNA expression with that of the baseline (the value obtained in the presence of a control siRNA and in the absence of dexamethasone and AICAR). Bars represent mean ± se values of the fold induction of GILZ mRNA expression in the presence or absence of dexamethasone. *, P < 0.01, n.s., not significant, compared with the conditions indicated.
Overexpression of a constitutively active form of the α1catalytic subunit of AMPK (AMPKα1-CA) also potentiated dexamethasone-stimulated GILZ mRNA expression in 3T3-L1 cells (Fig. 4D), whereas knockdown of AMPK α-subunit 1 and 2 abolished AICAR-mediated enhancement of dexamethasone-stimulated GILZ mRNA expression (Fig. 4E), Thus, AMPK positively regulates GR-induced transcriptional activity in 3T3-L1 cells, although the directions of AMPK-mediated regulation on glucocorticoid-induced transcriptional activity are tissue- and gene-specific in vivo (Figs. 2 and 3).
p38 MAPK mediates the effect of AMPK on GR-induced transcriptional activity
Because AMPK targeted GR for regulating its transcriptional activity, we searched potential AMPK phosphorylation sites in the amino acid sequence of the human GR. AMPK phosphorylates preferentially a sequence motif represented as Hyd-(Basic, X)-X-X-Ser/Thr-X-X-X-Hyd, where Hyd is a hydrophobic residue, Basic is a basic residue, and X is any amino acid (35). We could not find any sequence that matched to this consensus motif in the human GR. Instead, GR has one serine residue at amino acid 211, which perfectly matches to the phosphorylation motif of the p38 MAPK, a well-known substrate of AMPK, specifically phosphorylated by this kinase (13,29,30,41,42). Thus, we examined the involvement of this kinase in the AMPK-mediated regulation of GR-induced transcriptional activity (Fig. 5).
Figure 5.
AMPK regulates GR-induced transcriptional activity through the activation of p38 MAPKα. A, p38, but not p42, MAPK mediates AICAR-induced enhancement of dexamethasone-stimulated GILZ mRNA expression in 3T3-L1 cells. Left panel, The p38, but not p42, inhibitor abolishes the AICAR-induced enhancement of dexamethasone-stimulated GILZ mRNA expression in 3T3-L1 cells. 3T3-L1 cells were incubated with 2 mm of AICAR, 2 μm of SB202190 (p38 MAPK inhibitor), and/or 10 μm of PD098059 (p42 MAPK inhibitor) in the presence or absence of 10−6 m dexamethasone (Dex). Fold induction of GILZ mRNA expression was calculated by dividing the values of the mRNA expression with that of the baseline (the value obtained in the absence of the compounds and dexamethasone). Bars represent mean ± se values of the fold induction of GILZ mRNA expression in the presence or absence of dexamethasone. *, P < 0.01, n.s., not significant, compared with the condition indicated. Right panel, The p38 but not p42 inhibitor abolishes MKK3-CA-induced expression of FOXM1 mRNA expression in 3T3-L1 cells. 3T3-L1 cells were transfected with MKK3-CA-expressing plasmid and were incubated with 2 μm of SB202190 or 10 μm of PD098059. Fold induction of FOXM1 mRNA expression was calculated by dividing the values of the mRNA expression with that of the baseline (the value obtained in the presence of control plasmid and in the absence of the compounds). Bars represent mean ± se values of the fold induction of FOXM1 mRNA expression in the presence or absence of MKK3-CA expression. *, P < 0.01, n.s., not significant, compared with the condition obtained in the presence of MKK3-CA expression and in the absence of the compounds. B, p38 MAPKα mediates AICAR-induced enhancement of GR transcriptional activity in 3T3-L1 cells. Left panel, p38 MAPKα siRNA abolishes AICAR-induced enhancement of GR transcriptional activity in 3T3-L1 cells. 3T3-L1 cells were transfected with control or p38 MAPKα siRNA and were incubated with 2 mm of AICAR in the presence or absence of 10−6 m of dexamethasone. Fold induction of GILZ mRNA expression was calculated by dividing values of the mRNA expression with that of the baseline (the value obtained with transfection of control siRNA in the absence of AICAR and dexamethasone). Bars represent mean ± se values of the fold induction of GILZ mRNA expression in the presence or absence of dexamethasone. *, P < 0.01, n.s., not significant, compared with the conditions indicated. Right panels, p38 MAPKα siRNA reduces levels of p38 MAPKα mRNA and protein in 3T3-L1 cells. 3T3-L1 cells were transfected with control or p38 MAPKα siRNA and were incubated with 2 mm of AICAR. Fold induction of p38 MAPKα mRNA expression was calculated by dividing values of the mRNA expression with that of the baseline (the value obtained with transfection of control siRNA in the absence of AICAR). Bars represent mean ± se values of the fold induction of p38 MAPKα mRNA expression in the presence or absence of AICAR. *, P < 0.01, compared with the conditions transfected with control siRNA. Bottom panel demonstrates results of Western blots showing protein expression of p38 MAPKα (top gel) and actin (bottom gel) by running a fraction of samples used in the top panel and by detecting with anti-p38 MAPKα or -actin antibody. C and D, Knockdown of AMPKα1/α2 or p38 MAPKα attenuates AICAR-induced enhancement of dexamethasone-stimulated GILZ mRNA expression in the mouse liver. Mice were infected with control lentivirus particles or those expressing shRNA for AMPKα1/AMPKα2 or p38 MAPKα and were subsequently injected with AICAR (0.7 g/kg per animal) and/or dexamethasone (1.5 mg/kg per animal). Total RNA was further purified from their livers and mRNA expression of GILZ (C, top panel), G6Pase (C, middle panel), PEPCK (C, bottom panel), AMPKα1 (D, top panel), and p38 MAPKα (D, bottom panel) were examined. *, P < 0.01, n.s., not significant, compared with the condition indicated.
SB202190, an inhibitor of p38 MAPK, completely abolished AICAR-induced enhancement of dexamethasone-stimulated GILZ mRNA expression in 3T3-L1 cells, whereas the p42 MAPK inhibitor PD098058 did not have any effect (Fig. 5A, left panel). The MAPK kinase 3 (MKK3) is known to activate p38 MAPKα and stimulates mRNA expression of the p38 MAPK-responsive forkhead box M1 (FOXM1) gene in 3T3-L1 cells (43). SB202190, but not PD098058, efficiently abolished the MKK3 constitutively active form (CA)-induced FOXM1 mRNA expression, suggesting that SB202190 suppressed kinase activity of the p38 MAPKα in these cells. (Fig. 5A, right panel).
In agreement with these results, knockdown of p38 MAPKα attenuated AICAR-induced enhancement of dexamethasone effect on GILZ mRNA expression (Fig. 5B). These results indicate that p38 MAPK, but not the p42 form, mediates the effect of AMPK on GR-induced transcriptional activity.
Lentivirus-mediated knockdown of AMPKα1/AMPKα2 catalytic subunits or p38 MAPKα abolished AICAR-mediated down-regulation of GR-induced transcriptional activity in mouse liver
To further examine the influence of AMPK on the GR-induced transcriptional activity in vivo, we knocked down the AMPKα1/α2 catalytic subunits and/or p38 MAPKα in the mouse liver by injecting lentiviral particles expressing short hairpin (sh) RNA for these molecules and by treating these mice with dexamethasone and/or AICAR (Fig. 5, C and D). In the presence of control lentivirus particles, AICAR strongly suppressed dexamethasone-induced mRNA expression of GILZ, G6Pase, and PEPCK, whereas either of the lentivirus particles expressing shRNA for AMPKα1/α2 or p38 MAPKα effectively abolished this AICAR effect on GR transcriptional activity (Fig. 5C). The lentivirus particles used strongly suppressed mRNA expression of AMPKα1/α2 or p38 MAPKα (Fig. 5D). These results further suggest that AMPK modulates GR-induced transcriptional activity through activation of p38 MAPKα.
AMPK indirectly phosphorylates GR at serine 211 via p38 MAPK in vitro and in vivo
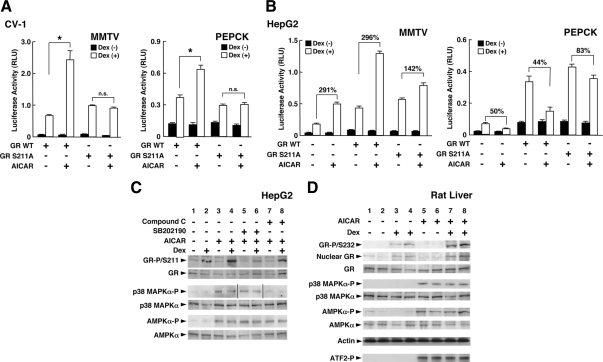
Because p38 MAPK is known to phosphorylate human GR at serine 211 (13,41), we examined the effect of AICAR on the transcriptional activity of a mutant GR, whose serine at amino acid 211 was replaced with alanine. AICAR enhanced wild-type GR-induced transcriptional activity of the mouse mammary tumor virus (MMTV) and PEPCK promoter in CV-1 cells, whereas it did not affect the transcriptional activity of this GR mutant (Fig. 6A).
Figure 6.
AMPK phosphorylates human GR at serine 211 (232 of rat GR) through p38 MAPKα and modulates GR-induced transcriptional activity by changing the attraction of coregulators to DNA-bound GR. A, Replacement of human GR serine 211 by alanine abolishes AICAR-induced enhancement of GR transcriptional activity in CV-1 cells. CV-1 cells were transfected with the plasmid expressing wild-type (WT) GR or the GR mutant defective in serine 211, together with pMMTV-Luc (left panel) or pPEPCK-Luc (right panel), and pSV40-β-Gal. They were then incubated with 2 mm of AICAR in the presence or absence of 10−6 m of dexamethasone (Dex). Bars represent mean ± se values of the luciferase activity normalized for the β-galactosidase activity in the presence or absence of dexamethasone. *, P < 0.01, n.s., not significant, compared with the condition indicated. B, Replacement of human GR serine 211 by alanine blunts AICAR-induced regulation of GR transcriptional activity in HepG2 cells. HepG2 cells were transfected with the plasmid expressing wild-type GR or the GR mutant defective in serine 211, together with pMMTV-Luc (left panel) or pPEPCK-Luc (right panel), and pSV40-β-Gal. They were then incubated with 2 mm of AICAR in the presence or absence of 10−6 m of dexamethasone. Bars represent mean ± se values of the luciferase activity normalized for the β-galactosidase activity in the presence or absence of dexamethasone. The numbers above comparison lines indicate the percent increase of the transcriptional activity between the two conditions indicated. C, AMPK phosphorylates human GR at serine 211 through the phosphorylation/activation of p38 MAPKα in HepG2 cells. The HepG2 cells were treated with 2 mm of AICAR, 2 μm of SB202190 (p38 MAPK inhibitor), and/or 40 μm of compound C (AMPK inhibitor) in the presence or absence of 10−6 m of dexamethasone. Phosphorylated forms and entire proteins of the GR, p38 MAPKα, and the AMPKα catalytic subunit were examined in Western blots using their specific antibodies. D, AICAR phosphorylates rat GR at serine 232 by activating AMPK and p38 MAPKα in rat liver. Rats were injected with AICAR (0.7 g/kg per animal) and/or dexamethasone (1.5 mg/kg), and their livers were harvested after 2 h. Whole homogenates and nuclear extracts were prepared, and the phosphorylated forms and entire proteins of GR, p38 MAPKα, and the AMPK α-subunit were examined in Western blots using their specific antibodies. The kinase activity of the p38 MAPKα was also examined by using ATF-2 as a substrate (bottom gel). Samples from two rats in each treatment were run on the gels.
We also performed similar experiments in human hepatoma-derived HepG2 cells, although these cells express endogenous functional GR (Fig. 6B). In these cells, AICAR enhanced dexamethasone-stimulated transcriptional activity of the MMTV promoter (Fig. 6B, left panel). Overexpression of wild-type GR further enhanced the AICAR effect, whereas expression of the GR mutant defective in serine 211 significantly blunted AICAR-mediated enhancement, suggesting that serine 211 mediated the positive effect of AICAR. Interestingly, AICAR suppressed dexamethasone-stimulated transcriptional activity of the PEPCK promoter in these cells (Fig. 6B, right panel), confirming that the effect of AICAR on GR-induced transcriptional activity is gene-specific. AICAR demonstrated a similar suppressive effect in the cells overexpressing wild-type GR, but this effect was significantly blunted in the cells with GRS211A overexpression. Taken together, these results indicate that serine 211 of the human GR mediates gene-specific positive and negative effects of AMPK on the transcriptional activity of this receptor.
We next examined whether AMPK phosphorylates the GR through activation of p38 MAPK in HepG2 cells and rat liver. In HepG2 cells, GR was phosphorylated at serine 211 upon addition of dexamethasone, consistent with previous reports (13) (Fig. 6C, top gel, lines 1 and 2). AICAR further enhanced dexamethasone-induced phosphorylation of serine 211 of the GR (top gel, lines 2 and 4). Addition of the p38 MAPK inhibitor SB202190 suppressed this AICAR-mediated enhancement of phosphorylation (Fig. 6, top gel, lines 4 and 6), whereas it did not affect AICAR-induced phosphorylation of the AMPKα catalytic subunit and p38 MAPKα (third top gel, lines 5 and 6, and second bottom gel, lines 5 and 6).
The AMPK inhibitor compound C reduced AICAR-mediated phosphorylation of GR and p38 MAPKα (top gel, lines 4 and 8, and third top gel, lines 7 and 8). These results indicated that AMPK enhanced GR-induced transcriptional activity by phosphorylating GR at serine 211 through phosphorylation/activation of p38 MAPKα. In the liver of rats treated with AICAR and/or dexamethasone for 2 h, AICAR also enhanced dexamethasone-induced phosphorylation of rat GR at serine 232, the rat residue orthologous to the human GR S211 (Fig. 6D, top gel, lines 3 and 4 and 7 and 8), whereas this compound induced phosphorylation of the AMPK α-subunit and p38 MAPKα (Fig. 6D, fifth and seventh top gels, lines 5–8).
AICAR stimulated kinase activity of p38 MAPKα detected by phosphorylation on an exogenous substrate, the activating transcription factor (ATF)-2 (Fig. 5G, bottom gel, lines 5–8). These results indicate that activation of AMPK phosphorylates GR at serine 211 (human) and 232 (rat) in a dexamethasone-dependent fashion both at cellular and whole animal levels.
AMPK altered GR-mediated attraction of transcriptional coregulators to the promoter region of glucocorticoid-responsive genes through phosphorylation of GR
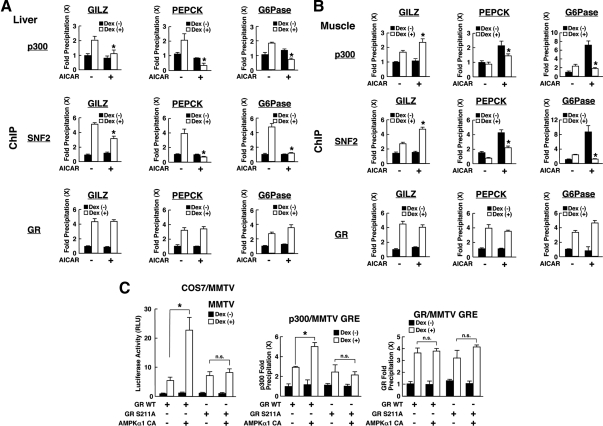
To further explore the mechanism underlying phosphorylation-mediated regulation of GR-induced transcriptional activity by AMPK, we examined attraction of coregulators p300 and SNF2 on the GRE-containing promoters, such as those of the GILZ, PEPCK, and G6Pase gene, in the liver and muscle samples obtained from the rats treated with AICAR and/or dexamethasone for 2 h (Fig. 7). p300 is a cointegrator of nuclear hormone receptors associating with these receptors and p160 type coactivators and acetylating histones and other molecules through its intrinsic histone acetyltransferase activity, whereas SNF2 is a component of the SWI/SNF complex, which alters the chromatin structure in an ATP-dependent fashion (44,45).
Figure 7.
AMPK differentially regulates dexamethasone-induced attraction of coregulators on the promoter region of glucocorticoid-responsive genes in a tissue- and gene-specific fashion through the phosphorylation of GR. A and B, AICAR differentially regulates dexamethasone (Dex)-induced attraction of p300 and SNF2 on GREs located in the promoter region of the GILZ, PEPCK, and G6Pase genes in a tissue- and gene-specific fashion. The samples obtained in Fig. 6D (liver and muscle) were fixed with 10% formaldehyde and were subsequently used for ChIP assays. ChIP assays were performed with anti-p300, -SNF2, -GR, or control antibodies. The portions of the GILZ, PEPCK, and G6Pase promoters that contain functional or putative GREs were amplified in SYBR Green-based real-time PCR. A and B, Results obtained in liver and muscle samples, respectively. Bars represent mean ± se values of fold precipitation of GILZ, PEPCK, or G6Pase GREs compared with the baseline. *, P < 0.01, compared with the baseline (the value obtained in the absence of AICAR and in the presence of dexamethasone). C, AMPK alters attraction of p300 to GREs of the MMTV promoter through the phosphorylation of GR in COS7/MMTV cells. COS7/MMTV cells were transfected with wild-type (WT) GR- or GRS211A-expressing plasmids in the presence or absence of AMPKα1-CA-expressing plasmid. Luciferase and β-galactosidase activities were measured in aliquots of the samples (left panel), whereas ChIP assays were performed in the rest of the samples by precipitating with anti-p300 (middle panel) or anti-GR (right panel) antibody and by amplifying MMTV GREs in SYBR Green-based real-time PCR. Bars represent mean ± se values of luciferase activity corrected for β-galactosidase activity (left panel) and fold precipitation of MMTV GREs compared with the baseline (middle and right panels). *, P < 0.01, n.s., not significant, compared with the conditions indicated.
In both the liver (Fig. 7A) and muscle (Fig. 7B), GR was attracted to GREs of these gene promoters in response to dexamethasone treatment. p300 and SNF2 were also attracted to these GREs in a dexamethasone-dependent fashion, whereas treatment with AICAR differentially altered dexamethasone-dependent attraction of these coregulators to the same direction observed in the induction of mRNA of the genes examined (Fig. 2).
We next transfected COS7 cells stably transformed with the MMTV promoter (COS7/MMTV) by using plasmids expressing wild-type GR or the GR mutant defective in serine 211 together with that for AMPKα1-CA (Fig. 7C). As expected, AMPKα1-CA enhanced wild-type GR-induced transcriptional activity of the MMTV promoter, whereas it did not influence that of GRS211A (Fig. 7C, left panel). In chromatin immunoprecipitation (ChIP) assays, AMPKα1 CA enhanced attraction of p300 to GREs of the MMTV promoter in wild-type GR-expressing cells but not in the cells expressing GRS211A (Fig. 7C, middle panel), suggesting that phosphorylation of GR at serine 211 is essential for AMPK-mediated alteration of cofactor accumulation on GRE-bound GR (Fig. 7C, right panel). Taken together, these results indicate that AMPK alters attraction of coregulators to promoter-bound GR through phosphorylation of GR.
AMPKα1 mRNA expression positively correlates with that of GILZ in peripheral blood mononuclear cells (PBMCs) from human subjects with different body mass indices (BMI)
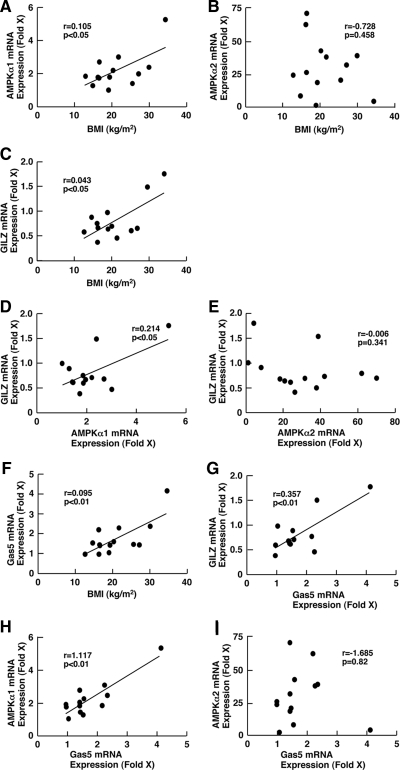
To examine the biological relevance of our results obtained at the animal and cellular levels to human pathophysiology, we purified total RNA from PBMCs obtained from human subjects with different BMIs ranging from 14.65 to 34.43 kg/m2 and measured mRNA expression of the AMPK catalytic subunits AMPKα1 and AMPKα2 and the glucocorticoid-responsive GILZ (Fig. 8). BMI correlated with mRNA expression of AMPKα1 (Fig. 8A), indicating that individuals with higher BMI have higher expression of AMPKα1 mRNA in PBMCs, in contrast to our original expectation that nutritional enrichment would suppress the expression of this gene.
Figure 8.
BMI, AMPKα1, and glucocorticoid-responsive GILZ mRNA expression is positively correlated with each other in the PBMCs of subjects with different BMIs. mRNA expression of AMPKα1, AMPKα2, Gas5, and GILZ was determined in PBMCs obtained from subjects of different BMIs, and their mutual correlations as well as correlations with BMI were examined. mRNA expression of the genes indicated was expressed as fold induction against the value of subject 1, who had normal BMI (18.98 kg/m2). The following comparisons were performed: A, BMI vs. AMPKα1; B, BMI vs. AMPKα2; C, BMI vs. GILZ; D, AMPKα1 vs. GILZ; E, AMPKα2 vs. GILZ; F, BMI vs. Gas5, G; Gas5 vs. GILZ; H, Gas5 vs. AMPKα1; and I, Gas5 vs. AMPKα2.
We surmise from this result that AMPK-mediated energy metabolism might be more active in PBMCs from subjects with higher BMI than those with lower BMI, in contrast to the liver, muscle, and fat, whereby we would have expected the opposite tendency in the regulation of AMPK activity. In addition, obesity is associated with increased risk of adipocyte damage by oxidative stress that might also play a role in the regulation of AMPK activity in this tissue (46). Interestingly, we found that GILZ mRNA expression also correlated positively with BMI, suggesting that subjects with higher BMI have elevated GR-induced transcriptional activity or hypersensitivity to glucocorticoids (Fig. 8C).
Comparison between AMPKα1 and GILZ mRNA expression revealed that these two parameters also correlated with each other (Fig. 8D), suggesting that AMPK may act as a positive regulator for tissue glucocorticoid action in PBMCs. These results are consistent with those of the animal study and cellular-based experiments that AMPK enhances GR-induced transcriptional activity in some tissues and cell types.
We further compared mRNA expression of AMPKα1 and AMPKα2 with that of the growth arrest-specific 5 (Gas5). Gas5 is a noncoding RNA, accumulating inside cells in response to starvation-related growth arrest, and functions as a transcriptional repressor for the GR by inhibiting the binding of GR to its cognate DNA sequences (47). Unexpectedly, Gas5 mRNA correlated positively with BMI and GILZ mRNA expression (Fig. 8, F and G), whereas it showed a strong positive correlation with mRNA expression of AMPKα1 but not of AMPKα2 (Fig. 8, H and I). These results suggest that the energy sensor/regulator AMPK system and the Gas5-mediated growth arrest/starvation-related regulatory pathway communicate with each other to adjust local glucocorticoid action.
Discussion
We showed that AMPK, a central player of the adaptive response to energy depletion, regulated GR-induced transcriptional activity by phosphorylating its serine residue at amino acid 211, through activation of its downstream substrate p38 MAPK. This indirect interaction between AMPK and GR observed at the cellular level was also evident at the whole animal level: AMPK reversed dexamethasone-induced steatosis in rat liver and suppressed elevation of blood glucose and hepatic glycogen by dexamethasone, most likely through regulating the action of this steroid on key glucose metabolic enzymes PEPCK and G6Pase in the liver. In mRNA expression analysis of glucocorticoid-responsive genes in various tissues and microarray analyses performed on rat liver, AMPK differentially regulated GR-induced transcriptional activity in a gene- and organ-specific fashion. Thus, our results suggest that the AMPK-mediated energy-sensing pathway communicates with the GR/glucocorticoid-mediated stress system through their molecular effectors AMPK and GR. Such pathway interaction may also be observed in human subjects with various BMI values.
Previous reports suggested that glucocorticoids exerted their metabolic effects, such as deposition of lipid in the liver, through activation of AMPK in this organ (48), whereas they stimulated mRNA expression of neuropeptide Y and the agouti-related peptide, which play important roles in the stimulation of appetite, through activation of AMPK in the arcuate nucleus of the hypothalamus (49). These reports indicate that glucocorticoids may stimulate the activity of AMPK, although the underlying molecular mechanisms have not been elucidated as yet.
Our results, together with these previous reports, suggest that glucocorticoids and AMPK influence each other’s activity. Such mutual regulation between the energy sensing and the stress-responsive systems would have been expected from the major overlap of their broad biological actions. For example, depletion of energy is an important stressor for humans and animals, whereas the adaptive response to stress includes major alterations of energy balance in the organism (2,4,50). The directions, as well as the duration and timing, of their mutual effects appear to be specific for the organs/tissues regulated, and the interactions between them appear to be at several steps of their signaling systems, including the communication between AMPK and GR that we showed here. AMPK was recently reported to regulate directly the promoter activity of the known glucocorticoid target proopiomelanocortin gene, another route through which energy and metabolism may be regulated (51).
We demonstrated the molecular mechanism through which AMPK regulates the transcriptional activity of the GR, i.e. phosphorylation of the GR at serine 211 through activation of the AMPK substrate p38 MAPKα. Serine 211 of the human GR is conserved in the rat and mouse GRs (serines 232 and 220, respectively) and is also phosphorylated by the yeast cyclin-dependent kinase p34CDC28 (18,22). In addition to serine 211, p38 MAPK is reported to phosphorylate serine 226 of the human GR (15), but the latter does not appear to be functional in AMPK-mediated regulation of GR transcriptional activity because replacement of serine 211 by alanine in the human GR completely abolished AICAR-mediated regulation of GR transcriptional activity. AMPK-mediated phosphorylation of serine 211 of the human GR resulted in altered accumulation of cofactors, p300 and the SNF2 component of the SWI/SNF complex, to ligand-activated GR bound on GREs of the glucocorticoid-responsive genes PEPCK, G6Pase, GILZ, and MMTV, possibly by preferential attraction/dissociation of phosphorylation site-specific cofactors.
These results are consistent with our previous report that showed that cyclin-dependent kinase 5, which plays important roles in the morphogenesis and functions of the nervous system and whose aberrant activation is associated with the development of neurodegenerative disorders, phosphorylated multiple serines of the human GR including serine 211, and the changed GR-induced transcriptional activity in a tissue- and gene-specific fashion (21,22).
We demonstrated that dexamethasone injection produced a severe steatotic change in the rat liver, whereas AICAR antagonized this dexamethasone effect. Steatosis is one of the most common complications of glucocorticoid excess in humans (4,7) and is characterized by massive accumulation of glycogen and lipids inside the hepatocytes (52). Dexamethasone also increased blood glucose levels, whereas AICAR strongly suppressed this change. Alteration of blood glucose levels and induction/attenuation of liver steatosis by dexamethasone and/or AICAR appear to be mediated by their differential regulations on the PEPCK and the G6Pase genes in the liver, muscle, and fat. Dexamethasone stimulated the mRNA expression of these genes in the liver and resulted in an increase in both the synthesis and release of glucose into the circulation.
AICAR strongly suppressed dexamethasone-stimulated mRNA expression of these genes, reducing blood glucose levels and antagonizing dexamethasone-induced accumulation of hepatic glycogen. AICAR, on the other hand, strongly stimulated G6Pase mRNA expression in the skeletal muscle, whereas simultaneous treatment of dexamethasone and AICAR strongly suppressed AICAR-stimulated G6Pase mRNA expression in this tissue. Taken together, these findings suggest that AMPK mainly suppresses the catabolic effects of glucocorticoids on glucose metabolism in the liver, skeletal muscle, and fat.
Diabetes mellitus type 2, hyperlipidemia, and liver steatosis are major metabolic complications associated with excess amounts or increased sensitivity of tissues to glucocorticoids (4,7,8,9). Thus, it is possible that pharmacological activation of AMPK, for example, by the use of metformin, thienopyridones, berberine, or AICAR-like nucleoside analogs, is a promising therapeutic target for eliminating these glucocorticoid-associated metabolic complications (53,54,55,56). However, AMPK and glucocorticoids both increase appetite by influencing the CNS appetite center (6,25); thus, selective AMPK activators functional only in peripheral tissues, especially in the liver, would be appropriate for suppressing metabolic action of glucocorticoids.
Our results in rat spleen and brain and human PBMCs strongly suggest the presence of functional interactions between AMPK and GR in these organs. Because glucocorticoids have numerous important actions in the regulation of the immune and the CNS (2,4), it would be interesting to examine further how the energy sensing system, represented by AMPK, alters glucocorticoid actions in these tissues. Indeed, AMPK can inhibit glucocorticoid-induced apoptosis in the thymus, an organ in which phosphorylation of GR by p38 MAPK is strongly present (13,28). It is also quite interesting to examine how the AMPK-mediated energy sensing system cooperates with other regulatory pathways, such as Gas5/starvation/growth arrest-mediated one, for controlling net glucocorticoid action at local tissues.
Materials and Methods
Plasmids and Reagents
pRShGRα, which expresses the human GR, and pRSVerbA−1, which contains the thyroid hormone receptor cDNA in an inverse orientation and was used as a negative control for pRShGRα, were both gifts from Dr. R. M. Evans (Salk Institute, La Jolla, CA) (57). pRShGRαS211A, which expresses a mutant GR having alanine at amino acid 211 instead of serine, was reported previously (21). pMMTV-luc and pPEPCK-Luc (original name: pCK2300-Luc), which express luciferase under the control of the glucocorticoid-responsive MMTV and PEPCK promoters, respectively, were gifts from Drs. G. L. Hager (National Cancer Institute, Bethesda, MD) and D. K. Granner (Vanderbilt University Medical School, Nashville, TN), respectively (37,57). pCDNA3-AMPKα1-CA, which expresses the CA of the α1-subunit of AMPK, was a gift from Dr. D. Carling (Imperial College, London, UK) (58). pRc/RSV-FLAG-MKK3 (glu) was purchased from Addgene, Inc. (Cambridge, MA). This plasmid expresses the CA of the human MKK3. MKK3 (glu) has replacements to glutamic acid at serine 189 and threonine 193 (59,60).
pCDNA3 and pRc/RSV, which are carrier plasmids for AMPKα1-CA and pRc/RSV-FLAG-MKK3 (glu), respectively, were purchased from Invitrogen (Carlsbad, CA), and were used as negative controls for pCDNA3-AMPKα1-CA and pRc/RSV-FLAG-MKK3 (glu), respectively. pSV40-β-Gal was purchased from Promega Corp. (Madison, WI). p38 MAPKα and control small interfering RNAs (siRNAs), and lentiviral particles expressing shRNA for the AMPKα1/AMPKα2 catalytic units or p38 MAPKα, and control viral particles were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). AICAR was purchased from Sigma-Aldrich (St. Louis, MO) and Toronto Research Chemicals Inc. (New York, Ontario, Canada). Compound C (AMPK inhibitor), dexamethasone, SB202190 (p38 MAPK inhibitor), and PD098059 (p42 MAPK inhibitor) were all purchased from Sigma-Aldrich. RU 486, a competitive GR antagonist, was reported previously (61).
Treatment of rats or mice with dexamethasone and/or AICAR
The following animal studies were approved by the National Institute of Child Health and Human Development Animal Care and Use Committee (protocol no. ASP07-016 and ASP09-030). Male Sprague Dawley rats (180–200 g) were injected with either AICAR (0.7 g/kg animal) or physiological saline into their peritoneal cavities. Animals in each group were also injected im with dexamethasone (1.5 mg/kg) or normal saline. Twenty-four hours after the injection, levels of blood glucose were measured using the Freestyle GlucoMeter (Abbott Laboratories, Abbott Park, IL) by sampling blood from their tails. Animals were subsequently euthanized using CO2, and their livers, skeletal muscles, sc fat, spleens, and brains were harvested for hematoxylin and eosin or PAS staining.
Sera were also obtained, and the levels of serum and liver cholesterol and triglycerides were determined by using their specific ELISA or calorimetric reactions. Serum insulin and liver glycogen levels were determined by using the rat insulin enzyme immunoassay kit (SPI Bio, Montigny le Bretonneux, France) and the glycogen assay kit (BioVision, Inc., Mountain View, CA), respectively. For Western blots examining the phosphorylation of GR in response to AICAR, the same experiments were performed, whereas the animals were euthanized 2 h after the injection of AICAR and/or dexamethasone. Their livers and whole homogenates were then harvested and were produced as previously reported (62). The extraction and purification of total RNA and/or separation of nuclear extracts for ChIP analyses were, respectively, performed in the indicated rat organs 8 and 2 h after the injection of these compounds.
C57BL/6J adult male mice (15–20 g/animal) were injected ip with 1 × 106 infectious units of virus per animal of control lentiviral particles or those expressing the shRNAs for the AMPKα1/AMPKα2 catalytic subunits and/or p38 MAPKα. Twenty-four hours after the injection of the lentiviral particles, animals were injected ip with dexamethasone (1.5 mg/kg) and/or AICAR (0.7 g/kg). After an additional 8 h, the mice were euthanized using CO2, and their livers were harvested for purification of total RNA.
SYBR Green-based real-time PCR
Total RNA was reverse transcribed into cDNA, as previously described (63,64). Real-time PCR was performed in triplicate using the SYBR Green PCR master mix (Applied Biosystems, Foster City, CA) in a 7500 real-time PCR system (Applied Biosystems), as previously described (63,64). Primer pairs used for the reactions are shown in Table 1. Obtained cycle threshold values of the target genes were normalized for those of the acidic ribosomal phosphoprotein P0 (RPLP0), and their relative mRNA expression was demonstrated as fold induction over the baseline. The dissociation curves of primer pairs used showed a single peak and samples after PCRs had a single expected DNA band in an agarose gel analysis (data not shown).
Table 1.
Primers used for SYBR Green-based real-time PCR
| Gene name | Primer sequence (5′–3′) |
|---|---|
| Mouse | |
| AMPKα1 | Forward, TGTTCCAGCAGATCCTTTCC |
| Reverse, ATAATTGGGTGAGCCACAGC | |
| FOXM1 | Forward, CCTTACTTTAAGCACATTGC |
| Reverse, GATTGGCACTTGGTGAATGG | |
| G6Pase | Forward, TTACCAAGACTCCCAGGACTG |
| Reverse, GAGCTGTTGCTGTAGTAGTCG | |
| GILZ | Forward, CAGCTGCACAATTTCTC |
| Reverse, CATCAGGTGGTTCTTCAC | |
| p38 MAPKα | Forward, CCCCAGAGATCATGCTGAAT |
| Reverse, TGAGATAAGCAGGGGGTGTC | |
| PEPCK | Forward, ATCTTTGGTGGCCGTAGACCT |
| Reverse, GCCAGTGGGCCAGGTATTT | |
| RPLP0 | Forward, GAGGACCTCACTGAGATTCG |
| Reverse, CTGGAAGAAGGAGGTCTTCTC | |
| Rat | |
| G6Pase | Forward, GGCTCACTTTCCCCATCAGG |
| Reverse, ATCCAAGTGCGAAACCAAACAG | |
| GILZ | Forward, GTGGCGGTCTATCAACTG |
| Reverse, CTGCTCAATCTTGTTGTC | |
| PEPCK | Forward, CCCAGACTAGAGATCCTGACAGAAT |
| Reverse, GCACAACGCTCTTTTCTTTTACC | |
| RPLP0 | Forward, GAGAAGACCTCTTTCTTC |
| Reverse, CAACATGTTCAGCAGTGTG | |
| Human | |
| AMPKα1 | Forward, GACTGCTACTCCACAGAGATCG |
| Reverse, TCAGCATCTGAATCACTCCTTT | |
| AMPKα2 | Forward, AACTGCAGAGAGCCATTCACTTT |
| Reverse, GGTGAAACTGAAGACAATGTGCTT | |
| Gas5 | Forward, AGCTGGAAGTTGAAATGG |
| Reverse, CAAGCCGACTCTCCATACC | |
| GILZ | Forward, GATGTGGTTTCCGTTAAGC |
| Reverse, CTCTCTCACAGCATACATCAG | |
| RPLP0 | Forward, GAGGACCTCACTGAGATTCG |
| Reverse, CTGGAAGAAGGAGGTCTTCTC |
Microarray analyses
Five milligrams of total RNA purified from the rat liver were used for producing probes with the one-cycle target labeling and control reagents kit (Affymetrix). The Rat Genome 230 2.0 tiled array (Affymetrix) was then labeled with the prepared probes, washed, and stained in the Affymetrix working station. Detailed data analysis steps were performed as previously described (21). Briefly, all hybridizations are normalized with the RMA method using Bioconductor package (http:/www.bioconductor.org). Followed by generation of gene expression ratios from treated vs. control samples, a one-sample Student t test was performed based on comparisons of gene expression values (log ratio) of the same probe set among all replicates with a critical value of P ≤ 0.05. Candidate genes were identified by using the z test by calculating the 95% cutoff interval. The Database for Annotation, Visualization and Integrated Discovery (DAVID) Bioinformatics Resources (http://david.abcc.ncifcrf.gov) was used to perform functional analysis (Fig. 3F) of all the candidate genes. The microarray data discussed in this publication have been deposited in the Gene Expression Omnibus (GEO) of the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO Series accession number GSE22647.
Cell culture, transfection, and treatment with chemical compounds
Murine preadipocyte 3T3-L1, human hepatoma HepG2 and African green monkey kidney CV-1 cells were all purchased from American Type Culture Collection (Manassas, VA). The 3T3-L1, HepG2, and CV-1 cells were maintained in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. The COS7/MMTV cells, which have stably transfected and integrated MMTV promoter, were described previously and were cultured in DMEM with the same supplements and 0.2 mg/ml neomycin (21,57). 3T3-L1 and HepG2 cells express endogenous, functional GR, whereas CV-1 and COS7/MMTV cells do not (21,57,61). 3T3-L1 cells were transfected with the indicated plasmids or siRNAs with the Nucleofector system (solution-R and program U-30; Amaxa GmbH, Cologne, Germany) with more than 80% transfection efficiency (data not shown). CV-1 and COS7/MMTV cells were transfected with the indicated plasmids with Lipofectin (Invitrogen), as previously described (61,64).
Cells were cultured with the compounds indicated in the presence or absence of 10−6 m of dexamethasone for 24 h. If transfection was applied, such compounds were added with dexamethasone into culture media 24 h after the transfection. Cells were subsequently lysed for purification of total RNA or measurement of the luciferase and β-galactosidase activities. Total RNA was purified using the RNeasy minikit (QIAGEN, Valencia, CA).
Luciferase and β-galactosidase assays
Luciferase and β-galactosidase activities were determined, as previously described (61).
Western blots
HepG2 cells were plated in 25-mm2 flasks at the density of 1 × 106 cells/flask and incubated with the compounds indicated in the presence or absence of 10−6 m of dexamethasone for 2 h. Cells were then lysed in buffer containing 50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 0.1% sodium dodecyl sulfate, 1% Nonidet P-40, 0.5% sodium deoxycholate, and one tablet per 50 ml Complete tablet (Roche Applied Science, Indianapolis, IN), and the cell extracts were obtained by centrifuging cell lysates at 10,000 × g for 15 min. Cell extracts were run on 4–20% SDS-PAGE gels, and separated proteins were blotted to nitrocellulose membranes. GR and its phosphorylated form at serine 211 or 232 were visualized with anti-GRα antibody (Santa Cruz Biotechnology) and an antibody specifically recognizing the human or rat GR phosphorylated at serine 211 or 232 (a gift from Dr. M. J. Garabedian, New York University, New York, NY), respectively.
p38 MAPKα, the AMPKα catalytic subunit, and their phosphorylated forms were detected with their specific antibodies (Santa Cruz Biotechnology). Nuclear extracts obtained from livers of the rats injected with dexamethasone and/or AICAR were also run on 4–20% SDS-PAGE gels and blotted on nitrocellulose membranes. GR, p38 MAPKα and the AMPKα catalytic subunit, and their phosphorylated forms were detected with their specific antibodies (Santa Cruz Biotechnology).
Kinase assay for p38 MAPK
Kinase activity of the p38 MAPK was measured using the p38 MAPK assay kit (Cell Signaling, Danvers, MA), as described previously (65). Briefly, livers of the rat treated with AICAR and/or dexamethasone were lysed, and phosphorylated p38 MAPK was precipitated with the antiphospho-p38 MAPK (threonine180/tyrosine 184) antibody. The precipitated proteins were then incubated with ATF-2, and phosphorylation of ATF-2 at threonine 71 was evaluated in Western blots using the antibody that reacted with the phosphorylated form.
ChIP assay
ChIP assay was performed in rat liver and muscle obtained in the animal study treated with AICAR and/or dexamethasone for 2 h or in COS7/MMTV cells. Samples were fixed in 10% formaldehyde, and DNA and bound proteins were cross-linked, and ChIP assays were performed by coprecipitating the DNA/protein complexes with anti-p300, anti-SNF2 or anti-GRα antibodies, or rabbit control IgG (Santa Cruz Biotechnology).
The −421 to −296, −447 to −281, −2452 to −2314, and −219 to −47 of the rat PEPCK, G6Pase, GILZ, and MMTV promoters containing functional or putative tandem GREs (37,38,66) were amplified from the prepared DNA samples using specific primer pairs (PEPCK promoter: 5′-GAATTAAAGGTGTATGC-3′ and 5′-CTTTGAACCTGGTATTTGC-3′, G6Pase promoter: 5′-CATCACTCTCCACAGTAGC-3′ and 5′-GCTTGACAGTGCTAAGG-3′, GILZ promoter: 5′-GAATTAAAGGTGTATGC-3′ and 5′-CTTTGAACCTGGTATTTGC-3′, MMTV promoter: 5′-AACCTTGCGGTTCCCAG-3′ and 5′-GCATTTACATAAGATTTGG-3′) in the SYBR Green-based real-time PCR using the SYBR Green PCR master mix (Applied Biosystems) and a 7500 real-time PCR system (Applied Biosystems) (57). The obtained cycle threshold values of ChIP samples were normalized for those of corresponding inputs and their relative precipitation were demonstrated as fold precipitation above the baseline.
Determination of AMPKα1/AMPKα2, Gas5, and GILZ mRNA expression in obese and anorectic lean individuals
Whole-blood samples were collected from subjects of different BMI (14.65–34.43 kg/m2, aged 8–14 yr) under a protocol approved by the Ethics Committee of the Children’s Hospital Aghia Sophia (Athens, Greece). Peripheral mononuclear cells were fractionated by using Ficoll-Paque PLUS (GE Healthcare Biosciences, Piscataway, NJ), and total RNA was purified by using TRIZOL reagent (Invitrogen). The mRNA levels of the AMPKα1 and AMPKα2 catalytic subunits, Gas5, glucocorticoid-responsive GILZ, and RPLP0 were measured with the SYBR Green-based real-time PCR, as described above. The fold induction of AMPKα1/AMPKα2 and GILZ mRNA expression was calculated against the values of subject 1 (BMI 18.98 kg/m2).
Statistical analysis
Statistical analyses were carried out by unpaired Student t test with the two-tailed P value or ANOVA, followed by a Student t test with Bonferroni correction for comparison of the results obtained in the presence of increased concentrations of AICAR (in Fig. 4B). Statistical analyses for examining correlations of mRNA expression and BMI in human samples were performed using the linear regression fit in the GraphPad PRISM 4 (GraphPad Software, Inc., La Jolla, CA).
Supplementary Material
Acknowledgments
We thank Drs. D. Carling, R. M. Evans, M. J. Garabedian, G. K. Granner, and G. L. Hager for providing their plasmids or antibody; Dr. D. Kleiner for the microscopic evaluation of liver histology; Drs. D. C. Edelman and P. S. Meltzer for supporting the microarray analyses; and Drs. A. Player and D. W. Mellon and Mr. E. K. Zachman for their superb technical assistance.
Footnotes
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, National Institutes of Health, and the University of Athens.
Disclosure Summary: All authors have nothing to disclose.
First Published Online July 21, 2010
Abbreviations: AICAR, 5-Aminoimidazole-4-carboxamide-1-β-d-ribonucleoside; AMPK, AMP-activated protein kinase; AMPKα1-CA, CA of the α1-catalytic subunit of AMPK; ATF, activating transcription factor; BMI, body mass index; CA, constitutively active form; ChIP, chromatin immunoprecipitation; CNS, central nervous system; FOXM1, forkhead box M1; Gas5, growth arrest-specific 5; GILZ, glucocorticoid-inducible leucine zipper protein; G6Pase, glucose-6-phosphatase; GR, glucocorticoid receptor; GRE, glucocorticoid response element; MKK3, MAPK kinase 3; MMTV, mouse mammary tumor virus; PAS, periodic acid Schiff; PBMC, peripheral blood mononuclear cell; PEPCK, phosphoenolpyruvate carboxykinase; RPLP0, acidic ribosomal phosphoprotein P0; sh, short hairpin; siRNA, small interfering RNA; SWI/SNF, mating-type switching/sucrose non-fermenting.
References
- Chrousos GP, Kino T 2007 Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress 10:213–219 [DOI] [PubMed] [Google Scholar]
- Kino T, Chrousos GP 2004 Glucocorticoid effect on gene expression. In: Steckler T, Kalin NH, Reul JMHM, eds. Handbook on stress and the brain, part 1. Amsterdam: Elsevier BV; 295–312 [Google Scholar]
- Kino T, De Martino MU, Charmandari E, Mirani M, Chrousos GP 2003 Tissue glucocorticoid resistance/hypersensitivity syndromes. J Steroid Biochem Mol Biol 85:457–467 [DOI] [PubMed] [Google Scholar]
- Chrousos GP 2001 Glucocorticoid therapy. In: Felig P, Frohman L, eds. Endocrinology and metabolism. 4th ed. New York: McGraw-Hill; 609–632 [Google Scholar]
- Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, Ehrhart-Bornstein M, O'Shea JJ, Chrousos GP, Bornstein SR 2002 Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J 16:61–71 [DOI] [PubMed] [Google Scholar]
- Adam TC, Epel ES 2007 Stress, eating and the reward system. Physiol Behav 91:449–458 [DOI] [PubMed] [Google Scholar]
- Dujovne CA, Azarnoff DL 1973 Clinical complications of corticosteroid therapy. A selected review. Med Clin North Am 57:1331–1342 [DOI] [PubMed] [Google Scholar]
- van Rossum EF, Russcher H, Lamberts SW 2005 Genetic polymorphisms and multifactorial diseases: facts and fallacies revealed by the glucocorticoid receptor gene. Trends Endocrinol Metab 16:445–450 [DOI] [PubMed] [Google Scholar]
- Chrousos GP 2004 The glucocorticoid receptor gene, longevity, and the complex disorders of Western societies. Am J Med 117:204–207 [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Kino T 2005 Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Sci STKE 2005:pe48 [DOI] [PubMed] [Google Scholar]
- Kino T, Chrousos GP 2004 Glucocorticoid and mineralocorticoid receptors and associated diseases. Essays Biochem 40:137–155 [DOI] [PubMed] [Google Scholar]
- Lonard DM, Lanz RB, O'Malley BW 2007 Nuclear receptor coregulators and human disease. Endocr Rev 28:575–587 [DOI] [PubMed] [Google Scholar]
- Miller AL, Webb MS, Copik AJ, Wang Y, Johnson BH, Kumar R, Thompson EB 2005 p38 mitogen-activated protein kinase (MAPK) is a key mediator in glucocorticoid-induced apoptosis of lymphoid cells: correlation between p38 MAPK activation and site-specific phosphorylation of the human glucocorticoid receptor at serine 211. Mol Endocrinol 19:1569–1583 [DOI] [PubMed] [Google Scholar]
- Ismaili N, Garabedian MJ 2004 Modulation of glucocorticoid receptor function via phosphorylation. Ann NY Acad Sci 1024:86–101 [DOI] [PubMed] [Google Scholar]
- Itoh M, Adachi M, Yasui H, Takekawa M, Tanaka H, Imai K 2002 Nuclear export of glucocorticoid receptor is enhanced by c-Jun N-terminal kinase-mediated phosphorylation. Mol Endocrinol 16:2382–2392 [DOI] [PubMed] [Google Scholar]
- Rogatsky I, Logan SK, Garabedian MJ 1998 Antagonism of glucocorticoid receptor transcriptional activation by the c-Jun N-terminal kinase. Proc Natl Acad Sci USA 95:2050–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmáry Z, Garabedian MJ, Vilcek J 2004 Inhibition of glucocorticoid receptor-mediated transcriptional activation by p38 mitogen-activated protein (MAP) kinase. J Biol Chem 279:43708–43715 [DOI] [PubMed] [Google Scholar]
- Krstic MD, Rogatsky I, Yamamoto KR, Garabedian MJ 1997 Mitogen-activated and cyclin-dependent protein kinases selectively and differentially modulate transcriptional enhancement by the glucocorticoid receptor. Mol Cell Biol 17:3947–3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Frederick J, Garabedian MJ 2002 Deciphering the phosphorylation code of the glucocorticoid receptor in vivo. J Biol Chem 277:26573–26580 [DOI] [PubMed] [Google Scholar]
- Webster JC, Jewell CM, Bodwell JE, Munck A, Sar M, Cidlowski JA 1997 Mouse glucocorticoid receptor phosphorylation status influences multiple functions of the receptor protein. J Biol Chem 272:9287–9293 [DOI] [PubMed] [Google Scholar]
- Kino T, Ichijo T, Amin ND, Kesavapany S, Wang Y, Kim N, Rao S, Player A, Zheng YL, Garabedian MJ, Kawasaki E, Pant HC, Chrousos GP 2007 Cyclin-dependent kinase 5 differentially regulates the transcriptional activity of the glucocorticoid receptor through phosphorylation: clinical implications for the nervous system response to glucocorticoids and stress. Mol Endocrinol 21:1552–1568 [DOI] [PubMed] [Google Scholar]
- Kino T 2007 Tissue glucocorticoid sensitivity: beyond stochastic regulation on the diverse actions of glucocorticoids. Horm Metab Res 39:420–424 [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG 2005 AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1:15–25 [DOI] [PubMed] [Google Scholar]
- Hardie DG 2007 AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol 47:185–210 [DOI] [PubMed] [Google Scholar]
- Kola B, Boscaro M, Rutter GA, Grossman AB, Korbonits M 2006 Expanding role of AMPK in endocrinology. Trends Endocrinol Metab 17:205–215 [DOI] [PubMed] [Google Scholar]
- Corton JM, Gillespie JG, Hawley SA, Hardie DG 1995 5-Aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229:558–565 [DOI] [PubMed] [Google Scholar]
- Sullivan JE, Brocklehurst KJ, Marley AE, Carey F, Carling D, Beri RK 1994 Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett 353:33–36 [DOI] [PubMed] [Google Scholar]
- Stefanelli C, Stanic I, Bonavita F, Flamigni F, Pignatti C, Guarnieri C, Caldarera CM 1998 Inhibition of glucocorticoid-induced apoptosis with 5-aminoimidazole-4-carboxamide ribonucleoside, a cell-permeable activator of AMP-activated protein kinase. Biochem Biophys Res Commun 243:821–826 [DOI] [PubMed] [Google Scholar]
- Lemieux K, Konrad D, Klip A, Marette A 2003 The AMP-activated protein kinase activator AICAR does not induce GLUT4 translocation to transverse tubules but stimulates glucose uptake and p38 mitogen-activated protein kinases α and β in skeletal muscle. FASEB J 17:1658–1665 [DOI] [PubMed] [Google Scholar]
- Xi X, Han J, Zhang JZ 2001 Stimulation of glucose transport by AMP-activated protein kinase via activation of p38 mitogen-activated protein kinase. J Biol Chem 276:41029–41034 [DOI] [PubMed] [Google Scholar]
- Witters LA, Gao G, Kemp BE, Quistorff B 1994 Hepatic 5′-AMP-activated protein kinase: zonal distribution and relationship to acetyl-CoA carboxylase activity in varying nutritional states. Arch Biochem Biophys 308:413–419 [DOI] [PubMed] [Google Scholar]
- Sullivan JE, Carey F, Carling D, Beri RK 1994 Characterisation of 5′-AMP-activated protein kinase in human liver using specific peptide substrates and the effects of 5′-AMP analogues on enzyme activity. Biochem Biophys Res Commun 200:1551–1556 [DOI] [PubMed] [Google Scholar]
- Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE 2001 5′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J Biol Chem 276:46912–46916 [DOI] [PubMed] [Google Scholar]
- Kiens B 2006 Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev 86:205–243 [DOI] [PubMed] [Google Scholar]
- Hardie DG, Scott JW, Pan DA, Hudson ER 2003 Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett 546:113–120 [DOI] [PubMed] [Google Scholar]
- Postic C, Dentin R, Girard J 2004 Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes Metab 30:398–408 [DOI] [PubMed] [Google Scholar]
- Imai E, Stromstedt PE, Quinn PG, Carlstedt-Duke J, Gustafsson JA, Granner DK 1990 Characterization of a complex glucocorticoid response unit in the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol 10:4712–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Morris DW, Chou JY 1998 Hepatocyte nuclear factor 1α is an accessory factor required for activation of glucose-6-phosphatase gene transcription by glucocorticoids. DNA Cell Biol 17: 967–974 [DOI] [PubMed] [Google Scholar]
- Blind RD, Garabedian MJ 2008 Differential recruitment of glucocorticoid receptor phospho-isoforms to glucocorticoid-induced genes. J Steroid Biochem Mol Biol 109:150–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin-Labat ML, David M, Biola-Vidamment A, Lecoeuche D, Zennaro MC, Bertoglio J, Pallardy M 2004 GILZ, a new target for the transcription factor FoxO3, protects T lymphocytes from interleukin-2 withdrawal-induced apoptosis. Blood 104:215–223 [DOI] [PubMed] [Google Scholar]
- Irusen E, Matthews JG, Takahashi A, Barnes PJ, Chung KF, Adcock IM 2002 p38 mitogen-activated protein kinase-induced glucocorticoid receptor phosphorylation reduces its activity: role in steroid-insensitive asthma. J Allergy Clin Immunol 109:649–657 [DOI] [PubMed] [Google Scholar]
- Shi Y, Gaestel M 2002 In the cellular garden of forking paths: how p38 MAPKs signal for downstream assistance. Biol Chem 383:1519–1536 [DOI] [PubMed] [Google Scholar]
- Behren A, Mühlen S, Acuna Sanhueza GA, Schwager C, Plinkert PK, Huber PE, Abdollahi A, Simon C 2010 Phenotype-assisted transcriptome analysis identifies FOXM1 downstream from Ras-MKK3-p38 to regulate in vitro cellular invasion. Oncogene 29:1519–1530 [DOI] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O'Malley BW 1999 Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev 20:321–344 [DOI] [PubMed] [Google Scholar]
- Rosenfeld MG, Lunyak VV, Glass CK 2006 Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev 20:1405–1428 [DOI] [PubMed] [Google Scholar]
- Eriksson JW 2007 Metabolic stress in insulin's target cells leads to ROS accumulation—a hypothetical common pathway causing insulin resistance. FEBS Lett 581:3734–3742 [DOI] [PubMed] [Google Scholar]
- Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP 2010 Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal 3:ra8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ-Crain M, Kola B, Lolli F, Fekete C, Seboek D, Wittmann G, Feltrin D, Igreja SC, Ajodha S, Harvey-White J, Kunos G, Müller B, Pralong F, Aubert G, Arnaldi G, Giacchetti G, Boscaro M, Grossman AB, Korbonits M 2008 AMP-activated protein kinase mediates glucocorticoid-induced metabolic changes: a novel mechanism in Cushing’s syndrome. FASEB J 22:1672–1683 [DOI] [PubMed] [Google Scholar]
- Shimizu H, Arima H, Watanabe M, Goto M, Banno R, Sato I, Ozaki N, Nagasaki H, Oiso Y 2008 Glucocorticoids increase neuropeptide Y and agouti-related peptide gene expression via AMP-activated protein kinase signaling in the arcuate nucleus of rats. Endocrinology 149:4544–4553 [DOI] [PubMed] [Google Scholar]
- Majzoub JA 1995 Adrenocorticotropin. In: Melmed S, ed. The pituitary. Cambridge, UK: Blackwell; 45–97 [Google Scholar]
- Iwasaki Y, Nishiyama M, Taguchi T, Kambayashi M, Asai M, Yoshida M, Nigawara T, Hashimoto K 2007 Activation of AMP-activated protein kinase stimulates proopiomelanocortin gene transcription in AtT20 corticotroph cells. Am J Physiol Endocrinol Metab 292:E1899–E1905 [DOI] [PubMed] [Google Scholar]
- Turkish AR 2008 Nonalcoholic fatty liver disease: emerging mechanisms and consequences. Curr Opin Clin Nutr Metab Care 11:128–133 [DOI] [PubMed] [Google Scholar]
- Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ 2002 Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 51:2074–2081 [DOI] [PubMed] [Google Scholar]
- Zhao G, Iyengar RR, Judd AS, Cool B, Chiou W, Kifle L, Frevert E, Sham H, Kym PR 2007 Discovery and SAR development of thienopyridones: a class of small molecule AMPK activators. Bioorg Med Chem Lett 17:3254–3257 [DOI] [PubMed] [Google Scholar]
- Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R, Adler A, Gagne G, Iyengar R, Zhao G, Marsh K, Kym P, Jung P, Camp HS, Frevert E 2006 Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab 3:403–416 [DOI] [PubMed] [Google Scholar]
- Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, Hohnen-Behrens C, Gosby A, Kraegen EW, James DE, Kim JB 2006 Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 55:2256–2264 [DOI] [PubMed] [Google Scholar]
- Kino T, Tiulpakov A, Ichijo T, Chheng L, Kozasa T, Chrousos GP 2005 G protein β interacts with the glucocorticoid receptor and suppresses its transcriptional activity in the nucleus. J Cell Biol 169:885–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel T, Carling D 2002 Functional analysis of mutations in the γ2 subunit of AMP-activated protein kinase associated with cardiac hypertrophy and Wolff-Parkinson-White syndrome. J Biol Chem 277:51017–51024 [DOI] [PubMed] [Google Scholar]
- Dérijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ 1995 Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science 267:682–685 [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Whitmarsh AJ, Barrett T, Dérijard B, Davis RJ 1996 MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol 16:1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T, Gragerov A, Kopp JB, Stauber RH, Pavlakis GN, Chrousos GP 1999 The HIV-1 virion-associated protein vpr is a coactivator of the human glucocorticoid receptor. J Exp Med 189:51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader N, Chrousos GP, Kino T 2009 Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications. FASEB J 23:1572–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichijo T, Chrousos GP, Kino T 2008 Activated glucocorticoid receptor interacts with the INHAT component Set/TAF-Iβ and releases it from a glucocorticoid-responsive gene promoter, relieving repression: implications for the pathogenesis of glucocorticoid resistance in acute undifferentiated leukemia with Set-Can translocation. Mol Cell Endocrinol 283:19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichijo T, Voutetakis A, Cotrim AP, Bhattachryya N, Fujii M, Chrousos GP, Kino T 2005 The Smad6-histone deacetylase 3 complex silences the transcriptional activity of the glucocorticoid receptor: potential clinical implications. J Biol Chem 280:42067–42077 [DOI] [PubMed] [Google Scholar]
- Kino T, Takatori H, Manoli I, Wang Y, Tiulpakov A, Blackman MR, Su YA, Chrousos GP, DeCherney AH, Segars JH 2009 Brx mediates the response of lymphocytes to osmotic stress through the activation of NFAT5. Sci Signal 2:ra5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan S, Sarabdjitsingh RA, Van Batenburg MF, Lachize SB, Li H, Dijkmans TF, Vreugdenhil E, de Kloet ER, Meijer OC 2008 Chromatin immunoprecipitation scanning identifies glucocorticoid receptor binding regions in the proximal promoter of a ubiquitously expressed glucocorticoid target gene in brain. J Neurochem 106:2515–2523 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.