Abstract
Activation of the LH receptor (LHR) on preovulatory granulosa cells stimulates the cAMP/protein kinase A (PKA) pathway to regulate expression of genes required for ovulation and luteinization. LHR signaling also initiates rearrangement of the actin cytoskeleton. Because disruption of the actin cytoskeleton has been causally linked to steroidogenesis in various cell models, we sought to identify the cellular mechanisms that may modulate reorganization of the actin cytoskeleton and to determine whether cytoskeletal reorganization is required for steroidogenesis. Herein we report that LHR signaling in preovulatory granulosa cells promotes rapid dephosphorylation of the actin-depolymerizing factor cofilin at Ser3 that is dependent on PKA. The LHR-stimulated dephosphorylation of cofilin(Ser3) switches on cofilin activity to bind actin filaments and enhance their dynamics. Basal phosphorylation of cofilin(Ser3) is mediated by active/GTP-bound Rho and downstream protein kinases; LHR signaling promotes a decrease in active/GTP-bound Rho by a PKA-dependent mechanism. LHR-dependent Rho inactivation and subsequent activation of cofilin does not involve ERK, epidermal growth factor receptor, or phosphatidylinositol 3-kinase pathways downstream of PKA. To understand the biological significance of cofilin activation, preovulatory granulosa cells were transduced with a mutant cofilin adenoviral vector in which Ser3 was mutated to Glu (S-E cofilin). Inactive S-E cofilin abolished LHR-mediated reorganization of the actin cytoskeleton and caused a 70% decrease in LHR-stimulated progesterone that is obligatory for ovulation. Taken together, these results show that LHR signaling via PKA activates a cofilin-regulated rearrangement of the actin cytoskeleton and that active cofilin is required to initiate progesterone secretion by preovulatory granulosa cells.
Activation of the LH receptor in preovulatory granulosa cells promotes the PKA-dependent activation of cofilin that is required to initiate progesterone secretion.
Final follicular maturation leading to ovulation and corpus luteum formation requires the gonadotropin LH. Activation of the LH receptor (LHR) (1), upon binding LH or the LH agonist human chorionic gonadotropin (hCG), in ovarian granulosa and theca cells initiates signaling cascades that regulate transcription of genes necessary for ovulation and luteinization. The activated LHR, a seven-transmembrane receptor that is preferentially coupled to the stimulatory guanine nucleotide-binding protein Gαs (1), stimulates adenylyl cyclase to promote a rapid increase in the second messenger, cAMP. cAMP then binds to the regulatory subunits of protein kinase A (PKA), resulting in release of active catalytic subunits. PKA catalyzes the phosphorylation of the cAMP response element binding protein (CREB) and histone H3 in preovulatory granulosa cells, both of which contribute to increased gene transcription (2,3). LHR through the actions of PKA also signals to activate the epidermal growth factor receptor (EGFR), ERK, and phosphatidylinositol 3 (PI3)-kinase pathways (reviewed in Ref. 4). Activation of the EGFR and its downstream target ERK is mediated indirectly via LHR/PKA-regulated synthesis and release of EGF-like factors amphiregulin, epiregulin, and betacellulin (5). The ERK pathway can also be activated through direct signaling cascades downstream of PKA (2). Conditional deletion of ERK1/2 from maturing granulosa cells has revealed that these kinases are required for cessation of the cell cycle; expansion of cumulus cells, induction of meiosis in oocytes, and ovulation; and terminal differentiation of granulosa into luteal cells to form the corpus luteum (6). In the absence of ERK1/2, LHR activation failed to promote both down-regulation of Lhcgr, Fshr, and aromatase (Cyp19a1) and up-regulation of cytochome P450 side chain cleavage (scc, Cyp11a1), steroidogenic acute regulatory protein (StAR, Star), prostaglandin synthase (Ptgs2), betacellulin (Btc), and progesterone receptor (Pgr) genes that are required for ovulation, cumulus cell expansion, and luteinization (6).
In contrast to the ERK pathway, the importance of the PI3-kinase pathway to the LHR response is less well understood. However, promoter/reporter studies have demonstrated that the PI3-kinase pathway contributes to the transcription of Star, low-density lipoprotein receptor (Ldlr), and Cyp11a1 genes (7).
Besides promoting signaling via the EGFR/ERK and PI3-kinase pathways to regulate gene transcription required for ovulation and luteinization, LHR signaling promotes dramatic alterations in the morphology of granulosa cells. In the absence of an LHR signal, preovulatory granulosa cells have a flat, epithelioid shape and are composed of many long actin filaments (8). Upon exposure to either LH or hCG, preovulatory granulosa cells become more rounded and exhibit a more spindle-like shape with many long, thin processes extending from the body of the cell (8). The cAMP signal transduction pathway has been implicated as the mediator of these LH/hCG-stimulated morphological changes based on the ability of forskolin (an adenylyl cyclase activator), cAMP analogs, and phosphodiesterase inhibitors to elicit these morphological changes (8,9,10,11).
LHR-induced morphological changes appear to involve the actin cytoskeleton, based on the ability of actin-disrupting agents such as cytochalasins D and B to mimic these morphological effects of LH/hCG (12,13). Chemical-induced disruption of the actin cytoskeleton has also been reported to inhibit LHR-stimulated progesterone production in hamster and avian preovulatory granulosa cell models (12,13). In other steroidogenic cell models, such as adrenal, luteal, and MA-10 tumor Leydig cells, trophic hormones or cAMP analogs consistently promote rearrangement of the actin cytoskeleton, causing cells to round (14), and chemical-induced disruption of the actin cytoskeleton often abrogates steroidogenesis, although in other instances, steroidogenesis was enhanced (15,16,17,18). Taken together, these studies suggest the rearrangement of the actin cytoskeleton that occurs in preovulatory granulosa cells in response to LH/hCG, and in other steroidogenic cells in response to trophic hormones/cAMP analogs, appears to be necessary but may not always be sufficient for the steroidogenic response.
Actin cytoskeletal dynamics are regulated by the Rho GTPase family of proteins that consist of Rho, Rac, and Cdc42. In many cell types, activation of Rho contributes to the formation of stress fibers and retraction of the lagging edge of the cell during migration, whereas activation of Rac causes the formation of lamellipoida and membrane protrusions at the leading edge of the cell during migration (reviewed in Ref. 19). Active Cdc42 causes filopodium formation and establishes cell polarity (reviewed in Ref. 19). These GTPases control actin cytoskeletal dynamics through a variety of signaling pathways that converge on proteins of the actin-depolymerizing factor/cofilin family (reviewed in Ref. 20), cofilin being the major isoform in steroidogenic cells. Deletion of cofilin is embryonically lethal and prevents proliferation and migration of neuronal precursor cells (21). Rho signaling to cofilin occurs via activation of Rho kinases 1 and 2 that in turn activate the cofilin kinase LIM-domain-containing kinase (LIMK). In some cells, Rac and Cdc42 signal to cofilin via activation of PAK (p21-activated kinase) and MRCK (myotonic dystrophy kinase-related Cdc42 binding kinase)-α, respectively, both of which cause subsequent LIMK activation. Activated LIMK phosphorylates cofilin on Ser3, a modification that blocks the ability of cofilin to bind actin (reviewed in Ref. 22). Cofilin’s effect on actin filament dynamics is concentration dependent (23). At low stoichiometry to actin, cofilin severs filaments and enhances filament dynamics by providing more barbed ends for assembly and more pointed ends for disassembly. At intermediate stoichiometry with actin, cofilin severs filaments rapidly but through cooperative binding saturates and stabilizes pieces of filamentous actin (F-actin) (24), and at high stoichiometry with actin, cofilin can nucleate new filament growth (23). Although the ratio of cofilin to actin subunits can be quite different in different regions of the same cell, the best characterized role of dephosphorylated cofilin is its ability to sever and enhance filament dynamics that are essential to lamellipodium and filopodium formation, cell migration, and overall actin cytoskeleton remodeling.
Based on circumstantial evidence that LHR-mediated changes in the actin cytoskeleton might contribute to granulosa cell functions such as steroidogenesis, we sought to examine the mechanism by which LH may regulate the actin cytoskeleton in preovulatory granulosa cells and whether this regulation of the actin cytoskeleton is required for progesterone accumulation by these cells. Our results show that in the absence of LHR signals, preovulatory granulosa cells maintain a pool of non-actin-binding cofilin phosphorylated on Ser3. This basal phosphorylation of cofilin is maintained by Rho kinase downstream of active GTP-bound Rho. LHR signals promote PKA-dependent inactivation of Rho and Rho kinase, resulting in the rapid dephosphorylation/activation of cofilin and consequent rearrangement of the actin cytoskeleton. Results using a dominant-negative cofilin demonstrate that cofilin activation within preovulatory granulosa cells is necessary for LHR-stimulated accumulation of progesterone, a critical steroid required for ovulation as well as subsequent support of pregnancy. Collectively, these results suggest that cofilin-mediated rearrangements of the actin cytoskeleton are crucial for granulosa cell functions leading to ovulation and thus fertility.
Results
LHR signaling transiently disrupts the actin cytoskeleton
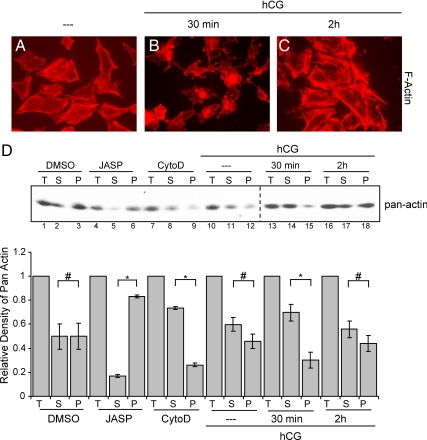
Initially, we sought to establish a time course of hCG-stimulated changes of the actin cytoskeleton in the preovulatory granulosa cell model. To this end, preovulatory granulosa cells were harvested from immature female rats 48 h after sc injection with 10 IU of pregnant mare serum gonadotropin (PMSG), plated on coverslips and allowed to incubate overnight. Cells were left untreated or treated with hCG and then fixed and permeabilized at specific time points after hCG treatment and stained with phalloidin-rhodamine. Phalloidin is a phallotoxin that binds only to F-actin and was used to observe changes in F-actin in response to LHR signaling. The actin cytoskeleton within untreated cells is composed of many long bundles of F-actin, and the overall cell shape is flattened and fibroblastic in appearance (Fig. 1A). As early as 5 min after hCG treatment (data not shown), the actin fibers begin to disappear, and by 30 min after treatment (Fig. 1B), long actin fibers are no longer visible. At this time point, the actin cytoskeleton is more punctate than fibrous, and the cells have a more dendritic phenotype with long thin cellular processes extending from the cell body. This effect of LHR signaling on the cytoskeleton is transient; by 2 h after hCG treatment, the cells exhibit actin fibers similar to those seen in untreated cells (Fig. 1C).
Figure 1.
LHR signaling disrupts the actin cytoskeleton. A–C, Preovulatory granulosa cells from PMSG-primed rats were left untreated (A) or treated for 30 min (B) or 2 h (C) with 1 IU/ml hCG. Cells were then fixed and permeabilized according to methods described in Materials and Methods. Cells were then stained with phalloidin linked to rhodamine (red labeling). Images shown are representative of three separate experiments. D, Cells were left untreated or treated with hCG for 30 min and 2 h. Controls were treated with DMSO for 1 h, 0.5 μm jasplakinolide (JASP) for 30 min, or 0.5 μg/ml cytochalasin D (CytoD) for 1 h. Pelleted/ cytoskeletal (P) and soluble (S) fractions were harvested according to methods described in Materials and Methods. Both P and S fractions along with total (T) cell lysates were loaded equally onto SDS-PAGE gels, and Western blots were performed to determine amount of actin in each fraction. Western results are representative of three separate experiments. The bar graph represents quantification of the S fraction, P fraction, and S+P fraction (T) where S+P fraction has been set to the value of one. Values are the mean ± se of three separate experiments. *, P < 0.01; #, P > 0.01.
To quantify this effect of hCG on the actin cytoskeleton, we examined the shift in the ratio of cytoskeletal to soluble actin in preovulatory granulosa cells. Cells were left untreated or treated with hCG for 30 min and 2 h. Cell lysates were harvested and separated by low-speed centrifugation into fractions containing either soluble (supernatant) actin (referred to as S) or pelleted/cytoskeletal actin (referred to as P) (25). In the absence of added myosin II to the lysis buffer to bind and pellet F-actin at low speeds, this assay does not separate globular actin and F-actin pools (25); however, it does effectively separate the cytoskeletal cross-linked actin [pelletable actin (P-actin)] from soluble unassembled or uncomplexed oligomers of actin [soluble actin (S-actin)], as we demonstrate below in control experiments. Two actin cytoskeleton-disrupting reagents, cytochalasin D and jasplakinolide, were used as positive controls. Jasplakinolide is a cell-permeable peptide isolated from the marine sponge, Jaspis johnstoni, that binds to and stabilizes F-actin in vitro (26). Jasplakinolide was used to induce assembly and stabilize F-actin and to provide a positive control for the amount of cytoskeletal actin (P-actin) that can form in preovulatory granulosa cells. Cytochalasins are cell-permeable cytotoxins that inhibit actin polymerization in vitro. These toxins bind to growing ends of F-actin and prevent addition of actin monomers to these sites, thus inducing depolymerization of dynamic actin filaments (27,28,29,30). Cytochalasin D was used to disrupt the actin cytoskeleton and to provide a positive control for the amount of S-actin that could be induced in preovulatory granulosa cells. Equal volumes of each fraction (S, P, and total actin) were assessed by Western blotting using a pan-actin antibody. Quantification of results is shown in Fig. 1D, bottom panel. Untreated and dimethylsulfoxide (DMSO)-treated cells showed no significant (P > 0.01) differences between S-actin and P-actin (Fig. 1D, lanes 2, 3 and 11, 12), whereas the difference between these fractions within cells was significant (P < 0.01) upon treatment with either jasplakinolide or cytochalasin D (Fig. 1D, lanes 5, 6 and 8, 9, respectively). By 30 min after hCG treatment, the amount of S-actin increased significantly (P < 0.01) in relation to P-actin (Fig. 1D, compare lanes 11, 12 to 14, 15). By 2 h of hCG treatment, the amount of S- and P-actin was no longer significantly different (P > 0.01) (Fig. 1D, lanes 17 and 18). This relative LHR-mediated increase in S-actin and corresponding decrease in P-actin coincides with changes observed in actin cytoskeleton morphology in response to LHR signaling in preovulatory granulosa cells.
LHR signaling promotes dephosphorylation/activation of cofilin
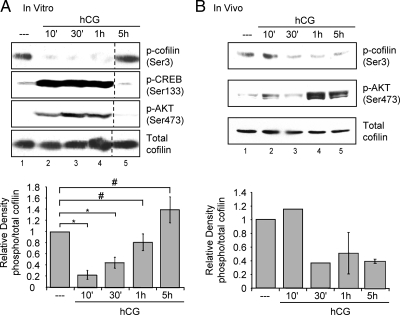
Based on our evidence that LHR signaling caused a quantitative decrease in cytoskeletal-associated actin in preovulatory granulosa cells, we sought to identify the cellular mechanism(s) responsible for this change. Cofilin is an actin-dynamizing protein whose ability to bind to actin and to initiate actin dynamics is inhibited by phosphorylation of Ser3 (20). Conversely, dephosphorylation of cofilin at Ser3 is activating and necessary for cofilin to participate in actin cytoskeleton remodeling. To determine whether LHR signaling affects cofilin phosphorylation, phosphorylation of cofilin(Ser3) was examined in preovulatory granulosa cells. Cells isolated from PMSG-primed rats were left untreated or treated with hCG, and total cell lysates were collected at specific time points after treatment. Western blots were performed using a phosphospecific antibody that detects cofilin phosphorylation on Ser 3 [anti-phospho-cofilin(Ser3)] as well as an antibody that detects total cofilin. Quantification of phospho-cofilin(Ser3) relative to total cofilin is shown in Fig. 2A, bottom panel. In untreated cells, a pool of cofilin is phosphorylated and therefore inactive (Fig. 2A, lane 1). Treatment with hCG caused a significant (P < 0.01) decrease in cofilin(Ser3) phosphorylation by 10 min (Fig. 2A, lane 2 compared with lane 1 and lower panel). This dephosphorylation of cofilin(Ser3) is still significant (P < 0.01) 30 min after hCG treatment and coincides with phosphorylation of the established LHR signaling targets, CREB at Ser133 and AKT at Ser473 (Fig. 2A, lane 3). The phosphorylation of cofilin(Ser3) is restored by 2 h (not shown) and maintained at 5 h after hCG addition (Fig. 2A, lane 5). Taken together, these data suggest that LHR signaling in vitro in preovulatory granulosa cells causes a rapid, albeit transient, dephosphorylation/activation of the actin-depolymerizing factor cofilin.
Figure 2.
LHR signaling decreases cofilin phosphorylation in vitro and in vivo. A, Preovulatory granulosa cells from PMSG-primed rats were isolated and plated overnight in complete medium. Cells were then left untreated (- - -) or treated with 1 IU/ml hCG for indicated times. Western blots are representative of five separate experiments. Graphs represent densitometric analysis of phospho (p)-cofilin Western values divided by total cofilin values to account for loading differences. Values are expressed as fold change from that of the untreated lysates, which has been set to a value of one. Values are the mean ± se of five separate experiments. *, P < 0.01; #, P > 0.01. B, PMSG-primed rats were injected with 50 IU hCG or not treated (0 h). Ovaries were harvested at specific times after injection, and whole ovarian extracts were prepared according to Materials and Methods. Clarified lysates were used for SDS-PAGE. Results for 10- and 30-min time points are from one experiment and results for 1- and 5-h time points are representative of two separate experiments. Graphs represent densitometric analysis of phospho-cofilin divided by total cofilin Western values to account for loading differences. Values are expressed as fold change from that of 0 h, which has been set to a value of one. Values are the mean ± sd of two separate experiments.
To confirm that this dephosphorylation event occurs in vivo, we examined phosphorylation of cofilin in whole ovarian extracts from PMSG-primed rats that were either left untreated or injected ip with 50 IU hCG. Ovaries were harvested at specific time points after ip injections, and Westerns blots were performed with anti-phospho-cofilin(Ser3). Quantification of phospho-cofilin(Ser3) relative to total cofilin is shown in Fig. 2B, bottom panel. Ovarian extracts from rats not injected with hCG exhibit basal phosphorylation of cofilin (Fig. 2B, lane 1). Reduced phosphorylation of cofilin(Ser3) is detected by 30 min after ip hCG injection and persists at 5 h after treatment (Fig. 2B, lanes 3–5). LHR-stimulated cofilin(Ser3) dephosphorylation after the ip injection of hCG appears to occur somewhat more slowly than the phosphorylation of AKT(Ser473) (Fig. 2B, lane 2). The modestly slower time course for the in vivo vs. in vitro responses to hCG (Fig. 2, A vs. B) reflects the additional minutes required for hCG to diffuse from the peritoneal cavity to the venous blood supply and to travel from the heart to the lungs and back to the heart. These results demonstrate that reduced cofilin phosphorylation in response to hCG occurs in a physiological context and may thus contribute to LH-induced ovulation and luteinization.
LHR-stimulated cofilin dephosphorylation is mediated by PKA
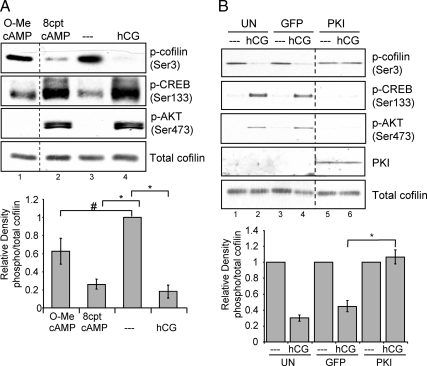
Initiation of LHR signaling causes an activation of adenylyl cyclase resulting in an increase in cAMP, subsequent activation of PKA (31), and potential activation of the Rap guanine exchange nucleotide factor EPAC (exchange protein activated by cAMP) (32,33). To determine whether cAMP mediates hCG-stimulated dephosphorylation of cofilin(Ser3), preovulatory granulosa cells were treated with a saturating concentration (34) of the cell-permeable cAMP analog that activates PKA and EPAC, 8–4-chlorophenylthio cAMP (8-cpt-cAMP) (35), for 10 or 30 min. Quantification of phospho-cofilin(Ser3) relative to total cofilin in total cell extracts is shown in Fig. 3A, bottom panel. Results indicate that treatment of cells with 8-cpt-cAMP caused a significant (P < 0.001) decrease in cofilin(Ser3) phosphorylation compared with untreated controls that is comparable to the effect of hCG on cofilin dephosphorylation of cofilin (Fig. 3A, lane 4 compared with lanes 2 and 3). Treatment with 8-cpt-cAMP also caused phosphorylation of CREB at Ser133 and AKT at Ser473 (Fig. 3A, lane 2).
Figure 3.
LHR signaling to cofilin is mimicked by cAMP and requires PKA. A, Preovulatory granulosa cells were left untreated (- - -) or treated with 1 IU/ml hCG, 500 μm 8-cpt-cAMP, or 500 μm 8-cpt-2′O-Me-cAMP for 10 or 30 min. Western blots are representative of five separate experiments. Graphs represent densitometric analysis of phospho (p)-cofilin Western values divided by total cofilin values to account for loading differences. Values are expressed as fold change from that of the untreated lysates, which has been set to a value of one. Values are the mean ± se of five separate experiments. *, P < 0.001; #, P < 0.01. B, Preovulatory granulosa cells were left untransduced or transduced overnight with either empty GFP or PKI adenovirus at a multiplicity of infection of 100. Cells were then left untreated (- - -) or treated with hCG for 10 min. Western blots are representative of three separate experiments. Graphs represent densitometric analysis of phospho-cofilin Western values divided by total cofilin values to account for loading differences. Values are expressed as fold change from that of the untreated controls, which has been set to a value of one. Values are the mean ± se of three separate experiments. *, P < 0.01.
Although the more established target of cAMP in preovulatory granulosa cells is PKA (31,34,36), EPAC is also a potential target of cAMP (32,33). A cell-permeable cAMP analog, 8-(4-chlorophenylthio)-2′-O-methyl cAMP (8-cpt-2′O-Me-cAMP), that activates only EPACs (37) and an adenoviral-expressed PKA catalytic subunit-specific inhibitor protein (PKI) (38,39), were used to determine whether one or both of these cAMP targets is involved in LHR signaling to cofilin. Treatment of cells with 8-cpt-2′O-Me-cAMP did not cause a significant decrease (P > 0.01) in cofilin(Ser3) phosphorylation compared with treatments with hCG or 8-cpt-cAMP (Fig. 3A, lane 1 compared with lanes 2 and 4). However, transduction of cells with an adenovirus expressing PKI (39) demonstrates that PKI fully blocked (P < 0.01) the ability of hCG to promote the dephosphorylation of cofilin(Ser3) (Fig. 3B, lanes 5 and 6). The complete blockade of LHR-stimulated cofilin dephosphorylation by adenoviral PKI suggests that PKA and not EPAC is the predominant downstream target of cAMP necessary for LHR-mediated cofilin dephosphorylation.
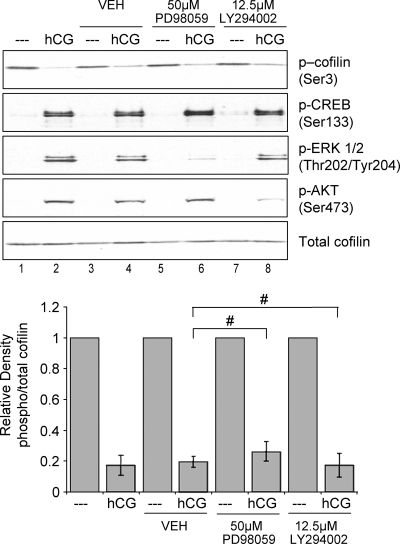
Dephosphorylation of cofilin in leukocytes and in a fibroblast cell line is mediated by the ERK and PI3-kinase pathways (40,41). In preovulatory granulosa cells, PKA activation not only promotes phosphorylation of direct PKA targets such as CREB but also leads to activation of the ERK, PI3-kinase, and EGFR signaling pathways (reviewed in Ref. 4). LHR signaling activates the ERK pathway both directly via PKA and by initiating synthesis and release of EGF-like factors amphiregulin, epiregulin, and betacellulin that activate the EGFR, which in turn activates the ERK pathway (2,42,43,44,45). LHR signaling also promotes phosphorylation of AKT, a downstream target of PI3-kinase (34). Thus, we hypothesized that one of these pathways may mediate the effect of PKA on cofilin phosphorylation in preovulatory granulosa cells. To this end, we used two different signal transduction pathway inhibitors to examine the contribution of these pathways to hCG-stimulated cofilin phosphorylation. PD98059 inhibits MAPK kinase (46), the ERK kinase, and was used to determine whether signaling from PKA and/or EGFR to the ERK signaling pathway is required for hCG-mediated cofilin dephosphorylation. LY294002 was used to block PI3-kinase signaling (47). Cells were preincubated with each inhibitor and then left untreated or treated with hCG for 10 or 30 min. Quantification of phospho-cofilin(Ser3) relative to total cofilin in total cell extracts is shown in Fig. 4, bottom panel. Results demonstrate that neither inhibitor affected basal phosphorylation of cofilin and that both inhibitors failed to significantly block LHR-dependent dephosphorylation of cofilin(Ser3) (Fig. 4, lanes 6 and 8 compared with lane 4, P > 0.01). However, PD98059 and LY294002 significantly reduced LHR-stimulated phosphorylation of ERK and AKT (P < 0.01), respectively, indicating that both inhibitors were able to inhibit signaling in their respective pathways (Fig. 4, compare lanes 6 and 8 with lane 2). Taken together, these data strongly suggest that LHR signaling to promote cofilin dephosphorylation does not involve the potentially intermediate PI3-kinase, ERK, or EGFR signaling pathways downstream of PKA.
Figure 4.
The ERK, PI3-kinase, and EGFR pathways are not involved in LHR signaling to cofilin. Preovulatory granulosa cells were not treated or treated with DMSO (VEH) for 1.5 h, 50 μm PD98059 (PD) for 1.5 h, or 12.5 μm LY294002 (LY) for 1 h and then left untreated (- - -) or treated with hCG for 10 or 30 min. Western blots are representative of three separate experiments. Graphs represent densitometric analysis of phospho (p)-cofilin Western values divided by total cofilin values to account for loading differences. Values are expressed as fold change from that of untreated controls, which has been set to a value of one. Values are the mean ± se of three separate experiments. #, P > 0.01.
LHR signals via PKA to promote inactivation of the Rho signaling pathway
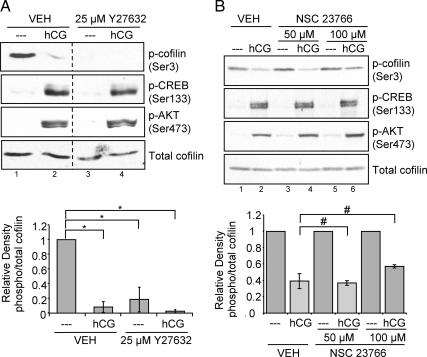
We next sought to evaluate how PKA promotes the dephosphorylation of cofilin. To this end, we initially investigated the participation of Rho GTPases in the basal phosphorylation status of cofilin. Rho GTPase activates the Rho kinases 1 and 2 that in turn activate the cofilin kinase LIMK. Rac GTPase activates p21-activated kinase, which also activates LIMK. Two inhibitors, Y27632 and NSC23766, were used to determine whether either of these pathways was involved in LHR signaling to cofilin. Y27632 selectively inhibits Rho kinases by binding to its active site and preventing it from accessing ATP (48). NSC23766 inhibits Rac1 GDP/GTP exchange activity by blocking the interaction of Rac with the Rac-specific guanine nucleotide exchange factors Trio and Tiam (49,50). Preovulatory granulosa cells were pretreated with vehicle (DMSO) or 25 μm Y27632 for 1 h and then left untreated or treated with hCG for 10 or 30 min. Quantification of phospho-cofilin(Ser3) relative to total cofilin is shown in Fig. 5, bottom panel. Pretreatment of cells with Y27632 significantly (P < 0.01) eliminated all basal phosphorylation of cofilin(Ser3), thus mimicking the effect of hCG on cofilin dephosphorylation (Fig. 5A, lane 3). This inhibitor did not affect hCG-stimulated CREB or AKT phosphorylation, indicating that this inhibitor did not compromise LHR signaling to other downstream PKA targets. Taken together, these results suggest that Rho kinase and therefore Rho signaling is necessary for the basal phosphorylation of cofilin(Ser3).
Figure 5.
Rho GTPase signaling is necessary for LHR-stimulated phospho-regulation of cofilin, whereas Rac GTPase signaling is not. A, Preovulatory granulosa cells were treated with DMSO (VEH) or 25 μm Y27632 for 1 h and then left untreated (- - -) or treated with 1 IU/ml hCG for 10 or 30 min. Western blots are representative of five separate experiments. Graphs represent densitometric analysis of phospho (p)-cofilin Western values divided by total cofilin values to account for loading differences. Values are expressed as fold change from that of untreated lysates, which has been set to a value of one. Values are the mean ± se of five separate experiments. *, P < 0.01. B, Preovulatory granulosa cells were treated with ddH2O (VEH) or with 50 or 100 μm NSC23766 for 1 h and then left untreated (- - -) or treated with 1 IU/ml hCG for 10 or 30 min. Western blots are representative of three separate experiments. Graphs represent densitometric analysis of phospho-cofilin Western values divided by total cofilin values to account for loading differences. Values are expressed as fold change from that of untreated controls, which has been set to a value of one. Values are the mean ± se of three separate experiments. #, P > 0.01.
To evaluate whether Rac GTPase may also participate in LHR signaling to cofilin, preovulatory granulosa cells were treated with vehicle (ddH2O) or the Rac inhibitor NSC23766 at concentrations of 50 and 100 μm for 1 h and then left untreated or treated with hCG for 10 or 30 min. Quantification of phospho-cofilin(Ser3) relative to total cofilin is shown in Fig. 5B, bottom panel. Neither concentration of NSC23766 significantly (P > 0.01) compromised the ability of hCG to reduce the phosphorylation of cofilin(Ser3) (Fig. 5B, lanes 4 and 6). In contrast to the Rho kinase inhibitor Y27632, NSC23766 did not affect basal cofilin phosphorylation (Fig. 5B, lanes 3 and 5). This result suggests that Rac does not participate in LHR signaling to cofilin and that Rac is not necessary for basal phosphorylation of cofilin(Ser3).
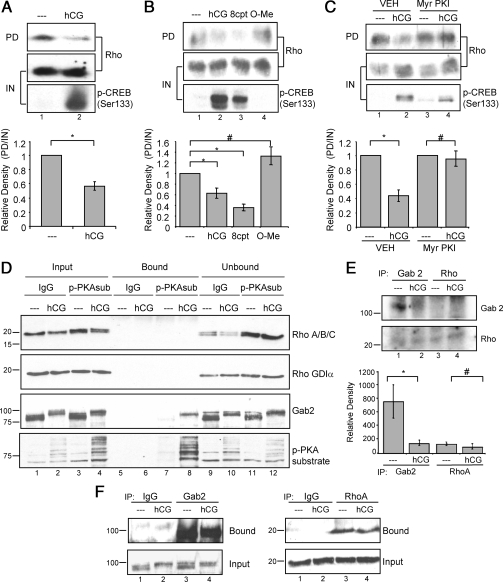
Based on the ability of the Rho kinase inhibitor to mimic LHR-stimulated dephosphorylation of coflin(Ser3), we hypothesized that stimulation of the LHR promotes Rho inactivation. Inactivation of Rho would prevent activation of Rho kinase and downstream LIMK, thereby halting phosphorylation of cofilin(Ser3). To test this hypothesis, we performed Rho-GTP pull-down assays to determine whether LHR signaling affects the active/GTP-bound state of Rho. Lysates from cells left untreated or treated with hCG were incubated with the agarose-conjugated Rho-binding domain of rhotekin. Rho is able to bind rhotekin only in its active/GTP-bound state; therefore, we used this reagent to determine whether LHR signaling regulates Rho activity. Quantification of active/GTP-bound Rho in the pull-down relative to total Rho input is shown in the bottom panel of Fig. 6A. Results show that Rho is active/GTP bound in untreated cells and that hCG treatment stimulated a significant (P < 0.001) decrease in the amount of active/GTP-bound Rho in preovulatory granulosa cells. Pull-down lysates were also probed for CREB(Ser133) phosphorylation as a positive control for PKA signaling. Taken together, these results suggest that LHR signaling leads to decreased Rho activity.
Figure 6.
LHR signaling via cAMP and PKA decreases Rho activity, but Rho is not phosphorylated directly by PKA. A, Preovulatory granulosa cells were left untreated (- - -) or treated with 1 IU/ml hCG for 10 or 30 min. Rho pull-down assays were performed as described in Materials and Methods. Western blots are representative of seven separate experiments. GTP-bound Rho levels were quantified by densitometric analysis of pull-down Western blot values divided by input to account for loading differences. Values for hCG-treated samples are expressed as a fold change from that of untreated controls, which has been set to a value of one. Values are the mean ± se of seven separate experiments. *, P < 0.001. B, Preovulatory granulosa cells were left untreated (- - -) or treated with 1 IU/ml hCG, 500 μm 8-cpt-cAMP (8cpt), or 500 μm 8-cpt-2′O-Me-cAMP (O-Me) for 10 or 30 min. Rho pull-down assays were performed as described in Materials and Methods. Western blots are representative of three separate experiments. GTP-bound Rho levels were quantified as described in A. Values are for hCG-treated or cAMP analog samples expressed as fold change from that of untreated controls, which has been set to a value of one. Values are the mean ± se of three separate experiments. *, P < 0.05; #, P > 0.05. C, Preovulatory granulosa cells were treated with ddH2O (VEH) or 50 μm Myr-PKI for 1 h and then left untreated or treated with 1 IU/ml hCG for 10 or 30 min. Rho pull-down assays were performed as described in Materials and Methods. Western blots are representative of three separate experiments. GTP-bound Rho levels were quantified as described in A. Values are expressed as a fold change from untreated controls with no inhibitor pretreatment. *, P < 0.01; #, P > 0.01. D, After treatment of preovulatory granulosa cells without or with 1 IU/ml hCG for 30 min, soluble cell extracts were subjected to coimmunoprecipitation using control IgG or anti-phospho-PKA substrate (p-PKAsub) antibody, as described in Materials and Methods. Input, unbound, and bound (immunoprecipitated) proteins were probed by Western blotting with indicated antibodies. The lower portion of the blot (below 37 kDa) was probed with RhoA/B/C and RhoGDIα antibodies; the upper portion of the blot was probed with phospho-PKA substrate and Gab2 antibodies. Molecular mass markers (kilodaltons) are indicated at the left. Input and unbound reflects 4% of total lysate; bound reflects 100% of immunoprecipitated proteins. Results are representative of four experiments. E, Granulosa cells were untreated or treated with 1 IU/ml hCG for 10 min. Soluble extracts were subjected to immunoprecipitation using antibodies to Gab2 or RhoA, and washed immunocomplexes were subjected to a back-phosphorylation assay in the presence of [γ-32P]ATP and the PKA catalytic subunit, as described in Materials and Methods. Results are representative of three separate experiments. Quantitation of signals is shown in the lower panel. *, P < 0.05; #, P > 0.05. F, After treatments of granulosa cells as described in E, soluble extracts were subjected to immunoprecipitation using indicated antibodies. Bound and input protein fractions were probed with indicated antibodies. Results are representative of 3 separate experiments.
To evaluate whether cAMP via PKA mediates the hCG-dependent inactivation of Rho, the amount of active/GTP-bound Rho was assessed in cells treated with the cell-permeable cAMP analogs 8-cpt-cAMP and 8-cpt-2′O-Me-cAMP and with myristoylated PKI peptide (Myr-PKI). Quantification of active/GTP-bound Rho in the pull-down relative to the input is shown in the bottom panel of Fig. 6B. Results show that 8-cpt-cAMP but not 8-cpt-2′O-Me-cAMP mimicked hCG and significantly (P < 0.05) reduced the level of active/GTP-bound Rho (Fig. 6B). This result suggests that PKA is responsible for the decrease in Rho activity and that EPAC is not involved in LHR signaling to Rho. To verify that PKA is necessary for hCG-stimulated Rho inactivation, Myr-PKI was used to block PKA activity. Results show that Myr-PKI abrogated the hCG-stimulated inactivation of Rho (Fig. 6C). We can conclude that the LHR-dependent conversion of Rho from its active, GTP-bound state to the inactive state is mediated by cAMP and PKA.
Although there are a number of possible mechanisms by which PKA can promote the inactivation of Rho, the most likely mechanism is the direct phosphorylation of Rho on Ser188 by PKA (51,52). This phosphorylation causes Rho to bind the guanine disassociation inhibitor-α (RhoGDIα) and to translocate away from the cell membrane, thereby excluding it from potential targets (reviewed in Ref. 53). However, we were not able to detect hCG-stimulated RhoA phosphorylation using a phospho-RhoA(Ser188) antibody either in total cell lysates or RhoA immunoprecipitates (not shown). Alternatively, we evaluated the PKA-dependent phosphorylation of Rho in cells treated without or with hCG for 30 min by immunoprecipitating with phospho-PKA substrate antibody vs. control IgG. The lower portion of blots was probed with a pan-Rho antibody that detects RhoA/B/C and an antibody that detects RhoGDIα; the upper portion of blots was probed both with phospho-PKA substrate antibody and with an antibody to the adaptor GRB2 (growth factor receptor-bound protein 2)-associated binding protein 2 (Gab2), which we recently identified as a direct PKA target (Hunzicker-Dunn, M., unpublished), as positive controls (Fig. 6D). Results show that neither RhoA/B/C nor RhoGDIα was immunoprecipitated by phospho-PKA substrate antibody despite the clear ability of this antibody to pull down phosphorylated PKA substrates as well as Gab2 in immunoprecipitations from cells treated with hCG but not with vehicle (Fig. 6D, compare lanes 7 and 8). Because the PKA phosphorylation sites on RhoA/B are atypical (KKKSG and RYGSQ, respectively, vs. RRXS/TX), we conducted a back-phosphorylation reaction in which RhoA or Gab2, as a positive control, was immunoprecipitated, and washed immunocomplexes were subjected to an in vitro phosphorylation reaction in the presence of exogenous PKA catalytic subunit. Proteins phosphorylated by PKA in the intact cell should be resistant to additional phosphorylation in the in vitro back-phosphorylation reaction. Results show that whereas Gab2 from untreated cells was readily phosphorylated in the back-phosphorylation reaction, Gab2 phosphorylation was not detected in cells treated with hCG (Fig. 6E, lanes 1 and 2 and lower panel), consistent with hCG-stimulated Gab2 phosphorylation in intact granulosa cells. In contrast, RhoA does not appear to be a PKA target in intact granulosa cells, based on the absence of signal in lane 3 vs. lane 4 in the back-phosphorylation assay. Results in Fig. 6F confirm that both Gab2 and RhoA are readily immunoprecipitated from granulosa cells. Based on these results, we conclude that negative regulation of Rho by LHR signaling in preovulatory granulosa cells does not occur via direct phosphorylation of Rho by PKA or via association of Rho with RhoGDIα.
The activation of cofilin is necessary for hCG-mediated effects on the actin cytoskeleton and to stimulate progesterone production by granulosa cells
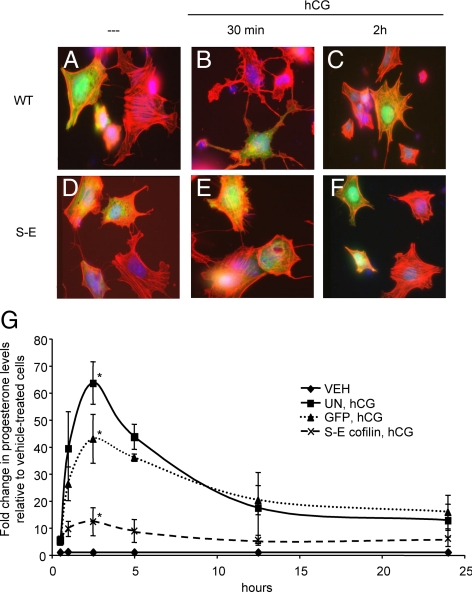
LHR signaling causes a rapid, transient rearrangement of the actin cytoskeleton that coincides with the dephosphorylation and activation of cofilin (see Fig. 1). To determine whether the activation of cofilin is necessary for actin cytoskeleton rearrangement observed in response to LHR signaling, we used an adenoviral construct that expresses a Xenopus cofilin in which the Ser3 has been mutated to glutamic acid (XAC1-SE-GFP) (54). This mutation of the Xenopus cofilin results in a phospho-mimetic cofilin that has been shown to function in primary rat neuronal cells in a dominant-negative manner (55). Preovulatory granulosa cells were plated on coverslips and transduced with XAC1-WT-GFP (WT) or XAC1-SE-GFP (S-E) adenovirus overnight. Transduced cells were then left untreated or treated with hCG for 30 min and 2 h and then stained with phalloidin-rhodamine to visualize F-actin. In untreated cells, both WT- and S-E-transduced cells exhibit similar actin cell morphology and contain many long bundles of F-actin in the cytosol (Fig. 7, A and D). Within 30 min of hCG treatment, WT-transduced cells undergo morphological changes in which their actin cytoskeleton becomes less filamentous and more punctate (Fig. 7B), consistent with results seen in Fig. 1A. The overall cell morphology also changes drastically, and the cells are more round and spindly. However, cells that express the mutated S-E cofilin maintain actin bundles even after 30 min of hCG treatment (Fig. 7E) comparable to those of untreated cells (Fig. 7, A and D). By 2 h after hCG treatment, WT-transduced cells again exhibit F- actin bundles in their cytosol, whereas actin bundles within S-E-transduced cells remain unchanged (Fig. 7, C and F). These data indicate that LHR-mediated dephosphorylation and activation of cofilin is required for the actin cytoskeleton rearrangement seen in response to LHR signaling in preovulatory granulosa cells.
Figure 7.
Constitutively inactive cofilin inhibits LHR-mediated actin cytoskeleton rearrangement. A–F, Preovulatory granulosa cells were transduced with XAC1-WT-GFP (WT; panels A–C) or XAC1-SE-GFP (S-E; panels D–F) overnight and then left untreated (- - -) or treated for 30 min (panels B and E) or 2 h (panels C and F) with 1 IU/ml hCG. Cells were then fixed and permeabilized according to methods described in Materials and Methods. Cells were then stained with phalloidin linked to rhodamine (red labeling). Images shown are representative of three separate experiments. G, Preovulatory granulosa cells were left untransduced (UN) or transduced with XAC1-GFP S-E (S-E) cofilin or empty GFP control (GFP) adenovirus overnight and then left untreated or treated with 1 IU/ml hCG. Cell culture medium was collected at specific time points after hCG and evaluated for progesterone via RIA. Concentration of progesterone from hCG-treated cells was evaluated as fold increase over progesterone levels in untreated cells (VEH). Values are the mean ± se of three separate experiments. P values for 2.5- and 5-h time points are shown: **, P < 0.01; *, P < 0.05; #, P > 0.05.
Next, we examined whether active cofilin was required for progesterone secretion by preovulatory granulosa cells because progesterone comprises a critical physiological LHR-stimulated target response necessary for follicles to progress to ovulation and thus fertility. To test this hypothesis, cells were transduced with S-E cofilin or empty GFP control adenovirus and then left untreated or treated with hCG for up to 24 h. Cell culture medium was collected at specific time points after hCG addition and evaluated for progesterone accumulation via RIA. Transduction of cells with the S-E cofilin caused a significant decrease in progesterone secretion at 2.5 and 5 h compared with both untransduced (2.5 h, P < 0.01; 5 h, P < 0.01) and GFP-transduced cells (2.5 h, P < 0.05; 5 h, P < 0.01) (Fig. 7G). At 2.5 h after hCG treatment, secretion of progesterone in S-E cofilin-transduced cells is 70% less than GFP-transduced cells and 80% less than untransduced cells. These results indicate that active/dephosphorylated cofilin is required for the initiation of hCG-stimulated progesterone secretion by preovulatory granulosa cells.
Discussion
Progesterone receptor knockout mice revealed a crucial role for this nuclear receptor and its ligand, progesterone, in the LHR-induced ovulatory response (56). Preovulatory granulosa cells transiently express the progesterone receptor and produce progesterone in response to the preovulatory LH surge (reviewed in Ref. 57). Transcriptional activation of the progesterone receptor by progesterone promotes expression of genes, such as a disintegrin and metalloproteinase with thrombospondin-like motifs (ADAMTS-1) and cathepsin L, that are required for rupture of the ovarian follicle (58,59,60). Deletion of genes required for progesterone production, such as Star or hepatic lipase (Lipc), results in an anovulatory or subovulatory phenotype, respectively (61,62). Therefore, it is important to understand the cellular mechanism(s) that regulate LHR-stimulated progesterone production.
Steroidogenesis in various cellular models has often been causally linked to the actin cytoskeleton, based on the inhibitory effects on steroidogenesis of chemicals that prevent reorganization of the actin cytoskeleton (12,13,15,17,18) and of placing cells on a substrate that prevents the trophic hormone-stimulated switch from a flattened to a rounded phenotype (8,9,10,11,12,18). We therefore sought to identify the cellular mechanism that may modulate reorganization of the actin cytoskeleton in steroidogenic cells and to determine whether reorganization of the actin cytoskeleton facilitated steroidogenesis in a physiologically relevant model of preovulatory granulosa cells.
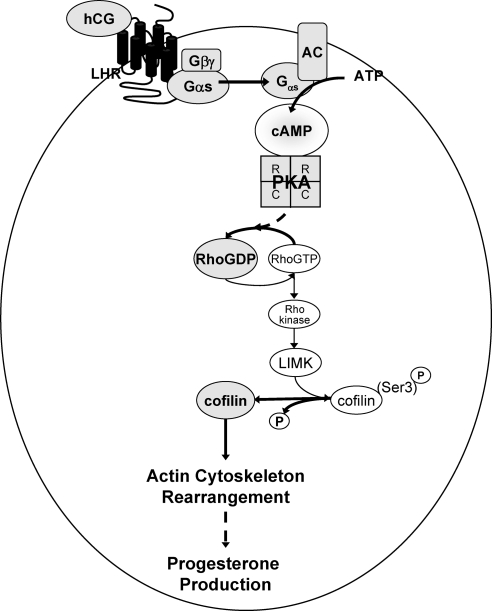
Herein we demonstrate that LHR signaling promotes the rapid, albeit transient, dephosphorylation/activation of the actin-depolymerizing factor cofilin, leading to remodeling of the actin cytoskeleton within and robust progesterone secretion by preovulatory granulosa cells (modeled in Fig. 8). This conclusion is based on our evidence that transduction of granulosa cells with a dominant-negative cofilin (XAC1-SE-GFP), in which Ser3 was mutated to Glu to generate a constitutively inactive cofilin mutant that cannot bind actin, prevented LHR-stimulated remodeling of the actin skeleton and markedly reduced LHR-stimulated progesterone secretion (by 70%).
Figure 8.
Model of LHR signaling to cofilin. The signaling pathway initiated by hCG binding to the LHR leading to cofilin dephosphorylation and resulting rearrangement of the actin cytoskeleton is depicted. Bold lines refer to consequences of LHR signals that promote the dephosphorylation of cofilin. AC, Adenylyl cyclase.
LHR-stimulated dephosphorylation of cofilin at Ser3 is mediated by cAMP via PKA and is independent of EGFR/ERK and PI3-kinase signaling pathways that PKA regulates in preovulatory granulosa cells. Cofilin dephosphorylation is triggered by the LHR-dependent inactivation of GTP-bound Rho to GDP-bound Rho, thereby reducing Rho kinase activity to eliminate the signaling necessary for cofilin phosphorylation. Consistent with our results, growth factors like lysophosphatidic acid that are recognized to activate Rho (63) inhibit gonadotropin-stimulated progesterone production (64) and the characteristic gonadotropin-stimulated shape change in luteal cells (65).
Basal phosphorylation of cofilin in preovulatory granulosa cells requires active, GTP-bound Rho and active Rho kinase; however, the physiological significance of an active GTP-bound Rho leading to inactive cofilin and the signaling pathway that maintains GTP-bound Rho in granulosa cells before the LH surge is not clear. Rho activation is mediated by at least 60 distinct guanine nucleotide exchange factors that promote the rate-limiting GDP release from Rho in response to signals from growth factor receptor tyrosine kinases, G protein-coupled receptors, and the extracellular matrix (63). Within the ovarian follicle, granulosa cells interact with laminin, fibronectin, and collagen of the basal lamina via β1 integrin (66); these interactions contribute to granulosa cell proliferation and differentiation (67,68). Based on evidence that β1 integrin can promote activation of RhoA in various cell models (69,70), the binding of extracellular matrix proteins in the basal lamina of the follicle could initiate β1 integrin signaling in granulosa cells that in turn activates Rho. The active Rho we observe before the LH surge may be needed for proper adherens junction arrangement and/or gap junction formation between granulosa cells or for gap junction formation between granulosa cells and the oocyte that is necessary for preserving meiotic arrest (71,72,73,74).
Although we have delineated that cAMP/PKA signaling events promote the inactivation of Rho in preovulatory granulosa cells, we have not identified the PKA phosphorylation target that accelerates the hydrolysis of Rho-GTP to Rho-GDP. Although in some cell models, PKA-catalyzed phosphorylation of RhoA is recognized to promote association of RhoA with GDIα, thereby inactivating Rho (reviewed in Ref. 53), we did not detect PKA-phosphorylated Rho or association of Rho with GDIα (see Fig. 6, D–F). However, Rho activity can also be regulated, directly or indirectly, by inactivating Rho guanine nucleotide exchange factors or by activating either Rho guanine nucleotide-activating proteins or Rho GDIs, many of which exhibit putative loci for PKA regulation (reviewed in Ref. 63). Identification of the specific PKA phosphorylation target in preovulatory granulosa cells that promotes inactivation of Rho thus requires future investigations beyond the scope of this report.
LHR signaling to cofilin occurs primarily at the level of inhibiting Rho signaling to Rho kinase and in turn to the cofilin kinase LIMK, and not by regulation of the cofilin phosphatases, slingshot and chronophin (reviewed in Ref. 75). We base this conclusion on the fact that a 1-h pretreatment of cells with the selective Rho kinase inhibitor Y27632, in the absence of LH, allows for total dephosphorylation of cofilin. This result suggests that the phosphatases responsible for eliminating cofilin phosphorylation are constitutively active and not regulated by LHR signaling. This basal activity of cofilin phosphatases contrasts with other cell models in which slingshot is often the primary site of hormone/growth factor regulation (reviewed in Ref. 75). However, we cannot rule out the contribution of a LIMK and/or cofilin phosphatase whose activity is enhanced by hCG.
LHR-dependent cofilin activation is obligatory for initiation of the progesterone response in preovulatory granulosa cells that is required for ovulation (see Fig. 7G). However, sustained progesterone production by granulosa cells in culture beyond 2 h is not associated with a detectable pool of actin-binding cofilin (i.e. dephosphorylated cofilin) or with sustained dissolution of the actin cytoskeleton. In the PMSG-primed immature rat model, serum progesterone levels peak by 2–4 h and then decline to approximately 50% of peak levels by 10 h after (iv) injection of LH (with ovulation beginning at 12 h) (76). We noted in ovaries from PMSG-primed immature rats that hCG-stimulated cofilin dephosphorylation persisted for at least 5 h (see Fig. 2B). Although active/dephosphorylated cofilin does not appear to be required for sustained progesterone production by granulosa cells in our in vitro model, the inability of the in vitro granulosa cell model to completely mimic preovulatory events leading to ovulation and luteinization (77,78) makes it difficult to determine whether the fall in progesterone levels in the granulosa cell model compared with the in vivo immature rat model is attributable to premature inactivation of cofilin or to some other deficiency.
It is interesting that although preantral granulosa cells produce progesterone in response to FSH, this response requires induction of cytochrome P450 side chain cleavage (P450scc) and other genes and is thus delayed by at least 24 h (79). Although FSH addition to primary granulosa cell cultures promotes cell rounding (10,80), FSH does not stimulate an acute cofilin dephosphorylation response (by 1 h after FSH; Karlsson, A. B., unpublished). These results suggest that a detectable pool of actin-binding cofilin is not required for the cytoskeletal changes that mediate FSH-dependent rounding of granulosa cells.
Cofilin-mediated dissolution of the actin filament cytoskeleton in response to LHR activation in preovulatory granulosa cells correlates with the initiation of progesterone production. Although cofilin activation is recognized to sever actin and enhance actin filament dynamics, our results do not formally prove that hCG-stimulated dissolution of the actin cytoskeleton per se is required for progesterone production. Indeed, we cannot rule out the possibility that cofilin is performing additional functions in granulosa cells. For example, cofilin has been reported as essential for the nuclear import of actin in mast cells (81). Nuclear actin is believed to bind chromatin remodeling complexes and RNA polymerases and thus to participate in transcriptional activation (82). Thus, cofilin could mediate a short-term effect on actin dissolution that modulates gene expression in preovulatory granulosa cells.
However, we favor the hypothesis that the cofilin-mediated dissolution of the actin cytoskeleton in preovulatory granulosa cells is required for the initiation of progesterone production based not only on our evidence that dominant-negative cofilin prevents both progesterone production and reorganization of the actin cytoskeleton in preovulatory granulosa cells but also on the bulk of evidence that chemicals that prevent reorganization of the actin cytoskeleton inhibit steroidogenesis (12,13,15,17,18). The initial enzyme-catalyzed step in steroidogenesis is the conversion of cholesterol to pregnenolone by P450scc located on the inner mitochondrial membrane (reviewed in Ref. 83). Because maximal steroidogenesis can be achieved in the absence of trophic hormones or cAMP when cells are provided with saturating concentrations of the hydrophobic cholesterol, 22R-hydroxycholesterol (reviewed in Refs. 83 and 84), delivery of cholesterol to the inner mitochondrial membrane is rate limiting. The most common source of cholesterol for steroidogenesis in adrenal and ovarian cells are high-density lipoproteins from which cholesterol esters are extracted via the scavenger receptor class B type I coordinately with hepatic lipase (62,85,86,87,88). High-density lipoprotein-derived cholesterol esters are either rapidly hydrolyzed to free cholesterol by a family of tissue-specific neutral cholesterol ester hydrolases, including hormone-sensitive lipase, or transferred as cholesterol esters to lipid droplets for storage (85,88,89,90). Trophic hormones/cAMP analogs increase the uptake of cholesterol esters (reviewed in Ref. 91).
Both StAR and translocator protein (TSPO) are required for steroidogenesis (92). PKA-phosphorylated StAR, which rapidly accumulates at the outer mitochondrial membrane in response to trophic hormone stimulation, appears to function, likely with the voltage-dependent anion channel 1, primarily to facilitate delivery of free cholesterol to the outer mitochondrial membrane from lipid droplets (83,84,93,94). TSPO upon binding the PKA anchor acyl-coenzyme A-binding domain-containing protein 3 (ACBD3) is believed to facilitate the transfer of cholesterol from the outer to the inner mitochondrial membrane for binding to P450scc to produce pregnenolone (reviewed in Ref. 84).
Thus, there are a number of possible sites in the steroidogenic pathway that may require a dynamic actin cytoskeleton. Dissolution of the actin cytoskeleton may be required for the recognized cAMP-dependent active transit of lipid droplets along microtubules (reviewed in Ref. 95) to the vicinity of the mitochondria, which has been visualized in Y-1 adrenal cells, based on evidence that this event is prevented in cells that have not rounded (14). Alternatively, dissolution of the actin cytoskeleton may be required for the relocation of the PKA-binding protein ACBD3 from the trans-Golgi network to the mitochondria to bind to TSPO to facilitate delivery of free cholesterol to the inner mitochondrial membrane (96). It is also possible that cofilin-dependent actin reorganization is required for StAR, possibly in association with a neutral cholesterol ester lipase, to facilitate hydrolysis of lipid droplet cholesterol esters at the outer mitochondrial membrane (97). Future studies are required to elucidate the specific pathways that require cofilin-dependent reorganization of the actin cytoskeleton. However, we hypothesize that the PKA-mediated cofilin-dependent reorganization of the actin cytoskeleton and the resulting rounding of cells is a universal requirement for steroidogenesis not only in preovulatory granulosa cells but also in luteal, Leydig, and adrenal cells.
In conclusion, we have demonstrated that LHR signaling in preovulatory granulosa cells promotes the activation of cofilin that is necessary for the initiation of progesterone production. We have also shown that activation of cofilin is necessary for actin cytoskeleton rearrangement coincident with cell rounding and progesterone production. To the best of our knowledge, our study is the first to delineate a mechanism by which trophic hormones promote rearrangement of the actin cytoskeleton that appears to be required for the steroidogenic response.
Materials and Methods
Materials
The following were purchased: hCG from Abraxis Pharmaceutical Products (Schaumburg, IL); DMEM/F12, fetal bovine serum, phalloidin, and 4′,6-diamidino-2-phenylindole (DAPI) stains from Invitrogen (Carlsbad, CA); transferrin, hydrocortisone, insulin, PMSG, and PKA catalytic subunit from Sigma-Aldrich (St. Louis, MO); human fibronectin from BD Biosciences (San Jose, CA); 8-cpt-cAMP and 8-cpt-2′O-Me-cAMP from Biolog Life Sciences Institute (Bremen, Germany); jasplakinolide from Alexis Biochemicals (San Diego, CA); Myr-PKI, PD98059, LY294002, Y27632, NSC23766, and cytochalasin D from EMD Biosciences/Calbiochem (La Jolla, CA); enhanced chemiluminescence reagents, Hybond-C Extra nitrocellulose membrane, and storage phosphor screen from Amersham Biosciences/GE Healthcare (Buckinghamshire, UK); Halt protease inhibitor cocktail from Thermo Scientific/Pierce (Rockford, IL); Criterion precast gels and Kaleidoscope marker from Bio-Rad (Hercules, CA); Rho GTPase activity assay, anti-phospho-CREB(Ser133) clone 10E9, and anti-Rho A/B/C clone 55 antibodies from Upstate Biotechnology/Millipore (Lake Placid, NY); anti-GDIα, RhoA (119) agarose conjugate antibodies, and Exactacruz preclearing matrix from Santa Cruz Biotechnology (Santa Cruz, CA); and anti-phospho-cofilin(Ser3), anti-total cofilin, anti-phospho-AKT(Ser 473), anti-phospho-p44/42 ERK 1/2(Thr202/Tyr204), anti-phospho-PKA substrate, anti-Gab2, and anti-pan-actin antibodies from Cell Signaling Technology (Danvers, MA). [γ-32P]ATP was purchased from PerkinElmer (Fremont, CA).
Animals
Sprague-Dawley rats (Charles River Laboratories, Inc., Portage, MI) were housed either at Northwestern University or Washington State University animal care facilities and maintained in accordance with the Guidelines for the Care and Use of Laboratory Animals by protocols approved by the Northwestern University or Washington State University Animal Care and Use Committees, respectively.
Granulosa cell culture and Western blot
Rats were injected sc with 10 IU/0.1 ml PMSG in PBS at 22 d of age to promote maturation of follicles to the preovulatory phenotype, and ovaries were harvested 48 h after injection. Ovaries were then trimmed to remove bursa, fat, and oviducts and incubated for 30 min in DMEM/F-12 containing 10 mm EGTA and 500 mm sucrose (pH 7.3). Granulosa cells were mechanically isolated from ovaries via penetration with a 30-gauge needle. Cells were plated at a density of 1.5 × 106 cells per 35-mm or six-well culture dish, at 3 × 106 cells per 60-mm dish, or at 8–10 × 106 cells per 100-mm culture dish. Cells were plated in complete medium for all experiments except as noted below. Complete medium consists of DMEM/F-12, 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 5 μg/ml transferrin, 4 ng/ml hydrocortisol, and 2 μg/ml insulin (98). Cells treated with Myr-PKI were plated on fibronectin-coated tissue culture plates in DMEM/F-12 serum-free medium supplemented with 10 nm 17β-estradiol, 100 U/ml penicillin, and 100 μg/ml streptomycin. Treatments were terminated by aspirating medium and rinsing cells once with 1× PBS. Total cell extracts were collected by scraping cells in 3× sodium dodecyl sulfate (SDS) sample buffer followed by heat denaturation (31). Because granulosa cells do not proliferate under these culture conditions, protein concentrations were controlled by plating identical cell numbers per plate in each experiment and then loading equal volumes of total cell extract per gel lane. Equal protein loading was confirmed by total cofilin Western blots, as indicated. Granulosa cell proteins were separated by SDS-PAGE and transferred to Hybond C-extra nitrocellulose. Blots were incubated with primary antibody overnight at 4 C, and antigen-antibody complexes were detected by enhanced chemiluminescence. Western signals were quantified with Molecular Analyst/PC Image Analysis software program (Bio-Rad), divided by the densitometric signal for control protein load (total cofilin), and expressed relative to the maximal signal. Results were analyzed for significance using Student’s t test (99).
Immunofluorescence
For immunofluorescence analysis, preovulatory granulosa cells were cultured in complete medium on coverslips at 1.5 × 106 cells per coverslip. Cells were cultured overnight and before treatments were serum starved for 4 h. After treatments, cells were washed with 1× PBS, fixed for 30 min in 4% formaldehyde/cytoskeleton-stabilizing buffer [10 mm 2-(N-morpholino) ethanesulfonic acid (pH 6.1), 138 mm KCl, 3 mm MgCl2, 10 mm EGTA, and 0.32 m sucrose], and then permeabilized for 10 min with 0.5% Triton X-100 (100). Coverslips were washed three times with 1× PBS and then incubated with phalloidin (1:100) and DAPI (1:1000) in a humidified chamber at 4 C overnight. Coverslips were washed three times with 1× PBS and mounted on glass microscope slides using polyvinyl alcohol mounting medium containing 100 mg/ml DABCO (1,4-diazabicyclo[2.2.2]octane) as antifading reagent. Slides were visualized using the Nikon Eclipse TE2000 inverted epifluorescence microscope at a magnification of ×60. Images were captured using the imaging software MetaMorph version 5 and processed in Adobe Photoshop CS3 Extended.
S-/P-actin assay
Preovulatory granulosa cells were plated in complete medium and cultured overnight. After treatments, cells were rinsed quickly with room-temperature 1× PBS. Lysis buffer [1% Triton X-100, 10 mm Tris, 2 mm MgCl2, 0.2 mm dithiothreitol (pH 7.4)] with 15% glycerol chilled to −10 C was added to the cell culture plate to promote cell lysis (at room temperature) (25). After 5 min, cell lysate was harvested using a rubber policeman and centrifuged at 10,000 × g for 1 min at room temperature. Supernatant (S-actin fraction) was removed and the remaining pellet (cytoskeletal P-actin fraction) was resuspended in an equal amount of lysis buffer. Total actin fraction was harvested from cell lysates of an identical plate to that used for S/P fractions in an equal volume of lysis buffer. SDS sample buffer was added to cell lysates, and lysates were boiled for 15 min to denature proteins (31). Actin in total, S, and P fractions was quantified by Western blot. Quantification was performed by adding the densitometric signal for S and P fractions and then dividing the densitometric signal of each fraction (S+P, S, and P) by the S+P densitometric signal.
Whole ovarian extract preparation
Sprague Dawley rats were injected sc with 10 IU/0.1 ml PMSG followed 48 h later by ip injections of 50 IU hCG or left untreated (34). Ovaries were harvested at various time points after injections; dissected free of bursa, fat, and oviducts; weighed; and homogenized (5:1 ratio of homogenization buffer to wet weight) in homogenization buffer [10 mm potassium phosphate (pH 7.0), 1 mm EDTA, 5 mm EGTA, 10 mm MgCl2, 50 mm β-glycerol phosphate, 1 mm sodium orthovanadate, 2 mm dithiothreitol, 40 μg/ml phenylmethylsulfonyl fluoride, 0.5% Nonidet P-40, and 0.1% deoxycholate] using 12 strokes with a ground-glass homogenizer (101). Homogenates were clarified by centrifugation at 10,000 × g at 4 C for 10 min. Supernatants were added to 0.5 × volume of 3× SDS sample buffer and denatured by boiling. Protein concentrations were determined by the Lowry protein assay using BSA as the standard (102).
Rho activation/pull-down assays
Rho pull-down assays were performed using Rho activation assay kits (Upstate Biotechnology) according to the manufacturer’s instructions. Briefly, preovulatory granulosa cells were stimulated as described in Results using approximately 20 million cells per treatment. After stimulation, cells were washed with ice-cold Tris-buffered saline and harvested on ice. Lysates were subjected to centrifugation for 5 min at 14,000 × g at 4 C to clear insoluble cellular debris. Supernatants were incubated for 45 min with agarose slurries containing the Rho-GTP-binding domain of rhotekin conjugated to agarose beads. Agarose beads were washed and then resuspended with 3× SDS sample buffer. Samples were boiled for 5 min to release bound Rho before Western blot analysis.
Immunoprecipitation
Cells were plated at 8–10 × 106 cells per 100-mm plate in complete medium overnight. After indicated treatments, cells were rinsed with 1× PBS and then harvested into 1 ml lysis buffer [50 mm HEPES (pH 7.0), 100 mm sodium chloride, 0.5% Nonidet P-40, 20 mm sodium fluoride, 2 mm sodium vanadate, 2 mm sodium diphosphate, 5 mm EGTA, 5 mm EDTA, 20 mm benzamidine, 10 μg/ml calpain, 50 μg/ml antipain, 50 μg/ml soybean trypsin inhibitor, 10 μm isobutylmethylxanthine, and Halt protease inhibitor cocktail] and sonicated on ice in 10-sec bursts for a total time of 1 min. Lysates were cleared by centrifugation at 10,000 × g for 5 min at 4 C. Supernatants (50 μl for input) were then incubated with rabbit preclearing matrix (Santa Cruz Biotechnology) for 1 h at 4 C with rotation followed by centrifugation for 1 min at 5,000 × g at 4 C. Supernatant was removed and incubated with anti-phospho-PKA substrate or rabbit IgG antibodies and protein A/G agarose overnight at 4 C with rotation. After centrifugation at 5000 × g for 1min at 4 C, the supernatant was saved (unbound fraction); the pellet was washed four times with wash buffer (20 mm HEPES, 150 mm sodium chloride, 10% glycerol, and 0.1% Triton-X). The final pellet was resuspended in 40 μl 3× SDS sample buffer and boiled for 5 min. Input, bound, and unbound fractions were subjected to SDS-PAGE; blots were assessed by Western blotting.
Back-phosphorylation assay
For back-phosphorylation assays, immunoprecipitations were performed according to previously stated methods with either Gab2 or RhoA agarose-conjugated antibodies. Back-phosphorylation assays were performed as described previously (103) with the following modifications. After immunoprecipitation, pellets were washed two times in kinase buffer [50 mm HEPES (pH 7.0), 10 mm magnesium chloride, 10 mm EGTA, 0.2% Triton X-100, and 2 mm sodium fluoride]. Pellets were then resuspended in 50 μl kinase buffer. To begin the assay, 50 μl reaction buffer (10 U PKA catalytic subunit, 10 μCi [γ-32P]ATP, and 10 μm ATP) was added to the suspension, and samples were incubated at 30 C for 5 min. Reactions were stopped by addition of 3× SDS sample buffer and boiled for 15 min. Samples were subjected to SDS-PAGE, and an autoradiograph was created by exposing gels to a storage phosphor screen for time periods ranging from 1 h to 1 wk. Phosphoimages were captured using GE Storm Scanner 845 and Image Quant TL software program.
Adenoviral transduction
After harvest, granulosa cells were allowed to plate for 4–6 h in complete medium before adenoviral transduction. Adenoviruses were added to the cell culture medium at a specified multiplicity of infection, and cells were transduced for 12–20 h before treatments. Transduction efficiency was at least 90%, based on the presence of GFP signal. The following adenoviruses were kindly provided by Dr. James Bamburg (Colorado State University, Ft. Collins, CO): XAC1-GFP SE and empty GFP (55). Adenoviral PKI was kindly provided by Dr. Marco Conti (University of California, San Francisco, San Francisco, CA) (39).
Progesterone RIA
Media samples were analyzed for progesterone by the Center for Reproductive Biology Assay Core at Washington State University (http://www.crb.wsu.edu/corelaboratories/AssayCore.html).
Footnotes
Disclosure Summary: The authors have nothing to declare.
This work was supported by National Institutes of Health (NIH) Grants HD-046955 and HD-62503 (to M.H.-D.), NIH Training Program in Reproductive Biology at North-western University (T32 HD 07068) (to A.B.K.), and NIH Medical Scientist Training Grant at Northwestern University (T32-GM08152) (to M.P.F.).
First Published Online July 7, 2010
Abbreviations: 8-cpt-cAMP, 8-4-Chlorophenylthio cAMP; 8-cpt-2′O-Me-cAMP, 8-(4-chlorophenylthio)-2′-O-methyl cAMP; CREB, cAMP response element-binding protein; DMSO, dimethylsulfoxide; EGFR, epidermal growth factor receptor; EPAC, exchange protein activated by cAMP; F-actin, filamentous actin; hCG, human chorionic gonadotropin; LIMK, LIM-domain-containing kinase; LHR, LH receptor; Myr-PKI, myristoylated PKI peptide; P-actin, pelletable actin; PI3, phosphatidylinositol 3; PKA, protein kinase A; PKI, PKA-specific protein kinase inhibitor; PMSG, pregnant mare serum gonadotropin; P450scc, cytochrome P450 side chain cleavage; RhoGDIα, Rho guanine nucleotide disassociation inhibitor-α; S-actin, soluble actin; SDS, sodium dodecyl sulfate; StAR, steroidogenic acute regulatory; TSPO, translocator protein.
References
- Rajagopalan-Gupta RM, Rasenick MM, Hunzicker-Dunn M 1997 Luteinizing hormone/choriogonadotropin-dependent, cholera toxin-catalyzed adenosine 5′-diphosphate (ADP)-ribosylation of the long and short forms of Gsα and pertussis toxin-catalyzed ADP-ribosylation of Giα. Mol Endocrinol 11:538–549 [DOI] [PubMed] [Google Scholar]
- Salvador LM, Maizels E, Hales DB, Miyamoto E, Yamamoto H, Hunzicker-Dunn M 2002 Acute signaling by the LH receptor is independent of protein kinase C activation. Endocrinology 143:2986–2994 [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Hsieh M, Doyle KH, Falender AE, Lo YK, Sharma SC 2002 Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog Horm Res 57:195–220 [DOI] [PubMed] [Google Scholar]
- Hunzicker-Dunn M, Mayo K 2006 Gonadotropin signaling in the ovary. In: Neill JD, ed. Knobil and Neill’s physiology of reproduction. Vol 1. 3rd ed. New York: Elsevier; 547–592 [Google Scholar]
- Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M 2007 Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol 27:1914–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS 2009 MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 324:938–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar N, Veldhuis JD 2001 Concerted transcriptional activation of the low density lipoprotein receptor gene by insulin and luteinizing hormone in cultured porcine granulosa-luteal cells: possible convergence of protein kinase A, phosphatidylinositol 3-kinase, and mitogen-activated protein kinase signaling pathways. Endocrinology 142:2921–2928 [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A, Amsterdam A 1989 Regulation of cytoskeletal protein organization and expression in human granulosa cells in response to gonadotropin treatment. Endocrinology 124:1033–1041 [DOI] [PubMed] [Google Scholar]
- Furger C, Pouchelet M, Zorn JR, Ferré F 1996 Cell shape change reveals the cyclic AMP-mediated action of follicle stimulating hormone, human chorionic gonadotrophin and vasoactive intestinal peptide in primary cultured human granulosa-lutein cells. Mol Hum Reprod 2:251–257 [DOI] [PubMed] [Google Scholar]
- Lawrence TS, Ginzberg RD, Gilula NB, Beers WH 1979 Hormonally induced cell shape changes in cultured rat ovarian granulosa cells. J Cell Biol 80:21–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto EA, Kliman HJ, Strauss 3rd JF, Paavola LG 1986 Gonadotropins and cyclic adenosine 3′,5′-monophosphate (cAMP) alter the morphology of cultured human granulosa cells. Biol Reprod 34:559–569 [DOI] [PubMed] [Google Scholar]
- Iczkowski KA, Hertelendy F 1991 Participation of the cytoskeleton in avian granulosa cell steroidogenesis. Gen Comp Endocrinol 82:355–363 [DOI] [PubMed] [Google Scholar]
- Silavin SL, Javitt NB, Strauss 3rd JF 1984 Reevaluation of the effects of cytochalasins on steroidogenesis: studies on hamster granulosa cells. Endocrinology 115:1511–1516 [DOI] [PubMed] [Google Scholar]
- Nan X, Potma EO, Xie XS 2006 Nonperturbative chemical imaging of organelle transport in living cells with coherent anti-stokes Raman scattering microscopy. Biophys J 91:728–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar S, Menon KM 1981 Receptor-mediated gonadotropin action in the ovary. Action of cytoskeletal element-disrupting agents on gonadotropin-induced steroidogenesis in rat luteal cells. Biochem J 194:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crivello JF, Jefcoate CR 1980 Intracellular movement of cholesterol in rat adrenal cells. Kinetics and effects of inhibitors. J Biol Chem 255:8144–8151 [PubMed] [Google Scholar]
- Nagy L, Freeman DA 1990 Effect of cholesterol transport inhibitors on steroidogenesis and plasma membrane cholesterol transport in cultured MA-10 Leydig tumor cells. Endocrinology 126:2267–2276 [DOI] [PubMed] [Google Scholar]
- Silavin SL, Moss GE, Niswender GD 1980 Regulation of steroidogenesis in the ovine corpus luteum. Steroids 36:229–241 [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A 2005 Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 21:247–269 [DOI] [PubMed] [Google Scholar]
- Van Troys M, Huyck L, Leyman S, Dhaese S, Vandekerkhove J, Ampe C 2008 Ins and outs of ADF/cofilin activity and regulation. Eur J Cell Biol 87:649–667 [DOI] [PubMed] [Google Scholar]
- Gurniak CB, Perlas E, Witke W 2005 The actin depolymerizing factor n-cofilin is essential for neural tube morphogenesis and neural crest cell migration. Dev Biol 278:231–241 [DOI] [PubMed] [Google Scholar]
- Bamburg JR, Wiggan OP 2002 ADF/cofilin and actin dynamics in disease. Trends Cell Biol 12:598–605 [DOI] [PubMed] [Google Scholar]
- Andrianantoandro E, Pollard TD 2006 Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol Cell 24:13–23 [DOI] [PubMed] [Google Scholar]
- Chan C, Beltzner CC, Pollard TD 2009 Cofilin dissociates Arp2/3 complex and branches from actin filaments. Curr Biol 19:537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heacock CS, Bamburg JR 1983 The quantitation of G- and F-actin in cultured cells. Anal Biochem 135:22–36 [DOI] [PubMed] [Google Scholar]
- Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED 1994 Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem 269:14869–14871 [PubMed] [Google Scholar]
- Flanagan MD, Lin S 1980 Cytochalasins block actin filament elongation by binding to high affinity sites associated with F-actin. J Biol Chem 255:835–838 [PubMed] [Google Scholar]
- Brenner SL, Korn ED 1979 Substoichiometric concentrations of cytochalasin D inhibit actin polymerization. Additional evidence for an F-actin treadmill. J Biol Chem 254:9982–9985 [PubMed] [Google Scholar]
- Brown SS, Spudich JA 1979 Cytochalasin inhibits the rate of elongation of actin filament fragments. J Cell Biol 83:657–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean-Fletcher S, Pollard TD 1980 Mechanism of action of cytochalasin B on actin. Cell 20:329–341 [DOI] [PubMed] [Google Scholar]
- Hunzicker-Dunn M 1981 Selective activation of rabbit ovarian protein kinase isozymes in rabbit ovarian follicles and corpora lutea. J Biol Chem 256:12185–12193 [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL 1998 Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396:474–477 [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM 1998 A family of cAMP-binding proteins that directly activate Rap1. Science 282:2275–2279 [DOI] [PubMed] [Google Scholar]
- Flynn MP, Maizels ET, Karlsson AB, McAvoy T, Ahn JH, Nairn AC, Hunzicker-Dunn M 2008 Luteinizing hormone receptor activation in ovarian granulosa cells promotes protein kinase A- dependent dephosphorylation of microtubule-associated protein 2D. Mol Endocrinol 22:1695–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopperud R, Krakstad C, Selheim F, Døskeland SO 2003 cAMP effector mechanisms. Novel twists for an ‘old’ signaling system. FEBS Lett 546:121–126 [DOI] [PubMed] [Google Scholar]
- Carr DW, Cutler Jr RE, Cottom JE, Salvador LM, Fraser ID, Scott JD, Hunzicker-Dunn M 1999 Identification of cAMP-dependent protein kinase holoenzymes in preantral- and preovulatory-follicle-enriched ovaries, and their association with A-kinase-anchoring proteins. Biochem J 344(Pt 2):613–623 [PMC free article] [PubMed] [Google Scholar]
- Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Døskeland SO, Blank JL, Bos JL 2002 A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol 4:901–906 [DOI] [PubMed] [Google Scholar]
- Scott JD, Fischer EH, Takio K, Demaille JG, Krebs EG 1985 Amino acid sequence of the heat-stable inhibitor of the cAMP-dependent protein kinase from rabbit skeletal muscle. Proc Natl Acad Sci USA 82:5732–5736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruss MD, Richter W, Horner K, Jin SL, Conti M 2008 Critical role of PDE4D in β2-adrenoceptor-dependent cAMP signaling in mouse embryonic fibroblasts. J Biol Chem 283:22430–22442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebl G, Fischer S, Penzel R, Samstag Y 2004 Dephosphorylation of cofilin is regulated through Ras and requires the combined activities of the Ras-effectors MEK and PI3K. Cell Signal 16:235–243 [DOI] [PubMed] [Google Scholar]
- Okada K, Takano-Ohmuro H, Obinata T, Abe H 1996 Dephosphorylation of cofilin in polymorphonuclear leukocytes derived from peripheral blood. Exp Cell Res 227:116–122 [DOI] [PubMed] [Google Scholar]
- Andric N, Ascoli M 2008 The luteinizing hormone receptor-activated extracellularly regulated kinase-1/2 cascade stimulates epiregulin release from granulosa cells. Endocrinology 149:5549–5556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigone S, Hsieh M, Fu M, Persani L, Conti M 2008 Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol 22:924–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M 2004 EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303:682–684 [DOI] [PubMed] [Google Scholar]
- Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS 2006 Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol 20:1352–1365 [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P 2000 Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351:95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P 2007 The selectivity of protein kinase inhibitors: a further update. Biochem J 408:297–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M, Narumiya S 2000 Pharmacological properties of Y-27632, a specific inhibitor of Rho-associated kinases. Mol Pharmacol 57:976–983 [PubMed] [Google Scholar]
- Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y 2004 Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA 101:7618–7623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Désiré L, Bourdin J, Loiseau N, Peillon H, Picard V, De Oliveira C, Bachelot F, Leblond B, Taverne T, Beausoleil E, Lacombe S, Drouin D, Schweighoffer F 2005 RAC1 inhibition targets amyloid precursor protein processing by γ-secretase and decreases Aβ production in vitro and in vivo. J Biol Chem 280:37516–37525 [DOI] [PubMed] [Google Scholar]
- Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J 1996 Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J 15:510–519 [PMC free article] [PubMed] [Google Scholar]
- Ellerbroek SM, Wennerberg K, Burridge K 2003 Serine phosphorylation negatively regulates RhoA in vivo. J Biol Chem 278:19023–19031 [DOI] [PubMed] [Google Scholar]
- Dovas A, Couchman JR 2005 RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem J 390:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meberg PJ, Ono S, Minamide LS, Takahashi M, Bamburg JR 1998 Actin depolymerizing factor and cofilin phosphorylation dynamics: response to signals that regulate neurite extension. Cell Motil Cytoskeleton 39:172–190 [DOI] [PubMed] [Google Scholar]
- Meberg PJ, Bamburg JR 2000 Increase in neurite outgrowth mediated by overexpression of actin depolymerizing factor. J Neurosci 20:2459–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery Jr CA, Shyamala G, Conneely OM, O'Malley BW 1995 Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278 [DOI] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O'Malley BW 2002 Reproductive functions of progesterone receptors. Recent Prog Horm Res 57:339–355 [DOI] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, Lydon JP, De Mayo FJ 2001 Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice. Mol Cell Endocrinol 179:97–103 [DOI] [PubMed] [Google Scholar]
- Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW, Richards JS 2000 Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci USA 97:4689–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriraman V, Sinha M, Richards JS 2010 Progesterone receptor-induced gene expression in primary mouse granulosa cell cultures. Biol Reprod 82:402–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, Fukami M, Sato N, Katsumata N, Sasaki G, Fukutani K, Morohashi K, Ogata T 2004 Testicular dysgenesis without adrenal insufficiency in a 46,XY patient with a heterozygous inactive mutation of steroidogenic factor-1. J Clin Endocrinol Metab 89:5930–5935 [DOI] [PubMed] [Google Scholar]
- Wade RL, Van Andel RA, Rice SG, Banka CL, Dyer CA 2002 Hepatic lipase deficiency attenuates mouse ovarian progesterone production leading to decreased ovulation and reduced litter size. Biol Reprod 66:1076–1082 [DOI] [PubMed] [Google Scholar]
- Buchsbaum RJ 2007 Rho activation at a glance. J Cell Sci 120:1149–1152 [DOI] [PubMed] [Google Scholar]
- Budnik LT, Brunswig-Spickenheier B 2005 Differential effects of lysolipids on steroid synthesis in cells expressing endogenous LPA2 receptor. J Lipid Res 46:930–941 [DOI] [PubMed] [Google Scholar]
- Budnik LT, Mukhopadhyay AK 2001 Lysophosphatidic acid antagonizes the morphoregulatory effects of the luteinizing hormone on luteal cells: possible role of small Rho-G-proteins. Biol Reprod 65:180–187 [DOI] [PubMed] [Google Scholar]
- Monniaux D, Huet-Calderwood C, Le Bellego F, Fabre S, Monget P, Calderwood DA 2006 Integrins in the ovary. Semin Reprod Med 24:251–261 [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Irving-Rodgers HF, Russell DL 2003 Extracellular matrix of the developing ovarian follicle. Reproduction 126:415–424 [DOI] [PubMed] [Google Scholar]
- Berkholtz CB, Lai BE, Woodruff TK, Shea LD 2006 Distribution of extracellular matrix proteins type I collagen, type IV collagen, fibronectin, and laminin in mouse folliculogenesis. Histochem Cell Biol 126:583–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMali KA, Wennerberg K, Burridge K 2003 Integrin signaling to the actin cytoskeleton. Curr Opin Cell Biol 15:572–582 [DOI] [PubMed] [Google Scholar]
- Danen EH, Sonneveld P, Brakebusch C, Fassler R, Sonnenberg A 2002 The fibronectin-binding integrins α5β1 and αvβ3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J Cell Biol 159:1071–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A 1987 The role of changes in cell shape and contacts in the regulation of cytoskeleton expression during differentiation. J Cell Sci Suppl 8:293–312 [DOI] [PubMed] [Google Scholar]
- Matsushita T, Oyamada M, Fujimoto K, Yasuda Y, Masuda S, Wada Y, Oka T, Takamatsu T 1999 Remodeling of cell-cell and cell-extracellular matrix interactions at the border zone of rat myocardial infarcts. Circ Res 85:1046–1055 [DOI] [PubMed] [Google Scholar]
- Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA 2008 Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development 135:3229–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochnow N, Dermietzel R 2008 Connexons and cell adhesion: a romantic phase. Histochem Cell Biol 130:71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TY, DerMardirossian C, Bokoch GM 2006 Cofilin phosphatases and regulation of actin dynamics. Curr Opin Cell Biol 18:26–31 [DOI] [PubMed] [Google Scholar]
- Goff AK, Henderson KM 1979 Changes in follicular fluid and serum concentrations of steroids in PMS treated immature rats following LH administration. Biol Reprod 20:1153–1157 [DOI] [PubMed] [Google Scholar]
- Hickey GJ, Krasnow JS, Beattie WG, Richards JS 1990 Aromatase cytochrome P450 in rat ovarian granulosa cells before and after luteinization: adenosine 3′,5′-monophosphate-dependent and independent regulation. Cloning and sequencing of rat aromatase cDNA and 5′ genomic DNA. Mol Endocrinol 4:3–12 [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Mayo K 2000 Regulation of inhibin subunit gene expression by gonadotropins and cAMP in ovarian granulosa cells. In: Shupnick MA, ed. Gene engineering in endocrinology. Totowa, NJ: Humana Press; 277–306 [Google Scholar]
- Goldring NB, Durica JM, Lifka J, Hedin L, Ratoosh SL, Miller WL, Orly J, Richards JS 1987 Cholesterol side-chain cleavage P450 messenger RNA: evidence for hormonal regulation in rat ovarian follicles and constitutive expression in corpora lutea. Endocrinology 120:1942–1950 [DOI] [PubMed] [Google Scholar]
- Maizels ET, Cottom J, Jones JC, Hunzicker-Dunn M 1998 Follicle stimulating hormone (FSH) activates the p38 mitogen-activated kinase pathway, induces small heat shock protein phosphorylation and cell rounding in immature rat ovarian granulosa cells. Endocrinology 139:3353–3356 [DOI] [PubMed] [Google Scholar]
- Pendleton A, Pope B, Weeds A, Koffer A 2003 Latrunculin B or ATP depletion induces cofilin-dependent translocation of actin into nuclei of mast cells. J Biol Chem 278:14394–14400 [DOI] [PubMed] [Google Scholar]
- Miralles F, Visa N 2006 Actin in transcription and transcription regulation. Curr Opin Cell Biol 18:261–266 [DOI] [PubMed] [Google Scholar]
- Miller WL 2007 Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochim Biophys Acta 1771:663–676 [DOI] [PubMed] [Google Scholar]
- Rone MB, Fan J, Papadopoulos V 2009 Cholesterol transport in steroid biosynthesis: role of protein-protein interactions and implications in disease states. Biochim Biophys Acta 1791:646–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly MA, Kellner-Weibel G, Rothblat GH, Williams DL 2003 SR-BI-directed HDL-cholesteryl ester hydrolysis. J Lipid Res 44:331–341 [DOI] [PubMed] [Google Scholar]
- Kraemer FB, Shen WJ, Harada K, Patel S, Osuga J, Ishibashi S, Azhar S 2004 Hormone-sensitive lipase is required for high-density lipoprotein cholesteryl ester-supported adrenal steroidogenesis. Mol Endocrinol 18:549–557 [DOI] [PubMed] [Google Scholar]
- Rigotti A, Miettinen HE, Krieger M 2003 The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other tissues. Endocr Rev 24:357–387 [DOI] [PubMed] [Google Scholar]
- Azhar S, Nomoto A, Leers-Sucheta S, Reaven E 1998 Simultaneous induction of an HDL receptor protein (SR-BI) and the selective uptake of HDL-cholesteryl esters in a physiologically relevant steroidogenic cell model. J Lipid Res 39:1616–1628 [PubMed] [Google Scholar]
- Reaven E, Tsai L, Azhar S 1995 Cholesterol uptake by the ‘selective’ pathway of ovarian granulosa cells: early intracellular events. J Lipid Res 36:1602–1617 [PubMed] [Google Scholar]
- Connelly MA, Williams DL 2004 SR-BI and HDL cholesteryl ester metabolism. Endocr Res 30:697–703 [DOI] [PubMed] [Google Scholar]
- Azhar S, Reaven E 2002 Scavenger receptor class BI and selective cholesteryl ester uptake: partners in the regulation of steroidogenesis. Mol Cell Endocrinol 195:1–26 [DOI] [PubMed] [Google Scholar]
- Hauet T, Yao ZX, Bose HS, Wall CT, Han Z, Li W, Hales DB, Miller WL, Culty M, Papadopoulos V 2005 Peripheral-type benzodiazepine receptor-mediated action of steroidogenic acute regulatory protein on cholesterol entry into Leydig cell mitochondria. Mol Endocrinol 19:540–554 [DOI] [PubMed] [Google Scholar]
- Bose M, Whittal RM, Miller WL, Bose HS 2008 Steroidogenic activity of StAR requires contact with mitochondrial VDAC1 and phosphate carrier protein. J Biol Chem 283:8837–8845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco DM 2000 Intramitochondrial cholesterol transfer. Biochim Biophys Acta 1486:184–197 [DOI] [PubMed] [Google Scholar]
- Martin S, Parton RG 2005 Caveolin, cholesterol, and lipid bodies. Semin Cell Dev Biol 16:163–174 [DOI] [PubMed] [Google Scholar]
- Liu J, Rone MB, Papadopoulos V 2006 Protein-protein interactions mediate mitochondrial cholesterol transport and steroid biosynthesis. J Biol Chem 281:38879–38893 [DOI] [PubMed] [Google Scholar]
- Shen WJ, Patel S, Natu V, Hong R, Wang J, Azhar S, Kraemer FB 2003 Interaction of hormone-sensitive lipase with steroidogenic acute regulatory protein: facilitation of cholesterol transfer in adrenal. J Biol Chem 278:43870–43876 [DOI] [PubMed] [Google Scholar]
- Park-Sarge OK, Mayo KE 1994 Regulation of the progesterone receptor gene by gonadotropins and cyclic adenosine 3′,5′-monophosphate in rat granulosa cells. Endocrinology 134:709–718 [DOI] [PubMed] [Google Scholar]
- Bender FE, Douglass LW, Kramer A 1982 Statistical methods for food and agriculture. Westport, CT: AVI Publishing Co., Inc.; 87–107 [Google Scholar]
- Garvalov BK, Flynn KC, Neukirchen D, Meyn L, Teusch N, Wu X, Brakebusch C, Bamburg JR, Bradke F 2007 Cdc42 regulates cofilin during the establishment of neuronal polarity. J Neurosci 27:13117–13129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador LM, Flynn MP, Avila J, Reierstad S, Maizels ET, Alam H, Park Y, Scott JD, Carr DW, Hunzicker-Dunn M 2004 Neuronal microtubule-associated protein 2D is a dual a-kinase anchoring protein expressed in rat ovarian granulosa cells. J Biol Chem 279:27621–27632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ 1951 Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275 [PubMed] [Google Scholar]
- Rossie S, Catterall WA 1987 Cyclic-AMP-dependent phosphorylation of voltage-sensitive sodium channels in primary cultures of rat brain neurons. J Biol Chem 262:12735–12744 [PubMed] [Google Scholar]