Abstract
The GnRH receptor (GnRHR), expressed at the cell surface of the anterior pituitary gonadotrope, is critical for normal secretion of gonadotropins LH and FSH, pubertal development, and reproduction. The signaling network downstream of the GnRHR and the molecular bases of the regulation of gonadotropin expression have been the subject of intense research. The murine LβT2 cell line represents a mature gonadotrope and therefore is an important model for the study of GnRHR-signaling pathways and modulation of the gonadotrope cell by physiological regulators. In order to facilitate access to the information contained in this complex and evolving literature, we have developed a pathway-based knowledgebase that is web hosted. At present, using 106 relevant primary publications, we curated a comprehensive knowledgebase of the GnRHR signaling in the LβT2 cell in the form of a process diagram. Positive and negative controls of gonadotropin gene expression, which included GnRH itself, hypothalamic factors, gonadal steroids and peptides, as well as other hormones, were illustrated. The knowledgebase contains 187 entities and 206 reactions. It was assembled using CellDesigner software, which provides an annotated graphic representation of interactions, stored in Systems Biology Mark-up Language. We then utilized Biological Pathway Publisher, a software suite previously developed in our laboratory, to host the knowledgebase in a web-accessible format as a public resource. In addition, the network entities were linked to a public wiki, providing a forum for discussion, updating, and error correction. The GnRHR-signaling network is openly accessible at http://tsb.mssm.edu/pathwayPublisher/GnRHR_Pathway/GnRHR_Pathway_ index.html.
A pathway-based knowledgebase of the LβT2 gonadotrope literature is openly web accessible. Entities link to a public wiki for discussion.
The GnRH receptor (GnRHR) plays a pivotal role in the neurohormonal control of reproductive function. Expressed at the surface of pituitary gonadotrope cells, the receptor binds hypothalamic neuropeptide GnRH and thus stimulates the synthesis and pulsatile release of gonadotropins LH and FSH; in turn, LH and FSH regulate gonadal functions, including gametogenesis, steroidogenesis, and ovulation. GnRH is secreted by hypothalamic neurons in a pulsatile manner, and the individual gonadotropin genes respond differentially to GnRH pulse frequency, as previously demonstrated in vivo (1,2). Thus, the intracellular signaling network activated upon binding of GnRH to its receptor must decipher the information received to ensure an appropriate physiological response of the gonadotrope cell. The pivotal role of GnRHR in the physiology of reproduction is further evidenced by the existence ofGnRHR mutations that cause hypogonadotropic hypogonadism in humans (3). Furthermore, GnRH analogs are widely used in the treatment of male and female infertility as well as hormone-dependent cancers, such as breast and prostate cancer.
The GnRHR belongs to the family of G protein-coupled receptors (GPCRs), the largest group of membrane receptors. GPCRs transduce extracellular stimuli via heterotrimeric GTP-binding proteins (G proteins). The GnRHR possesses the unique structural feature of lacking an intracellular carboxy-terminal domain. Therefore, in contrast to other GPCRs, it is characterized by poor internalization and lack of rapid desensitization (4,5).
The GnRHR intracellular signaling pathway has been the subject of intense experimental investigation for the past 25 yr, by means of both in vivo and in vitro approaches. In vitro models have mainly consisted of pituitary primary cultures, heterologous cell systems, and the gonadotrope cell lines αT3-1 and LβT2. Because pituitary gonadotropes account for only 5–10% of the anterior pituitary cell population, using primary cultures as a model for the study of gonadotrope-specific intracellular signaling cascades is challenging. The immortalized gonadotrope cell lines αT3-1 and LβT2 were obtained by directed oncogenesis in transgenic mice (6,7): the αT3-1 cell line represents an immature gonadotrope that expresses the glycoprotein hormone α-subunit gene, responds to GnRH, but does not express the LHβ and FSHβ subunits; in contrast, the LβT2 cell line displays the characteristics of a fully differentiated pituitary gonadotrope: in addition to the glycoprotein hormone α-subunit, it expresses LHβ, FSHβ, GnRHR, activin, inhibin, follistatin, and steroid receptors (8,9,10,11,12,13). Consequently, LβT2 cells represent a useful model for the study of GnRHR-mediated signaling, as well as intracellular transduction pathways triggered by physiological modulators of the gonadotropin response.
We have constructed a comprehensive, annotated map of the molecular interactions in LβT2 cells described in the literature as a resource for research on GnRHR signaling. We have made this knowledgebase readily accessible to the scientific community, with the goal of shared contribution by experimentalists through a wiki.
Results
General characteristics of the GnRHR-signaling map
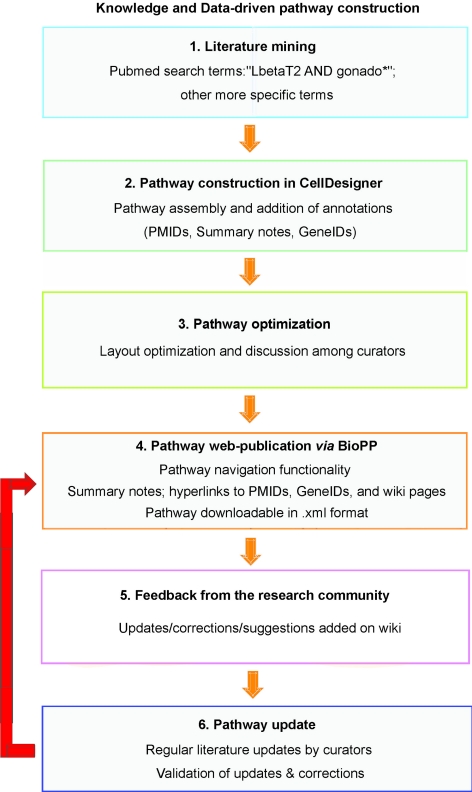
We manually curated a comprehensive pathway map for GnRHR-mediated signaling. The pathway construction workflow, which is summarized in Fig. 1, began with a literature-mining phase, using the general PubMed search terms gonado* ANDLbetaT2, more specific terms such as LbetaT2 AND PACAP, or searches by authors’ names. Following a pathway construction step, the curators discussed the contents of their diagrams, carefully reexamining the literature if necessary. This process led to a unified diagram, the layout of which was then optimized. Upon web publication of the pathway, we expect to receive feedback from experts in the research community through the gonadotrope pathway wiki pages. We will keep updating the pathway based on the latest publications in the field in addition to experts’ suggestions.
Figure 1.
Pathway construction workflow. A workflow diagram summarizes the main stages of pathway assembly from literature mining to pathway update. Boxes represent the pathway construction steps, which are connected by orange arrows. The red arrow indicates feedback to a previous construction step. In the initial phase of literature mining, more specific terms include: LbetaT2 AND PACAP, or searches by authors’ names.
The network diagram was initially created using CellDesigner (http://celldesigner.org/), a free process diagram editor for gene-regulatory and biochemical networks (14). This allows users to draw networks that are stored in Systems Biology Mark-up Language (SBML), a computer-readable format for representing models of biological processes (http://sbml.org/) (15). Furthermore, to enable real-time sharing of the GnRHR knowledgebase, we have used a tool previously developed in our laboratory, Biological Pathway Publisher (BioPP), which converts CellDesigner-SBML formatted pathways into a web-accessible format (16). Accordingly, the knowledgebase is deposited into a public repository endowed with a pathway navigator, which facilitates browsing of the uploaded pathway. Each species in the network is annotated with a complete list of interactions in which it participates, and PubMed references [PubMed Identifications (PMIDs)] supporting those interactions. Entities are also linked to National Center for Biotechnology Information (NCBI) Entrez Gene pages and to a public GnRH wiki, which is provided as a discussion forum for the community. The GnRH wiki pages are generated in an automated fashion by BioPP. Furthermore, users may download the GnRHR pathway diagram from the BioPP web site in an .xml format, which allows them to edit and/or expand it in CellDesigner, based upon their own experimental data or knowledge. The GnRHR-signaling pathway map is accessible at http://tsb.mssm.edu/pathwayPublisher/GnRHR_Pathway/GnRHR_Pathway_index.html.
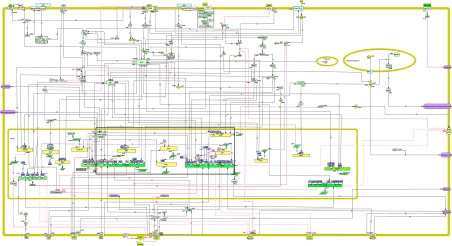
As illustrated in Fig. 2, the map includes G protein-mediated signal transduction pathways initiated in response to GnRH binding to its receptor, ultimately leading to the synthesis and secretion of gonadotropins LH and FSH. These intracellular signaling events include protein kinase C (PKC) via the activation of phospholipase Cβ, calcium mobilization, and the protein kinase A (PKA)-dependent pathway. GnRHR stimulation also results in activation of phospholipase D and phospholipase A2, the latter leading to the formation of leukotrienes and prostaglandins. Conventional PKCs activate MAPK cascades, whereas some novel PKC isoforms can activate the PKA-dependent pathway. MAPK cascades include ERK1/2, c-Jun N-terminal kinases 1/2, and p38 MAPKs. From a physiological standpoint, rapid gonadotropin secretion is primarily attributed to calcium mobilization by inositol triphosphate (17), whereas activation of MAPKs is thought to be involved in differential gonadotropin expression (18,19).
Figure 2.
Snapshot of the GnRHR-signaling pathway map. This map was created using CellDesigner version 4.0.1 and version 4.0.α (http://celldesigner.org/). A total of 187 species and 206 reactions were included. The main symbols are those implemented by CellDesigner version 4.0.1 and version 4.0.α. Interactions are color coded: black solid arrows, stimulatory reactions; red bar-headed lines, inhibitory reactions; black solid and white solid arrows with a bar, transport and trigger reactions, respectively; round-headed lines, catalysis reactions. The presence of a question mark or a dashed line denotes that, whether the reactions are direct or indirect, is unknown. Transcription reactions are represented by dashed and double-dotted lines, whereas translation reactions are symbolized by dashed and single-dotted lines. The following cellular compartments are illustrated on the diagram, as indicated: cytoplasm, endoplasmic reticulum, and nucleus. The size and color of each module were configured by us. Briefly, round angle green squares signify cell-signaling proteins, whereas green and yellow circles represent small molecules and ions, respectively; round angle blue squares symbolize transcription factors, and round angle purple squares strictly correspond to nuclear receptors; yellow/green rectangles designate genes, on which some regulatory regions or response elements are depicted as small white squares. The area of the signaling pathway map that is framed in black is shown in more detail in Fig. 3. The map can be best viewed on the web at http://tsb.mssm.edu/pathwayPublisher/GnRHR_Pathway/GnRHR_Pathway_index.html.
The current GnRHR-signaling network is based on the molecular interactions documented in more than 100 publications accessible from PubMed. It comprises 187 species or entities and 206 reactions or edges. A species is an entity that can participate in reactions. A reaction describes some transformation that can change the amount or state of one or more species. The database was limited to entities and transitions about which specific information existed in the literature. The following species participate in the GnRHR-signaling network: 119 proteins, three ions, 13 simple molecules, 16 RNAs, 16 genes, 10 complexes, one degraded entity, and seven phenotypes. Among the protein species, 16 are receptors, two are ion channels, and 34 are transcription factors, which are represented in blue or in light purple (nuclear receptors). The reactions can be classified into 101 state transitions (which include four catalyzes), 42 unknown transitions, five transport reactions, 21 inhibitions, nine unknown inhibitions, 16 transcriptions, and 12 translations.
Control of the GnRHR-signaling network
Activating processes are represented by black arrows, whereas inhibiting interactions are represented by red inhibition symbols. The biosynthesis and release of LH and FSH are regulated by hypothalamic factors, mainly GnRH, by gonadal steroids and peptides, as well as other hormones. The cell-signaling and molecular mechanisms that underlie the differential regulation of pituitary gonadotropins in various physiological situations, such as estrous cycle, childhood, and puberty, have been the subject of intense scrutiny and yet are still not fully understood. A number of modulators of the gonadotrope function are depicted on this pathway map. Steroid hormone feedback has been implicated in the regulation of gonadotropin gene expression. In fact, steroid hormone receptors are expressed in the gonadotropes (20,21,22). As illustrated here, estrogen, acting through the estrogen receptor, enhances GnRH-stimulated transcription of LHβ and α-subunit genes, by repressing the expression of the suppressive transcription factor Zeb1 (23). Androgen via its androgen receptor suppresses GnRH-stimulated LHβ transcription, whereas it activates FSHβ transcription (24,25,26,27). As shown in Fig. 3, whereas progesterone [PR (progesterone receptor)] and glucocorticoids [GR (glucocorticoid receptor)] directly activate FSHβ expression, they indirectly suppress LHβ gene expression (26,28).The circulating gonadal proteins, activin, inhibin, and follistatin are also key regulators of the gonadotrope function. These hormones are produced by the gonadotropes, suggesting some autocrine/paracrine effects on gonadotropin expression. It is shown here that activin stimulates the transcription of FSHβ, LHβ, and the GnRHR gene, and that activin action is blocked by follistatin and inhibin (29,30).
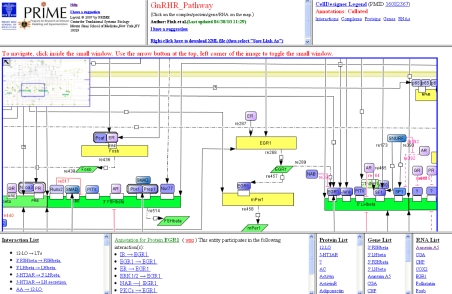
Figure 3.
Web-accessible GnRHR pathway navigator. This selected part of the GnRHR-signaling pathway map notably displays the promoters of the gonadotropin β-subunit genes (FSHβ and LHβ subunits), their response elements, and transcription factors that bind to them and regulate gene transcription. Indirect regulators of gene transcription are also illustrated. Entities are clickable, such that corresponding annotations, namely the interactions in which those entities are involved, and the related PubMed references are displayed in the bottom left-hand frame. Additionally, other frames include an interaction list, a protein list, a gene list, and an RNA list. Hyperlinks to the relevant NCBI Entrez Gene page(s) and GnRH wiki pages are also provided. A zoom rectangle located in the upper left corner of the image facilitates navigation through the pathway (16).
Pituitary adenylate cyclase-activating polypeptide (PACAP) is an important modulator of gonadotropin synthesis and secretion, acting both alone and in concert with GnRH (31). PACAP is not only secreted by hypothalamic neurons, but it is also expressed in the pituitary gonadotropes, suggesting its autocrine/paracrine role in the gonadotrope cells (32,33,34,35). Herein, we illustrate how PACAP, acting through its cell membrane receptor, activates the PKA pathway, thereby stimulating gonadotropin expression in cooperation with GnRH, as well as GnRHR gene expression. Expression of the PACAP gene itself is regulated by both PKC and PKA pathways.
Peroxisome proliferator-activated receptors (PPARs) are nuclear receptors that were found to be expressed in pituitary tumors and thus may represent a molecular target for treating patients with the disease (36). In LβT2 cells, PPARα and PPARγ down-regulate the transcription of the gonadotropin β-subunits as well as that of GnRHR (30).
Bone morphogenetic proteins, which belong to the TGF-β superfamily, play critical roles in the differentiation of pituitary gonadotropes (37). In contrast to PPARs, bone morphogenetic proteins activate FSHβ transcription (38,39).
Dopamine D2 receptor, which is highly expressed in the pituitary lactotropes, has been shown to be present in gonadotrope cells as well. Similarly, prolactin receptors are expressed in gonadotropes (40). Both dopamine and prolactin block GnRH-induced LHβ transcription (41). Moreover, dopamine acts as a negative regulator of the α-subunit gene transcription induced by the cAMP-dependent pathway (42).
The involvement of the epidermal growth factor receptor (EGFR) in the GnRHR-signaling network exemplifies the cross talk between GPCRs and receptor tyrosine kinases in gonadotrope cells. Studies revealed that members of the matrix metalloproteinase family are responsible for GnRH-mediated EGFR transactivation via activation of PKC, which subsequently results in rapid engagement of the ERK cascade (43,44).
Another example of cross talk is depicted on the network between the GnRHR- and Wnt-signaling pathways, wherein GnRH mediates the inactivation of glycogen synthase kinase 3, resulting in β-catenin stabilization and accumulation, T-cell factor activation, and subsequent up-regulation of Wnt target genes such as c-Jun (45). Furthermore, another study revealed that β-catenin is required for maximal activation of LHβ transcription in response to GnRH (46).
GnRH stimulates the induction of cyclooxygenase-2, a key enzyme involved in the synthesis of prostaglandins, which are autocrine regulators in many tissues (47). In the LβT2 cell line, prostaglandins have been shown to down-regulate GnRH-induced expression of GnRHR and LHβ (48).
GnRH increases the production of nitric oxide (NO) by NO synthase type I in LβT2 cells (49). In fact, chronic treatment with GnRH inhibits activin-induced expression of FSHβ via activation of NO synthase type I (50), which is consistent with the fact that chronic administration of GnRH inhibits FSHβ in vivo and in vitro (51,52).
Adiponectin, a factor secreted by the adipose tissue, is thought to be linked to reproduction, because a previous study demonstrated that female mice overexpressing adiponectin are infertile (53). In LβT2 cells, adiponectin binds to adiponectin receptors and attenuates LH secretion via the induction of AMP-activated protein kinase (54). This finding further supports the notion that adiponectin may be an important modulator of the gonadotrope function.
Graphic notations of the GnRHR-signaling map
The main symbols used to represent reactions and entities in the map are provided in the legend of Fig. 2, as well as on the web-accessible GnRHR-signaling map. Those symbols were obtained through CellDesigner version 4.0.1 and version 4.0.α and originally derive from the earlier proposals of Kitano et al. (15). They are also listed in Appendix 1 of the CellDesigner version 4.0 Startup Guide at http://celldesigner.org/documents/StartupGuide40.pdf.
Lists of entities, annotations, and links to wiki pages
The pathway navigator component of the BioPP suite allows users to browse the pathway and search for specific information. Lists of entities include hyperlinked lists of all proteins, genes, RNAs, and simple molecules/ions/phenotypes. As described above, annotations for entities include hyperlinked lists of interactions, which themselves provide relevant literature references in conjunction with their hyperlinked PMIDs. Entities may be associated with one or more GeneIDs, depending on the extent of experimentally based literature findings (Fig. 3). Finally, the rationale for associating the GnRHR-signaling pathway diagram with a mediawiki is to provide a community-based curation effort, allowing members of the scientific community to add comments or suggest new additions to the network. Hence, as new experimental data are being published, this editorial interface contributes to a perpetual improvement of the network.
Discussion
In this report, we present a comprehensive network map of the GnRHR signaling in the LβT2 gonadotrope cell line. Based on all the relevant articles published in the field, we manually curated a pathway map of GnRHR signaling in LβT2 cells. In addition to GnRH itself, regulators of the gonadotrope include gonadal and adrenal steroids, gonadal peptides, and hypothalamic neuropeptide PACAP. Steroid hormones are known to play a fundamental role in the control of GnRH expression in the hypothalamus, on the one hand, and in the regulation of expression of pituitary gonadotropins, on the other hand. Hence, under the influence of sex steroid feedback, transcription levels of the gonadotropin subunits vary during the estrous cycle in females (55,56,57). This network illustrates the intracellular cascades that are elicited by the interaction of GnRH and its receptor, as well as by other known regulators of gonadotrope function. The map is intended to be comprehensive and help researchers to unravel the signal transduction pathway and gene response mechanisms occurring in the pituitary gonadotrope. However, it is not necessarily exhaustive. We anticipate updating the map regularly using data drawn from newly published studies, as well as through exchanges with researchers whose area of expertise is the hypothalamic-pituitary-gonadal axis in general, and/or the pituitary gonadotrope cell in particular. Those exchanges will be made possible by the availability of the GnRH wiki, which will allow experts to suggest corrections or additions through their feedback and comments.
Building a thorough signaling network is challenging and thus may inevitably result in a visually dense and complex pathway diagram. For instance, the number of nodes and edges comprised in the signaling networks of the toll-like receptor [444 and 652, respectively; (58)] and of macrophage activation [295 and 272, respectively; (59)] are such that all interactions cannot be observed on the same page. This is in contrast with other existing GnRH-signaling networks, such as those of the KEGG PATHWAY database (http://www.genome.jp/kegg/pathway.html), and of Ingenuity Systems (a commercial knowledgebase; http://www.ingenuity.com/index.html). Our signaling network is considerably more detailed and referenced than its KEGG counterpart, because it notably depicts the effects of various hormonal regulators on the gonadotrope cell and numerous elements of the transcriptome. Although Ingenuity Systems offers a great diversity of annotations, including literature references from various biological models and many other database resources, it presents a fairly basic GnRH-signaling network comprised of only 50 entities; moreover, the Ingenuity network depicts a hypothetical GnRH-responsive cell rather than a true reflection of the gonadotrope cell: for instance, it displays the activation of transcription factor nuclear factor-κB by protein Gαi, a finding that was reported in human melanoma cells, but never in gonadotrope cells (60). Herein, we provide a web-published GnRHR-signaling diagram, which represents both an online resource and an opportunity for community-wide collaboration. Each node is clickable and links to a list of interactions in which it is involved in the LβT2 cell line; interactions themselves are supported by hyperlinked PMIDs; in the case of transcription factors, which typically bind to gene promoters, hyperlinked PMIDs are directly associated with those entities. Any entity that belongs to either a protein, RNA, or gene category is linked to a wiki page, which allows for contributions from experts in the field. Moreover, an .xml version of the pathway is available for download on the BioPP web site, allowing individual biologists to modify and expand it in CellDesigner in accordance with their own experimental observations. This .xml file (also available in the Supplemental data published on The Endocrine Society’s Journals Online web site http://mend.endojournals.org) includes the PMIDs supporting the interactions and the GeneIDs describing the entities. Finally, researchers who wish to generate a web-accessible version of their network diagrams are encouraged to do so by following the guidelines posted on the BioPP web site.
The concept of a knowledgebase that receives input from the research community is fairly novel. In fact, a new community-based platform termed “Payao” (http://www.payaologue.org) has just been developed by the Kitano laboratory for sharing pathway models. Payao provides a web-based interface for adding tags and comments to curated pathway models (61). However, whereas Payao assigns privileges to specific community members, the wiki scheme can record any changes made by contributors. We hope that our platform, as well as similar initiatives stemming from other groups, will contribute to the improvement of community-driven pathway enrichment (62).
To conserve space on the pathway map and for ease of readability, we intentionally omitted the following: 1) modification states of proteins, such as phosphorylation, acetylation, and ubiquitination, 2) ligands of nuclear receptors, e.g. steroid receptors. In some instances, a protein-protein interaction may be ambiguous due to conflicting literature results, in which case we typically choose to illustrate the interaction, which we believe is the most strongly supported by experimental data. Nevertheless, we include the contradictory paper(s) among the PubMed references. In fact, this type of scenario occurred with PACAP, which was demonstrated to have a stimulatory effect on glycoprotein hormone α-subunit (CGA) transcription in LβT2 cells (63), whereas another group failed to show any activation of the CGA promoter (64). The discrepancies in the results obtained from those two research teams might be due to a more than 300-bp difference in the length of the CGA gene promoter used in transfection studies, as well as distinct transfection methods and cell culture conditions.
We reckon that the LβT2 cell model offers a valuable representation of the GnRHR-signaling network and a reliable framework for integrating additional experimental data, as has occurred in other systems such as EGFR (65). We also recognize that the choice of a cellular model in a given research study may affect its experimental outcome; hence, Fujii et al. (66) formerly observed opposite results on FSHβ gene expression after treatment of primary pituitary cultures vs. clonal gonadotrope cell line LβT2 with either GnRH or PACAP: namely, FSHβ mRNAs were suppressed in GnRH- or PACAP-treated pituitary cultures and were increased in LβT2 cells; moreover, FSHβ gene transcription was stimulated in transiently transfected LβT2. Therefore, a cautious interpretation of experimental data based upon the selected model and an awareness of the limitations of this model are necessary. We would like to emphasize that the task of assembling a gonadotrope cell-signaling network is a work in progress, and that the support of the community in doing so is crucial. Hence, in the near future, we may envision to integrate data derived from 1) in vitro studies performed in cellular models other than the LβT2, such as other cell lines (αT3-1, etc.) and pituitary primary cultures, 2) in vivo experiments carried out in animals or transgenic rodents.
Materials and Methods
The GnRHR-signaling pathway map was created using CellDesigner version 4.0.1 and version 4.0.α (14), which provide graphic tools, pathway visualization, and navigation. The BioPP suite was employed to allow web publication of the pathway with an easily navigated user interface (16). Links to Entrez PubMed pages, Entrez Gene pages, and the appropriate pages of a public wiki-based discussion forum were supplied. An upgrade of BioPP was implemented in the context of the present work.
Supplementary Material
Acknowledgments
We thank Dr. Jeremy Seto for establishing the wiki.
Footnotes
This work was supported by National Institutes of Health Grant DK46943.
Disclosure Summary: M.Y.F., H.P., S.G.C., and G.N. have nothing to declare. S.C.S. is an inventor on US Patents 5,985,583 and 5,750,366 and has received royalties for these patents.
First Published Online June 30, 2010
Abbreviations: BioPP, Biological Pathway Publisher; CGA, glycoprotein hormone α-subunit; EGFR, epidermal growth factor; GnRHR, GnRH receptor; GPCR, G-protein-coupled receptor; NO, nitric oxide; PACAP, pituitary adenylate cyclase-activating polypeptide; PKA, protein kinase A; PKC, protein kinase C; PPAR, peroxisome proliferator-activated receptor; SBML, Systems Biology Mark-up Language.
References
- Burger LL, Dalkin AC, Aylor KW, Haisenleder DJ, Marshall JC 2002 GnRH pulse frequency modulation of gonadotropin subunit gene transcription in normal gonadotropes-assessment by primary transcript assay provides evidence for roles of GnRH and follistatin. Endocrinology 143:3243–3249 [DOI] [PubMed] [Google Scholar]
- Dalkin AC, Haisenleder DJ, Ortolano GA, Ellis TR, Marshall JC 1989 The frequency of gonadotropin-releasing-hormone stimulation differentially regulates gonadotropin subunit messenger ribonucleic acid expression. Endocrinology 125:917–924 [DOI] [PubMed] [Google Scholar]
- Layman LC, Cohen DP, Jin M, Xie J, Li Z, Reindollar RH, Bolbolan S, Bick DP, Sherins RR, Duck LW, Musgrove LC, Sellers JC, Neill JD 1998 Mutations in gonadotropin-releasing hormone receptor gene cause hypogonadotropic hypogonadism. Nat Genet 18:14–15 [DOI] [PubMed] [Google Scholar]
- Vrecl M, Heding A, Hanyaloglu A, Taylor PL, Eidne KA 2000 Internalization kinetics of the gonadotropin-releasing hormone (GnRH) receptor. Pflugers Arch 439:R19–R20 [PubMed] [Google Scholar]
- Willars GB, Heding A, Vrecl M, Sellar R, Blomenröhr M, Nahorski SR, Eidne KA 1999 Lack of a C-terminal tail in the mammalian gonadotropin-releasing hormone receptor confers resistance to agonist-dependent phosphorylation and rapid desensitization. J Biol Chem 274:30146–30153 [DOI] [PubMed] [Google Scholar]
- Alarid ET, Windle JJ, Whyte DB, Mellon PL 1996 Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development 122:3319–3329 [DOI] [PubMed] [Google Scholar]
- Windle JJ, Weiner RI, Mellon PL 1990 Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol Endocrinol 4:597–603 [DOI] [PubMed] [Google Scholar]
- Lawson MA, Li D, Glidewell-Kenney CA, López FJ 2001 Androgen responsiveness of the pituitary gonadotrope cell line LβT2. J Endocrinol 170:601–607 [DOI] [PubMed] [Google Scholar]
- Lewis KA, Gray PC, Blount AL, MacConell LA, Wiater E, Bilezikjian LM, Vale W 2000 Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature 404:411–414 [DOI] [PubMed] [Google Scholar]
- Pernasetti F, Vasilyev VV, Rosenberg SB, Bailey JS, Huang HJ, Miller WL, Mellon PL 2001 Cell-specific transcriptional regulation of follicle-stimulating hormone-β by activin and gonadotropin-releasing hormone in the LβT2 pituitary gonadotrope cell model. Endocrinology 142:2284–2295 [DOI] [PubMed] [Google Scholar]
- Schreihofer DA, Stoler MH, Shupnik MA 2000 Differential expression and regulation of estrogen receptors (ERs) in rat pituitary and cell lines: estrogen decreases ERα protein and estrogen responsiveness. Endocrinology 141:2174–2184 [DOI] [PubMed] [Google Scholar]
- Thomas P, Mellon PL, Turgeon J, Waring DW 1996 The L β T2 clonal gonadotrope: a model for single cell studies of endocrine cell secretion. Endocrinology 137:2979–2989 [DOI] [PubMed] [Google Scholar]
- Turgeon JL, Kimura Y, Waring DW, Mellon PL 1996 Steroid and pulsatile gonadotropin-releasing hormone (GnRH) regulation of luteinizing hormone and GnRH receptor in a novel gonadotrope cell line. Mol Endocrinol 10:439–450 [DOI] [PubMed] [Google Scholar]
- Funahashi A, Tanimura N, Morohashi M, Kitano H 2003 CellDesigner: a process diagram editor for gene-regulatory and biochemical networks. BioSilico 1:159–162 [Google Scholar]
- Kitano H, Funahashi A, Matsuoka Y, Oda K 2005 Using process diagrams for the graphical representation of biological networks. Nat Biotechnol 23:961–966 [DOI] [PubMed] [Google Scholar]
- Viswanathan GA, Nudelman G, Patil S, Sealfon SC 2007 BioPP: a tool for web-publication of biological networks. BMC Bioinformatics 8:168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse FW, Tse A, Hille B, Horstmann H, Almers W 1997 Local Ca2+ release from internal stores controls exocytosis in pituitary gonadotrophs. Neuron 18:121–132 [DOI] [PubMed] [Google Scholar]
- Naor Z, Benard O, Seger R 2000 Activation of MAPK cascades by G-protein-coupled receptors: the case of gonadotropin-releasing hormone receptor. Trends Endocrinol Metab 11:91–99 [DOI] [PubMed] [Google Scholar]
- Sundaresan S, Colin IM, Pestell RG, Jameson JL 1996 Stimulation of mitogen-activated protein kinase by gonadotropin-releasing hormone: evidence for the involvement of protein kinase C. Endocrinology 137:304–311 [DOI] [PubMed] [Google Scholar]
- Pelletier G, Labrie C, Labrie F 2000 Localization of oestrogen receptor α, oestrogen receptor β and androgen receptors in the rat reproductive organs. J Endocrinol 165:359–370 [DOI] [PubMed] [Google Scholar]
- Stefaneanu L 1997 Pituitary sex steroid receptors: localization and function. Endocr Pathol 8:91–108 [DOI] [PubMed] [Google Scholar]
- Turgeon JL, Waring DW 2000 Progesterone regulation of the progesterone receptor in rat gonadotropes. Endocrinology 141:3422–3429 [DOI] [PubMed] [Google Scholar]
- Kowase T, Walsh HE, Darling DS, Shupnik MA 2007 Estrogen enhances gonadotropin-releasing hormone-stimulated transcription of the luteinizing hormone subunit promoters via altered expression of stimulatory and suppressive transcription factors. Endocrinology 148:6083–6091 [DOI] [PubMed] [Google Scholar]
- Curtin D, Ferris HA, Häkli M, Gibson M, Jänne OA, Palvimo JJ, Shupnik MA 2004 Small nuclear RING finger protein stimulates the rat luteinizing hormone-β promoter by interacting with Sp1 and steroidogenic factor-1 and protects from androgen suppression. Mol Endocrinol 18:1263–1276 [DOI] [PubMed] [Google Scholar]
- Curtin D, Jenkins S, Farmer N, Anderson AC, Haisenleder DJ, Rissman E, Wilson EM, Shupnik MA 2001 Androgen suppression of GnRH-stimulated rat LHβ gene transcription occurs through Sp1 sites in the distal GnRH-responsive promoter region. Mol Endocrinol 15:1906–1917 [DOI] [PubMed] [Google Scholar]
- Thackray VG, McGillivray SM, Mellon PL 2006 Androgens, progestins, and glucocorticoids induce follicle-stimulating hormone β-subunit gene expression at the level of the gonadotrope. Mol Endocrinol 20:2062–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackray VG, Mellon PL 2008 Synergistic induction of follicle-stimulating hormone β-subunit gene expression by gonadal steroid hormone receptors and Smad proteins. Endocrinology 149:1091–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackray VG, Hunnicutt JL, Memon AK, Ghochani Y, Mellon PL 2009 Progesterone Inhibits basal and gonadotropin-releasing hormone induction of luteinizing hormone β-subunit gene expression. Endocrinology 150:2395–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suszko MI, Lo DJ, Suh H, Camper SA, Woodruff TK 2003 Regulation of the rat follicle-stimulating hormone β-subunit promoter by activin. Mol Endocrinol 17:318–332 [DOI] [PubMed] [Google Scholar]
- Takeda M, Otsuka F, Otani H, Inagaki K, Miyoshi T, Suzuki J, Mimura Y, Ogura T, Makino H 2007 Effects of peroxisome proliferator-activated receptor activation on gonadotropin transcription and cell mitosis induced by bone morphogenetic proteins in mouse gonadotrope LβT2 cells. J Endocrinol 194:87–99 [DOI] [PubMed] [Google Scholar]
- Culler MD, Paschall CS 1991 Pituitary adenylate cyclase-activating polypeptide (PACAP) potentiates the gonadotropin-releasing activity of luteinizing hormone-releasing hormone. Endocrinology 129:2260–2262 [DOI] [PubMed] [Google Scholar]
- Heinzlmann A, Kirilly E, Meltzer K, Szabó E, Baba A, Hashimoto H, Köves K 2008 PACAP is transiently expressed in anterior pituitary gland of rats: in situ hybridization and cell immunoblot assay studies. Peptides 29:571–577 [DOI] [PubMed] [Google Scholar]
- Kimura C, Ohkubo S, Ogi K, Hosoya M, Itoh Y, Onda H, Miyata A, Jiang L, Dahl RR, Stibbs HH, Arimura A, Fujino M 1990 A novel peptide which stimulates adenylate cyclase: molecular cloning and characterization of the ovine and human cDNAs. Biochem Biophys Res Commun 166:81–89 [DOI] [PubMed] [Google Scholar]
- Köves K, Kántor O, Scammell JG, Arimura A 1998 PACAP colocalizes with luteinizing and follicle-stimulating hormone immunoreactivities in the anterior lobe of the pituitary gland. Peptides 19:1069–1072 [DOI] [PubMed] [Google Scholar]
- Szabó E, Nemeskéri A, Heinzlmann A, Suzuki N, Arimura A, Köves K 2002 Cell immunoblot assay study demonstrating the release of PACAP from individual anterior pituitary cells of rats and the effect of PACAP on LH release. Regul Pept 109:75–81 [DOI] [PubMed] [Google Scholar]
- Heaney AP, Fernando M, Yong WH, Melmed S 2002 Functional PPAR-γ receptor is a novel therapeutic target for ACTH-secreting pituitary adenomas. Nat Med 8:1281–1287 [DOI] [PubMed] [Google Scholar]
- Paez-Pereda M, Giacomini D, Refojo D, Nagashima AC, Hopfner U, Grubler Y, Chervin A, Goldberg V, Goya R, Hentges ST, Low MJ, Holsboer F, Stalla GK, Arzt E 2003 Involvement of bone morphogenetic protein 4 (BMP-4) in pituitary prolactinoma pathogenesis through a Smad/estrogen receptor crosstalk. Proc Natl Acad Sci USA 100:1034–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HJ, Wu JC, Su P, Zhirnov O, Miller WL 2001 A novel role for bone morphogenetic proteins in the synthesis of follicle-stimulating hormone. Endocrinology 142:2275–2283 [DOI] [PubMed] [Google Scholar]
- Otsuka F, Shimasaki S 2002 A novel function of bone morphogenetic protein-15 in the pituitary: selective synthesis and secretion of FSH by gonadotropes. Endocrinology 143:4938–4941 [DOI] [PubMed] [Google Scholar]
- Tortonese DJ, Brooks J, Ingleton PM, McNeilly AS 1998 Detection of prolactin receptor gene expression in the sheep pituitary gland and visualization of the specific translation of the signal in gonadotrophs. Endocrinology 139:5215–5223 [DOI] [PubMed] [Google Scholar]
- Henderson HL, Townsend J, Tortonese DJ 2008 Direct effects of prolactin and dopamine on the gonadotroph response to GnRH. J Endocrinol 197:343–350 [DOI] [PubMed] [Google Scholar]
- Mutiara S, Kanasaki H, Harada T, Miyazaki K 2006 Dopamine D(2) receptor expression and regulation of gonadotropin α-subunit gene in clonal gonadotroph LβT2 cells. Mol Cell Endocrinol 259:22–29 [DOI] [PubMed] [Google Scholar]
- Grosse R, Roelle S, Herrlich A, Höhn J, Gudermann T 2000 Epidermal growth factor receptor tyrosine kinase mediates Ras activation by gonadotropin-releasing hormone. J Biol Chem 275:12251–12260 [DOI] [PubMed] [Google Scholar]
- Roelle S, Grosse R, Aigner A, Krell HW, Czubayko F, Gudermann T 2003 Matrix metalloproteinases 2 and 9 mediate epidermal growth factor receptor transactivation by gonadotropin-releasing hormone. J Biol Chem 278:47307–47318 [DOI] [PubMed] [Google Scholar]
- Gardner S, Maudsley S, Millar RP, Pawson AJ 2007 Nuclear stabilization of β-catenin and inactivation of glycogen synthase kinase-3β by gonadotropin-releasing hormone: targeting Wnt signaling in the pituitary gonadotrope. Mol Endocrinol 21:3028–3038 [DOI] [PubMed] [Google Scholar]
- Salisbury TB, Binder AK, Grammer JC, Nilson JH 2007 Maximal activity of the luteinizing hormone β-subunit gene requires β-catenin. Mol Endocrinol 21:963–971 [DOI] [PubMed] [Google Scholar]
- Hata AN, Breyer RM 2004 Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther 103:147–166 [DOI] [PubMed] [Google Scholar]
- Naor Z, Jabbour HN, Naidich M, Pawson AJ, Morgan K, Battersby S, Millar MR, Brown P, Millar RP 2007 Reciprocal cross talk between gonadotropin-releasing hormone (GnRH) and prostaglandin receptors regulates GnRH receptor expression and differential gonadotropin secretion. Mol Endocrinol 21:524–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Sakai T, Sakamoto S, Kato M, Inoue K 1999 Direct evidence of gonadotropin-releasing hormone (GnRH)-stimulated nitric oxide production in the L βT-2 clonal gonadotropes. Pituitary 2:191–196 [DOI] [PubMed] [Google Scholar]
- Shafiee-Kermani F, Han SO, Miller WL 2007 Chronic gonadotropin-releasing hormone inhibits activin induction of the ovine follicle-stimulating hormone β-subunit: involvement of 3′,5′-cyclic adenosine monophosphate response element binding protein and nitric oxide synthase type I. Endocrinology 148:3346–3355 [DOI] [PubMed] [Google Scholar]
- Bédécarrats GY, Kaiser UB 2003 Differential regulation of gonadotropin subunit gene promoter activity by pulsatile gonadotropin-releasing hormone (GnRH) in perifused LβT2 cells: role of GnRH receptor concentration. Endocrinology 144:1802–1811 [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Dalkin AC, Ortolano GA, Marshall JC, Shupnik MA 1991 A pulsatile gonadotropin-releasing hormone stimulus is required to increase transcription of the gonadotropin subunit genes: evidence for differential regulation of transcription by pulse frequency in vivo. Endocrinology 128:509–517 [DOI] [PubMed] [Google Scholar]
- Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, Nawrocki AR, Rajala MW, Parlow AF, Cheeseboro L, Ding YY, Russell RG, Lindemann D, Hartley A, Baker GR, Obici S, Deshaies Y, Ludgate M, Rossetti L, Scherer PE 2004 A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology 145:367–383 [DOI] [PubMed] [Google Scholar]
- Lu M, Tang Q, Olefsky JM, Mellon PL, Webster NJ 2008 Adiponectin activates adenosine monophosphate-activated protein kinase and decreases luteinizing hormone secretion in LβT2 gonadotropes. Mol Endocrinol 22:760–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortolano GA, Haisenleder DJ, Dalkin AC, Iliff-Sizemore SA, Landefeld TD, Maurer RA, Marshall JC 1988 Follicle-stimulating hormone β subunit messenger ribonucleic acid concentrations during the rat estrous cycle. Endocrinology 123:2149–2151 [DOI] [PubMed] [Google Scholar]
- Shupnik MA, Gharib SD, Chin WW 1989 Divergent effects of estradiol on gonadotropin gene transcription in pituitary fragments. Mol Endocrinol 3:474–480 [DOI] [PubMed] [Google Scholar]
- Zmeili SM, Papavasiliou SS, Thorner MO, Evans WS, Marshall JC, Landefeld TD 1986 α and Luteinizing hormone β subunit messenger ribonucleic acids during the rat estrous cycle. Endocrinology 119:1867–1869 [DOI] [PubMed] [Google Scholar]
- Oda K, Kitano H 2006 A comprehensive map of the toll-like receptor signaling network. Mol Syst Biol 2:2006.0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza S, Robertson KA, Lacaze PA, Page D, Enright AJ, Ghazal P, Freeman TC 2008 A logic-based diagram of signalling pathways central to macrophage activation. BMC Syst Biol 2:36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Duk Jung I, Gyo Park C, Han JW, Young Lee H 2006 Autotaxin stimulates urokinase-type plasminogen activator expression through phosphoinositide 3-kinase-Akt-nuclear [corrected] factor κB signaling cascade in human melanoma cells. Melanoma Res 16:445–452 [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Ghosh S, Kikuchi N, Kitano H 2010 Payao: a community platform for SBML pathway model curation. Bioinformatics 26:1381–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A 2010 Curators of the world unite: the International Society of Biocuration. Bioinformatics 26:991 [DOI] [PubMed] [Google Scholar]
- Harada T, Kanasaki H, Mutiara S, Oride A, Miyazaki K 2007 Cyclic adenosine 3′,5′monophosphate/protein kinase A and mitogen-activated protein kinase 3/1 pathways are involved in adenylate cyclase-activating polypeptide 1-induced common α-glycoprotein subunit gene (Cga) expression in mouse pituitary gonadotroph LβT2 cells. Biol Reprod 77:707–716 [DOI] [PubMed] [Google Scholar]
- Fowkes RC, Sidhu KK, Sosabowski JK, King P, Burrin JM 2003 Absence of pituitary adenylate cyclase-activating polypeptide-stimulated transcription of the human glycoprotein α-subunit gene in LβT2 gonadotrophs reveals disrupted cAMP-mediated gene transcription. J Mol Endocrinol 31:263–278 [DOI] [PubMed] [Google Scholar]
- Oda K, Matsuoka Y, Funahashi A, Kitano H 2005 A comprehensive pathway map of epidermal growth factor receptor signaling. Mol Syst Biol 1:2005.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y, Okada Y, Moore Jr JP, Dalkin AC, Winters SJ 2002 Evidence that PACAP and GnRH down-regulate follicle-stimulating hormone-β mRNA levels by stimulating follistatin gene expression: effects on folliculostellate cells, gonadotrophs and LβT2 gonadotroph cells. Mol Cell Endocrinol 192:55–64 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.