Abstract
Background
Sex disparities have been well documented in patients with ischemic stroke. Previous studies have suggested that female sex is a risk factor for delay in arrival time to the emergency department (ED) and may contribute to ineligibility for thrombolytic therapy. With the increase in education efforts targeting women, we investigated whether ED arrival times, rates of thrombolytic use, and functional outcomes continue to differ in men and women with acute ischemic stroke (AIS).
Methods
This study was a retrospective database analysis of patients with AIS (2001–2008). All AIS patients presenting within 24 hours with a known time of symptom onset and a documented admission National Institutes of Health Stroke Scale (NIHSS) were included. The Modified Barthel Index (MBI) assessed patients' functional status preadmission (historical), admission, and at 3 and 12 months poststroke.
Results
Included in the analysis were 480 (50.6%) women and 468 (49.4%) men. Women were significantly older than men (70.6 ± 0.7 vs. 65.3 years ± 0.6, p ≤ 0.001). Mean onset-to-ED time was not significantly different between the sexes (women 265 ± 283 vs. men 245 ± 300 minutes), nor was prestroke MBI. Logistic regression analysis demonstrated that female sex, increasing age, higher admission NIHSS, and longer onset-to-ED times all contributed to poorer functional status.
Conclusions
Women arrive at the ED at equivalent speed as men after AIS. Women had greater functional impairments at 3 months and 12 months poststroke despite equivalent prestroke MBI and admission NIHSS. Female sex contributes to poorer chronic functional outcomes after AIS.
Introduction
Stroke ranks as the third most frequent cause of death in the United States, trailing only acute myocardial infarction (AMI) and all forms of cancer.1 Even more importantly, stroke is the leading cause of disability in the aging American population—roughly 5.7 million U.S. stroke survivors are alive today. Historically, male sex has been considered a risk factor for stroke; however, because of their longer life expectancy, the majority of stroke deaths now occur in women.1,2 Furthermore, of the 5.7 million U.S. stroke survivors, 3.3 million are women and 2.4 million are men.1 Women consistently have worse acute functional outcomes after stroke compared with men regardless of whether or not intravenous or intra-arterial tissue plasminogen activator (tPA) therapy is used.3–5 Women also are more likely to self-report an incomplete recovery requiring a greater need for help than men.3 Therefore, the economic burden of stroke will continue to rise as our population ages and these sex differences are amplified.3–6
Despite advances in the public's awareness of stroke symptoms and of treatment options, stroke is often undertreated in the acute setting, as only a minority of patients arrive to medical care within the time frame for treatment.7 Several previous studies, including a recent meta-analysis,8 have shown that women are less likely to receive intravenous tPA therapy in the acute setting8–12 despite evidence that women may benefit more than men from tPA treatment.13–18 Diagnostic evaluation and acute hospital management may also be less active in women.15,19–21 One of the major limitations to widespread tPA use is its short time window. Earlier work found a 3-fold risk of delay in reaching the hospital in women experiencing a stroke,22 which could increase rates of ineligibility for thrombolytic treatment. Several studies tracking women's awareness of heart disease suggest that although awareness has improved, there still is a significant gap between the perceived and actual risk of cardiovascular disease (CVD) and stroke, as well as social and behavioral factors associated with delays to seeking treatment in women.23–25 The objectives of this study were to determine if women with acute ischemic stroke (AIS) arrive later to the ED compared with men (patient factors) and if treatment is equivalent in men and women on arrival at the emergency department (ED) (system factors) and to evaluate long-term functional outcomes in men and women after AIS in a community-based cohort.
Materials and Methods
This was an IRB-approved retrospective analysis of data collected from The Stroke Center at Hartford Hospital, which has been compiling data—demographic and medical information, clinical and imaging details of stroke, National Institutes of Health Stroke Scale (NIHSS) score at admission/discharge, and Barthel score (Modified Barthel Index [MBI]) at admission, discharge, and 3, 6, 9, and 12 months follow-up—on every stroke admission since May 2001. Data are collected in a standardized format for every stroke admission by a trained registered nurse at the time of presentation and subsequently by telephone interview and entered into a searchable stroke database. Data collected include but are not limited to onset of symptoms (date and time, demographics, type and location of stroke based on imaging), details of event, details of ED arrival and workup, symptoms, treatment, complications, medical history, and details of rehabilitation.
Participants
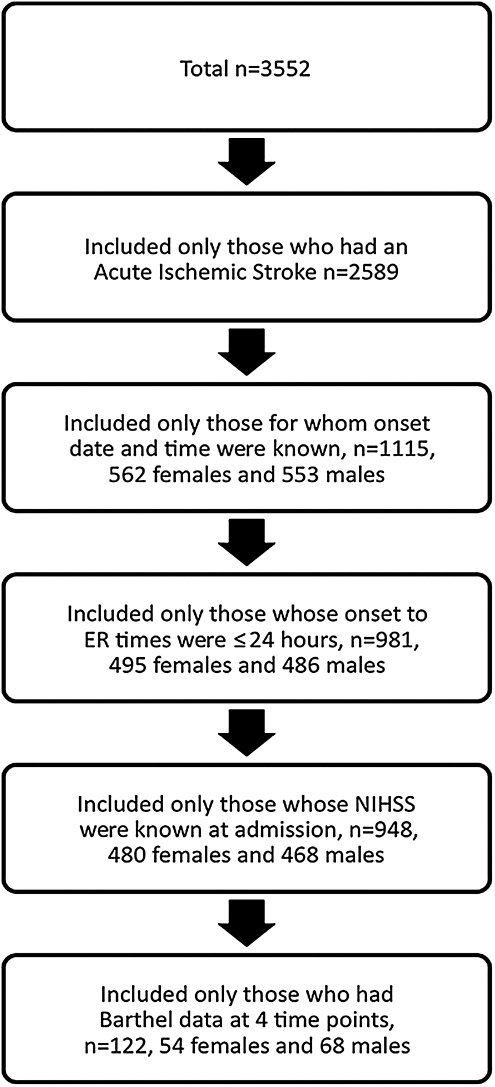
There were a total of 3552 entries in the stroke database (Fig. 1). Only those patients who had a diagnosis of AIS were included in this study; n = 2589, as these are the only patients eligible for thrombolysis/intervention. Those excluded had a diagnosis of transient ischemic attack (TIA), stroke rule-out, or hemorrhagic stroke (n = 963). Of the 2589 patients who had a diagnosis of AIS, only those who had a known stroke symptom onset date and time were included, (n = 1115), that is, only those patients for whom the time of symptom onset was definitively identified. In cases of wakeup stroke or where time of onset was not known, patients were excluded. Definitive symptom onset time is when either the patient or a witness was able to tell the time at which the first signs of stroke were seen. Those who woke up with stroke were assigned a time of midnight as the symptom onset time. The sample included patients of all ages who came to the ED from home, as well as from other hospitals (transfer patients), extended care facilities, and assisted living facilities. Because treatment options are limited in patients who arrive after 24 hours of AIS, we restricted the sample to those who came to the ED in ≤24 hours from onset of stroke symptoms, that is, 981 patients, with 486 men (49.5%) and 495 women (50.5%). Further, patients whose NIHSS score at admission was unknown were excluded (33 patients excluded) (n = 948, with 468 men (49.4%) and 480 women (50.6%). In later analysis, patients who did not have Barthel data at all four time points were excluded, as this information is required to perform within-subjects analysis. The final sample size for 12-month data in the final analysis was 122, with 54 women and 68 men for functional recovery measures at 12 months (Fig. 1).
FIG. 1.
Flow chart of patient exclusion criteria.
Measures
The NIHSS was used to assess stroke severity for all patients immediately on arrival at the ED. Information about patients' rehabilitation and functional status was collected at prestroke (historical), admission, and 3 months and 12 months poststroke using the universal MBI.26 A total of 831 patients had completed MBIs at prestroke and admission, only 754 patients had completed the MBI scale at 3 months, and a total of 715 patients completed the MBI scale at 12 months poststroke. Only 122 patients had completed MBI scale at all four time points. For the within-subjects analysis, we included only those who had completed MBI scale data for all time points. Information about age of the patient, date and time of onset of stroke symptoms, date and time of arrival at the ED, time seen by a physician, time seen by a neurology consultant, time at which a CT scan was done, and time of treatment (with tPA) were also collected in a standardized format by trained personnel. For patients for whom time of onset was unknown or when it was a wakeup stroke, the time of onset was left blank.
Statistical methods
Descriptive analyses were performed to evaluate distributions of continuous variables. Log transformation was used to normalize the distribution of positively skewed variables: onset-to ED, NIHSS admission scores, ED to thrombolytic treatment time, ED to neurology consultation time, ED to CT scan time, and ED to time seen by a physician. t Tests were done to examine sex differences in age at admission, log NIH admission score, log onset-to-ED time, log ED to tPA treatment time, log ED to neurology consultation time, log ED to CT scan time, and log ED to time seen by a physician, with correction for multiple comparisons. Generalized Linear Model (GLM) repeated measures analysis was used to explore the main effects of MBI at four time points (preadmission, admission, 3 months, and 12 months) by sex. The interaction of MBI and sex was also examined in this model. Similar models were run with patient age and NIHSS scores as covariates with sex (gender) in predicting the MBI scores at four time points. MBI data were available only for a subset of the participants (n = 122); 68 men (56%) and 54 women (44%) had MBI data at all time points. We used the MBI, a 10-item scale that is scored from 0 (death) to 20 (independent) and uses identical questions as the standard MBI (10-item scale from 0 to 100) for ease of use.26
In addition, two new variables were created, BarPre12mth and BarAdm12mth: BarPre12mth, MBI at 12 months − prestroke MBI; BarAdm12mth, MBI at 12 months − MBI at admission. These two variables assessed the change in functional status at 1 year poststroke in comparison to prestroke and admission functional status. These variables were further dichotomized based on the median value. BarPre12mthgroup value ≤ −1.0 (−1.0 was the median for BarPre12mth) was assigned a code of 1, and others were assigned a code of 2. BarAdm12mthgroup value ≤1.5 (1.5 was the median for BarAdm12mth) was assigned a code of 1, and others were assigned a code of 2. These two dichotomous variables were used as the outcome in a logistic regression model to examine whether gender, age, and NIHSS score at admission significantly predict what group (1 or 2) one falls into.
Results
There were 948 patients included in these analyses, 480 women and 468 men. Women were significantly older than men at admission; mean age for women = 70.6 ± 0.7 vs. mean age for men = 65.3 years ± 0.6, p ≤ 0.001). Mean onset-to-ED time was 245 ± 300 minutes for males and 265 ± 283 minutes for females. Onset-to-ED times and NIHSS scores at admission were positively skewed. A log transformation was used to normalize both these variables. Both log onset-to-ED and log NIHSS scores were not significantly different by sex. In addition, for ease of interpretation, NIHSS scores were categorized into four groups based on quartiles. The first quartile was assigned group number 1 (NIHSS ≤2), the second was assigned group number 2 (NIHSS >2 but ≤3), the third was assigned group number 3 (NIHSS >3 but ≤7), and the fourth was assigned group number 4 (NIHSS >7). The first quartile comprised 404 (42.6%) patients, 157 (16.6%) patients fell in the second quartile, 175 (18.5%) patients were in third quartile, and 212 (22.4%) patients were in the fourth quartile. Similar categorization was not performed for onset-to-ED because the cutoffs for quartiles for onset-to-ED did not relate to the time within which clinical treatment is warranted for AIS. t Tests for log-transformed patient care variables revealed no sex differences in arrival to tPA administration, arrival to time seen by physician, arrival to neurology consultation, and arrival to time of CT scan.
Repeated measures analysis results
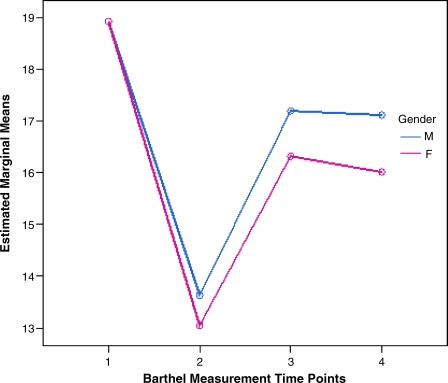
Significant sex differences in functional recovery were found. First, a GLM repeated measures analysis was conducted with MBI at four time points by sex, where 1 = males and 2 = females. The within-subjects test indicates that there is a significant time effect (Table 1); in other words, the MBI scores do change over time for both sexes, and they are worst at admission and improve at 3-month and 12-month follow-up. Table 2 shows test of within-subjects contrasts. Not surprisingly, MBI scores within subjects at admission and 3 months and 12 months poststroke are significantly different from MBI scores prestroke. The between-sex test indicated that sex is significant (Table 3 and Fig. 2). The two sexes diverge at three of the four time points. Further, the interaction of time and sex is significant (Table 2), demonstrating that the MBI scores for the sexes change over time but change in different ways, as evidenced by the nonparallel lines (Fig. 2). Although the MBI scores for sex are equivalent prestroke, demonstrating similar levels of prestroke function in both sexes, MBI scores decrease over time, and the lines progressively diverge, illustrating that women have a significantly lower functional status compared with men at 3 months and 12 months poststroke.
Table 1.
Test of Within-Subjects Effects
| Source | F | Significance |
|---|---|---|
| Barthel time | ||
| Sphericity assumed | 337.138 | 0.000 |
| Greenhouse-Geisser | 337.138 | 0.000 |
| Huynh-Feldt | 337.138 | 0.000 |
| Lower-bound | 337.138 | 0.000 |
| Barthel time * Gender | ||
| Sphericity assumed | 3.618 | 0.013 |
| Greenhouse-Geisser | 3.618 | 0.016 |
| Huynh-Feldt | 3.618 | 0.016 |
| Lower-bound | 3.618 | 0.057 |
GLM repeated measures analysis was conducted with MBI scores at four time points by gender, where 1 = males and 2 = females. The within-subjects test indicates that there is a significant time effect. MBI scores do change over time for both sexes, worst at admission and then improved at 3-month and 12-month follow-up.
MBI, Modified Barthel Index.
Table 2.
Test of Within-Subjects Contrasts
| Source | F | Significance |
|---|---|---|
| Barthel time | ||
| Level 2 vs. Level 1a | 826.792 | 0.000 |
| Level 3 vs. Level 1 | 186.672 | 0.000 |
| Level 4 vs. Level 1 | 225.317 | 0.000 |
| Barthel time * Gender | ||
| Level 2 vs. Level 1 | 2.280 | 0.131 |
| Level 3 vs. Level 1 | 7.638 | 0.006 |
| Level 4 vs. Level 1 | 12.331 | 0.000 |
MBI scores within subjects at admission and 3 months and 12 months poststroke are significantly different from MBI scores prestroke. The interaction of time and gender is significant.
Level 1, prestroke; Level 2, at admission; Level 3, 3 months poststroke; Level 4, 12 months poststroke.
Table 3.
Test of Between-Subjects Effects
| Source | F | Significance |
|---|---|---|
| Intercept | 20749.680 | 0.000 |
| Gender | 7.937 | 0.005 |
The between-genders test indicated that sex is significant.
FIG. 2.
GLM repeated measures plot for four time points of Modified Barthel Index (MBI), by sex. Estimated marginal means by gender show that although the MBI scores are equivalent in men and women prestroke, the MBI scores decrease over time in women. Women have a significantly lower functional status as measured by MBI at 3 months and 12 months poststroke.
Second, we performed a GLM repeated measures analysis that adjusted for age and NIHSS scores at admission in an attempt to control for factors that are known to influence functional outcome. The within-subjects test indicates that there is a significant time effect (Table 4). Therefore, MBI scores do change over time for both sexes, being worst at admission and then improving at 3-month and 12-month follow-up, after adjusting for age and NIHSS scores. Although the interaction of time and age, as well as time and NIHSS code, is significant, the interaction of time and sex is not. Tests of within-subjects contrasts (Table 5) reveal that within subjects, the MBI scores prestroke are significantly different from those at admission and at 3-month and 12-month follow-up.
Table 4.
GLM repeated Measures Analysis
| Source | F | Significance |
|---|---|---|
| Barthel time | ||
| Sphericity assumed | 20.146 | 0.000 |
| Greenhouse-Geisser | 20.146 | 0.000 |
| Huynh-Feldt | 20.146 | 0.000 |
| Lower-bound | 20.146 | 0.000 |
| Barthel time * Gender | ||
| Sphericity assumed | 1.296 | 0.274 |
| Greenhouse-Geisser | 1.296 | 0.275 |
| Huynh-Feldt | 1.296 | 0.275 |
| Lower-bound | 1.296 | 0.255 |
| Barthel time * Age | ||
| Sphericity assumed | 24.799 | 0.000 |
| Greenhouse-Geisser | 24.799 | 0.000 |
| Huynh-Feldt | 24.799 | 0.000 |
| Lower-bound | 24.799 | 0.000 |
| Barthel time * NIHAdmCode | ||
| Sphericity assumed | 45.242 | 0.000 |
| Greenhouse-Geisser | 45.242 | 0.000 |
| Huynh-Feldt | 45.242 | 0.000 |
| Lower-bound | 45.242 | 0.000 |
GLM repeated measures analysis was conducted adjusting for age and National Institutes of Health Stroke Score (NIHSS) scores at admission. The within-subjects test indicates there is a significant time effect. MBI scores do change over time for both genders; they get worse at admission and then better at 3-month and 12-month follow-up after adjusting for age and NIHSS scores. The interactions time and age and time and NIHSS code are significant, but the interaction of time and gender is not.
Table 5.
Test of Within-Subjects Contrasts
| Source | F | Significance |
|---|---|---|
| Barthel time | ||
| Level 2 vs. Level 1 | 5.387 | 0.021 |
| Level 3 vs. Level 1 | 67.983 | 0.000 |
| Level 4 vs. Level 1 | 39.748 | 0.000 |
| Barthel time * Gender | ||
| Level 2 vs. Level 1 | .681 | 0.409 |
| Level 3 vs. Level 1 | 1.884 | 0.170 |
| Level 4 vs. Level 1 | 4.935 | 0.027 |
| Barthel time * Age | ||
| Level 2 vs. Level 1 | 22.935 | 0.000 |
| Level 3 vs. Level 1 | 81.296 | 0.000 |
| Level 4 vs. Level 1 | 65.981 | 0.000 |
| Barthel time * NIHAdmCode | ||
| Level 2 vs. Level 1 | 118.019 | 0.000 |
| Level 3 vs. Level 1 | 49.015 | 0.000 |
| Level 4 vs. Level 1 | 22.039 | 0.000 |
Tests of within-subjects contrasts reveal that the MBI scores within subjects at prestroke are significantly different from those at admission and at 3-month and 12-month follow-up. Furthermore, the interaction of time and gender is not significantly different from prestroke to admission and 3-month and 12-month follow-up. The interactions of time and age and also time and NIHSS code are significantly different at admission, 3 months, and 12 months compared with prestroke.
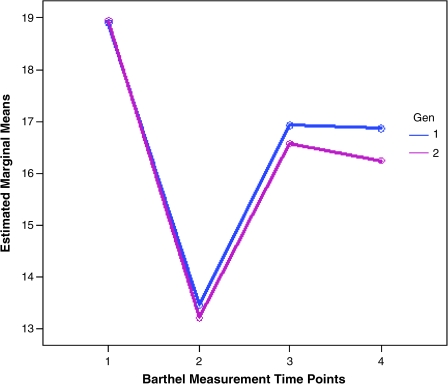
Furthermore, the interaction of time and sex is not significantly different from prestroke to admission or 3-month and 12-month follow-up. The interactions of time and age and also time and NIHSS code are significantly different at admission, and at 3 months and 12 months compared with prestroke. The between-sex test indicated that sex is not significant after adjusting for age and NIHSS scores except at 12 months (Fig. 3). t tests of log-transformed MBI scores showed that they are significantly different at 3-month and 12-month follow-up but not at prestroke and admission, after adjusting for age and NIHSS scores at admission.
FIG. 3.
GLM repeated measures plot for four time points of MBI, by sex after adjustment for age and NIHSS. Differences are seen between men and women at 12 months.
Regressions results
First, the logistic regression model included BarPre12mthgroup as the outcome and sex, age, NIHSS scores at admission, onset-to-ED times as the independent variables. Logistic regression results revealed that sex (male=1, females=2), age, NIHSS code at admission (value ranges from 1 to 4 based on quartiles), and log onset-to-ED all significantly predicted BarPreto12mthgroup (at p ≤ 0.05) (Table 6). As the coefficient for sex is positive, the probability of greater change in functional status from prestroke to 12 months poststroke increases for women (because sex = 1 is male and sex = 2 is female). Women are 1.42 times more likely than men to be in the second or higher category of BarPreto12mth change. This suggests that at 12 months poststroke, women are farther away from their prestroke functional status than are men.
Table 6.
Logistic Regression Model Predicting BarPre12mth Group
| B | Significance | Exp(B) | ||
|---|---|---|---|---|
| Step 1(a) | Gen(1) | 0.345 | 0.012 | 1.412 |
| Age | −0.025 | 0.000 | 0.975 | |
| NIHAdmCode | −0.256 | 0.000 | 0.774 | |
| Log onset-to-ED | −0.419 | 0.007 | 0.658 | |
| Constant | 2.939 | 0.000 | 18.899 |
The logistic regression model included BarPre12mthGrp as the outcome and gender, age, NIHSS scores at admission, onset-to-ED times as the independent variables. Logistic regression results revealed that gender (male = 1, females = 2), age, NIHSS code at admission (value ranges from 1 to 4 based on quartiles), and log onset-to-ED all significantly predicted BarPreto12mthGrp (at p ≤ 0.05). As the coefficient for gender is positive, the probability of greater change in functional status from prestroke to 12 months poststroke increases for women (because gender = 1 means males and gender = 2 means females). Women have a 1.42 times more chance than men of being in the second or higher category of BarPreto12mth change. This suggests that at 12 months poststroke, women are farther away from their prestroke functional status, than are men. The coefficients for age, NIHSS at admission, and onset-toED time were negative, suggesting that as age, NIHSS, and onset-to-ED increase, one is less likely to be in BArPre12-mthGrp = 1.
The coefficients for age, NIHSS at admission and onset-to-ED time were negative, suggesting that as age, NIHSS, and onset-to-ED increase, one is less likely to be in the BArPre12mthgroup = 1 (Table 5). With a unit increase in age, one is 0.975 times less likely to be in a higher category of BarPre12mth (BarPre12mth = 2 as opposed to being in the BarPre12mth = 1 category). With a unit increase in NIHAdmCode, one is 0.774 times less likely, and with a unit increase in onset-to-ED time, one is 0.658 times less likely to be in the higher category of BarPre12mth. Similar results were observed when log of NIHSS was used in the model. This suggests that for each unit increase in age, NIHAdmCode, and onset-to-ED, one's functional status at 12 months poststroke minus prestroke functional status is likely to be more negative. In other words, the greater the age, the higher the NIHSS score, and the longer the onset to ED time, the poorer will be the functional status at 12 months poststroke in both men and women.
Discussion
This study documents several important findings. First, women in this community-based cohort arrive at the ED at the same speed as men, in contrast to the findings of two earlier studies.22,25 Our data are in agreement with recent studies evaluating patients with TIA,27,28 where no sex differences have been seen in ED arrival times. It is possible that more women are recognizing stroke as an emergency, and this may reflect a growing awareness of the importance of early treatment and the success of recent educational programs targeting high-risk women (e.g., the Go Red campaign spearheaded by the American Heart Association29).
Second, in contrast to the findings of several recent studies,2,8–10,19,30 once women arrived in the ED, there were no differences in treatment rates for those eligible for tPA or in the speed with which women were treated. Several studies have suggested that women with acute stroke are more likely to have nontraditional stroke symptoms, such as chest pain or altered mental status.31,32 The presence of more atypical symptoms in women could cause a delayed diagnosis, longer in-hospital delays, and ultimately less tPA treatment in women. Atypical presentations were not documented in this study; however, as women and men were treated at the same speed once seen by a medical professional, these did not appear to contribute to any treatment delay in this cohort.
Third, we found no sex differences in rates of IV tPA use for eligible patients in this cohort. There have been conflicting reports in the literature about sex differences in thrombolytic administration. In two Michigan studies, eligible women were 46%–60% less likely to receive IV tPA than eligible men; however, these studies reported wide variations in the rates of eligibility and receipt of IV tPA across hospitals within the geographic region.10,12 Similar results were found in a recent meta-analysis of published literature.8 As our study was less geographically varied and involved a large tertiary care center, the large volume of stroke patients and presence of a stroke team could account for these differences. Interestingly, data from 32 academic medical centers found that women and men were equally likely to receive IV tPA in risk-adjusted analyses,33 suggesting that the gender gap may be closing, especially at larger academic centers.
Fourth, women with AIS were older than men, consistent with many larger studies.2,19 After controlling for age and initial stroke severity, however, sex remained a significant predictor of poor functional outcome at 12 months. Given the significantly higher age at which women experience AIS, it follows that their functional recovery and outcome would also be worse. Indeed, at both 3 months and 12 months after admission, women had a significantly lower BI score than men (Fig. 2). Others have found the odds of achieving activities of daily living (ADL) independence (defined as Barthel Index [BI] ≥95 or MBI of 18) in women 3 months after stroke were less than half that of men.10 Measures of quality of life, most notably in the physical function and mental health domains, are significantly lower in female patients 180 days after stroke even after correction for age and stroke severity.34,35 Sex-related differences have also been documented after active rehabilitation interventions.36 Men had a three times higher probability than women of obtaining independence their ADLs.36
In the current study, sex differences in MBI scores were not present preadmission, suggesting that functional status was similar between the sexes prior to stroke. Long-term functional outcome at 12 months was also assessed and remained significantly worse in women (Fig. 3). It should be recognized that complete data (all four time points) were available for only a small percentage of AIS patients coming to the ED within 24 hours (approximately 13%), and for this reason, our results should be interpreted with caution. These functional differences remained even after controlling for age and initial NIHSS, however, and are the largest dataset to date. These are the first reported data that examined sex differences in recovery this long after AIS.
There are several limitations to this study, and our results must be interpreted with these in mind. Despite the availability of a large, well-organized database that gave an adequate sample size, this was a retrospective study. This study was also performed at a single center with a large volume of stroke patients, an organized stroke team, and high treatment rates (>20%). Although this may add bias when trying to extrapolate findings to smaller hospitals without such resources, this also strengthens the dataset because of the excellent documentation of stroke severity (NIHSS) and long-term outcome data (MBI), which have limited analyses in previous studies. We attempted to contact every patient in our database, but we were able to get complete data on only 13%, which shows the difficulty in obtaining long-term follow-up information. Many patients move, enter nursing homes, leave the area, or die. Clearly, this large number of unobtainable data points could have a major impact on our findings. We are attempting to improve our database collection rates with more frequent call backs every 3 months.
Conclusions
Women with acute stroke symptoms arrive at the ED at the same speed as men. There were no differences in care once women reached the ED. Women with AIS were significantly older than men. Despite similar levels of prestroke function, women had worse functional outcomes at 3 months and 12 months poststroke. Even after adjusting for age and initial stroke severity, women continued to exhibit poorer functional recovery 12 months after stroke. The question remains: Why do women continue to have more severe functional impairments after stroke? This topic needs considerable attention and further study, as it could lead to the development of more effective treatment and rehabilitation interventions. Is it because women do not have the means to access treatment/rehabilitation compared with men (i.e., women are more likely to live at home alone)? Could we enhance functional recovery and independence by specifically targeting unique rehabilitation strategies to women? Should we be treating poststroke depression more aggressively? As women >80 years are the fastest growing cohort of stroke survivors, the vast burden of stroke-associated disability will continue to fall on the shoulders of elderly women.
Acknowledgments
This work was supported by the Department of Neurology Educational Research Grant Funding (to LDM). LDM is supported by grants from the National Institutes of Health, ROI NS050505 and ROI NS055215.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Post-stroke rehabilitation fact sheet. National Institute of Neurological Disorders and Stroke (NINDS) www.ninds.nih.gov/disorders/stroke/poststrokerehab.htm www.ninds.nih.gov/disorders/stroke/poststrokerehab.htm
- 2.Reeves MJ. Bushnell CD, et al. Sex differences in stroke: Epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chong JY. Lee HS. Boden-Albala B. Paik MC. Sacco RL. Gender differences in self-report of recovery after stroke: The Northern Manhattan Study. Neurology. 2006;67:1282–1284. doi: 10.1212/01.wnl.0000238161.71591.e9. [DOI] [PubMed] [Google Scholar]
- 4.Gargano JW. Reeves MJ. for the Paul Coverdell National Acute Stroke Registry Michigan Prototype Investigators. Sex differences in stroke recovery and stroke-specific quality of life. Stroke. 2007;38:2541–2548. doi: 10.1161/STROKEAHA.107.485482. [DOI] [PubMed] [Google Scholar]
- 5.Barrett KM. Brott TG. Brown RD, Jr, et al. for the ISGS Study Group. Sex differences in stroke severity, symptoms, and deficits after first-ever ischemic stroke. J Stroke Cerebrovasc Dis. 2007;16:34–39. doi: 10.1016/j.jstrokecerebrovasdis.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turtzo LC. McCullough LD. Sex differences in stroke. Cerebrovasc Dis. 2008;26:462–474. doi: 10.1159/000155983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleindorfer D. Lindsell CJ. Brass L. Koroshetz W. Broderick JP. National U.S. estimates of recombinant tissue plasminogen activator use: ICD-9 codes substantially underestimate. Stroke. 2008;39:924–928. doi: 10.1161/STROKEAHA.107.490375. [DOI] [PubMed] [Google Scholar]
- 8.Reeves M. Bhatt A. Jajou P. Brown M. Lisabeth L. Sex differences in the use of intravenous rt-PA thrombolysis treatment for acute ischemic stroke. A meta-analysis. Stroke. 2009;40:1743–1749. doi: 10.1161/STROKEAHA.108.543181. [DOI] [PubMed] [Google Scholar]
- 9.Lisabeth LD. Brown DL. Morgenstern LB. Barriers to intravenous tissue plasminogen activator for acute stroke therapy in women. Gend Med. 2006;3:270–278. doi: 10.1016/s1550-8579(06)80215-9. [DOI] [PubMed] [Google Scholar]
- 10.Gargano JW. Wehner S. Reeves M. Sex differences in acute stroke care in a statewide stroke registry. Stroke. 2008;39:24–29. doi: 10.1161/STROKEAHA.107.493262. [DOI] [PubMed] [Google Scholar]
- 11.Allen NB. Myers D. Watanabe E, et al. Utilization of intravenous tissue plasminogen activator for ischemic stroke: Are there sex differences? Cerebrovasc Dis. 2009;27:254–258. doi: 10.1159/000196824. [DOI] [PubMed] [Google Scholar]
- 12.Deng YZ. Reeves MJ. Jacobs BS. IV tissue plasminogen activator use in acute stroke: Experience from a statewide registry. Neurology. 2006;66:306–312. doi: 10.1212/01.wnl.0000196478.77152.fc. [DOI] [PubMed] [Google Scholar]
- 13.Savitz SI. Schlaug G. Caplan L. Selim M. Arterial occlusive lesions recanalize more frequently in women than in men after intravenous tissue plasminogen activator administration for acute stroke. Stroke. 2005;36:1447–1451. doi: 10.1161/01.STR.0000170647.42126.a8. [DOI] [PubMed] [Google Scholar]
- 14.Elkind MS. Prabhakaran S. Pittman J. Koroshetz W. Jacoby M. Johnston KC. Sex as a predictor of outcomes in patients treated with thrombolysis for acute stroke. Neurology. 2007;68:842–848. doi: 10.1212/01.wnl.0000256748.28281.ad. [DOI] [PubMed] [Google Scholar]
- 15.Di Carlo A. Lamassa M. Baldereschi M. et al. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: Data from a multicenter multinational hospital-based registry. Stroke. 2003;34:1114–1119. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- 16.Hill MD. Kent DM. Hinchey J, et al. Sex-based differences in the effect of intra-arterial treatment of stroke: Analysis of the PROACT-2 study. Stroke. 2006;37:2322–2325. doi: 10.1161/01.STR.0000237060.21472.47. [DOI] [PubMed] [Google Scholar]
- 17.Kent DM. Price LL. Ringleb P. Hill MD. Selker HP. Sex-based differences in response to recombinant tissue plasminogen activator in acute ischemic stroke: A pooled analysis of randomized clinical trials. Stroke. 2005;36:62–65. doi: 10.1161/01.STR.0000150515.15576.29. [DOI] [PubMed] [Google Scholar]
- 18.Kent DM. Buchan AM. Hill MD. The gender effect in stroke thrombolysis: Of cases, controls, and treatment-effect modification. Neurology. 2008;71:1080–1083. doi: 10.1212/01.wnl.0000316191.84334.bd. [DOI] [PubMed] [Google Scholar]
- 19.Reeves MJ. Fonarow GC. Zhao X. Smith EE. Schwamm LH. on behalf of the GWTG-Stroke Steering Committee & Investigators. Quality of care in women with ischemic stroke in the GWTG program. Stroke. 2009 doi: 10.1161/STROKEAHA.108.543157. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Arrich J. Müllner M. Lalouschek W. Greisenegger S. Crevenna R. Herkner H. Influence of socioeconomic status and gender on stroke treatment, diagnostics. Stroke. 2008;39:2066–2072. doi: 10.1161/STROKEAHA.107.506147. [DOI] [PubMed] [Google Scholar]
- 21.Smith MA. Lisabeth LD. Brown DL. Morgenstern LB. Gender comparisons of diagnostic evaluation for ischemic stroke patients. Neurology. 2005;65:855–858. doi: 10.1212/01.wnl.0000176054.72325.0f. [DOI] [PubMed] [Google Scholar]
- 22.Mandelzweig L. Goldbourt U. Boyko V. Tanne D. Perceptual, social, and behavioral factors associated with delays in seeking medical care in patients with symptoms of acute stroke. Stroke. 2006;37:1248–1253. doi: 10.1161/01.STR.0000217200.61167.39. [DOI] [PubMed] [Google Scholar]
- 23.Mosca L. Ferris A. Fabunmi R. Robertson RM. Tracking women's awareness of heart disease: An American Heart Association national study. Circulation. 2004;109:573–579. doi: 10.1161/01.CIR.0000115222.69428.C9. [DOI] [PubMed] [Google Scholar]
- 24.Dearborn JL. McCullough LD. Perception of risk and knowledge of risk factors in women at high risk for stroke. Stroke. 2009;40:1181–1186. doi: 10.1161/STROKEAHA.108.543272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menon SC. Pandey DK. Morgenstern LB. Critical factors determining access to acute stroke care. Neurology. 1998;51:427–432. doi: 10.1212/wnl.51.2.427. [DOI] [PubMed] [Google Scholar]
- 26.Shah S. Modified Barthel Index or Barthel Index (Expanded) In: Salek S, editor. Compendium of quality of life instruments Part II. Chichester: Wiley & Sons; 1998. [Google Scholar]
- 27.Giles MF. Flossman E. Rothwell PM. Patient behavior immediately after transient ischemic attack according to clinical characteristics, perception of the event, and predicted risk of stroke. Stroke. 2006;37:1254–1260. doi: 10.1161/01.STR.0000217388.57851.62. [DOI] [PubMed] [Google Scholar]
- 28.Sprigg N. Machili C. Otter ME. Wilson A. Robinson TG. A systematic review of delays in seeking medical attention after transient ischaemic attack. J Neurol Neurosurg Psychiatry. 2009;80:871–875. doi: 10.1136/jnnp.2008.167924. [DOI] [PubMed] [Google Scholar]
- 29.www.goredforwomen.org/ www.goredforwomen.org/
- 30.Gargano JW. Wehner S. Reeves MJ. Do presenting symptoms explain sex differences in emergency department delays among patients with acute stroke? Stroke. 2009;40:1114–1120. doi: 10.1161/STROKEAHA.108.543116. [DOI] [PubMed] [Google Scholar]
- 31.Labiche LA. Chan W. Saldin KR. Morgenstern LB. Sex and acute stroke presentation. Ann Emerg Med. 2002;40:453–460. doi: 10.1067/mem.2002.128682. [DOI] [PubMed] [Google Scholar]
- 32.Lisabeth LD. Brown DL. Hughes R. Majersik JJ. Morgenstern LB. Acute stroke symptoms. Comparing women and men. Stroke. 2009;40:2031–2036. doi: 10.1161/STROKEAHA.109.546812. [DOI] [PubMed] [Google Scholar]
- 33.Allen NB. Myers D. Watanabe E, et al. Utilization of intravenous tissue plasminogen activator for ischemic stroke: Are there sex differences? Cerebrovasc Dis. 2009;27:254–258. doi: 10.1159/000196824. [DOI] [PubMed] [Google Scholar]
- 34.Gray LJ. Sprigg N. Bath PM, et al. TAIST Investigators. Sex differences in quality of life in stroke survivors: Data from the Tinzaparin in Acute Ischaemic Stroke Trial (TAIST) Stroke. 2007;38:2960–2964. doi: 10.1161/STROKEAHA.107.488304. [DOI] [PubMed] [Google Scholar]
- 35.Gargano JW. Reeves MJ. Paul Coverdell National Acute Stroke Registry Michigan Prototype Investigators. Sex differences in stroke recovery and stroke-specific quality of life: Results from a statewide stroke registry. Stroke. 2007;38:2541–2548. doi: 10.1161/STROKEAHA.107.485482. [DOI] [PubMed] [Google Scholar]
- 36.Paolucci S. Bragoni M. Coiro P, et al. Is sex a prognostic factor in stroke rehabilitation? A matched comparison. Stroke. 2007;38:1860–1863. doi: 10.1161/01.STR.0000248456.41647.3d. [DOI] [PubMed] [Google Scholar]