Abstract
Because rearing rats in large litters (LLs) protects them from becoming obese, we postulated that LL rearing would protect rats selectively bred to develop diet-induced obesity (DIO) from becoming obese by overcoming their inborn central leptin resistance. Male and female DIO rats were raised in normal litters (NLs; 10 pups/dam) or LLs (16 pups/dam) and assessed for anatomical, biochemical, and functional aspects of leptin sensitivity at various ages when fed low-fat chow or a 31% fat high-energy (HE) diet. LL rearing reduced plasma leptin levels by postnatal day 2 (P2) and body weight gain by P8. At P16, LL DIO neonates had increased arcuate nucleus (ARC) binding of leptin to its extracellular receptors and at P28 an associated increase of their agouti-related peptide and α-MSH axonal projections to the paraventricular nucleus. Reduced body weight persisted and was associated with increased ARC leptin receptor binding and sensitivity to the anorectic effects of leptin, reduced adiposity, and enhanced insulin sensitivity in LL DIO rats fed chow until 10 wk of age. The enhanced ARC leptin receptor binding and reduced adiposity of LL DIO rats persisted after an additional 5 wk on the HE diet. Female LL DIO rats had similar reductions in weight gain on both chow and HE diet vs. normal litter DIO rats. We postulate that LL rearing enhances DIO leptin sensitivity by lowering plasma leptin levels and thereby increasing leptin receptor availability and that this both enhances the ARC-paraventricular nucleus pathway development and protects them from becoming obese.
Large litter rearing increases the leptin sensitivity of male diet-induced obesity neonates, and this is associated with reduced adiposity whether they are fed chow or high energy diet as adults.
A multitude of perinatal factors can alter the metabolic fate of offspring. This is particularly true of metabolic and neural systems that regulate energy homeostasis. In rodents, a variety of postnatal factors can either increase or decrease the propensity to become obese as adults. Kennedy (1) first demonstrated that raising pups in small litters from birth caused them to overeat and become obese. On the other hand, raising pups in large litters (LLs) reduced their intake and resulted in them becoming leaner as adults. Although neonates do make small amounts of their own leptin, they also ingest and absorb leptin (and insulin) up to at least postnatal day (P) 12 from maternal milk (2,3,4,5,6). Thus, reduced intake of maternal milk in pups raised in LLs might be expected to reduce their plasma levels of both leptin and insulin. Both are critical neurotrophic and neurotropic factors, and leptin has been demonstrated to be essential for the normal axonal outgrowth of neuropeptide Y/agouti-related peptide (AgRP) and proopiomelanocortin neurons from their cell bodies in the arcuate nucleus (ARC) to the hypothalamic paraventricular nucleus (PVN) during the first 12 postnatal days (7,8). A lack of leptin or deficient leptin signaling causes the underdevelopment of both AgRP and α-MSH fiber densities in the PVN (9,10). In addition, postnatal nutritional manipulations can also produce long-term changes in hypothalamic leptin sensitivity by altering the mRNA expression, plasma membrane localization, and/or downstream signaling of the leptin receptor (11,12,13,14,15,16). All of these can have major effects on the ability of offspring to develop or resist obesity, particularly when challenged with a high-fat diet.
To facilitate the study of factors that alter the relationship between postnatal genetic and environmental factors that affect the propensity to become obese, we selectively bred outbred Sprague Dawley rats to develop polygenic substrains of rats, which are highly prone to develop diet-induced obesity (DIO) or to be diet resistant (DR) when fed a high-energy (HE) diet with moderate densities of fat (31%), sucrose (25%), and calories (17). A major distinguishing feature of the DIO rat is its early, possibly inborn, central resistance to leptin. Compared with DR rats, DIO rats have decreased hypothalamic expression of leptin receptor mRNA (18) and binding of leptin to its receptors (19). They also have reduced leptin-induced activation of signaling pathways downstream of the leptin receptor (10) and leptin-responsive neurons (20). Presumably as a consequence of this reduced leptin sensitivity, their ARC-PVN AgRP and α-MSH fiber pathways are underdeveloped (10), and they are less responsive to the anorectic and thermogenic effects of leptin. Thus, it is likely that the failure of rising leptin levels associated with increased adipose stores to inhibit further food intake is a major contributor to the development of obesity in DIO rats when they are fed HE diet (18,21,22). Given the known ability of LL rearing to protect rodents from becoming obese as adults, we postulated that LL rearing would similarly protect DIO rats from developing diet-induced obesity, possibly by increasing their reduced leptin sensitivity.
Materials and Methods
Animals and diet
Animal usage was in compliance with Institutional Animal Care and Usage Committee of the East Orange Veterans Affairs Medical Center (East Orange, NJ). Male and female rats bred selectively to express the DIO trait when fed a diet moderately high in fat and calories (HE diet) (17) were raised in our in-house colony and used for all studies. Colony rats were individually housed after weaning at 23–24 C on a reversed 12-h light, 12-h dark cycle (lights off at 0900 h) and fed Purina rat chow (no. 5001; St. Louis, MO) containing 3.30 kcal/g with 23.4% as protein, 4.5% as fat, and 72.1% as carbohydrate, which is primarily in the form of complex polysaccharide (23). For breeding, two chow-fed female DIO rats were bred to a single chow-fed male DIO rat. Offspring were weighed at birth and, at P2, were randomized by weight and fostered to dams in normal litters (NLs; n = 10 pups/dam, six males, four females) or LLs (n = 16 pups/dam, 10 males, six females). Pups were weaned onto chow at P21 and singly housed. In the majority of studies, they were kept on chow until 10 wk of age and then were placed onto HE diet (no. C11024F; Research Diets, New Brunswick, NJ) containing 4.47 kcal/g with 21% of the metabolizable energy content as protein, 31% as fat, and 48% as carbohydrate, 50% of which is sucrose (23) for 4–5 wk, depending on the experiment.
Experiment 1: early postnatal effects of litter size on body weight and plasma leptin levels
These studies used the offspring of a total of 30 female and 15 male DIO rats, which were weighed at birth, randomized by weight, and placed into NLs or LLs at P1. At the end of the light and dark phase of P3, one male pup from each of four litters was killed and trunk blood was collected for plasma leptin levels. The pups that had been used for those studies from NLs and LLs were replaced by pups from donor NL and LL dams to maintain litter size. A similar procedure was carried out on four additional NL and LL pups at the end of the dark and light phases at P8 and P14. Additional sets of pups from four NLs and four LLs were removed from their dams at dark onset. One set of four pups from each NL and LL group was decapitated and trunk blood was collected for baseline leptin levels. Two other sets of four pups from NLs and LLs were gavaged with 0.3 ml saline containing 100 ng leptin. One set of four pups each was decapitated 2 h later, and a second set was decapitated 4 h later with collection of trunk blood for measurement of plasma leptin levels.
Experiment 2: effect of LL rearing on energy homeostasis and leptin sensitivity
These studies used a total of 26 DIO female rats bred to 13 male DIO rats. Food intake and body weight of dams were followed up at weekly intervals throughout gestation and lactation. At birth, pups from 13 litters were fostered to 16 dams to compose eight NL and eight LL groups. Male and female pups were weighed again at P8, P14, and P21 and then weaned onto chow with weekly body weight and food intake measures. At various intervals, single offspring from each litter were assessed for a variety of end point measures such that the experimental number of animals for each end point was n = 1 per litter. When a pup was removed from a litter, they were replaced by same-sex pups from donor dams with equivalent litter size to maintain the NL and LL litters at 10 and 16 pups/dam, respectively.
The following end point measures were made from this initial cohort of NL and LL pups. First, at P16, eight NL and eight LL male pups had their brains removed and processed for leptin receptor binding autoradiography. Retroperitoneal, mesenteric, inguinal, and epididymal fat pads were removed and weighed, and trunk blood was assessed for plasma leptin and insulin levels. Second, at P21, plasma samples were taken by tail nip for leptin and insulin levels, and nasoanal length was assessed in male pups. A cohort of eight NL and eight LL females was killed for trunk blood leptin and insulin levels and retroperitoneal, mesenteric, inguinal, retroperitoneal, and ovarian fat pad weights. All remaining male and female all pups were weaned onto chow. Third, at P28, eight NL and eight LL males were anesthetized with pentobarbital and had their nasoanal length measured. They were then perfused with a 4% paraformaldehyde solution for the determination of PVN, AgRP, and α-MSH fiber density. Fourth, at 9–10 wk of age, eight NL and eight LL chow-fed males were assessed for their anorectic responses to leptin by having cumulative 4- and 24-h food intake assessed after injections of either 0.5 ml saline or 0.5 ml saline containing rat leptin (5 mg/kg, ip; National Hormone and Peptide Program, Torrance, CA) spaced 3 d apart. Fifth, during the 10th wk on chow, eight NL and eight LL males underwent an oral glucose tolerance test (OGTT) and then 2 d later. they were killed for removal of brains for leptin receptor binding autoradiography, trunk blood leptin and insulin levels, and carcass composition. And finally, after leptin testing, the remaining rats were switched to the HE diet for 4–5 wk. During the fourth week, eight NL and eight LL male rats were given an OGTT, and then 2 d later, they and eight NL and eight NL female rats were killed for hypothalamic leptin receptor binding autoradiography (males), trunk blood leptin and insulin levels, and carcass composition (males).
Quantitative leptin receptor binding autoradiography
Leptin receptor binding autoradiography was used to assess binding of leptin to its receptor by methods previously established in our laboratory (19). Rat brains were quick frozen on dry ice and then cut using a cryostat in six sets of 10-μm sections, which were then freeze thawed onto gel-coated slides and dried. They were then incubated with radiolabeled 125I-leptin (PerkinElmer Life Sciences, Boston, MA), with or without an excess of unlabeled competing ligand for the receptor to assess specific binding. Slides were then dried and apposed to x-ray film. Binding of 125I-leptin was carried out with 0.25 nm murine 125I-leptin, Tris HCl (pH 7.2), 1% BSA (Sigma, St. Louis, MO), 0.05% leupeptin (Sigma), and 0.001% pepstatin (Sigma). Nonspecific binding was defined as that seen in the presence of a 1000-fold excess of unlabeled murine leptin (National Hormone and Peptide Program) and accounted for less than 30% of total binding (19). The resulting autoradiograms were quantitated by an experimentally blinded observer using computer-assisted densitometry (Drexel University, Philadelphia, PA) by outlining the area to be analyzed on a computer image of the cresyl violet-stained section used to generate the autoradiogram and reading the exposed autoradiogram density from the overlaid image. Film density was equated to density of known radiolabeled standards placed on each film and binding was calculated directly from density (24,25,26).
Immunocytochemistry for AgRP and α-MSH
AgRP and α-MSH immunocytochemistries were performed to assess the density of arcuate projections to the PVN. Brains were sectioned at 35 μm and processed for immunofluorescence as described elsewhere (10). Briefly, sections were incubated for 72 h in a rabbit antihuman AgRP (Phoenix Pharmaceuticals, Burlingame, CA; 1:4,000) or a sheep anti-α-MSH (Chemicon, Temecula, CA; 1:40,000). For the α-MSH staining, sections were then incubated in Alexa Fluor 568 donkey antisheep IgG (Invitrogen, Carlsbad, CA; 1:200) for 1 h. For the AgRP staining, the primary antibodies were localized with a biotinylated goat antirabbit IgG (Vector Laboratories, Burlingame, CA; 1:5,000). Tyramide signal amplification was accomplished by placing the sections in an avidin-biotin solution (Vector Laboratories) for 1 h, followed by incubation in tyramide signal amplification solution for 20 min according to the manufacturer’s instruction (TSA-Indirect kit; PerkinElmer). Deposited biotin was detected with Alexa 568-conjugated streptavidin (Invitrogen; 1:1000). Sections were then counterstained using SYTOX green nuclear stain (Invitrogen; 1:3000) to visualize cell nuclei and coverslipped with buffered glycerol (pH 8.5).
Quantification of PVN fiber density
The density of AgRP and α-MSH innervation of the PVN was determined by quantitative confocal microscopy using previously published methods (10). For each animal, two sections through the PVN were acquired using a Leica SP confocal microscope (Heidelberg, Germany) equipped with a ×10 objective (numerical aperture 0.40; working distance 360 μm). Image analysis was performed using Image J analysis software (version 1.39t; National Institutes of Health, Bethesda, MD). Each image plane was binarized to isolate the labeled fibers from the background as well as compensate for differences in fluorescence intensity and was then skeletonized so that each fiber segment was 1 pixel thick. The integrated intensity was then calculated for each image, which reflects the total number of pixels in the skeletonized image and was proportional to the total length of labeled fibers in the image. This procedure was carried out on each image plane in the stack, and the values for all image planes in a stack was summed. The resulting value is an accurate index of fiber density in the volume sampled.
OGTT and assay of plasma leptin, insulin, and glucose levels
After an overnight fast, a baseline blood sample (∼0.3 ml) was taken by tail nick for glucose and insulin levels into EDTA-coated tubes. Rats were then gavaged with 0.5 g/kg glucose, and blood was sampled by tail nick at 30, 60, 90, and 120 min. Plasma insulin and leptin levels were analyzed by RIA using antibodies authentic to rat insulin and leptin (Linco, St. Charles, MO). Plasma glucose levels were measured using an Analox glucometer (San Diego, CA).
Carcass composition
Terminal carcass composition was assessed by quantitative magnetic resonance imaging (27,28) at the University of Alabama (Birmingham, AL) by Dr. Tim Nagy.
Statistics
Comparisons of body weight gain, fat pad weights, plasma hormones, area under the curve (AUC) for oral glucose tolerance values, and receptor binding data were carried out using one-way ANOVA. Serial measures of food intake and body weight gain were carried out by repeated-measures, one-way ANOVA. When significant differences were found, post hoc analysis was performed using Bonferroni corrections (P ≤ 0.05). For OGTTs, the AUCs for insulin and glucose levels during the testing period were calculated using the trapezoidal rule. Insulin sensitivity index was calculated as 10,000/square root [(fasting glucose × fasting insulin) × (mean glucose × mean insulin AUC during OGTT)] according to Matsuda and DeFronzo (29).
Results
Dam food intake and body weight gain during gestation and lactation
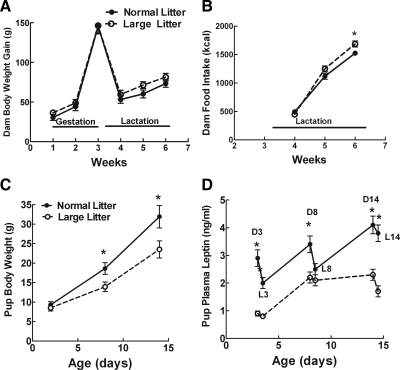
Dam body weights progressively increased during gestation and then fell precipitously during lactation, regardless of litter size (Fig. 1A). Despite their comparable weight gain, dams with LLs ate 8% more than those with NLs (LLs, 3383 ± 130 kcal; NLs, 3137 ± 125 kcal; P = 0.05; Fig. 1B) during lactation.
Figure 1.
A, Dam body weight gain during gestation and lactation. B, Dam food intake during lactation. C, Male pup body weight gain from P2–14. D, Male pup plasma leptin levels during dark (D) and light (L) phases from P2 to P14. *, P ≤ 0.05 by repeated-measures ANOVA followed by post hoc t test.
Preweaning effects of raising male offspring in LLs
From P2 to P14, pups raised in LLs gained 26% less body weight than those raised in NLs (Fig. 1C). Rather than demonstrating a surge in leptin levels during the second week of life as has been reported in mice (30), plasma leptin levels rose progressively by 40% from P2 to P14 during the dark and by 90% during the light phases. Levels were also consistently higher during the dark than light phases of the diurnal cycle in NL DIO pups (Fig. 1D). In parallel with their reduced body weight gain, plasma leptin levels in pups raised in LLs were also lower than those from NLs, averaging 33, 65, and 56% of NL pup levels at P3, P8, and P14 during the dark cycle, respectively. Along with their lowered leptin levels, LL DIO pups also had significant reductions in both dark- and light-phase insulin levels compared with NL DIO pups at these same ages (Supplemental Fig. 1, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). To demonstrate that leptin absorbed from maternal milk is capable of raising plasma leptin levels in LL DIO pups, we gavaged them with 100 ng leptin and were able to raise their levels to those of NL DIO pups for up to 4 h (Supplemental Fig. 2).
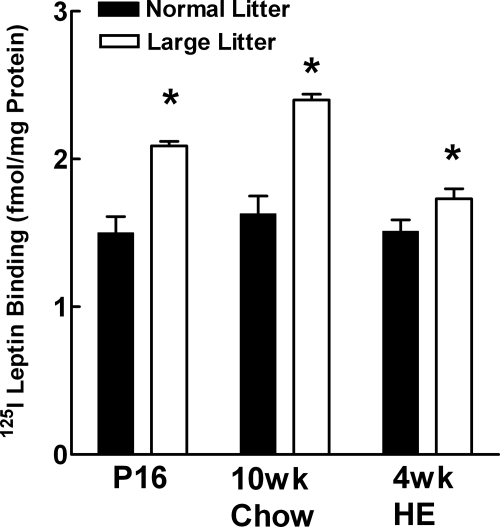
By P16, pups from LLs weighed 20% less, had 37% less total fat pad weight as a percent of carcass weight, and 48% lower leptin levels than those from NLs (Table 1). Despite their reduced weights, LL-reared pups had similar nasoanal lengths to those reared in NLs, suggesting that they had no stunting of their linear growth. As opposed to their lower plasma leptin levels at P14, P16 pups reared in LLs had 40% higher binding of 125I leptin to its receptors in the ARC (Fig. 2), whereas binding did not differ from that in NL pups in the ventromedial nucleus (VMN) or dorsomedial nucleus (DMN) (data not shown). In association with their enhanced ARC leptin receptor binding, P28 pups raised in LLs had a 71% greater density of immunoreactive fibers for α-MSH and 79% greater density of AgRP fibers in their PVN (Fig. 3).
Table 1.
Morphometric and biochemical data for male DIO rats raised in NLs (10 pups/dam) and LLs (16 pups/dam) from P2
| NL | LL | |
|---|---|---|
| P16 | ||
| Carcass mass (g) | 29.8 ± 1.7 | 24.0 ± 1.7a |
| Retroperitoneal (mg) | 28.6 ± 6.3 | 13.0 ± 2.8a |
| Mesenteric (mg) | 74.4 ± 12.2 | 50.8 ± 11.6a |
| Inguinal (mg) | 109.7 ± 15.2 | 60.5 ± 9.4a |
| Epididymal (mg) | 23.7 ± 3.8 | 15.0 ± 2.4a |
| Total fat pad weight (mg) | 256.3 ± 14.6 | 136.0 ± 22.6a |
| Total fat pad/body weight (%) | 0.81 ± 0.04 | 0.52 ± 0.09a |
| Leptin | 2.1 ± 0.6 | 1.1 ± 0.3a |
| Insulin | 1.0 ± 0.7 | 0.4 ± 0.1 |
| 4-wk nasoanal length (cm) | 14.6 ± 0.1 | 13.9 ± 0.2 |
| 10 wk on chow | ||
| Body weight gain (g) | 355 ± 4 | 319 ± 8a |
| Carcass mass (g) | 347 ± 5 (9) | 315 ± 16 (9)a |
| Lean mass (g) | 288 ± 2 | 268 ± 5a |
| Fat mass (g) | 35.5 ± 0.9 | 28.3 ± 0.9a |
| Lean (%) | 84.2 ± 0.2 | 85.3 ± 0.3a |
| Fat (%) | 10.3 ± 0.2 | 9.0 ± 0.2a |
| Leptin (ng/ml) | 5.10 ± 0.48 | 3.70 ± 0.49a |
| Insulin (ng/ml) | 3.19 ± 2.0 | 2.35 ± 1.2 |
| Nasoanal length (cm) | 23.6 ± 0.2 | 22.9 ± 0.2 |
| Glucose AUC (mg/dl per 120 min) | 3404 ± 473 (10) | 2940 ± 514 (10) |
| Insulin AUC (mg/dl per 120 min) | 169 ± 17 | 127 ± 14a |
| Insulin sensitivity index | 7.3 ± 0.6 | 9.0 ± 0.6a |
| 4–5 wk on HE diet | ||
| Body weight gain (g) | 140 ± 5 | 111 ± 4a |
| Food intake (kcal) | 3372 ± 119 | 3004 ± 101a |
| Carcass mass (g) | 479 ± 10 (13) | 432 ± 9 (13)a |
| Lean mass (g) | 341 ± 7 (13) | 314 ± 6 (13)a |
| Fat mass (g) | 107 ± 3 (9) | 90 ± 4 (9)a |
| Lean (%) | 71.3 ± 0.4 | 72.7 ± 0.6 |
| Fat (%) | 22.3 ± 0.04 | 20.6 ± 0.6a |
| Leptin (ng/ml) | 12.4 ± 0.6 | 12.3 ± 1.1 |
| Insulin (ng/ml) | 8.0 ± 0.4 | 7.0 ± 0.5 |
| Glucose AUC (mg/dl per 120 min) | 16879 ± 518 | 16007 ± 548 |
| Insulin AUC (mg/dl per 120 min) | 219 ± 28 | 217 ± 23 |
| Insulin sensitivity index | 4.5 ± 0.3 | 4.9 ± 0.4 |
Insulin sensitivity index calculations (17) are provided in Materials and Methods. Group numbers range from 8 to 14 per group. Numbers in parentheses indicate animals/group.
P ≤ 0.05 when data from LL offspring were compared with those from NL offspring.
Figure 2.
Binding of 125I-leptin to its receptors in the ARC of male offspring raised in LLs vs. NL at P16, at 10 wk of age in offspring fed chow from weaning and in offspring fed chow for 10 wk and then the HE diet for an additional 4 wk. *, P ≤ 0.05, LLs vs. NLs by unpaired t test.
Figure 3.
Examples of the relative densities of AgRP and α-MSH-immunoreactive fibers (α-MSH-IR) in the paraventricular nuclei (PVH) of male DIO pups raised in NLs vs. those from P28 pups raised in LLs (left panels). V3, Third ventricle. **, P = 0.01, LLs vs. NLs by unpaired t test for comparisons of quantitation of fiber densities.
Postweaning effects of raising male offspring in LLs
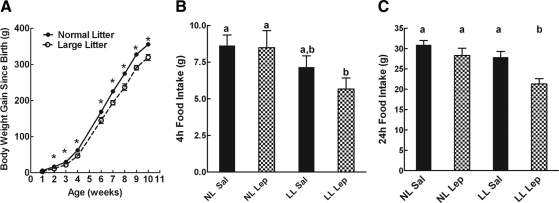
When weaned onto chow, male DIO pups reared in LLs gained 10% less body weight over their first 10 wk of life than those raised in NLs (Table 1). In addition, they had 13% less carcass fat and 1% more carcass lean body mass than did NL offspring. In keeping with their reduced fat mass, LL offspring also had 27% lower plasma leptin levels than did NL offspring. When subjected to an OGTT, LL offspring had similar glucose AUC but 25% lower insulin AUC. This resulted in a 23% higher insulin sensitivity index in LL offspring, suggesting that they were more insulin sensitive than NL offspring (Table 1). At 10 wk of age, LL offspring had 33% higher 125I-leptin binding to their ARC receptors (Fig. 2), whereas binding did not differ in the VMN or DMN compared with NL offspring (data not shown). When tested for their anorectic response to leptin (5 mg/kg, ip) at 9 wk of age, significant decreases in chow intake were seen only at 24 h in which leptin reduced intake by 24% in LLs but had no effect on NL offspring intake (Fig. 4).
Figure 4.
A, Body weight gain for male rats raised in NLs and LLs and fed chow from weaning until 10 wk of age. *, P ≤ 0.05 when groups were compared by repeated-measures ANOVA followed by post hoc t test. Four-hour (B) and 24-h (C) food intake for NL and LL offspring injected with saline (Sal) or leptin (Lep; 5 mg/kg, ip) are shown. Groups in each panel that differ from each other by P ≤ 0.05 have differing superscript letters.
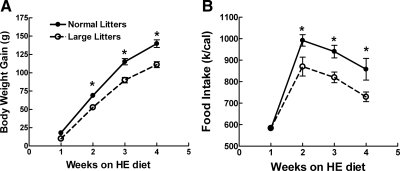
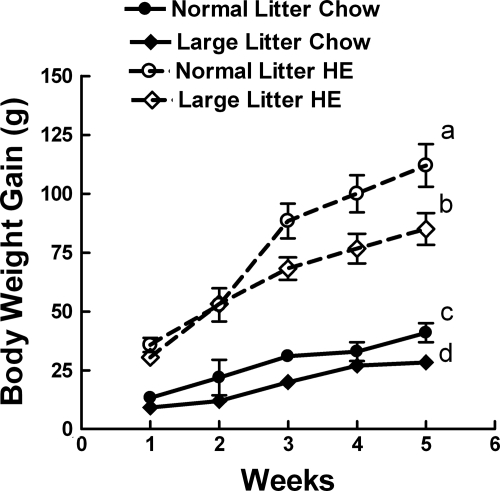
When additional chow-fed, 10-wk-old rats were fed the HE diet for 4 wk, LL offspring ate 11% less, gained 21% less body weight, and had 8% less carcass fat than did NL offspring (Table 1 and Fig. 5). However, there were no differences in plasma leptin or insulin levels. Neither were their differences in glucose or insulin AUC or insulin sensitivity indices between the groups during an OGTT (Table 2). At this time, LL offspring also had 13% higher ARC 125I-leptin receptor binding (Fig. 2). Again, there were no differences in VMN or DMN binding between the groups (data not shown).
Figure 5.
Body weight gain (A) and food intake over 4 wk on the HE diet in male NL and LL offspring, which had previously been fed chow from weaning until 10 wk of age. *, P ≤ 0.05 by repeated-measures ANOVA followed by post hoc t test.
Table 2.
Morphometric and biochemical data for female DIO rats raised in NLs (10 pups/dam) and LLs (16 pups/dam) from P2
| NL chow | LL chow | NL, 5 wk HE | LL, 5 wk HE | |
|---|---|---|---|---|
| Weaning | ||||
| Carcass weight (g) | 92.0 ± 1.9 | 80.5 ± 3.0 | ||
| Ovarian (mg) | 99.6 ± 13 | 84.3 ± 11 | ||
| Mesenteric (mg) | 352 ± 41 | 303 ± 35 | ||
| Inguinal (mg) | 553 ± 66 | 373 ± 38a | ||
| Retroperitoneal (mg) | 246 ± 62 | 102 ± 16a | ||
| Total fat pad weight (mg) | 1251 ± 130 | 862 ± 82a | ||
| Total fat pad/carcass weightt (%) | 0.01 ± 0.001 | 0.01 ± 0.001 | ||
| Leptin (ng/ml) | 1.6 ± 0.3 | 0.8 ± 0.2a | ||
| Insulin (ng/ml) | 2.9 ± 0.6 | 2.6 ± 0.3 | ||
| 10 wk chow | ||||
| Body weight (g) | 257 ± 7 | 232 ± 8a | ||
| Glucose AUC (mg/dl per 120 min) | 1985 ± 459 (7) | 1939 ± 205 | ||
| Insulin AUC (mg/dl per 120 min) | 193 ± 21 | 199 ± 45 | ||
| Insulin sensitivity index | 9.2 + 0.8 | 9.1 + 0.8 | ||
| 5 wk chow/HE | ||||
| Body weight (g) | 309 ± 11 | 265 ± 4a | 365 ± 15 | 315 ± 12a |
| Body weight gain (g) | 41 ± 1 | 28 ± 1a | 112 ± 9 | 85 ± 6a |
Group numbers range from 8 to 14 per group.
P ≤ 0.05 when data from LL offspring were compared with those from NL offspring.
Postweaning effects of raising female offspring in LLs
At weaning, female offspring raised in LLs had 31% less fat than did NL offspring. They also had 50% lower plasma leptin levels than did NL offspring (Table 2). All rats were fed chow from weaning until 10 wk of age. At that point, LL offspring weighed 10% less than NL offspring, and separate groups of rats were then continued on chow or were switched to the HE diet for an additional 5 wk. Over that 5-wk period, LL females fed chow gained 32% less, and those fed the HE diet gained 24% less body weight than did NL offspring (Table 2 and Fig. 6). Unlike the male LL rats, the females showed no improvement in insulin sensitivity index by OGTT when fed on chow.
Figure 6.
Body weight gain in NL and LL female offspring fed the HE diet for 5 wk after being fed chow for 10 wk from weaning. Groups that differ from each other by P ≤ 0.05 have different letters.
Effects of LL rearing on male DR rat body weight and adiposity
Five groups of DR male neonates were placed in NLs (10 pups/dam) or LLs (16 pups/dam) at P2 and fed chow from weaning to 10 wk of age and then fed the HE diet for 5 wk. Unlike DIO offspring, rearing DR pups in LLs had no effect on body weight gain, adiposity, plasma leptin or insulin levels, or the glucose response to an OGTT (Supplemental Table 1).
Discussion
These studies were undertaken to test the hypothesis that LL rearing would selectively protect DIO rats from developing DIO on an HE diet, much as it reduces adiposity in other rodent strains (1,31,32). We used DIO and DR rats to assess the effect of genotype on the obesity-sparing effect of LL rearing because of their genetically inherited predispositions to be obesity prone or resistant on HE diets, respectively (17). In addition, the DIO rat allowed us to examine the effects of LL rearing on leptin sensitivity because it has an inborn reduction in leptin responsiveness (10,19,22,33). We postulated that LL rearing would reduce the intake of maternal milk and thereby reduce their plasma leptin levels, leading to an early enhancement of their leptin sensitivity. An early postnatal increase in leptin sensitivity should, in turn, both improve their aberrant development of the ARC-PVN AgRP and α-MSH fiber projections (10) and protect them from becoming obese on the HE diet. We found that rearing DIO pups in LLs did, in fact, lower their plasma leptin levels as early as P3, increase their ARC leptin receptor binding by at least P16, and markedly improve their defective ARC-PVN AgRP and α-MSH fiber pathway development, most of which occurs in the first 12 d of life in the rat and mouse (7,8).
Presumably, as a result of their increased ARC leptin receptor binding and index of increased leptin sensitivity, adult LL DIO rats had increased sensitivity to the anorectic effects of leptin and reduced body weight gain and adiposity on both chow and the HE diet and improved insulin sensitivity on chow in males. The reduction in body weight of male LL DIO rats occurred as early as P8 and was associated with reduced adiposity as early as P16. Their reduced adiposity was still present at 10 wk of age after they were weaned onto chow. In fact, chow-fed DIO males have no more carcass adiposity than do DR males by 10 wk of age, even though they weigh more (34). Thus, LL rearing had an additive effect on reducing adiposity, even in chow-fed DIO males. Importantly, this occurred without apparent stunting because their nasoanal lengths were not reduced. More importantly, the enhanced leptin sensitivity of LL DIO rats is likely to be an important contributor to their obesity resistance on the HE diet. The picture was slightly different for LL females. Although they did have lower body weight and leptin levels, their fat pad weights as a percent of total carcass weight did not differ at weaning. When weaned onto chow for 10 wk and then fed either chow or the HE diet after an additional 5 wk, the LL females gained significantly less body weight. Unfortunately, because these females were kept alive for further studies (not included here), fat pad weights were not assessed after this period. Thus, we have no measure of carcass adiposity.
Importantly, the LL effect on body weight and adipose gain was selective to DIO rats. Rearing DR male rats in LLs neither reduced their adiposity on chow nor further enhanced their inborn resistance to become obese on the HE diet. This is likely due to the fact that these rats are already highly obesity resistant from birth (17). Thus, although others have demonstrated a protective effect of LL rearing in other rodent strains and species (1,31,32), these are the first studies to examine the interaction of litter size and genotype on the development of obesity and the first to identify enhanced leptin sensitivity as a likely important contributor to the obesity resistance of LL DIO male rats.
From these observations, we postulate that the early reduction in plasma leptin was the proximate cause of the increase in ARC leptin receptor numbers and that this led to increased leptin sensitivity in association with an increased neurotrophic effect of leptin on the outgrowth of ARC-PVN fibers in male DIO rats (9,10). To produce such an effect, increased leptin sensitivity would have to have occurred between P3 and P12 because this pathway’s development is largely complete by P12 in rodents (7,8). Also, leptin administration during this critical window can similarly correct the ARC-PVN pathway development of leptin-deficient ob/ob mice (9). However, it is unlikely that improvement in this pathway development alone is critical for the later increase in leptin sensitivity and obesity resistance of the LL DIO rats because leptin-deficient ob/ob mice with aberrant pathway development still are exquisitely sensitive to leptin given in adult life (35,36). Furthermore, LL rearing equally enhanced both of the opposing catabolic α-MSH and anabolic AgRP projections to the PVN. Thus, the importance of the enhanced ARC-PVN pathway development is that it serves as an important index of an early increase in leptin sensitivity of the LL DIO rats.
There are a number of factors that might have contributed to the early increase in neonatal leptin sensitivity of LL DIO offspring. The most likely cause would be the reduction in plasma leptin levels that occurred within 1 d of putting pups in LLs. This phenomenon has been reported previously (37), and we confirm here that these lowered levels for plasma leptin, as well as for insulin, occurred within 1 d of being placed in LLs and persisted up to P14. Although neonates can make their own leptin, they also ingest and absorb leptin (and insulin) from maternal milk up to the 12th day of life (2,3,4,5,6). It is important to point out that DIO neonates raised in NLs have comparable plasma leptin levels with NL-reared DR rats (34). We have also confirmed that LL DIO pups, like other rodents (38), can have their plasma leptin levels increased by gavage of exogenous leptin. Importantly, LL pups eat less than NL pups (1,39), presumably because of increased competition for a limited number of teats and because dams do not increase the amount of milk they produce in response to the increased litter size (40). Thus, a combination of reduced ingestion of leptin from maternal milk and reduced leptin production due to caloric restriction imposed by LL rearing (41,42,43) probably accounts for the lowered leptin levels of LL DIO neonates. Because leptin receptor expression has an inverse relationship to plasma leptin levels (44,45), we postulate that this may be the proximate cause of their increased leptin receptor binding and apparent early increased leptin sensitivity. Although it is uncertain why such an early correction in the reduced leptin responsiveness and signaling of the DIO rat should persist into adulthood, it has been clearly demonstrated that the administration of leptin during the early postnatal period leads to the opposite effect, i.e. reduced leptin sensitivity and obesity (12,13,14,15). It may well be that such early interventions lead to epigenetic changes in genes involved in leptin receptor production, intracellular trafficking, and/or downstream signaling. Obviously these ideas remain to be tested.
There are other potential factors associated with LL rearing that might contribute to the increased leptin sensitivity demonstrated here. For example, LL rearing necessarily causes dams to spend less time with their pups. This means that the pups are likely to spend more time exposed to vivarium temperatures that are well below their thermoneutral zone (46). Before 1 wk of age, pups are largely immobile at or near the temperatures used in our vivarium (22–23 C) and depend on retrieval by the dam for warmth and feeding (47). It is possible that such early hypothermia might affect their leptin sensitivity. Finally, altering the amount of exposure of pups to their dams can alter physiological responsiveness in association with altered methylation of genes (48,49,50). This phenomenon might also contribute to the early increases in leptin sensitivity of LL DIO offspring.
Although we have focused on enhanced leptin sensitivity as a likely cause of the induction of obesity resistance in LL DIO offspring, it is possible that enhanced insulin signaling might also contribute to their obesity resistance as a result of low plasma insulin levels during the first 2 postnatal weeks of life. Exposure of the hypothalamus to elevated insulin levels during the postnatal period results in adult obesity (51). Also, increased insulin exposure from maternal milk insulin during this critical period of hypothalamic neural development may also contribute to the development of obesity in adult DR rats fostered by obese DIO dams during the neonatal period (11). Although we did not assess central insulin sensitivity, there was an increase in the peripheral insulin sensitivity index, a surrogate for insulin sensitivity (29), in the chow-fed LL DIO males. Finally, although LL rearing did reduce body weight and adiposity on chow and prevented obesity in LL DIO males, it did not have a similar effect on DR rats, which have normal leptin sensitivity (22). Because the protective effects of LL rearing in DIO rats with regard to obesity is similar to what is seen in other rat strains (52,53,54), the failure of DR rats to respond similarly suggests that they are already maximally protected against the development of DIO obesity.
Finally, there is an important caveat to the interpretation of our results. First, we used immunocytochemistry and densitometry measures to estimate the ARC-PVN development of AgRP and α-MSH fiber pathways. However, differences in the apparent densities of these terminal fields could represent differences in the amount of each neuropeptide present in the terminals rather than a difference in the number of terminals present. Also, we assessed these densities at P28, well after the completion of projection outgrowth from the ARC to PVN (7,8). Thus, differences in leptin sensitivity might have altered the amount of neuropeptide present in the terminals rather than the actual density of these terminals after the critical period of their development. It is possible that examination of these fibers earlier in development might have provided a different outcome. Thus, although these results do not confirm an early increase in leptin sensitivity acting to correct the aberrant ARC-PVN projections in DIO rats, they are compatible with such a hypothesis.
Summary and conclusion
The current studies demonstrate that LL rearing increases the leptin sensitivity of male DIO neonates and that this is associated with reduced adiposity, whether they are fed chow or the HE diet as adults. The increased leptin sensitivity is also associated with improved α-MSH and AgRP fiber density in the PVN, which suggests that it occurs early in the postnatal period while these ARC-PVN pathways are developing. It may be that the chronically reduced plasma leptin levels resulting from LL rearing are responsible for the increased ARC leptin receptor population and enhance sensitivity to the anorectic effects of leptin. However, the long-lasting increase in sensitivity may well be due to epigenetic changes that occur in the first 2 wk of postnatal life. Because these effects on body weight gain were selective for DIO compared with DR rats, this suggests that early reductions in leptin levels brought on by LL rearing has an important genotype-specific effect on the ability to resist the development of obesity.
Supplementary Material
Acknowledgments
We thank Charlie Salter and Antoinette Moralishvilli for the expert technical assistance. We thank the University of Alabama at Birmingham Small Animal Phenotyping Core for body composition.
Footnotes
This work was supported by National Institutes of Health Grant F31-NS-050903 (to C.M.P.), a March of Dimes grant, Agence Nationale de la Recherche Grant ANR-08-JCJC-0055-01 (to S.G.B.), the Research Service of the Veterans Administration (to A.A.D.-M., B.E.L.), and National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1-30066 (to S.P. and A.A.D.-M., B.E.L.).
Disclosure Summary: C.M.P., S.G.B., S.P., B.G.I., A.A.D.-M., and B.E.L. have nothing to declare.
First Published Online July 28, 2010
Abbreviations: AgRP, Agouti-related peptide; ARC, arcuate nucleus; AUC, area under the curve; DIO, diet-induced obesity; DMN, dorsomedial nucleus; DR, diet resistant; HE, high-energy; LL, large litter; NL, normal litter; OGTT, oral glucose tolerance test; P, postnatal day; PVN, paraventricular nucleus; VMN, ventromedial nucleus.
References
- Kennedy GC 1957 The development with age of hypothalamic restraint upon the appetite of the rat. J Endocrinol 16:9–17 [DOI] [PubMed] [Google Scholar]
- Grosvenor CE, Picciano MF, Baumrucker CR 1993 Hormones and growth factors in milk. Endocr Rev 14:710–728 [DOI] [PubMed] [Google Scholar]
- Mosinger B, Placer Z, Koldovsky O 1959 Passage of insulin through the wall of the gastro-intestinal tract of the infant rat. Nature 184(Suppl 16):1245–1246 [DOI] [PubMed] [Google Scholar]
- Casabiell X, Pineiro V, Tomé MA, Peinó R, Diéguez C, Casanueva FF 1997 Presence of leptin in colostrum and/or breast milk from lactating mothers: a potential role in the regulation of neonatal food intake. J Clin Endocrinol Metab 82:4270–4273 [DOI] [PubMed] [Google Scholar]
- Sánchez J, Priego T, Palou M, Tobaruela A, Palou A, Picó C 2008 Oral supplementation with physiological doses of leptin during lactation in rats improves insulin sensitivity and affects food preferences later in life. Endocrinology 149:733–740 [DOI] [PubMed] [Google Scholar]
- Koldovský O, Illnerová H, Macho L, Strbák V, Stěpánková R 1995 Milk-borne hormones: possible tools of communication between mother and suckling. Physiol Res 44:349–351 [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB 2004 Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci 24:2797–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove KL, Allen S, Grayson BE, Smith MS 2003 Postnatal development of the hypothalamic neuropeptide Y system. Neuroscience 116:393–406 [DOI] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB 2004 Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304:108–110 [DOI] [PubMed] [Google Scholar]
- Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB 2008 Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab 7:179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J, Dunn-Meynell AA, Hartman TG, Levin BE 2006 Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. Am J Physiol Regul Integr Comp Physiol 291:R768–R778 [DOI] [PubMed] [Google Scholar]
- Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M 2008 The effect of neonatal leptin treatment on postnatal weight gain in male rats Is dependent on maternal nutritional status during pregnancy. Endocrinology 149:1906–1913 [DOI] [PubMed] [Google Scholar]
- Toste FP, de Moura EG, Lisboa PC, Fagundes AT, de Oliveira E, Passos MC 2006 Neonatal leptin treatment programmes leptin hypothalamic resistance and intermediary metabolic parameters in adult rats. Br J Nutr 95:830–837 [DOI] [PubMed] [Google Scholar]
- Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, Kawamura M, Takemura M, Kakui K, Ogawa Y, Fujii S 2005 Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab 1:371–378 [DOI] [PubMed] [Google Scholar]
- de Oliveira Cravo C, Teixeira CV, Passos MC, Dutra SC, de Moura EG, Ramos C 2002 Leptin treatment during the neonatal period is associated with higher food intake and adult body weight in rats. Horm Metab Res 34:400–405 [DOI] [PubMed] [Google Scholar]
- Patterson CM, Bouret SG, Dunn-Meynell AA, Levin BE 2009 Three weeks of postweaning exercise in DIO rats produces prolonged increases in central leptin sensitivity and signaling. Am J Physiol Regul Integr Comp Physiol 296:R537–R548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE 1997 Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol 273:R725–R730 [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Ricci MR, Cummings DE 2003 Abnormalities of leptin and ghrelin regulation in obesity-prone juvenile rats. Am J Physiol Endocrinol Metab 285:E949–E957 [DOI] [PubMed] [Google Scholar]
- Irani BG, Dunn-Meynell AA, Levin BE 2007 Altered hypothalamic leptin, insulin and melanocortin binding associated with moderate fat diet and predisposition to obesity. Endocrinology 148:310–316 [DOI] [PubMed] [Google Scholar]
- Irani BG, Le Foll C, Dunn-Meynell AA, Levin BE 2009 Ventromedial nucleus neurons are less sensitive to leptin excitation in rats bred to develop diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 296:R521–R527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA 2002 Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 283:R941–R948 [DOI] [PubMed] [Google Scholar]
- Gorski JN, Dunn-Meynell AA, Levin BE 2007 Maternal obesity increases hypothalamic leptin receptor expression and sensitivity in juvenile obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 292:R1782–R1791 [DOI] [PubMed] [Google Scholar]
- Levin BE, Hogan S, Sullivan AC 1989 Initiation and perpetuation of obesity and obesity resistance in rats. Am J Physiol 256:R766–R771 [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Levin BE 1997 Location and effect of obesity on putative anorectic binding sites in the rat brain. Obes Res 5:201–207 [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA 2002 Maternal obesity alters adiposity and monoamine function in genetically predisposed offspring. Am J Physiol Regul Integr Comp Physiol 283:R1087–R1093 [DOI] [PubMed] [Google Scholar]
- Levin BE, Israel P, Lattemann DP 1998 Insulin selectively down-regulates α2-adrenoceptors in the arcuate and dorsomedial nucleus. Brain Res Bull 45:179–181 [DOI] [PubMed] [Google Scholar]
- Tinsley FC, Taicher GZ, Heiman ML 2004 Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obes Res 12:150–160 [DOI] [PubMed] [Google Scholar]
- Johnson MS, Smith Jr DL, Nagy TR 2008 Validation of the quantitative magnetic resonance instrument for determination of body composition in rats. Int J Body Compos Res 6:62–68 [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA 1999 Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470 [DOI] [PubMed] [Google Scholar]
- Ahima RS, Prabakaran D, Flier JS 1998 Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest 101:1020–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippel U, Plagemann A, Davidowa H 2003 Altered action of dopamine and cholecystokinin on lateral hypothalamic neurons in rats raised under different feeding conditions. Behav Brain Res 147: 89–94 [DOI] [PubMed] [Google Scholar]
- Knittle JL, Hirsch J 1968 Effect of early nutrition on the development of rat epididymal fat pads: cellularity and metabolism. J Clin Invest 47:2091–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll C, Irani BG, Magnan C, Dunn-Meynell A, Levin BE 2009 Effects of maternal genotype and diet on offspring glucose and fatty acid-sensing ventromedial hypothalamic nucleus neurons. Am J Physiol Regul Integr Comp Physiol 297:R1351–R1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci MR, Levin BE 2003 Ontogeny of diet-induced obesity in selectively bred Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 285:R610–R618 [DOI] [PubMed] [Google Scholar]
- Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB, Flier JS 1995 Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med 1:1311–1314 [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F 1995 Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269:540–543 [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Rake A, Waas T, Melchior K, Ziska T, Rohde W, Dörner G 1999 Observations on the orexigenic hypothalamic neuropeptide Y-system in neonatally overfed weanling rat. J Neuroendocrinol 11:541–546 [DOI] [PubMed] [Google Scholar]
- Sánchez J, Oliver P, Miralles O, Ceresi E, Picó C, Palou A 2005 Leptin orally supplied to neonate rats is directly uptaken by the immature stomach and may regulate short-term feeding. Endocrinology 146:2575–2582 [DOI] [PubMed] [Google Scholar]
- Morag M, Popliker F, Yagil R 1975 Effect of litter size on milk yield in the rat. Lab Anim 9:43–47 [DOI] [PubMed] [Google Scholar]
- Russell JA 1980 Milk yield, suckling behaviour and milk ejection in the lactating rat nursing litters of different sizes. J Physiol 303:403–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P, Thomas ME, Duncan JS, Rayner DV 1995 Effects of fasting and refeeding on ob gene expression in white adipose tissue of lean and obese (ob/ob) mice. FEBS Lett 368:488–490 [DOI] [PubMed] [Google Scholar]
- Hardie LJ, Rayner DV, Holmes S, Trayhurn P 1996 Circulating leptin levels are modulated by fasting, cold exposure and insulin administration in lean but not Zucker (fa/fa) rats as measured by ELISA. Biochem Biophys Res Commun 223:660–665 [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA 2000 Defense of body weight against chronic caloric restriction in obesity-prone and -resistant rats. Am J Physiol Regul Integr Comp Physiol 278:R231–R237 [DOI] [PubMed] [Google Scholar]
- Martin RL, Perez E, He YJ, Dawson Jr R, Millard WJ 2000 Leptin resistance is associated with hypothalamic leptin receptor mRNA and protein down-regulation. Metabolism 49:1479–1484 [DOI] [PubMed] [Google Scholar]
- Hindlet P, Bado A, Kamenicky P, Deloménie C, Bourasset F, Nazaret C, Farinotti R, Buyse M 2009 Reduced intestinal absorption of dipeptides via PepT1 in mice with diet-induced obesity Is associated with leptin receptor down-regulation. J Biol Chem 284:6801–6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deviterne D, Desor D, Krafft B 1990 Maternal behavior variations and adaptations, and pup development within litters of various sizes in Wistar rat. Dev Psychobiol 23:349–360 [DOI] [PubMed] [Google Scholar]
- Kleitman N, Satinoff E 1982 Thermoregulatory behavior in rat pups from birth to weaning. Physiol Behav 29:537–541 [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ 2004 Epigenetic programming by maternal behavior. Nat Neurosci 7:847–854 [DOI] [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M 2005 Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci 25:11045–11054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M, Weaver IC, Champagne FA, Diorio J, Meaney MJ 2005 Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front Neuroendocrinol 26:139–162 [DOI] [PubMed] [Google Scholar]
- Plagemann A, Heidrich I, Göotz F, Rohde W, Dörner G 1992 Lifelong enhanced diabetes susceptibility and obesity after temporary intrahypothalamic hyperinsulinism during brain organization. Exp Clin Endocrinol 99:91–95 [DOI] [PubMed] [Google Scholar]
- Faust IM, Johnson PR, Hirsch J 1980 Long-term effects of early nutritional experience on the development of obesity in the rat. J Nutr 110:2027–2034 [DOI] [PubMed] [Google Scholar]
- Remmers F, Fodor M, Delemarre-van de Waal HA 2008 Neonatal food restriction permanently alters rat body dimensions and energy intake. Physiol Behav 95:208–215 [DOI] [PubMed] [Google Scholar]
- Remmers F, Schreuder MF, Gemke RJ, Delemarre-van de Waal HA 2008 Energy intake and resting energy expenditure in adult male rats after early postnatal food restriction. Br J Nutr 99:1149–1156 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.