Abstract
The serotonin molecule plays a multifunctional role in mammalian homeostasis serving as a neurotransmitter in the central nervous system, a gut-derived mediator of peristalsis, and a circulating hormone that regulates appetite, cardiovascular function, and hemostasis. Recent evidence from the clinic and the bench highlight an unexpected target for serotonin action, the skeleton. Clinically, two classes of drugs, the second generation antipsychotic drugs (SGAs) and selective serotonin reuptake inhibitors (SSRIs), which modulate central and peripheral serotonin signaling, have been shown to alter bone remodeling although the mechanism is not clear. In contrast, genetically engineered mouse models have demonstrated a bimodal control system whereby gut-derived serotonin under the control of the Wnt/Lrp/β-catenin system acts systemically to suppress bone formation, whereas CNS serotonin activated by leptin modulates sympathetic outflow to the skeleton. In this brief review, we will summarize recent findings linking serotonin to the skeleton and discuss future directions for this new but challenging aspect of this multidimensional molecule.
Serotonin is a novel regulator for skeletal metabolism and may be a therapeutic target for the treatment of osteoporosis.
Serotonin (5-hydroxytryptamine, 5-HT) has confounded physiologists and endocrinologists for decades. It has defied our attempts to classify it as a hormone, in part because it is a gut-derived paracrine factor, a modulator of gastrointestinal motility, a neurotransmitter, an appetite mediator, a regulator of circadian rhythmicity, a determinant of platelet contraction and hemostasis, an integrator of energy modulation, and a molecule that when altered in central nervous system (CNS) synapses is related to depression (1,2,3). Indeed, serotonin defies a single classification; rather it is a simple, but evolutionarily conserved molecule with pleiotropic effects on multiple target tissues. And, remarkably, investigators have recently found another target for serotonin, the skeleton.
Bone remodeling is necessary to maintain skeletal mass but requires the tight coupling of bone formation to resorption. In certain physiological states as well as in several pathologic situations, the remodeling sequence can become imbalanced by alterations in energy status (4). For example, during chronic calorie deprivation such as anorexia nervosa, the skeleton adapts to profound deficiencies in nutrient status by switching to a catabolic state with increased bone resorption and suppression of bone formation (4). However, that switch is complex and demands hypothalamic integration from both peripheral energy depots and central neuronal input (5). Leptin, an adipokine secreted in response to expansion of triglyceride storage in the adipocyte, is the principal afferent signal to those higher centers in the hypothalamus; its actions are to regulate appetite and energy expenditure principally through activation of the sympathetic nervous system as an efferent modulator of energy depots (6). Importantly, but not surprisingly, the skeleton is also targeted by hypothalamic output. The Karsenty laboratory has been out front in efforts to demonstrate that control of skeletal mass relative to energy status occurs through the leptin-hypothalamic-sympathetic nervous system, although other neurotransmitters such as neuropeptide Y have also been implicated in both central and peripheral regulation of bone mass (7,8,9).
Serotonin is found in high concentrations in certain portions of the CNS acting as a neurotransmitter to regulate metabolic rate and appetite in hypothalamic nuclei, thus making it another potential modulator of energy status (3). Serotonin also influences cognitive and psychological function in higher centers of the brain (1,3). Drugs that target serotonin receptors or transporters have been developed to treat a wide range of psychiatric disorders from depression to schizophrenia to the autism complex. Two classes of drugs that have effects on CNS serotonin are the second generation antipsychotic agents (SGAs) and the selective serotonin reuptake inhibitors (SSRIs). Use of these agents has increased recently, and with wider utilization has come recognition of significant adverse metabolic and skeletal events. Dramatic weight gain, often associated with the metabolic syndrome, bone loss, and greater fracture prevalence have startled clinicians and provided an impetus for studying the serotonin-metabolic-CNS-skeletal connection.
Serotonin’s peripheral effects occur through local synthesis in the enterochromaffin cells of the gut. Its principal function is to regulate intestinal peristalsis, and its secretion is triggered by food in the gut. The enzyme responsible for serotonin synthesis in the gut is Tph1, and an inhibitor of this enzyme is currently in phase II trials for the treatment of irritable bowel syndrome. The surprising discovery that Tph1 expression was one of the most markedly up-regulated genes in a Lrp5-deficient mouse with low bone mass led to a series of studies demonstrating serotonin signaling in the regulation of bone mass. In this brief review we will summarize recent findings both from human and mouse models regarding the central regulation of bone remodeling and discuss the limitations of current studies as well as the immense potential for targeting serotonin either centrally or peripherally for the treatment of osteoporosis.
Serotonin Physiology
Serotonin (5-Hydroxytryptamine; 5-HT) is a neurotransmitter and governs a wide range of physiological functions from complex behavioral activities to gastrointestinal peristalsis depending on its site of synthesis (10,11,12). Serotonin is generated from l-tryptophan, which is hydroxylated to 5-hydroxy-l-tryptophan (5-HTP). This reaction is catalyzed by the rate-limiting enzyme, tryptophan hydroxylase (Tph). 5-HTP is then converted into serotonin (13). Circulating serotonin is produced in the enterochromaffin cells of the duodenum by Tph1 and is released into the circulation, to be taken up principally by platelets through the 5-hydroxytrophan transporter (5-HTT) (13). Gut-derived serotonin accounts for 95% of total serotonin in the body, but it does not cross the blood-brain barrier (11). Platelet serotonin is an important regulator of cellular contraction and serves as a modulator of hemostasis once the clotting system is activated (3). In the CNS, serotonergic neurons mainly reside in the dorsal, median, and caudal raphe nuclei of the brainstem (2,3). These project to almost of all parts of the brain including the hypothalamus (2,3). In contrast to Tph1, Tph2 is responsible for the production of local production of serotonin in the CNS (14). Serotonin receptors consist of at least seven major families and at lease 14 subtypes, and these have been found in many tissues both centrally and in the periphery (2). Serotonin in the brain is involved in the regulation of food intake as evidenced by mouse models lacking 5-HT1AR, 5-HT1BR, or 5-HT2cR, all of which have been shown to exhibit increased food intake (1). With respect to the role of serotonin in the regulation of skeletal mass, there is consistent evidence that 5-HT and 5-HTT are expressed in osteoblasts as are the serotonin receptors (15,16,17). In addition, 5-HTT is also expressed in RANKL-induced osteoclasts in vitro (18). Genetic models that induce functional changes in the receptors or transporters have been shown to affect peak bone mass in mice (19,20,21). In humans, at least one study has shown that serum serotonin is weakly but inversely correlated with total body and spine areal bone mineral density and with femur neck total and trabecular volumetric bone mineral density (22). These skeletal phenotypic differences were associated with alterations in trabecular number, and trabecular thickness at the radius assessed by high-resolution peripheral quantitative CT. Although body weight is a confounder in these regression analyses, several key parameters were still significantly different following the adjustment for body mass index (22).
Serotonin regulation of skeleton: indirect implications from clinical observations
SGAs such as risperidone have been widely used for the treatment of behavioral problems in autism spectrum disorders and acute psychotic symptoms because of their effectiveness and low adverse event rates compared with earlier first generational antipsychotic drugs (23). However, accumulating evidence suggests there may be serious long-term adverse metabolic effects from these drugs, including weight gain, nighttime eating, and the metabolic syndrome (24). With respect to the skeleton, bone loss has been associated with schizophrenic individuals taking psychotropic medication (25). Howard et al. (26) reported a higher risk of hip fractures among individuals using antipsychotic medications. Because the SGAs act as a potent blocker for the 5-HT2A receptor and are a weaker inhibitor of the dopamine D2 receptor (27), these data suggest that blockade of serotonin signaling through 5-HT receptor could have a negative effect on skeletal accrual. However, the SGAs also cause various degrees of hyperprolactinemia, which can accelerate bone resorption by suppressing gonadotropin secretion (28). Prolactin is produced by lactotrophs in the anterior pituitary and is negatively regulated by dopamine signaling from tuberoinfundibulum neurons in arcuate nuclei in the hypothalamus through dopamine D2 receptors (23,28). The SGAs also inhibit dopamine D2 receptors as well as the 5-HT2A receptors (27), so it is conceivable that the SGAs cause hyperprolactinemia through inhibition of dopamine signaling. Nevertheless, it is still unclear whether the inhibition of 5-HT2A receptor by SGAs, which is operative within the CNS, has any role in the pathogenesis of bone loss. Interestingly, we recently observed that pubertal male C57BL/6J mice treated with risperidone had significant trabecular bone loss due to increased bone resorption (29). Surprisingly, these mice did not gain weight but rather showed an adipose redistribution phenotype with enhanced marrow adiposity and significant hepatic lipid deposition. Thus it is likely that the bone loss with these agents is multifactorial and relates to both central and peripheral alterations in serotonin, prolactin, and the gonadotropins.
Selective serotonin reuptake inhibitors in human and mouse models
SSRIs, which have been widely used for depression, have recently been associated with bone loss and an increased risk for fracture (30,31,32,33,34,35,36,37). In support of this finding, 5-HTT knockout mice exhibit reduced bone mass (19). In line with this, treatment of SSRIs has been reported to reduce bone mass, although the effect of SSRIs on bone mass in experimental models is not clear (38,39 and see Table 1). SSRIs are inhibitors of 5-HTT and increase the local concentration of serotonin at the pericellular space, although its chronic use reduces serotonin content in the platelets by 80–95% (40). Therefore, one has to be cautious in interpreting these clinical findings because SSRIs are likely to inhibit 5-HTT function at multiple levels including the CNS, platelets, and the skeletal microenvironment. In that respect, as noted, osteoblasts and osteoclasts express serotonin receptors and 5-HTT (13,15,16,17,18), making it likely that serotonin plays some role in lineage allocation and/or osteoblast and osteoclast function/differentiation. It is conceivable that the SSRIs increase the skeletal micro-concentration of serotonin through inhibition of uptake by osteoblasts thereby prolonging serotonin exposure, although even in platelets it is still controversial how a reduction of serotonin content or alterations in reuptake in platelets directly affects their function. In addition, there are certain to be other mechanisms. Peripheral serotonin synthesis is catalyzed by Tph1 in the gut, and circulating serotonin can have a direct negative effect on skeletal mass through activation of osteoblastic serotonin receptors, although these findings need to be confirmed independently (21). Thus the SSRIs may decrease bone mass by enhancing the local concentration of serotonin.
Table 1.
Genetically engineered mouse models for analyzing skeletal action of serotonin
| Mouse | Skeletal phenotype | Reference | |
|---|---|---|---|
| 5-HTT−/− | Global knock out of serotonin transporter | Low bone mass, low bone formation | 20 |
| Tph1gut−/− | Gut-specific Tph1 knock out under villin promoter | Reduced serotonin levels in the gut High bone mass, increased bone formation | 21 |
| Tph1do−/− | Osteoblasts specific Tph1 knock out under al(I)Col promoter | Minimal increase in bone mass | 21 |
| Htr1b−/− | Global knock out of Htr1b. Htr1b is the most abundantly expressed serotonin receptor in osteoblasts | High bone mass, increased bone formation | 21 |
| Tph2−/− | Global knock out of Tph2 | No detectable serotonin in the CNS Low bone mass, low bone formation, increased bone resorption | 19 |
| Htr2c−/− | Global knock out of Htr2c. Htr2c is most abundantly expressed in the hypothalamus | Low bone mass, low bone formation, increased bone resorption High sympathetic activity | 19 |
5-HTT, 5-HT(5-hydroxytryptamine) transporter; Tph, tryptophan hydroxylase; Htr, 5-HT receptor.
However serotonin pharmacology is not simple. The SSRIs cross the blood brain barrier and block reuptake of serotonin in the CNS, thus increasing local serotonin concentrations. Serotonin may activate its receptors in the ventromedial hypothalamus, which in turn could modulate sympathetic outflow and increase bone mass (19). The discrepancy between patients treated with SSRIs and mouse models may come from the site and context specific nature of expression profiles for 5-HTT. 5-HTT expression is limited to serotonergic neurons in the raphe nuclei of adults, but broader expression profiles of 5-HTT are observed in the fetal brain of rodents (41,42). Thus, use of SSRIs in adults may have a distinctly different effect on central serotonergic regulation of bone mass from those observed in genetically engineered mouse models. Furthermore, an appreciation of an increasing level of complexity and redundancy in the serotonin system continues to confound our understanding of the role of SSRIs in modulating bone mass. For example, Yadav and colleagues (21) demonstrated that inhibition of Tph1 in the gut (it is not expressed in the brain) resulted in high skeletal mass and this enzyme was under the control of Lrp5, a coreceptor for Wnt signaling. Conditional deletion of Tph1 in the gut confirmed there was increased bone formation and high bone mass, with very low circulating serotonin, suggesting a peripheral effect of endocrine secreted serotonin on bone mass (21). Notwithstanding these findings, it is still difficult to extrapolate from mouse models to define precisely how the SSRIs mediate their effects on bone mass.
Regulation of skeletal mass by serotonin in the brain
To further understand the role of serotonin in the CNS and its interaction with skeletal acquisition independent of the gut, Yadav et al. (19) took a genetic approach. They analyzed Tph2−/− mice because Tph2 is the rate-limiting enzyme for the production of serotonin in the CNS. Tph2−/− mice showed almost complete absence of serotonin in the brain despite normal blood serotonin levels. Skeletal phenotyping revealed low bone mass both in long bones and vertebrae accompanied by impaired bone formation and increased bone resorption, implying that serotonin in the CNS has a significant positive role in the regulation of skeletal accrual. Based on their observation that the skeletal phenotype in Tph2−/− mice is the mirror image of the phenotype observed in β-adrenergic receptor (Adrβ2) knockout mice (8) the authors hypothesized that the low bone mass phenotype in Tph2−/− mice was caused by increased sympathetic tone. To test this hypothesis, Yadav et al. (19) generated Tph2−/−;Adrβ2+/− mice and found the low bone mass phenotype in Tph2−/− mice was rescued in the absence of one allele of Adrβ2. These data suggest that serotonin in the CNS regulates skeletal acquisition through suppression of sympathetic tone, thereby implicating the hypothalamus as a principal target for serotonin.
This same group next investigated the central mechanism by which serotonin affected bone mass. Because VMH neurons are critical integrators of energy metabolism through modulation of sympathetic tone, the authors used genetically engineered mouse models to demonstrate that serotonergic neurons targeted the VMH thereby affecting sympathetic output. First they identified that neurons in the brain stem projected to the VMH area in the hypothalamus. Second, deletion of Htr2c, which is the most abundantly expressed serotonin receptor in the hypothalamus, caused low bone mass accompanied by increased sympathetic activity. The double Tph2+/−;Htr2c+/− mice exhibited a low bone mass phenotype with high sympathetic tone despite normal skeletal mass and sympathetic tone in Tph2+/− and Htr2c+/− mice. Third, rescuing the Htr2c expression in the VMH neurons on the Htr2c−/− background reversed the low bone mass phenotype of Htr2c−/− mice. These findings were provocative but left a more important question: where did leptin fit in this homeostatic scheme?
Leptin regulation of brain serotonin synthesis and bone mass
Leptin is produced by adipocytes and regulates a wide range of physiological functions including energy homeostasis, appetite, reproductive capacity, and bone accrual (6). The complexity of leptin’s actions on skeletal accrual lies in the difference between the direct effects of leptin on bone and its indirect actions through hypothalamus (43). It is now well established that leptin decreases vertebrae trabecular bone mass through indirect activation of the sympathetic nervous system via VMH neurons (7,9). Takeda et al. (9) reported that chemical lesioning of VMH neurons by gold thioglucose increased bone mass, thereby recapitulating the skeletal phenotype observed in ob/ob mice. But intriguingly, conditional knockouts of the leptin receptor in VMH neurons using the SF-1 promoter did not show a skeletal phenotype (44). These data pointed to an important role for leptin mediation of sympathetic activity but paradoxically not directly through VMH neurons. This conclusion, however, was counter to previous evidence suggesting that leptin after crossing the blood-brain barrier directly targets the hypothalamus. Yadav et al. (19) suggested that the VMH was the ultimate target for leptin but that there was an intermediate step in the CNS process. These authors found that Tph2 expression was increased in ob/ob mice and leptin ICV infusion decreased Tph2 expression. Leptin strongly suppressed the activity of serotonergic neurons in wild-type mouse, but not in mice lacking the leptin receptor in serotonergic neurons. Moreover, these mice showed a high bone mass phenotype. These lines of evidence together with the studies showing a pro-osteogenic activity of serotonin in the CNS through its receptor in VMH nuclei suggest that effect of leptin on skeletal accrual is mediated through its receptor expressed in serotonergic neurons residing in the brain stem. It appears from these studies that CNS serotonin mediates leptin’s effect by binding to its receptors in VMH neurons, completing a feedback loop for afferent signaling through the brain stem.
Summary: More Questions than Answers
The likelihood that serotonin regulates bone mass through both central and peripheral mechanisms pose as many questions as answers (Fig. 1). It also provides tremendous research opportunities. Although there are convincing data in conditional mouse models, there are still inconsistencies between animal models and clinical observations. For example, the osteoporotic phenotype has not been reported in patients with serotonin producing cartinoids despite high concentrations of free and platelet serotonin (45,46). Future studies will be needed to fully define this complex regulatory system in humans; for example: how is the balance maintained between the pro-osteogenic effects of central control vs. the negative effects of circulating gut-derived serotonin? Is there a threshold effect so that at a critical circulating serotonin level an increase in bone formation occurs? If so, why is this necessary for skeletal maintenance? And furthermore, what do circulating serotonin concentrations really mean, particularly because 90% or more is taken up by platelets. If leptin’s central effects are mediated through the serotoninergic system originating in the brain stem, how close is this connection to the VMH centers for appetite and energy metabolism? How does the suprachiastmatic nucleus, which modulates light-dark signals from the retina and energy intake, relate to serotonin production and modulation of bone mass? Can we develop anabolic skeletal agents that selectively block serotonin production in the periphery or enhance it in the brain to stimulate bone formation? How closely do the mouse models of conditional deletions in the serotonergic regulatory system recapitulate human metabolism and skeletal turnover? These and other questions provide fertile ground for determining whether there is a skeleton in serotonin’s closet and, if so, how this system can be modulated therapeutically to treat osteoporosis.
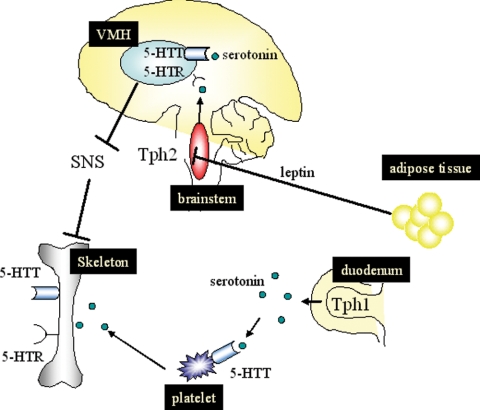
Figure 1.
Schematic model of serotonin action on skeleton. Adipose tissue-derived adipokine, leptin, suppresses Tph2 expression in the brain stem, resulting in the decreased amount of serotonin in the brain. Brain-derived serotonin, catalyzed by Tph2, binds to serotonin receptor (5-HTR) in the ventral medial hypothalamic (VMH), inactivates tone from the sympathetic nervous system (SNS), and enhances skeletal acquisition. Thus, leptin induces bone loss by suppressing serotonin signaling in the VMH. Gut-derived serotonin, catalyzed by Tph1, is up-taken by platelet through 5-HTT and has been shown to suppress skeletal accrual. Osteoblasts and osteoclasts express 5-HTR and serotonin transporter (5-HTT), and serotonin network is likely to be operative at the skeletal environment. Expression of 5-HTT in the brain is developmental stage-dependent,, and the hypothalamic expression of 5-HTT needs to be determined. Tph, Tryptophan hydroxylase; 5-HTR, 5-HT(5-hydroxytryptamine) receptor; 5-HTT, 5-HT transporter.
Footnotes
This work was supported by U.S. National Institutes of Health Grant NIH 1R24DK084970-01 (to C.J.R.).
Disclosure Summary: The authors have nothing to declare.
First Published Online July 21, 2010
Abbreviations: 5-HT, 5-Hydroxytryptamine; 5-HTP, 5-hydroxy-l-tryptophan; 5-HTT, 5-hydroxytrophan transporter; CNS, central nervous system; SGA, second generation antipsychotic agent; SSRI, elective serotonin reuptake inhibitor; Tph, tryptophan hydroxylase.
References
- Garfield AS, Heisler LK 2009 Pharmacological targeting of the serotonergic system for the treatment of obesity. J Physiol 587:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L 2003 The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci 4:1002–1012 [DOI] [PubMed] [Google Scholar]
- Berger M, Gray JA, Roth BL 2009 The expanded biology of serotonin. Annu Rev Med 60:355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen CJ 2008 Bone remodeling, energy metabolism, and the molecular clock. Cell Metab 7:7–10 [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Klibanski A 2009 Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med 122:409–414 [DOI] [PubMed] [Google Scholar]
- Friedman JM 2009 Leptin at 14 y of age: an ongoing story. Am J Clin Nutr 89:973S–979S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G 2000 Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207 [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G 2005 Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434:514–520 [DOI] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G 2002 Leptin regulates bone formation via the sympathetic nervous system. Cell 111:305–317 [DOI] [PubMed] [Google Scholar]
- Fuller RW, Wong DT 1990 Serotonin uptake and serotonin uptake inhibition. Ann NY Acad Sci 60:68–78; discussion 79–80 [DOI] [PubMed] [Google Scholar]
- Furness JB, Costa M 1982 Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: their projections in the guinea-pig small intestine. Neuroscience 7:341–349 [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hörtnagl H, Voits M, Fink H, Bader M 2003 Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299:76 [DOI] [PubMed] [Google Scholar]
- Jonnakuty C, Gragnoli C 2008 What do we know about serotonin? J Cell Physiol 217:301–306 [DOI] [PubMed] [Google Scholar]
- Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG 2004 Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science 305:217 [DOI] [PubMed] [Google Scholar]
- Westbroek I, van der Plas A, de Rooij KE, Klein-Nulend J, Nijweide PJ 2001 Expression of serotonin receptors in bone. J Biol Chem 276:28961–28968 [DOI] [PubMed] [Google Scholar]
- Bliziotes M, Eshleman A, Burt-Pichat B, Zhang XW, Hashimoto J, Wiren K, Chenu C 2006 Serotonin transporter and receptor expression in osteocytic MLO-Y4 cells. Bone 39:1313–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliziotes MM, Eshleman AJ, Zhang XW, Wiren KM 2001 Neurotransmitter action in osteoblasts: expression of a functional system for serotonin receptor activation and reuptake. Bone 29:477–486 [DOI] [PubMed] [Google Scholar]
- Battaglino R, Fu J, Späte U, Ersoy U, Joe M, Sedaghat L, Stashenko P 2004 Serotonin regulates osteoclast differentiation through its transporter. J Bone Miner Res 19:1420–1431 [DOI] [PubMed] [Google Scholar]
- Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G 2009 A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 138:976–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden SJ, Robling AG, Sanders MS, Bliziotes MM, Turner CH 2005 Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology 146:685–693 [DOI] [PubMed] [Google Scholar]
- Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schütz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G 2008 Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135:825–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modder UI, Achenbach SJ, Amin S, Riggs BL, Melton LJ, Khosla S 2010 Relation of serum serotonin levels to bone density and structural parameters in women. J Bone Miner Res 25:414–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki T, Nagao N, Nakahara T 2008 Neuropharmacology of second-generation antipsychotic drugs: a validity of the serotonin-dopamine hypothesis. Prog Brain Res 172:199–212 [DOI] [PubMed] [Google Scholar]
- Gründer G, Hippius H, Carlsson A 2009 The ‘atypicality’ of antipsychotics: a concept re-examined and re-defined. Nat Rev Drug Discov 8:197–202 [DOI] [PubMed] [Google Scholar]
- Halbreich U, Palter S 1996 Accelerated osteoporosis in psychiatric patients: possible pathophysiological processes. Schizophr Bull 22:447–454 [DOI] [PubMed] [Google Scholar]
- Howard L, Kirkwood G, Leese M 2007 Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry 190:129–134 [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, Lee JC 1989 Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J Pharmacol Exp Ther 251:238–246 [PubMed] [Google Scholar]
- Bostwick JR, Guthrie SK, Ellingrod VL 2009 Antipsychotic-induced hyperprolactinemia. Pharmacotherapy 29:64–73 [DOI] [PubMed] [Google Scholar]
- Dick-de-Paula I, Welch G, Kawai M, Maloney A, Bornstein S, Sikich L, Rosen CJ Risperidone, a second generation antipsychotic alters fat distribution and has a deleterious effect on bone mass in young C57BL6 male mice. Program of the 92nd Annual Meeting of The Endocrine Society, San Diego, CA, 2010 [Google Scholar]
- Vestergaard P, Rejnmark L, Mosekilde L 2008 Selective serotonin reuptake inhibitors and other antidepressants and risk of fracture. Calcif Tissue Int 82:92–101 [DOI] [PubMed] [Google Scholar]
- Diem SJ, Blackwell TL, Stone KL, Yaffe K, Haney EM, Bliziotes MM, Ensrud KE 2007 Use of antidepressants and rates of hip bone loss in older women: the study of osteoporotic fractures. Arch Intern Med 167:1240–1245 [DOI] [PubMed] [Google Scholar]
- Liu B, Anderson G, Mittmann N, To T, Axcell T, Shear N 1998 Use of selective serotonin-reuptake inhibitors or tricyclic antidepressants and risk of hip fractures in elderly people. Lancet 351:1303–1307 [DOI] [PubMed] [Google Scholar]
- Lewis CE, Ewing SK, Taylor BC, Shikany JM, Fink HA, Ensrud KE, Barrett-Connor E, Cummings SR, Orwoll E 2007 Predictors of non-spine fracture in elderly men: the MrOS study. J Bone Miner Res 22:211–219 [DOI] [PubMed] [Google Scholar]
- Bolton JM, Metge C, Lix L, Prior H, Sareen J, Leslie WD 2008 Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol 28:384–391 [DOI] [PubMed] [Google Scholar]
- Richards JB, Papaioannou A, Adachi JD, Joseph L, Whitson HE, Prior JC, Goltzman D 2007 Effect of selective serotonin reuptake inhibitors on the risk of fracture. Arch Intern Med 167:188–194 [DOI] [PubMed] [Google Scholar]
- Calarge CA, Zimmerman B, Xie D, Kuperman S, Schlechte JA 2010 A cross sectional evaluation of the effect of risperidone and selective serotonin reuptake inhibitors on bone mineral density in boys. J Clin Psychiatry 71:338–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziere G, Dieleman JP, van der Cammen TJ, Hofman A, Pols HA, Stricker BH 2008 Selective serotonin reuptake inhibiting antidepressants are associated with an increased risk of nonvertebral fractures. J Clin Psychopharmacol 28:411–417 [DOI] [PubMed] [Google Scholar]
- Warden SJ, Hassett SM, Bond JL, Rydberg J, Grogg JD, Hilles EL, Bogenschutz ED, Smith HD, Fuchs RK, Bliziotes MM, Turner CH 2010 Psychotropic drugs have contrasting skeletal effects that are independent of their effects on physical activity levels. Bone 46:985–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglino R, Vokes M, Schulze-Späte U, Sharma A, Graves D, Kohler T, Müller R, Yoganathan S, Stashenko P 2007 Fluoxetine treatment increases trabecular bone formation in mice. J Cell Biochem 100:1387–1394 [DOI] [PubMed] [Google Scholar]
- Maurer-Spurej E, Pittendreigh C, Solomons K 2004 The influence of selective serotonin reuptake inhibitors on human platelet serotonin. Thromb Haemost 91:119–128 [DOI] [PubMed] [Google Scholar]
- Homberg JR, Schubert D, Gaspar P 2010 New perspectives on the neurodevelopmental effects of SSRIs. Trends Pharmacol Sci 31:60–65 [DOI] [PubMed] [Google Scholar]
- Narboux-Nême N, Pavone LM, Avallone L, Zhuang X, Gaspar P 2008 Serotonin transporter transgenic (SERTcre) mouse line reveals developmental targets of serotonin specific reuptake inhibitors (SSRIs). Neuropharmacology 55:994–1005 [DOI] [PubMed] [Google Scholar]
- Kawai M, Devlin MJ, Rosen CJ 2009 Fat targets for skeletal health. Nat Rev Rheumatol 5:365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua Jr S, Elmquist JK, Lowell BB 2006 Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49:191–203 [DOI] [PubMed] [Google Scholar]
- Kema IP, de Vries EG, Schellings AM, Postmus PE, Muskiet FA 1992 Improved diagnosis of carcinoid tumors by measurement of platelet serotonin. Clin Chem 38:534–540 [PubMed] [Google Scholar]
- Meijer WG, van der Veer E, Jager PL, van der Jagt EJ, Piers BA, Kema IP, de Vries EG, Willemse PH 2003 Bone metastases in carcinoid tumors: clinical features, imaging characteristics, and markers of bone metabolism. J Nucl Med 44:184–191 [PubMed] [Google Scholar]