Abstract
17β-Estradiol (E2) both inhibits and excites GnRH neurons via presynaptic as well as postsynaptic mechanisms. Although it has been demonstrated that E2 can alter the excitability of GnRH neurons via direct actions, the intracellular signaling cascades mediating these actions are not well understood. Previously we have shown that the activity of one of the critical ion channels needed for maintaining GnRH neurons in a hyperpolarized state, the ATP-sensitive potassium channel (KATP) channel, is augmented by E2 in ovariectomized females. However, the mRNA expression of the KATP channel subunits Kir6.2 and SUR1 are unchanged with in vivo E2 treatment. Therefore, to elucidate the cellular signaling mechanism(s) modulating the channel activity, we did whole-cell patch-clamp recording of enhanced green fluorescent protein-GnRH neurons from ovariectomized female mice to study the acute effects of E2. E2 dose-dependently (EC50 = 0.6 nM) enhanced the diazoxide (channel opener)-activated KATP channel currents by 1.2- to 2.0-fold, which was antagonized by ICI 182,780. E2-BSA was equally as effective as E2, whereas E2 had no effect. The protein kinase A (PKA) activator forskolin mimicked the effects of E2, whereas the PKA inhibitor H89 and the protein kinase C (PKC) inhibitor bisindolylmaleimide I blocked the effects of E2. Similar to E2, STX, a membrane estrogen receptor (ER) agonist that does not bind to ERα or ERβ, also potentiated the diazoxide-induced KATP channel current by 1.5-fold. Therefore, E2 can potentiate KATP channel activity in GnRH neurons through a membrane ER-activated PKC-PKA signaling pathway.
17β-estradiol potentiates KATP channel activity in GnRH neurons through a membrane estrogen receptor-activated PKC-PKA signaling pathway.
Control of the female reproductive cycle involves complex interactions between the gonads, pituitary, and brain. GnRH, the primary neural signal involved in activation of LH release from the pituitary, is synthesized and secreted episodically by hypothalamic GnRH neurons into the hypothalamo-hypophysial portal system (1,2,3,4). It is widely believed that burst firing of GnRH neurons underlies the episodic release of GnRH. Although a depolarizing afterpotential appears to be involved (5), the ensemble of intrinsic conductances that contribute to the burst firing have not been fully elucidated in GnRH neurons. 17β-Estradiol (E2) appears to be the primary humoral signal responsible for modulating GnRH neuronal excitability, and E2 inhibits and excites GnRH neurons by both direct and indirect (presynaptic) mechanisms (6,7,8,9,10,11). It is generally believed that the estrogen receptor (ER)-β, and not ERα, is expressed in GnRH neurons (12,13,14,15). E2 appears to increase the excitatory drive to GnRH neurons via activation of neurons presynaptic to GnRH neurons in an ERα-dependent manner (9,16,17,18). In addition, E2 has both inhibitory and excitatory actions that appear to be directly on GnRH neurons, but the E2 signaling cascade has not been elucidated (6,7,8,10,11,19,20).
Previously we found that in vivo E2 treatment increased an ATP-sensitive potassium channel (KATP) current in GnRH neurons (21). However, the mRNA levels of the KATP channel subunits Kir6.2 and SUR1 were unchanged by E2 treatment, suggesting that E2 may enhance KATP channel activity through an intracellular signaling mechanism. Therefore, in the present study, we investigated the mechanism by which E2 acutely regulates the activity of KATP channels in GnRH neurons.
Materials and Methods
Animals and treatments
All animal treatments described in this study are in accordance with institutional guidelines based on National Institutes of Health standards and were performed with institutional Animal Care and Use Committee approval at the Oregon Health and Science University (Portland, OR). Transgenic female mice expressing enhanced green fluorescent protein under the control of the GnRH promoter, originally provided by Dr. Suzanne Moenter and colleagues (22) were used in these studies. Animals were group housed until surgery at which time they were housed individually. All animals were maintained under controlled temperature and photoperiod (lights on at 0600 h and off at 1800 h) and given free access to food and water. Adult females were ovariectomized under a ketamine/xylazine anesthesia (10 and 2 mg, respectively), implanted with an oil capsule for 1–2 wk. The oil capsule implantation was used to compare the current animal treatment with that of our previous study (21).
Preparation of preoptic area-GnRH slices
Mice were killed quickly by decapitation at 1000–1100 h. The brain was rapidly removed from the skull and a block containing the diagonal band-preoptic area (DB-POA) was immediately dissected. The DB-POA block was submerged in cold (4 C) oxygenated (95% O2, 5% CO2) high-sucrose cerebrospinal fluid (CSF; in mmol): 208 sucrose; 2 KCl; 26 NaHCO3; 10 glucose; 1.25 NaH2PO4; 2 MgSO4; 1 MgCl2; 10 HEPES, pH 7.4; 290 mOsm. Coronal slices (200 μm) from the DB-POA were cut on a vibratome during which time (10 min) the slices were bathed in high-sucrose CSF at 4 C. The slices were then transferred to an auxiliary chamber in which they were kept at room temperature (25 C) in artificial CSF (aCSF) consisting of (in mM): 124 NaCl; 5 KCl; 2.6 NaH2PO4; 2 MgCl2; 2 CaCl2; 26 NaHCO3; 10 HEPES; 10 glucose, pH 7.4; 310 mOsm until recording (recovery for 2 h). A single slice was transferred to the recording chamber at a time and was kept viable by continually perfusing with warm (35 C), oxygenated aCSF at 1.5 ml/min.
Visualized whole-cell recording
Whole-cell patch clamp recordings were made under a Axioskop FS upright microscope (Zeiss, Thornwood, NY) equipped with fluorescence (fluorescein isothiocyanate filter set) and infrared differential interference contrast imaging devices. GnRH neurons were identified by the method described in our previous paper (21). Patch pipettes (A-M Systems, Seattle, WA; 1.5 mm outer diameter borosilicate glass) were pulled on a Brown/Flaming puller (Sutter Instrument Co., Navato, CA; model P-97) and were filled with the following solution (in mmol): 128 potassium gluconate; 10 NaCl; 1 MgCl2; 11 EGTA; 10 HEPES; 4 ATP; 0.25 GTP adjusted to pH 7.3 with KOH; 290 mOsm. For a subset of experiments to study the firing of GnRH neurons, the following pipette solution was used (in mmol): 140 potassium gluconate; 10 NaCl; 1 MgCl2; 1 EGTA; 0.6 CaCl2; 10 HEPES; 1 ATP; 0.25 GTP adjusted to pH 7.4 with KOH; 290 mOsm. The calculated free calcium with Winmaxc.exe version 2.0 software was 100 nm (http://www.stanford.edu/∼cpatton/maxc.html). Pipette resistances were 4–5 mΩ when filled with the above pipette solutions.
In the whole-cell configuration, access resistance was 10–25 mΩ. Voltage-clamp experiments were performed with an Axopatch 1D amplifier (2 kHz low pass filter; Axon Instruments, Foster City, CA). Steady-state current/voltage plots were constructed with step command potentials from −50 to −120 mV with a step of 5 mV (holding potential was −60 mV) and duration of 0.5 sec. Time-dependent KATP channel currents were measured at a holding potential of −60 mV. Electrophysiological signals were digitized with Digidata 1322A (Axon Instruments), and the data were analyzed using Clampfit 9.2 software (Axon Instruments).
Electrophysiological solutions/drugs
aCSF was used in all cases for electrophysiological recording. In whole-cell voltage-clamp recordings, tetrodotoxin was used to isolate the effect of presynaptic input. Different drug stock solutions were diluted at least 1000-fold in aCSF to their final concentrations in 20 ml reservoirs and were delivered by a Mini-Plus pump (Gilson, Middleton, WI) with a perfusion rate of 1.5 ml/min. All chemicals used were purchased from Sigma (St. Louis, MO) unless otherwise specified: E2 was purchased from Steraloids (Wilton, NH), recrystallized to ensure purity, and dissolved in 100% ethanol to a stock concentration of 1 mm. 17α-estradiol (Steraloids) was also recrystallized and dissolved in 100% ethanol to a stock concentration of 1 mm. E2 17-hemisuccinate-BSA (E2-BSA) (1 mm; Steraloids) was dissolved in H2O (23). The KATP channel opener diazoxide (100–300 mm), the KATP channel blocker tolbutamide (100 mm), the protein kinase A (PKA) inhibitor H-89 dihydrochloride (10 mm), the PKA activator forskolin (5 mm), the protein kinase C (PKC) inhibitors bisindolylmaleimide I hydrochloride (BIS; 1 mm) were dissolved in dimethylsulfoxide. STX, a novel membrane ER agonist, was dissolved in ethanol and diluted in aCSF by 1000-fold (24). Tetrodotoxin (1 mm) was purchased from Alomone Laboratories (Jerusalem, Israel). Controls were conducted in the presence of 0.1% ethanol or 0.1% dimethylsulfoxide. GnRH neurons were exposed to E2 for brief periods (<15 min), and also E2-BSA was used to ensure a nongenomic effect based on our previous studies in arcuate neurons (23,24,25).
Electrophysiology data analysis
Data were analyzed using Clampfit 9.2 software (Axon Instruments). Comparisons between different treatments were performed using an unpaired Student’s t test or a one-way ANOVA. Differences were considered significant if the probability of error was less than 5%. All data were presented as mean ± se. The concentration-response curve in Fig. 1 was fitted with a logistic equation (Sigma Plot 8; Systat Software Inc., San Jose, CA).
Figure 1.
E2 increases the whole-cell KATP channel current in GnRH neurons. A, A representative recording showing that 100 nm E2 acutely enhanced the diazoxide-induced KATP channel current by 2.7-fold. The diazoxide (50 μm)-induced currents were measured at a holding potential of −60 mV. After the first applications of diazoxide for 8 min, cells were washed with aCSF for 15 min and then treated with E2 for 15 min before the second application of diazoxide. Tolbutamide was applied at the end to verify that the current was from the opening of KATP (Kir6.2/SUR1) channels (21). B, A representative recording showing that 1 nm E2 acutely enhanced the diazoxide-induced KATP channel current by 1.5-fold. C, Summary of the acute effects of E2 on the diazoxide-activated KATP channel currents. The potentiating effects of E2 on the KATP currents were expressed as the ratio of the second diazoxide application-induced current to the first one. *, P < 0.05; **, P < 0.01, compared with control. Cell numbers are indicated on the bars. D, The concentration-response relationship from C was fitted with logistic equation (r2 = 0.999), which yielded an EC50 of 0.60 nm.
Results
E2 increases a KATP channel current in GnRH neurons
Our previous studies indicated that in vivo E2 treatment increased the KATP channel current in GnRH neurons. However, the mRNA expression of KATP channel subunits Kir6.2 and SUR1 were not altered by E2 treatment (21). Therefore, we hypothesized that E2 may increase the diazoxide-induced KATP current through nontranscriptional events directly altering channel function. To test this hypothesis, we examined the acute effects of E2 on the diazoxide-activated KATP current using 50 μm diazoxide (EC50 concentration) (26) and the established protocols for studying the acute effects of E2 (24). This protocol was developed to allow a direct comparison between the before and after drug effects on the outward potassium current, which increased robustness of the analysis. As shown in Fig. 1, nanomolar concentrations (0.3–100 nm) of E2 rapidly increased the diazoxide-induced KATP currents within 15 min in a concentration-dependent manner in GnRH neurons. E2 at 0.3, 1, and 100 nm potentiated the diazoxide-induced currents by 1.18 ± 0.07- (n = 6, P < 0.05), 1.68 ± 0.24- (n = 12, P < 0.05), and 2.02 ± 0.27 (n = 8, P < 0.01)-fold, respectively. A lower concentration of E2 (100 pm) had no significant effect on the diazoxide-induced KATP current (Fig. 1D). The EC50 for E2 in enhancing KATP channel activity was 0.60 nm, which is well within the physiological range.
E2 enhances the KATP channel activity through activation of PKA
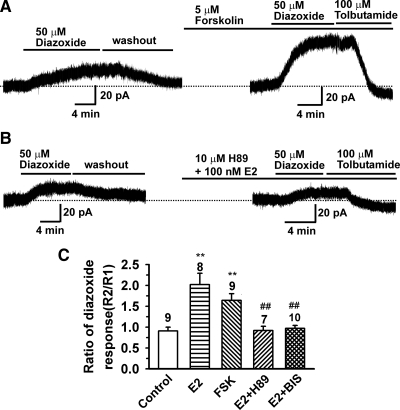
Our previous studies in hypothalamic proopiomelaocortin (POMC) neurons indicated that E2 rapidly attenuated baclofen-activated G protein-coupled inwardly-rectifying potassium (GIRK) current through a Gq-PKC-PKA pathway (23,24). Because the KATP channel activity can be enhanced by PKA and PKC activation (27,28), we tested the effects of PKA activators and inhibitors on E2’s actions on the KATP channel activity in GnRH neurons. As shown in Fig. 2, A and C, the PKA activator forskolin (5 μm) enhanced the diazoxide-induced KATP channel current by 1.64 ± 0.16 (n = 9, P < 0.01)-fold, which was similar to the effects of E2. However, if GnRH neurons were pretreated with forskolin (10 μm), there was no additional effect of E2 (100 nm; 0.91 ± 0.05, n = 3 cells), i.e. forskolin occluded the effects of E2. On the other hand, the PKA inhibitor H89 (10 μm) blocked the effects of E2 (0.92 ± 0.10, n = 7) (Fig. 2, B and C). As predicted from our previous studies (23), the PKC inhibitor BIS (1 μm) also inhibited E2’s effects (0.97 ± 0.03, n = 10) (Fig. 2C). Therefore, the cumulative evidence indicates that E2 may enhance the KATP channel activity through activation of PKC and PKA, which together with our previous findings is evidence for a G protein-mediated E2 response (29).
Figure 2.
E2 enhances the KATP channel activity through activation of PKA. A, A representative recording showing that treatment of GnRH cells with 5 μm forskolin for 15 min enhanced the diazoxide-induced KATP current by 2.4-fold. B, Pretreatment with 10 μm H89 for 10 min before E2 (100 nm) treatment prevented the potentiating effects of E2 on the KATP channel activity. C, Summary of the effects of forskolin (FSK; 5 μm), H89 (10 μm) and BIS (1 μm) on the E2 (100 nm)-induced potentiation of the KATP channel activity in GnRH neurons from oil-treated mice. **, P < 0.01, compared with control group; ##, P < 0.01, compared with E2 group. Cell numbers are indicated on the bars.
E2-BSA and the membrane ER (mER) agonist STX enhances the KATP channel activity
Recently multiple membrane estrogen signaling mechanisms in central nervous system (CNS) neurons have been identified (30). To examine whether E2 modulates KATP channel activity through a membrane ER, the membrane impermeable E2-BSA (100 nm) and the electrophysiological inactive stereoisomer 17α-estradiol (100 nm) were tested. As shown in Fig. 3, E2-BSA was as efficacious as E2, whereas 17α-estradiol had no effect on the KATP channel activity. The E2 receptor antagonist ICI 182,780 has been used to distinguish G protein-coupled receptor (GPR)-30 from other membrane ERs because ICI 182,780 is an agonist or partial agonist for GPR30 but an antagonist for other membrane ERs (8,20,23,31). Therefore, we examined the effects of ICI 182,780 on E2’s rapid action on the KATP channel activity. As shown in Fig. 3, ICI 182,780 antagonized the effects E2 on the KATP channel activation, whereas ICI182,780 alone had no effect (R2/R1 = 0.96 ± 0.09, n = 5), which indicates that the acute effects of E2 are not mediated by GPR30. Previously we identified a mER selective agonist, STX (a diphenylacrylamide compound that does not bind to ERα or ERβ) that activates a Gq-coupled signaling pathway (24). Therefore, we examined the effects of STX on the KATP channel activity. Similar to E2, STX (20 nm) significantly increased the KATP channel activity by 1.53 ± 0.15-fold (n = 9, P < 0.05), which indicates that E2 may enhance the KATP channel activity, at least in part, through acting via a Gq-coupled mER.
Figure 3.
E2 enhances the KATP channel activity through a mER. The membrane-impermeable E2-BSA (100 nm) was as efficacious as E2, whereas 17α-estradiol (17α-E2; 100 nm) had no effect. ICI 182,780 (ICI; 1 μm) antagonized the potentiating effects of E2 (1 nm) on the KATP channel activity, whereas ICI itself had no effect. STX (20 nm), a mER agonist mimicked the effects of E2. *, P < 0.05, compared with control group. Cell numbers are indicated above each bar.
Functional implication of KATP channel activation in GnRH neurons
Previously we showed that in vivo E2 treatment enhanced the KATP channel activity and E2 enhanced the tolbutamide-induced firing (21). To examine the acute actions of E2 on KATP channel activity, KATP channels were activated by 50 μm diazoxide, and the depolarization-induced firing rate was measured before and after diazoxide application with or without E2 (100 nm) pretreatment. As shown in Fig. 4, application of the KATP channel opener diazoxide strongly inhibited the firing rate of GnRH neurons, and E2 pretreatment enhanced the inhibitory effect of diazoxide.
Figure 4.
E2 enhances the inhibitory effects of diazoxide on the firing of GnRH neurons. A–C, Representative recordings of the induced firing under control conditions (A) and in the presence of diazoxide before (B) and after treatment with 100 nm E2 for 15 min (C). D, Current-clamp protocol used to induce firing. Cells were initially held at −60 mV, and a series of current pulses of 5–45 pA with a duration of 2 sec were applied to induce firing. E, Summary of the enhancing effects of E2 on the ability of diazoxide (50 μm) to inhibit the induced firing from six GnRH neurons. **, P < 0.01 compared with control group; #, P < 0.05 and ##, P < 0.01 compared with diazoxide group.
Furthermore, KATP channel blockade can facilitate the N-methyl-d-aspartate-induced episodic or burst firing of CNS neurons (32). This would suggest that KATP currents can sculpture the firing pattern of CNS neurons. To test this idea in GnRH neurons, cells were dialized with 1 mm ATP and the change in firing rate was measured after blockade of the KATP channel. As shown in Fig. 5A, application of the KATP channel antagonist tolbutamide depolarized GnRH neurons and induced burst firing. When cells were clamped at −70 mV, a 1-sec depolarizing step induced a higher firing rate (3.7 ± 0.6 Hz, control vs. 11 ± 2.3 Hz, tolbutamide; n = 5, P < 0.05) in the presence of tolbutamide (Fig. 5, B and C). This would indicate that opening of Kir channels in GnRH neurons, which hyperpolarizes the cells, can recruit low-voltage activated ion channels (e.g. T-type calcium channels) that facilitate bursting or episodic firing.
Figure 5.
Blockade of KATP channels enhances the excitability of GnRH neurons. A, A representative recording showing that blockade of KATP channels with tolbutamide depolarized a GnRH neuron and induced burst firing. B, Another cell was held at −70 mV by injecting a constant current (−15 pA) (bottom shows the current clamp protocol), and a current pulse (1 sec, 10 pA) was delivered to the cell. The induced firing increased from 4 to 9 Hz after blocking the KATP channel with tolbutamide. C, Summary of the effects of tolbutamide on the induced firing rate from five GnRH neurons. *, P < 0.05, tolbutamide compared with the control group.
Discussion
Presently we have shown that E2 acutely enhances KATP channel activity in GnRH neurons in a concentration- and ICI 182,780-dependent manner. These effects were dependent on a PKC-PKA signaling cascade because blockers of PKC and PKA abrogated these acute effects of E2. Moreover, activation of this pathway with E2-BSA or the mER ligand STX mimicked the effects of E2. Therefore, E2 can potentiate KATP channel activity through a PKC-PKA signaling pathway that is dependent, at least in part, on a STX-sensitive membrane ER.
Mechanism of acute estradiol actions in GnRH neurons
Previously we found that in vivo E2 treatment increased the whole-cell KATP current in GnRH neurons without altering the mRNA expression of the KATP channel subunits Kir6.2 and SUR1 (21). Therefore, we hypothesized that E2 enhances KATP channel activity, at least in part, through signaling cascades that phosphorylate Kir 6.2 and increase channel activity. Indeed, acutely applied E2 augmented the KATP channel activity within 15 min. The membrane-impermeable E2-BSA was equally as efficacious as E2 (whereas 17α-estradiol had no effect), which indicates that E2 may act via a membrane ER. Moreover, the effects of E2 were abrogated by the PKA catalytic subunit inhibitor H89 and mimicked by the upstream adenylyl cyclase activator forskolin. Interestingly, coperfusion of PKC inhibitor BIS was also efficacious to block E2 signaling, indicative that a Gq-signaling pathway is involved (23).
GnRH neurons, similar to pancreatic β-cells, express Kir 6.2 and SUR1 subunits (21). However, in contrast to its action in GnRH neurons, E2 inhibits KATP channel activity in pancreatic β-cells via ERβ activation of a cGMP-dependent kinase (33,34). Therefore, the actions of E2 on KATP channels in the pancreas are different from its actions in GnRH neurons. Phosphorylation of Kir6.2 by PKA in oocytes and HEK cells augments channel activity by increasing the open probability and decreasing the ATP-mediated inhibition (27,35). Thus, KATP channel activity can be enhanced by phosphorylation of Kir6.2 via GPRs that could be a mechanism by which E2 augments KATP channel activity in GnRH neurons. However, the specific targets of PKA phosphorylation in GnRH neurons need to be elucidated.
Previously we characterized a membrane ER that is Gq coupled to a phospholipase C-PKC-PKA pathway in hypothalamic POMC and dopamine neurons (23,24). This mER has subnanomolar affinity for estradiol based on our pharmacological (Schild) analysis (25). In addition, we developed a novel nonsteroidal ligand, STX, which is more selective for the mER and with even greater affinity (23,24). The activation of this pathway by E2 and STX in POMC and dopamine neurons, but not in GnRH neurons, desensitizes γ-aminobutyric acid (GABA)B and μ-opioid receptors and enhances POMC and dopamine neuronal excitability (6,24,25,36). In GnRH neurons, this mER signaling pathway appears to enhance KATP channel activity and membrane hyperpolarization.
Presently the molecular identity of the hypothalamic mER is not known. Recently an orphan GPR (GPR30) has been identified in breast tumor cell lines that binds estradiol, and GPR30 mRNA has been localized in the rat hypothalamus by in situ hybridization (37,38,39). The GPR30 agonist G-1 has been shown to increase Ca2+ oscillations and GnRH release from cultured GnRH neurons derived from the monkey olfactory placode region (20). However, STX does not activate GPR30 in native hypothalamic neurons (40). Therefore, the effects of STX are mediated via a receptor (mER) that is distinct from ERα, ERβ, or GPR30.
We found previously that E2 hyperpolarized guinea pig GnRH neurons via activation of an inwardly rectifying K+ (Kir) channel, and this could contribute to the negative feedback actions of the hormone (6). In mouse GnRH neurons, we do not see a change in the holding current (indicative of a membrane hyperpolarization) but rather an augmentation of the KATP channel opener (diazoxide)-activated current. The apparent direct activation of Kir channels in the guinea pig vs. the augmentation of a Kir channel activity in the mouse is most likely due to the different recording conditions (sharp electrode recordings in the guinea pig vs. the whole-cell patch recordings in the mouse GnRH neurons) or a subtle species difference. Regardless, in both species E2 acutely augments a Kir current that results in a membrane hyperpolarization, which inhibits cell activity but also critically sets the stage for recruiting excitatory channels (41).
Recently Chu et al. (11) found that E2 (0.1–100 nm) rapidly increased the neuronal firing of GnRH neurons (resting at −51 to −53 mV) by an ERβ-dependent mechanism. E2 reduced the afterhyperpolarization amplitude by about 8% but increased a slow afterdepolarization by 20%. The overall effect was a doubling of firing rate. Therefore, E2 via potentially two independent signaling pathways can modulate the excitability of GnRH neurons through hyperpolarizing the cell to recruit excitatory conductances for burst firing and inhibiting repolarizing potentials during active firing.
Role of Kir channels in modulating the excitability of GnRH neurons
It is well known that Kir channels are critical for maintaining excitable cells in a hyperpolarized resting state closer to the Nernst equilibrium potential for potassium ions, EK+, but once cells are depolarized, the channels allow for the quick transition to long depolarizing responses because of their inwardly rectifying properties (42). Indeed, KATP and GIRK channels appear to be the critical Kir channels in GnRH neurons for maintaining the membrane in a relatively negative resting state (21,43). With a greater membrane hyperpolarization of GnRH neurons, there is an increase in the number of Na+ spikes during rebound excitation (41). Blocking KATP with the sulfonylurea tolbutamide significantly depolarizes GnRH neurons, which is indicative of tonic KATP activity that is significantly augmented with E2 treatment (21). In addition, we found that KATP activity is directly regulated by E2 via a kinase signaling pathway. We also found that GABAergic input via GABAB receptors activates GIRK channels to hyperpolarize GnRH neurons (6,43). However, we know that kisspeptin inhibits Kir channel (KATP and GIRK) activity, which allows the rapid transition to an excitatory state. This combination of membrane hyperpolarization would allow for the recruitment of multiple excitatory conductances (e.g. T-type calcium current) that are critical for generating burst firing in CNS neurons (44,45,46). Alternatively, as previously hypothesized (6), the activation of Kir channels may represent a mechanism for a purely inhibitory action of GnRH neurons by E2 during the ovulatory cycle (21).
In summary, we believe that the membrane hyperpolarization generated directly by E2-mediated potentiation of KATP activity sets the stage for recruiting excitatory channels such as the T-type calcium channels that are critical for burst firing of GnRH neurons (46,47). However, future studies need to be directed toward elucidating the expression of other channels and how the integrative sum of these contribute to the burst firing activity in GnRH neurons (5,11,47,48).
Acknowledgments
We thank Ms. Elizabeth Rick for her excellent technical assistance.
Footnotes
This work was supported by Public Health Service Grants NS 38809, NS 43330, and DK 68098.
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 21, 2010
Abbreviations: aCSF, Artificial CSF; BIS, bisindolylmaleimide I hydrochloride; CNS, central nervous system; CSF, cerebrospinal fluid; DB-POA, diagonal band-preoptic area; E2, 17β-estradiol; ER, estrogen receptor; GABA, γ-aminobutyric acid; GIRK, G protein-coupled inwardly-rectifying potassium; GPR, G protein-coupled receptor; KATP, ATP-sensitive potassium channel; mER, membrane ER; PKA, protein kinase A; PKC, protein kinase C; POMC, proopiomelaocortin.
References
- Levine JE, Ramirez VD 1982 Luteinizing hormone-releasing hormone release during the rat estrous cycle and after ovariectomy, as estimated with push-pull cannulae. Endocrinology 111:1439–1448 [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Fink G 1980 Luteinizing hormone releasing factor in pituitary stalk plasma from long-term ovariectomized rats: effects of steroids. J Endocrinol 86:511–524 [DOI] [PubMed] [Google Scholar]
- Ching M 1982 Correlative surges of LHRH, LH and FSH in pituitary stalk plasma and systemic plasma of rat during proestrus. Neuroendocrinology 34:279–285 [DOI] [PubMed] [Google Scholar]
- Moenter SM, Brand RC, Karsch FJ 1992 Dynamics of gonadotropin-releasing hormone (GnRH) secretion during the GnRH surge: insights into the mechanism of GnRH surge induction. Endocrinology 130:2978–2984 [DOI] [PubMed] [Google Scholar]
- Kuehl-Kovarik MC, Partin KM, Handa RJ, Dudek FE 2005 Spike-dependent depolarizing afterpotentials contribute to endogenous bursting in gonadotropin releasing hormone neurons. Neuroscience 134:295–300 [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Rønnekleiv OK, Kelly MJ 1995 Estradiol-17β and μ-opioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback? Endocrinology 136:2341–2344 [DOI] [PubMed] [Google Scholar]
- Temple JL, Laing E, Sunder A, Wray S 2004 Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J Neurosci 24:6326–6333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple JL, Wray S 2005 BSA-estrogen compounds differentially alter gonadotropin-releasing hormone-1 neuronal activity. Endocrinology 146:558–563 [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne HJ, Todman MG, Korach KS, Greiner E, Pérez CA, Schütz G, Herbison AE 2006 Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H, Keen KL, Terasawa E 2008 Rapid action of estrogens on intracellular calcium oscillations in primate LHRH-1neurons. Endocrinology 149:1155–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z, Andrade J, Shupnik MA, Moenter SM 2009 Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J Neurosci 29:5616–5627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszán T, Carpenter CD, Liposits Z, Petersen SL 2000 Detection of estrogen receptor-β messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 141:3506–3509 [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabás K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z 2001 Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 142:3261–3264 [DOI] [PubMed] [Google Scholar]
- Herbison AE, Skynner MJ, Sim JA 2001 Lack of detection of estrogen receptor-α transcripts in mouse gonadotropin-releasing hormone neurons. Endocrinology 142:493 [DOI] [PubMed] [Google Scholar]
- Herbison AE, Pape JR 2001 New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol 22:292–308 [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E, Bridson WE 1978 Persistent estrus and blockade of progesterone-induced LH release follows lesions which do not damage the suprachiasmatic nucleus. Endocrinology 102:1645–1648 [DOI] [PubMed] [Google Scholar]
- Ronnekleiv OK, Kelly MJ 1986 Luteinizing hormone-releasing hormone neuronal system during the estrous cycle of the female rat: effects of surgically induced persistent estrus. Neuroendocrinology 43:564–576 [DOI] [PubMed] [Google Scholar]
- Petersen SL, Cheuk C, Hartman RD, Barraclough CA 1989 Medial preoptic microimplants of the antiestrogen, keoxifene, affect luteinizing hormone-releasing hormone mRNA levels, median eminence luteinizing hormone-releasing hormone concentrations and luteinizing hormone release in ovariectomized estrogen-treated rats. J Neuroendocrinol 1:279–283 [DOI] [PubMed] [Google Scholar]
- Abrahám IM, Han SK, Todman MG, Korach KS, Herbison AE 2003 Estrogen receptor β mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci 23: 5771–5777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E 2009 Involvement of G-protein couple receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol 23:349–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Levine JE, Rønnekleiv OK, Kelly MJ 2007 Gonadotropin-releasing hormone neurons express KATP Channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci 27:10153–10164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM 2000 Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology 141:412–419 [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ 2003 Rapid signaling of estrogen in hypothalamic neurons involves a novel G protein-coupled estrogen receptor that activates protein kinase C. J Neurosci 23:9529–9540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Krust A, Graham S, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Rønnekleiv OK, Kelly MJ 2006 A G protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci 26:5649–5655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange AH, Ronnekleiv OK, Kelly MJ 1997 Modulation of G protein-coupled receptors by an estrogen receptor that activates protein kinase A. Mol Pharmacol 51:605–612 [DOI] [PubMed] [Google Scholar]
- Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Rønnekleiv OK, Low MJ, Kelly MJ 2003 Hypothalamic proopiomelanocortin neurons are glucose-responsive and express K-ATP channels. Endocrinology 144:1331–1340 [DOI] [PubMed] [Google Scholar]
- Lin YF, Jan YN, Jan LY 2000 Regulation of ATP-sensitive potassium channel function by protein kinase A-mediated phosphorylation in transfected HEK293 cells. EMBO J 19:942–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribalet B, Eddlestone GT 1995 Characterization of the G protein coupling of a somatostatin receptor to the K+ATP channel in insulin-secreting mammalian HIT and RIN cell lines. J Physiol 485:73–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Wagner EJ 1999 Estrogen modulation of G-protein-coupled receptors. Trends Endocrinol Metab 10:369–374 [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Rønnekleiv OK 2008 Membrane-initiated estrogen signaling in hypothalamic neurons. Mol Cell Endocrinol 290:14–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton Jr AR, Bland KI 2002 Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol 16:70–84 [DOI] [PubMed] [Google Scholar]
- Shen KZ, Johnson SW 2010 Ca2+ influx through NMDA-gated channels activates ATP-sensitive K+ currents through a nitric oxide-cGMP pathway in subthalamic neurons. J Neurosci 30:1882–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropero AB, Fuentes E, Rovira JM, Ripoll C, Soria B, Nadal A 1999 Non-genomic actions of 17β-oestradiol in mouse pancreatic β-cells are mediated by a cGMP-dependent protein kinase. J Physiol 521:397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano S, Ropero AB, Alsonso-Magdelena P, Ripoll C, Quesada I, Gassner B, Kuhn M, Gustafsson JA, Nadal A 2009 Rapid regulation of KATP channel activity by 17β-estradiol in pancreatic β-cells involves the estrogen receptor β and the atrial natriuretic peptide receptor. Mol Endocrinol 23:1973–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguin P, Nagashima K, Nishimura M, Gonoi T, Seino S 1999 PKA-mediated phosphorylation of the human KATP channel: separate roles of Kir6.2 and SUR1 subunit phosphorylation. EMBO J 18:4722–4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Loose MD, Ronnekleiv OK 1992 Estrogen suppresses μ-opioid and GABAB-mediated hyperpolarization of hypothalamic arcuate neurons. J Neurosci 12:2745–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J 2005 Identity of an estrogen membrane receptor coupled to a G-protein in human breast cancer cells. Endocrinology 146:624–632 [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER 2005 A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630 [DOI] [PubMed] [Google Scholar]
- O'Dowd BF, Nguyen T, Marchese A, Cheng R, Lynch KR, Heng HH, Kolakowski Jr LF, George SR 1998 Discovery of three novel G-protein-coupled receptor genes. Genomics 47:310–313 [DOI] [PubMed] [Google Scholar]
- Qiu J, Rønnekleiv OK, Kelly MJ 2008 Modulation of hypothalamic neuronal activity through a novel G-protein coupled estrogen membrane receptor. Steroids 73:985–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Rick EA, Kelly MJ, Rønnekleiv OK 2009 17β-Estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci 29:10552–10562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B 2001 Potassium channels and chloride channels. In: Hille B, ed. Ion channels of excitable membranes. 1st ed. Sunderland, MA: Sinaur; 131–165 [Google Scholar]
- Zhang C, Bosch MA, Rønnekleiv OK, Kelly MJ 2009 GABAB receptor mediated inhibition of GnRH neurons is suppressed by kisspeptin-GPR54 signaling. Endocrinology 150:2388–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard JR, McCormick DA 1992 Simulation of the currents involved in rhythmic oscillations in thalamic relay neurons. J Neurophysiol 68:1373–1383 [DOI] [PubMed] [Google Scholar]
- Erickson KR, Ronnekleiv OK, Kelly MJ 1993 Role of a T-type calcium current in supporting a depolarizing potential, damped oscillations, and phasic firing in vasopressinergic guinea pig supraoptic neurons. Neuroendocrinology 57:789–800 [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Wagner EJ 2002 GnRH neurons and episodic bursting activity. Trends Endocrinol Metab 13:409–410 [DOI] [PubMed] [Google Scholar]
- Kuehl-Kovarik MC, Pouliot WA, Halterman GL, Handa RJ, Dudek FE, Partin KM 2002 Episodic bursting activity and response to excitatory amino acids in acutely dissociated gonadotropin-releasing hormone neurons genetically targeted with green fluorescent protein. J Neurosci 22:2313–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CB, Hemond P, Suter KJ 2008 Synaptic integration in hypothalamic gonadotropin releasing hormone (GnRH) neurons. Neuroscience 154:1337–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]