Abstract
Obesity induced by high-fat (HF) feeding is associated with low-grade inflammation in peripheral tissues that predisposes to insulin resistance. Recent evidence suggests the occurrence of a similar process in the hypothalamus, which favors weight gain through impairment of leptin and insulin signaling. In addition to its implications for obesity pathogenesis, this hypothesis suggests that centrally targeted antiinflammatory therapies may prove effective in prevention and treatment of this disorder. This article highlights molecular and cellular mechanisms by which hypothalamic inflammation predisposes to diet-induced obesity.
Hypothalamic inflammation is a novel mechanism contributing to obesity.
As the first decade of the new millennium comes to a close, obesity remains a leading public health problem due to its association with diabetes, cardiovascular disease, and other comorbidities. Although rapid, recent increases of overweight/obesity prevalence may finally be slowing (1), 68% of U.S. adults have a body mass index greater than 25 kg/m2 and collectively account for an estimated 10% of annual health care costs (∼$147 billion in 2008) (2). Exacerbating this problem is a lack of effective obesity treatment options. Neither diet- and exercise-based lifestyle interventions nor pharmacological therapies have met with large-scale success (3,4), and although bariatric surgical procedures yield sustained reductions of morbidity and mortality in randomized trials (5,6), they carry significant risk and can realistically be offered to only a small number of obese individuals. New strategies to combat the obesity epidemic are urgently needed, but gaps in our understanding of obesity pathogenesis continue to limit progress toward this goal.
Energy homeostasis and obesity pathogenesis
Although it seems intuitively obvious that switching to a highly palatable, energy-dense diet would favor weight gain, diet-induced obesity (DIO) in both humans and rodent models involves a change in energy homeostasis characterized by an increase in the defended level of body fat stores (7,8,9), and models forwarded to explain obesity pathogenesis must take this phenomenon into account. After the cloning of leptin in 1994 and subsequent characterization of its actions in the hypothalamus, insight into molecular pathways governing energy homeostasis has grown at a rapid pace (reviewed in Refs. 10 and 11). Leptin, secreted by adipocytes in proportion to body fat mass, serves as a circulating signal of energy stores in part by providing feedback inhibition of hypothalamic orexigenic pathways [e.g. neurons that express neuropeptide Y and agouti-related peptide (AgRP)] and stimulating anorexigenic neurons, including those that express proopiomelanocortin (POMC). Although rare forms of monogenic obesity stem from genetic defects in leptin or POMC signaling pathways (12), human obesity more commonly results from complex interactions between a large number of gene variants and a host of environmental/lifestyle variables. These interactions set the stage for two cardinal features of obesity: energy intake in excess of energy requirements and the biological defense of an elevated level of body fat mass.
Although the mechanisms responsible for the latter phenomenon remain uncertain, acquired leptin resistance is implicated as a factor that predisposes to DIO in rodent models. Obesity is strongly associated with hyperleptinemia in both humans and rodents placed on a high-fat diet (HFD), and exogenous leptin is relatively ineffective in reducing food intake or body weight in both species once obesity is established. These observations support a model in which DIO arises at least in part from a failure of key hypothalamic neurocircuits to respond to the stop signal provided by leptin, analogous to the central and peripheral insulin resistance that occurs in this setting (13,14,15). Indeed, mechanisms underlying obesity-induced insulin resistance at the cellular level can also impair leptin signaling (13,14,15,16,17). What has proven challenging, however, is to determine whether leptin resistance truly causes common forms of obesity or is merely a consequence of excess weight gain. Recent work reviewed herein has begun to resolve this question.
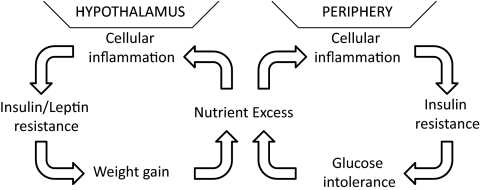
In peripheral tissues, the deleterious metabolic consequences of obesity arise in part via cellular inflammation triggered by nutrient excess (Fig. 1) (14,15,16,17,18,19). Excess visceral adiposity is accompanied by chronic low-grade inflammation affecting liver, adipose tissue, skeletal muscle, and the vasculature and is ultimately accompanied by increased circulating levels of proinflammatory cytokines and acute-phase reactants. Although this inflammatory process is initiated via cell-autonomous mechanisms, the subsequent infiltration of immune cells such as macrophages, dendritic cells, and T cells into key metabolic tissues generates an inflammatory milieu that further disrupts insulin receptor signal transduction (20,21,22). In addition, signals from Toll-like receptors (TLRs), evolutionarily conserved pattern recognition molecules critical for detecting pathogens, amplified through signaling intermediates such as MyD88 activate the inhibitor of κB-kinase-β (IKKβ)/nuclear factor-κB (NF-κB), c-Jun N-terminal kinase (Jnk) and other intracellular inflammatory signals in response to stimulation by circulating saturated fatty acids (23), exacerbating the inflammatory response and associated insulin resistance. Thus ensues a vicious cycle of inflammation and impaired nutrient use that produces progressive, systemic metabolic impairment predisposing to diabetes, steatohepatitis, and atherosclerosis (Fig. 1).
Figure 1.
The vicious cycle of obesity and diabetes. Energy intake in excess of energy requirements leads to a state of chronic nutrient excess that causes cellular inflammation in both peripheral tissues and the hypothalamus. The resulting activation of inflammatory pathways generates insulin and leptin resistance ultimately promoting obesity and diabetes. Therapies that prevent hypothalamic inflammation may disrupt these interlinked vicious cycles with consequent improvements in energy and glucose homeostasis.
In the context described above, failure of metabolic homeostasis arises as a result of obesity and does not play a causal role in excess weight gain itself. Thus, successful antiinflammatory interventions targeted to macrophages or peripheral organs result in dissociation of obesity and insulin resistance rather than resolution of both (15). In this review, we present evidence supporting the hypothesis that, in contrast to the peripheral response, hypothalamic inflammation resulting from HFD consumption contributes to obesity pathogenesis through the development of central leptin and insulin resistance.
High-fat (HF) feeding and hypothalamic inflammation
In 2005 evidence first emerged that inflammatory changes are detectable in the brain of HFD-fed animals. A 20-wk HFD-feeding study reported increased reactive oxygen species and prostaglandin E2 production along with up-regulation of NF-κB signaling in the rat cerebral cortex (24). Focusing on the hypothalamus, De Souza et al. (25) demonstrated that immune-related molecules, including the canonical proinflammatory cytokines, IL-1β, TNFα, and IL-6, represent the largest class of genes with altered hypothalamic expression levels after 16 wk of HFD. Underlying these responses are activation of both Jnk (25) and the IKKβ/NF-κB pathway as well as induction of endoplasmic reticulum (ER) stress (26,27,28,29) over a time frame that parallels the onset of reduced hypothalamic leptin sensitivity in rodent models of DIO (30,31). The association of DIO with both higher serum levels of insulin and leptin and increased activation of inflammatory signaling pathways raises the possibility that these two alterations are causally linked. However, animals lacking leptin signaling are obese and hyperphagic and manifest an even greater degree of inflammatory changes in the periphery and hypothalamus than do DIO animals (29). Thus, changes in hormone levels appear to be a response to weight gain-associated resistance rather than a driver of HFD-associated inflammation and obesity.
Hypothalamic inflammation contributes to HFD-induced obesity
Consistent with a causal role for hypothalamic inflammation in HFD-induced obesity, neuron-specific disruption of either the TLR4/MyD88 or IKKβ/NF-κB pathways protects against DIO, hypothalamic leptin resistance, and systemic insulin resistance (29,32). Moreover, viral strategies to either delete IKKβ or overexpress a dominant-negative IKKβ isoform specifically in mediobasal hypothalamic neurons also reduce food intake and weight gain during HF feeding, confirming that the phenotypes of congenital knockouts are not a result of altered hypothalamic development (29). In genetically normal animals, central infusion of either a specific inhibitor of IKKβ or antibodies to TLR4 can reduce food intake (and body weight in the latter case) in rats made obese by HFD (26,28) but not in controls fed standard chow. These studies identify hypothalamic inflammation as an important, potentially reversible cause of HFD-induced weight gain (Fig. 1).
Complementing these findings is evidence that interventions that augment hypothalamic inflammation predispose to HFD-induced obesity. For one, neuronal expression of a constitutively active IKKβ isoform (29) increases food intake. Furthermore, we have found that infusion of the Th2 cytokine IL-4 directly into the brain of rats fed HFD exerts a paradoxically proinflammatory effect on the hypothalamus (which is not observed in rats fed standard chow) that exacerbates weight gain in an IKKβ-dependent manner (33). These data collectively suggest that hypothalamic inflammation is both necessary and sufficient for initial and sustained weight gain during HF feeding and thus represents an important new target for obesity therapeutics. Interestingly, in a recent retrospective case-control study, use of antiinflammatory therapy (statin or aspirin) was associated with a 2-fold increase in the likelihood of weight loss in patients with type 2 diabetes at 1 yr of follow-up (34).
Mechanisms linking hypothalamic inflammation to HFD-induced weight gain
As described above, DIO is hypothesized to result in part from hypothalamic resistance to the weight-reducing effects of leptin and insulin. In this regard, inflammatory signaling can influence hypothalamic leptin and insulin action in several ways. Although ligands for structurally distinct receptors, both leptin and insulin signal through common downstream pathways including the insulin receptor substrate protein (IRS)-phosphatidylinositol-3 kinase and MAPK pathways (11,13). In addition, leptin (but not insulin) alters gene expression through activation of the Janus kinase (Jak)-signal transducer and activator of transcription (STAT)-3 pathway. In peripheral tissues, IKKβ and Jnk1 can serine-phosphorylate IRS molecules rendering them unable to transduce signals downstream of insulin receptors (14,15,16). Similarly, hypothalamic overexpression of a constitutively active IKKβ isoform reduces both insulin and leptin signaling (29); conversely, intracerebroventricular administration of an IKKβ inhibitor reverses HFD-induced hypothalamic insulin resistance (28), and neuron-specific deletion of either IKKβ or MyD88 maintains leptin and insulin sensitivity in HFD-fed mice (29,32).
By comparison, the role of neuronal Jnk1 in HFD-induced obesity remains unclear. Although hypothalamic Jnk1 is induced by HF feeding (32) and total body Jnk1 knockouts are resistant to DIO, neuron-specific Jnk1 knockout mice were reported by one group to have growth retardation and improved insulin sensitivity but intact susceptibility to HFD-induced leptin resistance and weight gain (32,35), whereas another group found them to be DIO resistant due to up-regulation of the hypothalamus-pituitary-thyroid axis (36). Further studies on the function of neuronal Jnk1 in energy balance regulation await targeted approaches that preserve pituitary Jnk1 activity.
Another mechanism through which hypothalamic inflammation is linked to leptin and insulin resistance is via up-regulation of suppressor of cytokine signaling (SOCS)-3. A member of a protein family originally characterized as negative feedback regulators of inflammation (13,37), SOCS3 inhibits insulin and leptin signaling both by direct binding to their cognate receptors and targeting IRS proteins for proteasomal degradation (13,37). HF feeding increases SOCS3 expression specifically within the arcuate nucleus of the hypothalamus coincident with the onset of leptin resistance selectively in this brain area (31). The mechanism underlying increased SOCS3 expression during HF feeding is uncertain because it can be induced via either leptin-Jak/STAT or IKKβ/NF-κB pathways.
Conversely, both SOCS3 haploinsufficiency and neuron-specific SOCS3 deletion protect mice from DIO by increasing leptin sensitivity (38,39), whereas overexpression of SOCS3 in POMC neurons (either directly or by increasing STAT3 activation) results in hyperphagia and obesity on a chow diet (40,41). Linking SOCS3 to inflammation, HFD-resistant neuronal IKKβ knockout mice show greatly reduced SOCS3 expression in the hypothalamus, whereas misexpression of SOCS3 in the mediobasal hypothalamus abolishes the protection from HFD-induced obesity in AgRP-neuron-specific IKKβ knockout animals (29). Future experiments are needed to identify mechanisms underlying the hypothalamus-specific increase of SOCS3 expression observed during high-fat feeding (31) and to determine whether HFD-induced weight gain caused by augmenting hypothalamic inflammation requires functional SOCS3 signaling.
Like SOCS3, the protein tyrosine phosphatase (PTP)-1B is a signal termination molecule that inhibits both leptin and insulin signaling. The mechanism underlying these effects involves its ability to dephosphorylate the insulin receptor, Jak2, and more distal components of both pathways (42), and available data suggest HF feeding increases PTP1B expression in several tissues including the hypothalamus (42,43). That this effect is recapitulated by systemic TNFα administration suggests that functional interactions exist between inflammatory signaling and PTP1B activation (42). Pan-neuronal (44) or POMC neuron-specific (45) PTP1B knockout mice are resistant to DIO due to enhanced hypothalamic leptin and insulin sensitivity, but whether and how this response might be related to altered hypothalamic inflammatory signaling awaits further study. Because both pan-neuronal PTP1B knockout mice and rats with hypothalamic PTP1B knockdown show equivalent reductions of food intake whether fed chow or HFD (44,46), this protein may favor weight gain via mechanisms in addition to those involving hypothalamic inflammation.
The unfolded protein response (UPR), an important inflammation-associated mechanism induced by ER stress, may also contribute to both HFD-induced hypothalamic inflammation and associated leptin and insulin resistance. Augmentation of the UPR in response to nutrient excess is a major cause of HFD-induced dysregulation of peripheral tissue metabolism (19), and a similar process may occur in the hypothalamus (26,27,29). In the periphery, components of the UPR trigger both Jnk and IKKβ/NF-κB activation, further fueling the inflammatory effect of HFD (19), and inflammatory signals may potentially feed back to exacerbate ER stress, although this issue remains unresolved. In support of this concept is evidence that expression of a constitutively active IKKβ isoform in mediobasal hypothalamus increases ER stress, whereas neuron-specific IKKβ knockout mice manifest a reduced UPR (29), although these effects are potentially confounded by associated changes of food intake and body weight. Importantly, ER stress inhibitors restore leptin sensitivity to HFD-fed mice and lower both food intake and body weight in obese animals (27,29). Conversely, induction of ER stress using the antibiotic tunicamycin or by deleting X-box binding protein-1 in neurons results in hyperleptinemia, obesity, hyperphagia, and reduced metabolic rate associated with severe hypothalamic leptin resistance (27). If ER homeostasis is not restored (as might be expected with continued HFD exposure), the UPR culminates with the induction of apoptosis, a finding recently described in hypothalamus (47), although both the functional relevance of this finding and its link to ER stress remain untested.
Although beyond the scope of this review, it is clear that inflammation in the hypothalamus can have widely varying behavioral outcomes, depending on the context, time frame, and amplitude of the response. Thus, substantial elevations of hypothalamic cytokine levels as observed in animal models of sepsis produce profound anorexia in both leptin-dependent and -independent manners (48,49). However, low-grade inflammation generated by HF feeding or experimentally by central administration of low levels of TNFα, for example, correlates with impaired rather than increased leptin and insulin-mediated anorexia (50). The nature of this divergent response remains unclear but may depend more on the different cellular origins and/or sites of action of inflammation rather than the specific signals involved [reviewed by these authors here (51)].
Mechanisms linking diet with inflammation
A cohesive picture has yet to emerge from attempts to determine how HFD induces hypothalamic inflammation. Indeed, the question of whether hypothalamic inflammation simply results from consumption of energy in excess of caloric needs irrespective of diet composition has yet to be definitively addressed. One potential mechanism that has received attention is the effect of saturated, but not unsaturated, fatty acids to activate TLR4/NF-κB signaling. The saturated fatty acid palmitate (16:0) induces NF-κB signaling through a TLR4-dependent mechanism both when administered in neuronal cell culture and after infusion directly into the brain (32,52,53), and the latter intervention also induces leptin and insulin resistance. However, studies using central infusion of saturated fatty acid treatments must be interpreted with caution because saturated fatty acids can have considerable cellular toxicity, even when administered at relatively physiological concentrations (∼200–500 μm).
Despite this support for palmitic acid as a putative mediator of hypothalamic inflammation, a recent comprehensive survey of long-chain fatty acids infused into the brain found no effect of palmitic acid (16:0), whereas 18:0 (stearic) and longer saturated fatty acids as well as linolenic acid (18:3) increased proinflammatory cytokines, ER stress markers, and TLR4 activation (26). Likewise, a recent study using palmitic acid treatment of hypothalamic cell lines revealed no evidence of increased inflammatory signaling but instead robust induction of ER stress (54). Combined with a large number of studies showing that rodents gain equivalent amounts of weight on oleic acid/vegetable fat-enriched diets vs. diets rich in saturated fat, questions persist about the differential inflammatory properties of distinct fatty acid constituents of the diet and their implications for energy homeostasis (26,55). Although HFD has been shown to increase endogenous endotoxin levels, a potential contributor to DIO (33), it is unclear whether this effect is limited to fat-rich diets. Nevertheless, animals exposed to HFD have an exaggerated response to endotoxin administration with increases in hypothalamic expression of proinflammatory cytokines and SOCS3, a potential mechanism linking HFD, hypothalamic inflammation, and weight gain (56).
Central nervous system (CNS) cell types involved in HFD-induced inflammation
One key unresolved question regarding HFD-induced inflammation in the CNS is the nature of the cell types and brain regions involved in the response. This information is vital not only to understanding the mechanisms underlying obesity pathogenesis and identifying potential targets for obesity therapeutics but also for efforts to understand how inflammation during HF feeding (which is accompanied by increased food intake and body weight) differs from that induced by sickness or brain injury (typically associated with negative energy balance and weight loss) (51). Although some evidence suggests inflammation within specific hypothalamic neurons (e.g. AgRP neurons) (29) is critical for disrupting leptin and insulin signaling and favoring weight gain, a thorough analysis of the cell types responsible for initiating and propagating this inflammatory response has yet to be conducted. In addition, both the regional specificity and the time course of this response within the CNS remain to be characterized.
A key unanswered question is whether hypothalamic inflammation induced by HFD originates within neurons or whether immune cells such as microglia may play a role. If HFD-induced inflammation is restricted to neurons, what factors confer susceptibility to this response? Certainly not all neurons are susceptible. Although IKKβ signaling in discrete neuronal subsets appears to be required for both hypothalamic inflammation and excess weight gain to occur during HF feeding (29), whether this inflammation is prompted by microglial activation (analogous to the recruitment and activation of adipose tissue macrophages) remains an open question.
Going forward, cell-specific approaches using conditional mutants and cell separation techniques should help to clarify the role of the various CNS cell types in the genesis of hypothalamic inflammation induced by distinct stimuli. Of interest in this regard is the paradoxical observation that hyperphagia and weight gain occur when hypothalamic inflammation is induced by HF feeding, yet when it occurs in response to systemic or local inflammatory processes (e.g. administration of endotoxin), anorexia and weight loss are the rule. A plausible but untested hypothesis to explain this paradox proposes that neurons are the primary target of HFD-induced hypothalamic inflammation, whereas immune cells are the first responders to other, more potent inflammatory stimuli.
Summary
HF feeding induces inflammatory signaling in not only a multitude of peripheral tissues, resulting in insulin resistance, but also the hypothalamus, causing local resistance to both insulin and leptin. Unlike the situation in peripheral tissues, interventions that limit hypothalamic inflammatory signaling can prevent obesity from developing, implicating the latter as cause rather than just a consequence of obesity. Pharmacological interventions that block hypothalamic inflammation during HF feeding therefore have potential in obesity prevention and treatment (Fig. 1). To date, antiinflammatory treatments have failed to produce significant changes in body weight, although some have improved insulin sensitivity (57,58). These efforts have perhaps been limited by difficulties inherent in disrupting IKKβ signaling in the CNS and therapeutic approaches that target individual cytokines rather than cellular processes. As the mechanisms linking hypothalamic inflammatory signaling to leptin and insulin resistance become better understood, including activation of TLR4/IKKβ/NF-κB signaling and induction of SOCS3 and ER stress, so too will their potential as targets for obesity treatment and prevention.
Footnotes
This work was supported by National Institutes of Health Grants DK068384, DK052989, and DK083042 (to M.W.S.) and fellowships from the American Diabetes Association and National Institutes of Health (DK007247) (to J.P.T.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 23, 2010
Abbreviations: AgRP, Agouti-related peptide; CNS, central nervous system; DIO, diet-induced obesity; ER, endoplasmic reticulum; HF, high fat; HFD, high-fat diet; IKK, inhibitor of κB-kinase; IRS, insulin receptor substrate protein; Jak, Janus kinase; Jnk, c-Jun N-terminal kinase; NF-κB, nuclear factor-κB; POMC, proopiomelanocortin; PTP, protein tyrosine phosphatase; SOCS, suppressor of cytokine signaling; STAT, signal transducer and activator of transcription; TLR, Toll-like receptor; UPR, unfolded protein response.
References
- Flegal KM, Carroll MD, Ogden CL, Curtin LR 2010 Prevalence and trends in obesity among U.S. adults, 1999–2008. JAMA 303:235–241 [DOI] [PubMed] [Google Scholar]
- Finkelstein EA, Trogdon JG, Cohen JW, Dietz W 2009 Annual medical spending attributable to obesity: payer- and service-specific estimates. Health Aff (Millwood) 28:w822–w831 [DOI] [PubMed] [Google Scholar]
- Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, Vollmer WM, Gullion CM, Funk K, Smith P, Samuel-Hodge C, Myers V, Lien LF, Laferriere D, Kennedy B, Jerome GJ, Heinith F, Harsha DW, Evans P, Erlinger TP, Dalcin AT, Coughlin J, Charleston J, Champagne CM, Bauck A, Ard JD, Aicher K 2008 Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA 299:1139–1148 [DOI] [PubMed] [Google Scholar]
- Bessesen DH 2008 Update on obesity. J Clin Endocrinol Metab 93:2027–2034 [DOI] [PubMed] [Google Scholar]
- Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Agren G, Carlsson LM 2007 Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357:741–752 [DOI] [PubMed] [Google Scholar]
- Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC 2007 Long-term mortality after gastric bypass surgery. N Engl J Med 357:753–761 [DOI] [PubMed] [Google Scholar]
- Leibel RL 2008 Molecular physiology of weight regulation in mice and humans. Int J Obes (Lond) 32(Suppl 7):S98–S108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL 2002 Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab 87:2391–2394 [DOI] [PubMed] [Google Scholar]
- Levin BE, Keesey RE 1998 Defense of differing body weight set points in diet-induced obese and resistant rats. Am J Physiol 274:R412–R419 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte Jr D, Seeley RJ, Baskin DG 2000 Central nervous system control of food intake. Nature 404:661–671 [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW 2006 Central nervous system control of food intake and body weight. Nature 443:289–295 [DOI] [PubMed] [Google Scholar]
- Farooqi S, O'Rahilly S 2006 Genetics of obesity in humans. Endocr Rev 27:710–718 [DOI] [PubMed] [Google Scholar]
- Myers MG, Cowley MA, Münzberg H 2008 Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 70:537–556 [DOI] [PubMed] [Google Scholar]
- Schenk S, Saberi M, Olefsky JM 2008 Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 118:2992–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB 2006 Inflammation and insulin resistance. J Clin Invest 116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS 2006 Inflammation and metabolic disorders. Nature 444:860–867 [DOI] [PubMed] [Google Scholar]
- Wisse BE, Kim F, Schwartz MW 2007 Physiology. An integrative view of obesity. Science (New York, NY) 318:928–929 [DOI] [PubMed] [Google Scholar]
- Unger RH, Scherer PE 2010 Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol Metab 21:345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Erbay E 2008 Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol 8:923–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR 2007 Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 56:16–23 [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante Jr AW 2003 Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H 2003 Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler MB, Rudel LL, Brown JM 2009 Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr Opin Lipidol 20:379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Dong F, Ren J, Driscoll MJ, Culver B 2005 High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp Neurol 191:318–325 [DOI] [PubMed] [Google Scholar]
- De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA 2005 Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146:4192–4199 [DOI] [PubMed] [Google Scholar]
- Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME, Takahashi HK, Curi R, Oliveira HC, Carvalheira JB, Bordin S, Saad MJ, Velloso LA 2009 Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci 29:359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers Jr MG, Ozcan U 2009 Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab 9:35–51 [DOI] [PubMed] [Google Scholar]
- Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, Pennathur S, Baskin DG, Heinecke JW, Woods SC, Schwartz MW, Niswender KD 2009 Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab 296:E1003–E1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D 2008 Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell 135:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fam BC, Morris MJ, Hansen MJ, Kebede M, Andrikopoulos S, Proietto J, Thorburn AW 2007 Modulation of central leptin sensitivity and energy balance in a rat model of diet-induced obesity. Diabetes Obes Metab 9:840–852 [DOI] [PubMed] [Google Scholar]
- Münzberg H, Flier JS, Bjørbaek C 2004 Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145:4880–4889 [DOI] [PubMed] [Google Scholar]
- Kleinridders A, Schenten D, Könner AC, Belgardt BF, Mauer J, Okamura T, Wunderlich FT, Medzhitov R, Brüning JC 2009 MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab 10:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R 2007 Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56:1761–1772 [DOI] [PubMed] [Google Scholar]
- Boaz M, Lisy L, Zandman-Goddard G, Wainstein J 2009 The effect of anti-inflammatory (aspirin and/or statin) therapy on body weight in type 2 diabetic individuals: EAT, a retrospective study. Diabet Med 26:708–713 [DOI] [PubMed] [Google Scholar]
- Belgardt BF, Mauer J, Wunderlich FT, Ernst MB, Pal M, Spohn G, Brönneke HS, Brodesser S, Hampel B, Schauss AC, Brüning JC 2010 Hypothalamic and pituitary c-Jun N-terminal kinase 1 signaling coordinately regulates glucose metabolism. Proc Natl Acad Sci USA 107:6028–6033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio G, Cavanagh-Kyros J, Barrett T, Jung DY, Ko HJ, Ong H, Morel C, Mora A, Reilly J, Kim JK, Davis RJ 2010 Role of the hypothalamic-pituitary-thyroid axis in metabolic regulation by JNK1. Genes Dev 24:256–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JK, Flier JS 2006 Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab 17:365–371 [DOI] [PubMed] [Google Scholar]
- Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjørbaek C, Flier JS 2004 Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med 10:734–738 [DOI] [PubMed] [Google Scholar]
- Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A 2004 Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med 10:739–743 [DOI] [PubMed] [Google Scholar]
- Reed AS, Unger EK, Olofsson LE, Piper ML, Myers Jr MG, Xu AW 2010 Functional role of Socs3 up-regulation in hypothalamic leptin resistance and long-term energy homeostasis. Diabetes 59:894–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst MB, Wunderlich CM, Hess S, Paehler M, Mesaros A, Koralov SB, Kleinridders A, Husch A, Münzberg H, Hampel B, Alber J, Kloppenburg P, Brüning JC, Wunderlich FT 2009 Enhanced Stat3 activation in POMC neurons provokes negative feedback inhibition of leptin and insulin signaling in obesity. J Neurosci 29:11582–11593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabolotny JM, Kim YB, Welsh LA, Kershaw EE, Neel BG, Kahn BB 2008 Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem 283:14230–14241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CL, Whittington A, Barnes MJ, Wang Z, Bray GA, Morrison CD 2009 HF diets increase hypothalamic PTP1B and induce leptin resistance through both leptin-dependent and -independent mechanisms. Am J Physiol Endocrinol Metab 296:E291–E299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB 2006 Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med 12:917–924 [DOI] [PubMed] [Google Scholar]
- Banno R, Zimmer D, De Jonghe BC, Atienza M, Rak K, Yang W, Bence KK 2010 KK PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J Clin Invest 120:720–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picardi PK, Calegari VC, Prada Pde O, Moraes JC, Araújo E, Marcondes MC, Ueno M, Carvalheira JB, Velloso LA, Saad MJ 2008 Reduction of hypothalamic protein tyrosine phosphatase improves insulin and leptin resistance in diet-induced obese rats. Endocrinology 149:3870–3880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes JC, Coope A, Morari J, Cintra DE, Roman EA, Pauli JR, Romanatto T, Carvalheira JB, Oliveira AL, Saad MJ, Velloso LA 2009 High-fat diet induces apoptosis of hypothalamic neurons. PloS One 4:e5045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagurski M, Norquay L, Farhang J, D'Aquino K, Copps K, White MF 2010 Human IL6 enhances leptin action in mice. Diabetologia 53:525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, Choi SJ, Sajan MP, Ogimoto K, Nguyen HT, Matsen M, Benoit SC, Wisse BE, Farese RV, Schwartz MW 2009 Atypical protein kinase C activity in the hypothalamus is required for lipopolysaccharide-mediated sickness responses. Endocrinology 150:5362–5372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanatto T, Cesquini M, Amaral ME, Roman EA, Moraes JC, Torsoni MA, Cruz-Neto AP, Velloso LA 2007 TNF-α acts in the hypothalamus inhibiting food intake and increasing the respiratory quotient—effects on leptin and insulin signaling pathways. Peptides 28:1050–1058 [DOI] [PubMed] [Google Scholar]
- Thaler JP, Choi SJ, Schwartz MW, Wisse BE 2010 Hypothalamic inflammation and energy homeostasis: resolving the paradox. Front Neuroendocrinol 31:79–84 [DOI] [PubMed] [Google Scholar]
- Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW 2007 Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res 100:1589–1596 [DOI] [PubMed] [Google Scholar]
- Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME 2008 Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity 16:1248–1255 [DOI] [PubMed] [Google Scholar]
- Choi SJ, Kim F, Schwartz MW, Wisse BE 2010 Cultured hypothalamic neurons are resistant to inflammation and insulin resistance induced by saturated fatty acids. Am J Physiol Endocrinol Metab 298:E1122–E1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit SC, Kemp CJ, Elias CF, Abplanalp W, Herman JP, Migrenne S, Lefevre AL, Cruciani-Guglielmacci C, Magnan C, Yu F, Niswender K, Irani BG, Holland WL, Clegg DJ 2009 Palmitic acid mediates hypothalamic insulin resistance by altering PKC-θ subcellular localization in rodents. J Clin Invest 119:2577–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl J, Woodside B, Luheshi GN 2009 Changes in hypothalamically mediated acute-phase inflammatory responses to lipopolysaccharide in diet-induced obese rats. Endocrinology 150:4901–4910 [DOI] [PubMed] [Google Scholar]
- Goldfine AB, Silver R, Aldhahi W, Cai D, Tatro E, Lee J, Shoelson SE 2008 Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci 1:36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY 2007 Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 356:1517–1526 [DOI] [PubMed] [Google Scholar]