Abstract
Evidence suggests that release of oxytocin in the nucleus tractus solitarius (NTS) of the hindbrain from descending projections that originate in the paraventricular nucleus can inhibit food intake by amplifying the satiety response to cholecystokinin (CCK). To further evaluate this mechanism in rats, we used a novel cytotoxin, saporin conjugated to oxytocin (OXY-SAP), a compound designed to destroy cells that express oxytocin receptors (OXYr). OXY-SAP was injected directly into the NTS to lesion neurons that express OXYr and that are implicated in potentiating CCK’s satiety effects. The control consisted of injection of saporin conjugated to a nonsense peptide. We found that OXY-SAP was cytotoxic to human uterine smooth muscle cells in vitro, demonstrating that OXY-SAP can lesion cells that express OXYr. Using laser capture microdissection and real-time quantitative PCR, we demonstrated that OXYr mRNA levels were reduced in the NTS after OXY-SAP administration. Moreover, we found that OXY-SAP attenuated the efficacy of CCK-8 to reduce food intake and blocked the actions of an OXYr antagonist to stimulate food intake. The findings suggest that OXY-SAP is an effective neurotoxin for in vivo elimination of cells that express OXYr and is potentially useful for studies to analyze central nervous system mechanisms that involve the action of oxytocin on food intake and other physiological processes.
Stereotaxic injection of oxytocin-saporin into the hindbrain lowers oxytocin receptor mRNA levels in the nucleus of the solitary tract and attenuates the satiety action of cholecystokinin.
Several lines of indirect evidence support a central nervous system (CNS) anorexigenic mechanism that involves oxytocin release in the hindbrain from neuronal projections that descend from parvocellular neurons in the paraventricular nucleus. The evidence suggests that release of oxytocin in the nucleus tractus solitarius (NTS) of the hindbrain can inhibit food intake by amplifying the satiety response to cholecystokinin (CCK), resulting in smaller meals (1,2). For example, oxytocin neuronal projections from the paraventricular nucleus are anatomically positioned to interact with NTS neurons that respond to CCK (1), and intracerebroventricular (ICV) administration of oxytocin induces Fos in the NTS (3). Furthermore, oxytocin receptors (OXYr) are expressed by NTS cells (4), and ICV administration of an OXYr antagonist attenuates the satiety effect of CCK-8 and the ability of ICV leptin to enhance the hindbrain neuronal response to CCK-8 (5), implying a role for oxytocin in CNS neurocircuits linking the feeding actions of leptin and CCK.
Whereas these and other findings are consistent with a role for NTS oxytocin release in the homeostatic mechanisms that regulate food intake and satiation, this hypothesis would be strengthened if a reduction of oxytocin-receptive cells in the NTS were shown to attenuate the feeding effects of peripherally administered CCK-8. To investigate this possibility, we used a novel cytotoxin, saporin conjugated to oxytocin (OXY-SAP), that selectively targets and destroys cells displaying OXYr. Saporin inactivates ribosomes, resulting in cessation of protein synthesis and cell death, when it enters cells (6). It has been shown to be cytotoxic to neurons and other cells when coupled with ligands that are internalized (7,8,9).
Here we used the strategy of injecting OXY-SAP into the NTS to lesion neurons that express OXYr and are implicated in CCK’s satiety effects and measured the satiety response to ip administration of CCK-8 relative to controls receiving an equimolar amount of a control mock peptide-saporin conjugate (CON-SAP). We proposed that compared with CON-SAP, OXY-SAP treatment would result in reduced expression of OXYr mRNA in the NTS and attenuation of the satiety effects of CCK.
The novel aspect of this report is the validation of the usefulness of OXY-SAP to lesion oxytocin-receptive cells. We also show that OXY-SAP is cytotoxic to human uterine myometrial cells that express oxytocin receptors in vitro and that OXYr mRNA levels are reduced in the NTS after OXY-SAP administration. In behavioral studies, we show that OXY-SAP attenuates the efficacy of CCK-8 to reduce food intake and blocks the actions of an OXYr antagonist to stimulate food intake. The findings suggest that OXY-SAP is a novel neurotoxin that targets cells expressing OXYr and is potentially useful for studies to analyze the CNS effect of oxytocin on food intake and other mechanisms.
Materials and Methods
Cytotoxic reagent
OXY-SAP and the control CON-SAP were provided by Advanced Targeting Systems (San Diego, CA). OXY-SAP consists of oxytocin conjugated to saporin, a protein with N-glycosidase activity derived from the seeds of Saponaria officinalis. The CON-SAP consisted of saporin (6) conjugated to a scrambled 11-amino acid peptide that is derived from α-MSH and has no known cellular targets or biological activity.
Cell culture study
A control for the cytotoxicity of OXY-SAP was performed by incubating the reagent with uterine myometrial cells. These were cultured from a line of human uterine myometrial cells that are known to express OXYr (10). The cells were grown at 37 C in humidified 6% CO2 in a standard culture media (10) on eight-chamber Lab-Tek slides (Nalge-Nunc International, Rochester, NY). After initial plating at a density of 1 × 104 cells/chamber, the media were changed every 2–3 d, and the cells reached confluence after 5–7 d. Doses of OXY-SAP or CON-SAP (both at final concentrations of 5 and 50 nm) were based on previous studies (8) and the EC50 of oxytocin (11). Media without OXY-SAP or CON-SAP were additional controls. After 72 h incubation, cells were fixed in 4% paraformaldehyde in 10 mm PBS, 0.05% Tween 20, 0.3% and Triton X-100 for 15 min and counterstained with Hoechst 33258 (Sigma-Aldrich, St. Louis, MO) to visualize nuclei for counting. Cell counts were done using a Zeiss Axioplan fluorescence microscope (New York, NY) and a ×20 objective lens. Cells were counted in four to six chambers per treatment and data pooled for statistical analysis.
Animals
The Animal Research Committee of the Seattle Veterans Affairs Puget Sound Medical Center approved all experimental protocols. Adult male Wistar rats (322–403 g) were used in all studies (Charles River Laboratories, Wilmington, MA). Animals used in feeding studies were housed individually in Plexiglas cages at 22 ± 2 C under a 12-h light, 12-h dark cycle. Animals were adapted to lights off at 1500 h and were fed a standard rat chow diet (PMI LabDiet 5001; Animal Specialties, Hubbard, OR). Deionized water was freely available.
Laser capture microdissection (LCM)
The Arcturus AutoPix LCM system (Molecular Devices, Sunnyvale, CA) was used to collect bilateral samples of NTS tissue. Slide-mounted cryostat sections (10 μm) of hindbrain at the level of the area postrema (AP) and rostral to the AP were selected at 200-μm intervals (n = 8 slides/brain), dehydrated in ethanol and xylene, and then air dried (12). Hindbrain sections were imaged in the AutoPix and the medial region of the NTS (mNTS) was outlined on a monitor using the mouse-controlled cursor. The AutoPix then transferred the circumscribed NTS tissue to a plastic substrate for analysis. All microdissected NTS samples from a single brain were pooled for RNA extraction and PCR analysis. Samples of the AP and dorsal motor nucleus of the vagus (DMV), which adjoin the NTS, were sampled separately by LCM as controls for the localization of the lesion.
Real-time PCR
RNA extracted from the pooled samples of NTS, AP, and DMV was analyzed using the Arcturus Picopure RNA isolation kit (Molecular Devices) followed by reverse transcription into cDNA using a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA). Quantitative analysis for relative levels of oxytocin mRNA in the RNA extracts was measured in triplicate by real-time PCR on a Prism 7000 sequence detection system (Applied Biosystems) and normalized to the cycle threshold value of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA in each sample. Primers and probes were designed using Primer Express (version 2.0.0) from TAQMAN (Applied Biosystems). The primer sequences used for RT-PCR were: rat GAPDH forward primer, 5′-GCCAGCCTCGTCTCATAGACA-3′; rat GAPDH reverse primer, 5′-GTCCGATACGGCCAAATCC-3′; and rat GAPDH probe primer, VIC-5′-ATGGTGAAGGTCGGTGTG-3′. The probe and primers for rat OXYr (catalog no. Rn00563503_m1), TNF-α (catalog no. Rn99999017_m1) and IL-1β (catalog no. Rn00580432_m1) were acquired from Applied Biosystems.
Administration of OXY-SAP or CON-SAP
OXY-SAP was injected directly into the NTS region and allowed to lesion oxytocin-sensitive cells before administration of an OXYr antagonist to test the hypothesis that destroying NTS neurons that express OXYr would attenuate the effects of the OXYr antagonist on food intake. For the injections, guide cannulas were surgically installed into the third ventricle (3V) and temporarily installed into the NTS to enable administration of the OXY-SAP or CON-SAP after standard procedures (5). Angiotensin II (Sigma-Aldrich) was injected at 20 ng/μl to assess 3V cannula patency 48 h before the start of the feeding experiments. All animals used in the subsequent analysis of the data drank at least 5 ml of water during these tests. In the tests that used saporin treatment, animals received bilateral injections (0.5 μl injection volume per side) directed toward the mNTS via a 33-gauge injector over 30 sec of either OXY-SAP (0.016, 0.034, 0.06 μg/μl) or equimolar doses of CON-SAP dissolved in PBS (Sigma-Aldrich). These doses were based on effective doses of targeted saporin toxins reported in other studies (7,9).
The stereotaxic coordinates for the mNTS were −5.5 mm posterior to interaural line, +0.7 mm lateral to cerebral vein, and −7.1 mm ventral to surface of skull (13). Animals were given a highly palatable diet to aid in recovery from surgery and used in the experiments once body weight stabilized to presurgery values, which was approximately 3 wk after surgery, consistent with the onset of experiments conducted after administration of other saporin neurotoxins (7,14). At this time, animals were food deprived for 6 h before the onset of the dark cycle, and 30–45 min before the start of the dark cycle, each received a 3V injection of the OXYr antagonist [d(CH(2)51,Try (Me)2, Orn8)]-oxytocin (Bachem, Belmont, CA) (10 μg per 2 μl). Food intake was measured at 0.5, 1, 2, 3, and 4 h starting at the onset of the dark cycle as previously described (5). Cumulative food intake at 24 h was recorded in a subset of these animals. At the end of the experiment, each brain was immediately frozen for measuring OXYr mRNA levels and confirming histological cannula placements. A subset of animals treated with either OXY-SAP or CON-SAP was perfused with 4% paraformaldehyde for histological confirmation of cannula placements.
CCK-8 injections
To determine the effect of OXY-SAP on the hindbrain response to satiety signaling by CCK, rats were adapted to a 6-h fast immediately before the start of the dark cycle when they were given access to standard laboratory rat chow. CCK-8 (Bachem) was administered by ip injection immediately before the start of the dark cycle, when the animals normally begin eating and when CCK-8 has a potent effect on reducing food intake. Each animal received either CCK-8 or the saline vehicle in randomized fashion at 48-h intervals. CCK-8 was administered at two different doses (0.96 and 2.75 nmol/kg) in separate experiments using the same animals. Food intake was measured at 30 min and 24 h, starting at the onset of the dark cycle as previously described (1).
Statistics
Grouped results are expressed as means ± sem. Comparisons between multiple groups in a within-subject design were made using a repeated-measures ANOVA followed by repeated-measures Tukey’s multiple comparison test as a post hoc test. Comparisons between multiple groups in a between-subject design were made using ANOVA followed by Fisher’s least significant-difference test as a post hoc test. Analyses were performed using the statistical program SYSTAT (Systat Software, Inc., Point Richmond, CA). Differences were considered significant at P < 0.05.
Results
In vitro studies of OXY-SAP cytotoxicity
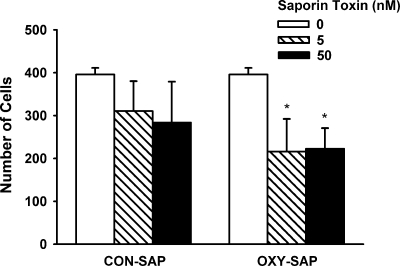
To verify that the OXY-SAP is cytotoxic to cells that express OXYr, we incubated human uterine myometrial cells with 0, 5, and 50 nm OXY-SAP and CON-SAP doses (added to the culture wells) for 72 h and then counted the density of surviving cells by microscopy. There was a significant main effect of OXY-SAP treatment to decrease human uterine myometrial cell numbers (P < 0.05). The data show that that the effect of adding OXY-SAP to the media reduced cell numbers by 45% at 5 nm (P < 0.05) and 44% at 50 nm (P < 0.05) (Fig. 1). Adding CON-SAP to the media had no effect (P > 0.05).
Figure 1.
Cytotoxic effect of OXY-SAP on human uterine myometrial cells after 72 h in culture. Measurements of cell counts were made on four to six culture wells for each treatment condition. Data represent means ± sem. *, P < 0.05 vs. media (no saporin).
In vivo PCR studies of OXY-SAP cytotoxicity
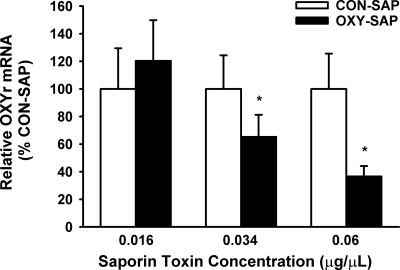
To verify that OXY-SAP is effective for inducing a lesion of NTS cells that express OXYr, relative levels of mRNA for OXYr in the NTS were compared by PCR after direct bilateral injection of either OXY-SAP and CON-SAP into the NTS region. Compared with CON-SAP treatment, the OXY-SAP injections were accompanied by a significant reduction in the relative levels of OXYr mRNA as measured by PCR of the microdissected NTS tissue (Fig. 2). The 0.034- and 0.06-μg/μl doses produced a significant stepwise decrease (as much as 2.5-fold) in levels of OXYr mRNA (P < 0.05). The 0.016-μg/μl mg dose of OXY-SAP was ineffective. Injections of OXY-SAP into the NTS did not change the relative levels of OXYr mRNA (compared with the CON-SAP injections) in the neighboring AP and DMV (P > 0.05) (data not shown). As a control for possible inflammatory effects, we also examined the effects of OXY-SAP and CON-SAP on markers of inflammation, such as TNF-α and IL-1β. The 0.034 and 0.06 μg/μl doses produced a significant increase (as much as 22-fold) in levels of TNF-α (P < 0.05) and IL-1β (P < 0.05) (Supplemental Fig. 1, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org) compared with NTS tissue that was injected with PBS or untreated NTS tissue.
Figure 2.
Effect of NTS injection of OXY-SAP on OXYr mRNA levels in NTS, compared with NTS injected with CON-SAP. Graph shows that OXY-SAP reduced OXYr mRNA expression in NTS (n = 4–9/group). Data represent means ± sem. *, P < 0.05.
Studies with OXYr antagonist
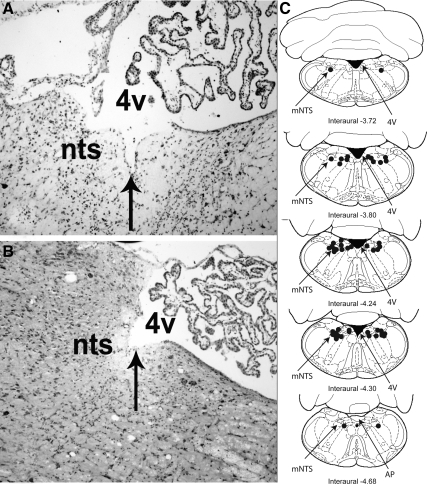
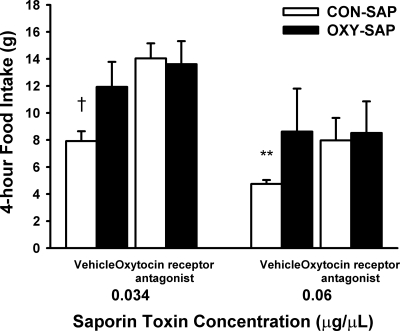
To confirm the utility of the OXY-SAP to lesion NTS oxytocin-receptive cells that are hypothesized to contribute to the regulation of food intake, we examined the effect of the OXYr antagonist, [d(CH(2)51,Try (Me)2, Orn8)]-oxytocin (with saline as a vehicle control) to stimulate food intake in rats that had been treated 3 wk earlier with direct injection of CON-SAP or OXY-SAP in the NTS to destroy NTS cells that express OXYr. Representative histological images of the injection sites and the mapping of their locations are shown in Fig. 3. When the OXYr antagonist was administered into the 3V at the 0.034-μg/μl dose 3 wk after the OXY-SAP treatment, the expected stimulation of food intake that normally follows administration of the OXYr antagonist (5,15,16) was not observed (P > 0.05). In contrast, the OXYr antagonist treatment resulted in elevated food intake at 4 h by 77% (P < 0.01) in the CON-SAP-treated animals (Fig. 4), consistent with blocking the effects of endogenous oxytocin to reduce food intake in response to the OXYr antagonist. Mildly increased food intake was also seen in vehicle-treated animals that received OXY-SAP (0.06 μg/μl) compared with CON-SAP-treated animals observed over a 4-h period (P = 0.053). This observation implies a physiological role for oxytocin-sensing neurons in the NTS to limit spontaneous food intake. Whereas this pattern was also evident after NTS administration of a lower concentration of OXY-SAP, the effect on food intake was not significantly increased beyond that of CON-SAP-treated animals (P = 0.16).
Figure 3.
Histological verification and distribution of injection sites in mNTS. Representative photomicrographs taken of an injection site from an OXY-SAP (A) and a CON-SAP-treated animal (B). The location of the injection site is centered in the mNTS, rostral to the AP. Coronal diagrams of the rat brain based on the atlas of Paxinos and Watson (22) show the anatomical distribution of injection sites for groups of animals with cannulae aimed at the mNTS (C). The solid circles indicate sites of injections in the medial NTS. 4V, Fourth ventricle.
Figure 4.
Effect of 3V administration of the OXYr antagonist [d(CH(2)51,Try (Me)2, Orn8)]-oxytocin on food intake in OXY-SAP and CON-SAP-treated animals. Graph shows that NTS administration of OXY-SAP eliminated the stimulation of food intake in response to 3V administration of the OXYr antagonist (10 μg per 2 μl) (n = 4–10/group). Data represent means ± sem. **, 0.05 less than P < 0.1 vs. vehicle; †, P < 0.01 vs. vehicle.
Studies on CCK-8-induced inhibition of food intake
To determine whether NTS-injected OXY-SAP attenuates the satiety effect of CCK, we examined the effects of bilateral NTS injections (0.5 μl volumes) of three different concentrations of OXY-SAP and CON-SAP on the ability of either a low (0.96 nmol/kg) or high (2.75 nmol/kg) dose of CCK-8 to reduce food intake. The results are summarized in Table 1. CON-SAP at all doses failed to suppress the satiety effect of CCK-8 at 30 min when it was administered at either 0.96 or 2.75 nmol/kg. Similar to CON-SAP treatment, OXY-SAP administered into the NTS at either of its two lower doses did not attenuate the inhibition of food intake produced by either the high or low dose of CCK-8 (P < 0.01). In contrast, OXY-SAP at its highest dose (0.06 μg/μl) was effective in attenuating the satiety effect of CCK-8 administered at its highest concentration (P > 0.05).
Table 1.
Effect of OXY-SAP and CON-SAP on CCK-8-induced inhibition of food intake
| Saporin treatment group and CCK-8 dose | Saporin dose (μg/μl) | Food intake (g)
|
|
|---|---|---|---|
| Vehicle | CCK-8 | ||
| CCK-8 (0.96 nmol/kg) | |||
| OXY-SAP | 0.060 | 4.4 ± 0.8 | 3.8 ± 0.7 |
| CON-SAP | 0.060 | 5.3 ± 0.7 | 2.9 ± 0.8a |
| OXY-SAP | 0.034 | 6.3 ± 0.5 | 4.1 ± 0.3b |
| CON-SAP | 0.034 | 5.3 ± 0.5 | 2.3 ± 0.6b |
| OXY-SAP | 0.016 | 6.3 ± 1.2 | 3.4 ± 0.8c |
| CON-SAP | 0.016 | 6.8 ± 0.7 | 4.3 ± 0.7a |
| CCK-8 (2.75 nmol/kg) | |||
| OXY-SAP | 0.060 | 3.7 ± 0.7 | 3.4 ± 0.9 |
| CON-SAP | 0.060 | 5.1 ± 0.9 | 1.5 ± 0.7b |
| OXY-SAP | 0.034 | 5.5 ± 0.5 | 3.2 ± 0.7a |
| CON-SAP | 0.034 | 5.8 ± 0.8 | 2.0 ± 0.4b |
| OXY-SAP | 0.016 | 6.9 ± 1.0 | 3.2 ± 0.7b |
| CON-SAP | 0.016 | 6.1 ± 0.6 | 2.4 ± 0.5b |
NTS administration of OXY-SAP dose-dependently attenuated the effect of CCK-8 to reduce 30-min food intake compared with CON-SAP-treated animals. Data represent means ± sem (n = 5–10/group).
P < 0.05 vehicle vs. CCK-8.
P < 0.01 vehicle vs. CCK-8.
0.05 less than P < 0.1 vehicle vs. CCK-8.
Discussion
These findings indicate that OXY-SAP has a cytotoxic action on cells that express OXYr and shed light on functional consequences of deleting these cells in the NTS. Using cultured human uterine smooth muscle cells that are known to exhibit oxytocin-specific binding as well as OXYr protein and mRNA (10), we found that OXY-SAP lesioned cells in a situation where the compound has direct access to its receptor targets. Whereas the OXY-SAP significantly reduced the numbers of cultured myometrial cells compared with the CON-SAP control, it did not completely eliminate all of the cultured myometrial cells. It was not expected to do so, however, because not all of the cells may have expressed OXYr under the culture conditions used, and moreover, the cells were cultured for only 72 h after treatment. Because saporin kills cells by destroying ribosomes and blocking protein synthesis (6), a longer exposure to the toxin in culture or higher doses of the toxin would probably have destroyed more cells. The relevant interpretation from the in vitro study therefore is that OXY-SAP can be cytotoxic to cells that express OXYr, which supports its use as a potential toxin for OXYr in the CNS.
To determine whether OXY-SAP can destroy CNS cells that express OXYr in vivo, we tested the compound in a behavioral paradigm that has been previously shown to require intact oxytocin signaling in the hindbrain. A similar strategy was used to analyze the effects of CNS oxytocin on salt appetite in rats using an oxytocin-ricin A chain conjugate (17). Administration of an OXYr antagonist via 3V or fourth ventricle in rats and mice results in deficiency of CNS oxytocin signaling and increases the food consumption 2–4 h after a brief fast (5,16).
Previous studies that attenuated oxytocin signaling using OXYr antagonists used ventricular routes of administration (2,5,15,16,18,19). Due to the unknown extent of diffusion of an antagonist within the ventricles as well as within brain parenchyma, the neuroanatomical resolution of the blockade of endogenous oxytocin action was somewhat uncertain in these earlier studies. Here we show that ICV administration of an OXYr antagonist at a dose that increases food intake in control rats was ineffective in producing hyperphagia when administered after OXY-SAP treatment directly into the NTS. A plausible interpretation of this result is that NTS cells that express OXYr (and presumably contribute to the satiety effects of oxytocin in the hindbrain) were destroyed by the OXY-SAP, and thus, no OXYr remained for the OXYr antagonist to block. Although we cannot rule out the possibility that some of the results of this study could reflect loss of OXYr cells in areas adjacent to the NTS, these findings identify this hindbrain area as a key mediator of the feeding response induced by pharmacological blockade of OXYr.
As an additional confirmation of the ability of OXY-SAP to lesion NTS neurons that influence feeding, we asked whether OXY-SAP would alter the inhibition of food intake caused by peripheral administration of CCK-8 because previous studies provide indirect evidence that endogenous oxytocin action in the NTS contributes to the satiety response to CCK-8 (1,5). We found that the OXY-SAP lesioning protocol was effective in blocking the satiety action of CCK-8, albeit only at the highest dose of OXY-SAP. Lower doses produced results similar to those of the CON-SAP controls, which were expected to be ineffective. This result was perhaps not unexpected because the distribution of the injected OXY-SAP and its local concentrations in the NTS region were uncontrolled and unknown. The higher dose may in fact be a minimally effective dose for lesioning cells expressing OXYr in the NTS under these experimental conditions. Thus, although the PCR data from the microdissected samples of NTS indicate that OXY-SAP caused a dose-related loss of OXYr mRNA in the NTS, supporting the interpretation that the OXY-SAP destroyed cells that express OXYr, we cannot know the stoichiometry between OXYr mRNA expression and numbers of cells destroyed in this experiment. We can only conclude that the loss of OXYr mRNA in the NTS is consistent with the conclusion that OXY-SAP resulted in lesioning of NTS cells that express OXYr.
Analysis of injection sites indicated that we were successful in targeting the OXY-SAP and CON-SAP compounds to the NTS. Although the injection volumes were only 0.5 μl, which should have limited diffusion of the injectate to the NTS, AP, and DMV regions, the possibility of its spread to other regions cannot be precluded. We also cannot rule out the possibility that local release of proinflammatory cytokines resulting from tissue trauma as a consequence of cannula placements and injections as well as from nonspecific inflammatory actions of saporin, could have influenced the data. Our PCR data indicate that TNF-α and IL-1β genes were activated by increasing concentrations of both CON-SAP and OXY-SAP (but not by injection of PBS or sham injected controls), thereby likely contributing to an apparent inhibition of baseline food intake similar to a previous report (20). This apparent inflammatory reaction does not explain, however, the marked differences in food intake and OXYr mRNA expression between the OXY-SAP and CON-SAP treatments because both produced equivalent increases of mRNA expression, respectively, for TNF-α and IL-1β. Moreover, whereas the mechanism of OXY-SAP cytotoxicity presumably involves binding to the OXYr, we cannot eliminate the possibility that either the CON-SAP or OXY-SAP (or both) could have cytotoxic effects independent of a mechanism mediated by OXYr and thus potentially affect other cells as well.
Although not a primary objective of this study, the findings indicate that oxytocin released in the area of the NTS plays an important role in the regulation of short-term food intake. Moreover, they are consistent with a current hypothesis that oxytocin receptive neurons in the NTS regulate food intake by enhancing the satiety response to CCK-8 (1,2,5). Because oxytocin is implicated in CNS mechanisms that regulate the hindbrain response to satiety signals from the intestinal tract (1,2,5,16,18,19,21), future studies are required to identify the phenotype of NTS neurons that express OXYr and play a role in food intake. The OXY-SAP neurotoxin, which appears to be effective as a cytotoxic agent for destroying neurons that have the molecular substrate for responding to oxytocin, has the potential to be useful for this research.
Supplementary Material
Acknowledgments
We gratefully acknowledge the work of Brendan Thatcher, Matilda Pham, David Caldwell, and Dr. Tami Wolden-Hanson.
Footnotes
This work was supported by resources from the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, including the Department of Veterans Affairs Career Development Program, Merit Review Research Program, and the Career Scientist Program. D.G.B. is the recipient of a Department of Veterans Affairs Senior Research Career Scientist Award at the Veterans Affairs Puget Sound Health Care System. The research was also supported by the biomedical research core programs, particularly the Cellular and Molecular Imagine Core of the National Institutes of Health (NIH) Diabetes Endocrinology Research Center and the NIH Clinical Nutrition Research Unit at the University of Washington. The research in our laboratory has been supported by a Pilot and Feasibility grant from the University of Washington NIH Clinical Nutrition Research Unit, NIH Grants DK17047, P30 DK035816, P30 DK017047, and PO1 DK068384.
Disclosure Summary: B.J.R. is employed by Advanced Targeting Systems. All other authors have nothing to disclose.
First Published Online July 7, 2010
Abbreviations: AP, Area postrema; CCK, cholecystokinin; CNS, central nervous system; CON-SAP, control mock peptide-saporin conjugate; DMV, dorsal motor nucleus of the vagus; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ICV, intracerebroventricular; LCM, laser capture microdissection; mNTS, medial region of the NTS; NTS, nucleus tractus solitarius; OXYr, oxytocin receptor; OXY-SAP, saporin conjugated to oxytocin; 3V, third ventricle.
References
- Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG 2003 Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res 993:30–41 [DOI] [PubMed] [Google Scholar]
- Olson BR, Drutarosky MD, Stricker EM, Verbalis JG 1991 Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake. Endocrinology 129:785–791 [DOI] [PubMed] [Google Scholar]
- Olson BR, Freilino M, Hoffman GE, Stricker EM, Sved AF, Verbalis JG 1993 c-Fos expression in rat brain and brainstem nuclei in response to treatments that alter food intake and gastric motility. Mol Cell Neurosci 4:93–106 [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Pizzolato G, Dreifuss JJ 1989 Localization of oxytocin binding sites in the human brainstem and upper spinal cord: an autoradiographic study. Brain Res 500:223–230 [DOI] [PubMed] [Google Scholar]
- Blevins JE, Schwartz MW, Baskin DG 2004 Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol 287:R87–R96 [DOI] [PubMed] [Google Scholar]
- Thorpe PE, Brown AN, Bremner Jr JA, Foxwell BM, Stirpe F 1985 An immunotoxin composed of monoclonal anti-Thy 1.1 antibody and a ribosome-inactivating protein from Saponaria officinalis: potent antitumor effects in vitro and in vivo. J Natl Cancer Inst 75:151–159 [PubMed] [Google Scholar]
- Bugarith K, Dinh TT, Li AJ, Speth RC, Ritter S 2005 Basomedial hypothalamic injections of neuropeptide Y conjugated to saporin selectively disrupt hypothalamic controls of food intake. Endocrinology 146:1179–1191 [DOI] [PubMed] [Google Scholar]
- Lappi DA, Maher PA, Martineau D, Baird A 1991 The basic fibroblast growth factor-saporin mitotoxin acts through the basic fibroblast growth factor receptor. J Cell Physiol 147:17–26 [DOI] [PubMed] [Google Scholar]
- Madden CJ, Ito S, Rinaman L, Wiley RG, Sved AF 1999 Lesions of the C1 catecholaminergic neurons of the ventrolateral medulla in rats using anti-DβH-saporin. Am J Physiol 277:R1063–R1075 [DOI] [PubMed] [Google Scholar]
- Rauk PN, Friebe-Hoffmann U 2000 Interleukin-1β down-regulates the oxytocin receptor in cultured uterine smooth muscle cells. Am J Reprod Immunol 43:85–91 [DOI] [PubMed] [Google Scholar]
- Monga M, Ku CY, Dodge K, Sanborn BM 1996 Oxytocin-stimulated responses in a pregnant human immortalized myometrial cell line. Biol Reprod 55:427–432 [DOI] [PubMed] [Google Scholar]
- Williams DL, Schwartz MW, Bastian LS, Blevins JE, Baskin DG 2008 Immunocytochemistry and laser capture microdissection for real-time quantitative PCR identify hindbrain neurons activated by interaction between leptin and cholecystokinin. J Histochem Cytochem 56:285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Carrive P, Wang H, Wang P-Y 1999 Chemoarchitectonic atlas of the rat brainstem. Orlando, FL: Academic Press [Google Scholar]
- Rinaman L 2003 Hindbrain noradrenergic lesions attenuate anorexia and alter central cfos expression in rats after gastric viscerosensory stimulation. J Neurosci 23:10084–10092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arletti R, Benelli A, Bertolini A 1989 Influence of oxytocin on feeding behavior in the rat. Peptides 10:89–93 [DOI] [PubMed] [Google Scholar]
- Blouet C, Jo YH, Li X, Schwartz GJ 2009 Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci 29:8302–8311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn RE, Samson WK, Fulton RJ, Stricker EM, Verbalis JG 1993 Central oxytocin inhibition of salt appetite in rats: evidence for differential sensing of plasma sodium and osmolality. Proc Natl Acad Sci USA 90:10380–10384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokrantz CM, Uvnäs-Moberg K, Kaplan JM 1997 Effects of central oxytocin administration on intraoral intake of glucose in deprived and nondeprived rats. Physiol Behav 62:347–352 [DOI] [PubMed] [Google Scholar]
- Rinaman L, Rothe EE 2002 GLP-1 receptor signaling contributes to anorexigenic effect of centrally administered oxytocin in rats. Am J Physiol Regul Integr Comp Physiol 283:R99–R106 [DOI] [PubMed] [Google Scholar]
- Plata-Salamán CR 1996 Anorexia induced by activators of the signal transducer gp130. Neuroreport 7:841–844 [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Sclafani A, Nilaver G 1988 Histochemical identification of a PVN-hindbrain feeding pathway. Physiol Behav 42:529–543 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C 1986 The rat brain in stereotaxic coordinates. Orlando, FL: Academic Press [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.