Abstract
Excess mineralocorticoid signaling is deleterious for cardiovascular functions, as demonstrated by the beneficial effects of mineralocorticoid receptor (MR) antagonism on morbidity and mortality in patients with heart failure. However, the understanding of signaling pathways after MR activation in the heart remains limited. We performed transcriptomic analyses in the heart of double-transgenic mice with conditional, cardiomyocyte-specific, overexpression of the MR (MRcardio mice) or the glucocorticoid receptor (GR; GRcardio mice). Some of the genes induced in MRcardio mice were selected for comparative evaluation (real time PCR) in vivo in the heart of mice and ex vivo in the MR-expressing cardiomyocyte H9C2 cell line after aldosterone or corticosterone treatment. We demonstrate that chronic MR overexpression in the heart results in a limited number of induced (n = 24) and repressed (n = 22) genes compared with their control littermates. These genes are specifically modulated by MR because there is limited overlap (three induced, four repressed) with the genes that are regulated in the heart of GRcardio mice (compared with control mice: 70 induced, 73 repressed). Interestingly, some MR-induced genes that are up-regulated in vivo in mice are also induced by 24-h aldosterone treatment in H9C2 cells, such as plasminogen activator inhibitor 1 and Serpina-3 (α1-antichymotrypsin). The signaling pathways that are affected by long-term activation of MR may be of particular interest to design novel therapeutic targets in cardiac diseases.
This study identifies target genes specifically modulated in the heart by long-term in vivo activation of the mineralocorticoid receptor.
Pathological activation of the mineralocorticoid receptor (MR) is involved in the adverse cardiovascular effects of aldosterone in humans and animal models (1). Deleterious consequences of enhanced mineralocorticoid signaling has been highlighted by clinical trials that showed a major benefit of addition of MR antagonists on top of standard treatment in patients with heart failure (2,3). The MR is a ligand-dependent transcription factor belonging to the superfamily of nuclear receptors that includes steroid receptors and a number of other receptors (4). The MR is always coexpressed with the closely related glucocorticoid receptor (GR) in epithelial cells of the distal nephron and also in cardiomyocytes, neurons, and other nonclassical targets like keratinocytes and adipocytes (5,6). The definition of MR-mediated vs. GR-mediated cellular events is often difficult because these two receptors share common ligands. In particular, MR binds, with similar high affinity (dissociation constant 0.5–1 nm), aldosterone as well as glucocorticoids, which are 100- to 1000-fold more abundant in the plasma than aldosterone. The GR has an equal (lower) affinity for both hormones (dissociation constant 10–30 nm) (5,6). In classical aldosterone target cells such as the renal collecting ducts, permanent MR occupancy by glucocorticoids is prevented because the hormone is metabolized by the enzyme 11β-hydroxysteroid dehydrogenase-2 (HSD2) (7). In cardiomyocytes, HSD2 is expressed at low levels and MR occupancy by glucocorticoids may predominate (5,6,8).
The effects of aldosterone on cardiac cells have been documented experimentally. A direct relationship between plasma aldosterone and cardiac L-type calcium current has been established (9), and up-regulation of such currents by corticosteroids is mediated by the MR, not the GR (10). It has recently been shown that aldosterone regulates intracellular calcium movements and activates the ryanodine receptor (11). To discriminate between MR- and GR-dependent pathophysiological roles in vivo, we produced double-transgenic mice with cardiomyocyte-specific MR (12) or GR (13) overexpression (MRcardio and GRcardio mice). These mice display distinct cardiac phenotypes: MRcardio mice present ventricular arrhythmias, whereas GRcardio mice have atrioventricular conduction defects, and sodium and potassium currents partly differ in cardiomyocytes isolated from these two mouse models. However, the signaling pathways and mechanisms whereby excess mineralocorticoids may lead to cardiac pathology are not yet elucidated. To address these questions, some studies searched for differentially expressed genes in response to aldosterone treatment (14,15,16,17). Among the up-regulated genes that were detected, some are related to extracellular matrix regulation, signaling, and regulation of vascular tone and inflammation, thus susceptible to interfere with cardiovascular functions. Whereas these analyses focused on the early response to the hormone (a few hours at most), much less is known about the genes that may be regulated by aldosterone/MR in the long term in vivo. Identification of genes that are involved in adaptation or compensatory events should enhance our understanding of the pathways that are altered after MR activation in pathological situations.
In this study, we performed a transcriptomic analysis to search for cardiac genes that could be affected by chronic increase in MR expression. We used mice with conditional MR or GR overexpression in cardiomyocytes (12,13). The expression levels of some genes identified in our array analyses together with some of the early aldosterone-induced genes identified previously (16) were evaluated both in the heart of MRcardio and GRcardio mice as well as in a cardiomyocyte cell line treated with aldosterone. Real-time PCR analysis showed that some genes are up-regulated both in the initial phase of aldosterone action in the cell model and in the heart of MRcardio mice.
Materials and Methods
H9C2/MR+ cell culture
H9C2/MR+ cells represent a clonal cell line of cardiomyocytes derived from the embryonic rat ventricle that stably express the MR (16); H9C2/MR+ cells exhibit features of cardiomyocytes as they express the Ca channel ICaL and α-myosin heavy chain (MHC) (Supplemental Fig. 1, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). They were grown in DMEM/F12 medium supplemented with 10% heat-inactivated fetal bovine serum plus an antibiotic mixture (penicillin-streptomycin; Invitrogen, Carlsbad, CA; and 12.5 μg/ml tylosin; Sigma-Aldrich, St. Louis, MO) to approximately 40% of confluence. For experiments, cells were grown in steroid-free medium 2 d before stimulation. Cells were treated with 10−8 mol/liter aldosterone (Sigma), 10−8 mol/liter corticosterone (Sigma), or vehicle for 24 h. To investigate the specificity of action of these steroids, the MR antagonist RU28318 (10−6 mol/liter; Tocris Bioscience, Bristol, UK) or GR antagonist RU38486 (10−6 mol/liter; Sigma) was used.
Cardiac MR or GR conditional mouse models
The previously characterized tetO-human (h) MR and tetO-hGR mouse strains were crossed with the αMHC-tTA transactivator mouse strain to obtain double-transgenic αMHC-tTA/tetO-hMR (MRcardio) (12) or αMHC-tTA/tetO-hGR (GRcardio) (13) mice with conditional cardiomyocyte-specific hMR or hGR expression. Doxycycline (2 mg/ml in drinking water; Sigma) was given to the pregnant mothers until birth of the progeny to avoid hMR expression in the embryos and so prevent the embryonic lethality previously reported in MRcardio mice. Six-week-old mice were used for this study. To switch off the transgene expression in 3-month-old MRcardio mice, doxycycline was administered for 4 wk before the animals were killed. To antagonize the MR, potassium canrenoate (100 mg/kg·d in drinking water; Sigma) was provided to MRcardio mice and their controls (1 month old) for 8 wk. The use of animals was in accordance with the guidelines of the European Community and approved by our Institutional Animal Care and Use Committee.
DNA microarray analysis
Total RNA was isolated from ventricles from 10 mice of the hMR conditional model (five controls and five MRcardio) and 18 mice of the hGR conditional model (nine controls and nine GRcardio) using TRIZOL reagent (Invitrogen). mRNA was isolated using the Oligotex mRNA kit (QIAGEN, Courtaboeuf, France). RNA and mRNA quality was assessed using an Agilent 2100 bioanalyzer (Agilent Technologies France, Massy, France). The mRNA from MRcardio mice was pooled and labeled with Cy5 in three separate reactions; mRNA from their controls was pooled and labeled with Cy3 in three separate reactions. The same procedure was used for GRcardio mice and their controls. Cy3- and Cy5-labeled cDNA was prepared using the CyScribe cDNA postlabeling kit (GE Healthcare Europe, Saclay, France). Using this kit, Cy3 and Cy5 are not incorporated during cDNA synthesis but are chemically coupled to aminoallyl-deoxyuridine 5-triphosphate that has been incorporated into all cDNA samples. This strategy leads to an even incorporation of both Cy3 and Cy5 and minimizes experimental variation caused by uneven incorporation of labels. Labeling reactions were performed separately for each microarray. Three microarray hybridizations were performed for the MR transgenic mice and six for the GR transgenic mice. The hybridization mixture was preincubated with human Cot-I DNA (i.e., the fraction of genomic DNA consisting largely of highly repetitive sequences), yeast tRNA, and polyA RNA and hybridized to microarrays.
Microarrays were prepared in-house using 50-mer oligonucleotide probes (Eurofins MWG Operon, Ebersberg, Germany). These microarrays have been previously validated (18,19,20,21). The probes were spotted onto epoxy-silane-coated glass slides using the Lucidea array spotter (GE Healthcare Europe). The 5419 genes that were represented on the microarrays have been selected for their involvement in cardiovascular and/or skeletal muscle normal and pathological functioning. Selection was based on the following: 1) substrative hybridization experiments (22), 2) genome-wide microarray hybridizations, and 3) literature data (23). The total number of analyzed oligonucleotides was 3459. Each gene probe was spotted in triplicate (for more information, see http://cardioserve.nantes.inserm.fr/ptf-puce/spip.php?article59).
Hybridized arrays were scanned by fluorescence confocal microscopy (Scanarray 4000XL; GSI-Lumonics, Warwickshire, UK). Fluorescence signal measurements were obtained separately for each fluorochrome at a 10-μm/pixel resolution. Hybridization and background signal intensities and quality control parameters were measured using GenePix Pro 5.0 (Molecular Devices, Sunnyvale, CA). A Lowess normalization procedure (24) was performed to correct for technical biases. The procedure was applied channel by channel as described previously (25). For each microarray, Cy3 and Cy5 signal intensities were individually normalized to a prototype defined as the median profile of all Cy3 or Cy5 signal intensities. The data discussed in this publication have been deposited in the National Centre for Biotechnology Information’s Gene Expression Omnibus and are accessible through Gene Expression Omnibus series accession no. GSE20525 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE20525).
Quantitative RT-PCR
Total RNA was extracted from cells (four to 16 wells per condition) and frozen hearts (individual samples from three to eight mice per group from experimental series distinct from those used for microarrays) using TRIZOL reagent (Invitrogen), according to the manufacturer’s protocol, and deoxyribonuclease treated. Reverse transcription was performed with 2 μg of total RNA, random primers (Invitrogen), and Superscript II reverse transcriptase (Invitrogen). Transcript levels were analyzed by real-time PCR in an iCycler iQ apparatus (Bio-Rad Laboratories, Marnes La Coquette, France) with SYBR Green I detection. The reactions were performed in duplicate for each sample using a quantitative PCR master mix plus SYBR with fluorescein (Eurogentec, Angers, France) with 300 nmol/liter of each sense and antisense primer and 15 ng of cDNA in 25 μl total volume.
The thermal cycling parameters were: initial denaturation at 95 C for 10 min, followed by 40 cycles at 95 C for 15 sec and 60 C for 1 min. Relative expression of the mRNA was quantified using the equation described by Pfaffl (26): ratio = (Etarget)Ĉttarget(mean control-sample)/(Eref)Ĉtref(mean control-sample); E represents the efficiency of the PCR, and Ct represents the number of cycles at which the amplified product reach threshold. The mRNA levels were normalized for β-actin mRNA in samples obtained from culture cells or ubiquitin C (ubc) mRNA in mouse heart. Values in control conditions were set as 1 for each gene, and fold changes are provided in the figures. The sequences of the specific primers are detailed in Supplemental Table 1.
Western blot analysis
Heart samples were homogenized in lysis buffer (50 mm Tris, pH 8; 150 mm NaCl; 1% Nonidet P-40; 1% Na deoxycholate; 0.1% sodium dodecyl sulfate; 1 m EDTA; 1 mm Na3VO4; 50 mm NaF; 2 mm phenylmethylsulfonyl fluoride) containing a protease inhibitor cocktail (Roche Diagnostics, Meylan, France); lysates were subjected to SDS-PAGE, and after blockade with BSA, Western blot was performed using a polyclonal anti-Serpina-3 (1:500; AVIVA Systems Biology, San Diego, CA) followed by antirabbit IgG (1:10,000; GE Health Care Europe). Antibody was visualized by using ECL Plus reagents (GE Health Care) and signals were acquired in a Las4000 DarkBox (Fuji Photo Film Europe GMBH, Düsseldorf, Germany). β-Actin (1:500; Abcam, Paris, France) was used as internal control for protein loading.
Statistical analysis
Linear model for microarray data (27) was used to identify genes with statistically significant differential expression (adjusted P < 0.0001) between transgenic and control mice. High-Throughput GoMiner (28) was used to identify functional categories that were overrepresented among the differentially expressed genes compared with all analyzed genes.
Results of quantitative PCR are provided as mean ± sem. The nonparametric Mann-Whitney U test was used to assess statistical differences between two experimental groups. Differences among more than two experimental conditions were tested by the ANOVA one-way test and Newman and Keuls test (StatEl software; AdScience, Paris, France). P < 0.05 was considered significant.
Results
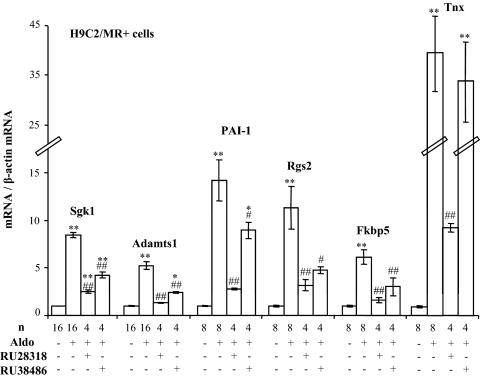
In the cardiomyocyte H9C2 cell line stably transfected with the MR (referred as H9C2/MR+), several early aldosterone-regulated genes have previously been identified after short-term (2 h) aldosterone treatment (16). To document the receptor specificity (MR vs. GR occupancy) of this hormonal response, we selected six gene products identified in this study: 1) the serum- and glucocorticoid-induced kinase Sgk1 that is regulated by corticosteroid hormones in kidney cells; 2) three genes of the extracellular matrix: the metalloprotease Adamts1, the plasminogen activator inhibitor (PAI)-1, and tenascin X (Tnx); 3) regulator of G protein signaling 2 (Rgs2); and 4) the FK506 binding protein (Fkbp5). Twenty-four hours of treatment of H9C2/MR+ cells with aldosterone increased Sgk1, Adamts1, PAI-1, Rgs2, Fkbp5, and Tnx mRNAs (Fig. 1), confirming our earlier observations for Adamts1, Rgs2, and PAI-1 (16). The up-regulation of all these genes was reduced by the MR antagonist RU28318 and the GR antagonist RU38486. Thus, these genes appear to be regulated via the MR and also via the endogenous GR (Fig. 1).
Figure 1.
Effect of steroid receptor antagonists on aldosterone-induced genes in H9C2/MR+ cells. Cells were treated with 10−8 m aldosterone (Aldo) alone for 24 h or in association with MR antagonist RU28318 (10−6 m) or GR antagonist RU38486 (10−6 m). Values of mRNA levels (real time PCR) were normalized for β-actin expression. Values in control (nontreated) cells were set as 1 for each gene, and fold changes are shown on the figure; data are provided as mean ± sem (n = 4–16 wells per condition). *, P < 0.05, **, P < 0.01 treatment vs. control; #, P < 0.05, ##, P < 0.01 treatment vs. Aldo alone (ANOVA analysis, Newman and Keuls test).
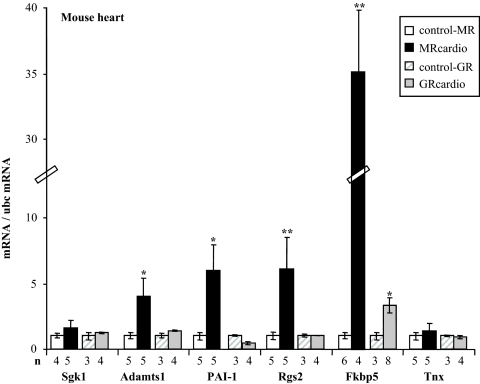
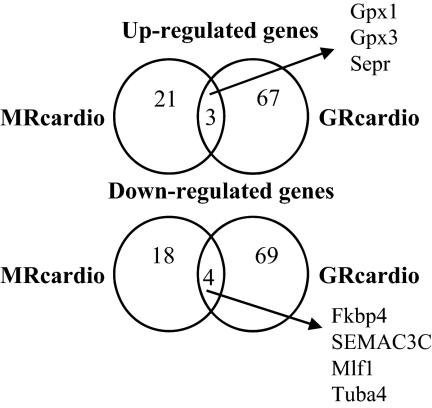
To evaluate the effect of long-term receptor activation in vivo, we took advantage of our mouse models with cardiomyocyte-specific overexpression of the MR (12) or the GR (13). MRcardio and GRcardio mice exhibit a 5-fold increase in MR or GR mRNA expression, respectively, compared with their control littermates (Supplemental Fig. 2). These mice were studied in steady-state basal situation, i.e. in the presence of normal endogenous corticosteroid plasma levels. Interestingly, some of the gene products identified as early aldosterone-induced genes in the cell model were also up-regulated in vivo in the heart of MRcardio mice compared with their control littermates: Adamts1, PAI-1, Rgs2, and Fkbp5 mRNAs were significantly increased in the heart of mice with cardiac overexpression of MR, whereas Sgk1 and Tnx levels did not differ (Fig. 2). In contrast, cardiac GR overexpression did not modify these genes, except for Fkbp5 that was moderately induced in the heart of GRcardio mice (its induction was 10-fold lower than in MRcardio mice). To identify more specifically genes that are regulated in vivo by corticosteroids in the heart, we investigated MR- or GR-dependent changes in the cardiac transcriptome by microarray analyses of the two mouse models with cardiac overexpression of MR or GR compared with their respective controls. A list of MR- and GR-regulated genes in the heart of each mouse model (each compared with their control littermates) is provided in Table 1 (MR regulated genes) and Supplemental Table 2 (GR regulated genes). We found that cardiac MR overexpression resulted in less regulated genes than did GR overexpression. MR overexpression in cardiomyocytes resulted in 24 transcripts that were significantly up-regulated, and 22 that were down-regulated in the heart of MRcardio mice compared with their control littermates. These transcripts correspond mainly to structural proteins, regulatory enzymes, transcription factors, and stress response genes. Cardiac GR overexpression led to the up-regulation of 70 transcripts and down-regulation of 73 transcripts (Supplemental Table 2); however, the changes were smaller than those observed in the MRcardio mice. Interestingly, only few modified cardiac genes were common to the MRcardio and GRcardio mouse models (only three up-regulated genes and four down-regulated genes), indicating that the two receptors control different transcription networks (Fig. 3 and Supplemental Table 3).
Figure 2.
Induced genes in the heart of mice overexpressing the MR (MRcardio) or the GR (GRcardio). Some genes that have been shown to be induced by aldosterone in H9C2/MR+ cells have been measured in the heart from transgenic mice. Values of mRNA levels (real time PCR) were normalized for ubc expression. Values in control mice were set as 1 for each gene, and fold changes are shown on the figure; data are provided as mean ± sem (n = 3–8 mice per condition). *, P < 0.05, **, P < 0.01 transgenic mice compared with their own controls (Mann-Whitney U test).
Table 1.
List of cardiac transcripts that differ between mice overexpressing the MR (MRcardio) and their control littermates
| Symbol | Accession no. | Full name | Fold |
|---|---|---|---|
| Up-regulated genes | |||
| Serpina-3n | NM_009252 | Serine (or cysteine) proteinase inhibitor, clade A, member 3N | 8.57 |
| Tnnt3 | L49018 | Troponin T3, skeletal, fast | 3.43 |
| Nr4a2 | NM_013613 | Nuclear receptor subfamily 4, group A, member 2 | 2.83 |
| MT1H | NM_005951 | Metallothionein 1H | 2.81 |
| Sepr | NM_013759 | Selenoprotein R | 2.77 |
| Gsta4 | NM_010357 | Glutathione S-transferase, α 4 | 2.60 |
| Cnn3 | BC005788 | Calponin 3, acidic | 2.58 |
| GPR72 | AF236081 | G protein-coupled receptor 72 | 2.43 |
| COL1A1 | NM_000088 | Collagen, type I, α1 | 2.38 |
| Apoc1 | NM_007469 | Apolipoprotein C-I | 2.17 |
| COL1AOI | M32790 | α-1 Collagen type 1 (osteogenesis imperfecta allele) | 2.08 |
| Gpx3 | AK002219 | Glutathione peroxidase 3 | 2.03 |
| TACC2 | NM_021314 | Transforming, acidic coiled-coil containing protein 2 | 1.83 |
| Nrip3 | NM_020610 | Nuclear receptor interacting protein 3 | 1.82 |
| BC017540 | RIKEN cDNA 2610102M01 gene | 1.82 | |
| Fn1 | XM_129845 | Fibronectin 1 | 1.80 |
| H2-Ab1 | M13539 | Histocompatibility 2, class II antigen A, β 1 | 1.80 |
| Des | AJ250633 | Desmin | 1.77 |
| Hmox2 | NM_010443 | Hemeoxygenase (decycling) 2 | 1.74 |
| AC167659 | Mus musculus chromosome 7, clone RP23-165I8, complete sequence | 1.68 | |
| S3–12 | NM_020568 | Plasma membrane-associated protein, S3-12 | 1.60 |
| Nfia | D90173 | Nuclear factor I/A | 1.58 |
| Ptprcap | NM_016933 | Protein tyrosine phosphatase, receptor type, C Polypeptide-associated protein | 1.43 |
| Gpx1 | NM_008160 | Glutathione peroxidase 1 | 1.42 |
| Down-regulated genes | |||
| Abcc9 | NM_021041 | ATP-binding cassette, subfamily C (CFTR/MRP), member 9 | 0.40 |
| Mlf1 | NM_010801 | Myeloid leukemia factor 1 | 0.41 |
| FLJ10948 | AK001810 | Hypothetical protein FLJ10948 | 0.42 |
| Reg3g | NM_011260 | Regenerating islet-derived 3γ | 0.43 |
| AK018837 | RIKEN cDNA 1700016D08 gene | 0.45 | |
| CYFIP2 | AF160973 | Cytoplasmic FMR1 interacting protein 2 | 0.55 |
| SEMA3C | NM_006379 | Sema domain, immunoglobulin domain (Ig), short basic domain, secreted, (semaphorin) 3C | 0.55 |
| AK010194 | RIKEN cDNA 2310076E16 gene | 0.56 | |
| Fkbp4 | NM_010219 | FK506 binding protein 4 | 0.57 |
| Hrc | AF132218 | Histidine-rich calcium binding protein | 0.57 |
| Pdhb | NM_024221 | Pyruvate dehydrogenase (lipoamide)-β | 0.60 |
| VLDLR | S73849 | Very low-density lipoprotein receptor | 0.60 |
| Idh3b | NM_130884 | Isocitrate dehydrogenase 3 (NAD+)-β | 0.62 |
| AK004874 | RIKEN cDNA 5730402K07 gene | 0.63 | |
| Ghr | NM_010284 | GH receptor | 0.63 |
| PPAT | U00238 | Phosphoribosyl pyrophosphate amidotransferase | 0.63 |
| Hspa9a | NM_010481 | Heat shock protein, A | 0.68 |
| PPIB | X58990 | Peptidylprolyl isomerase B (cyclophilin B) | 0.68 |
| Tuba4 | NM_009447 | Tubulin-α4 | 0.68 |
| VEGFA | AF022375 | Vascular endothelial growth factor | 0.70 |
| Gucy1a3 | NM_021896 | Guanylate cyclase 1, soluble, α3 | 0.71 |
| Rrad | NM_019662 | Ras-related associated with diabetes | 0.71 |
Figure 3.
Comparison of cardiac transcriptomes in the heart of mice overexpressing the MR (MRcardio) or the GR (GRcardio). For each mouse model (MRcardio or GRcardio), the circles refer to genes that are up- or down-regulated in the heart of transgenic mice compared with their own control littermates. The lists of differentially expressed genes in each mouse model are provided in Table 1 and Supplemental Tables 2 and 3. See tables for the abbreviations.
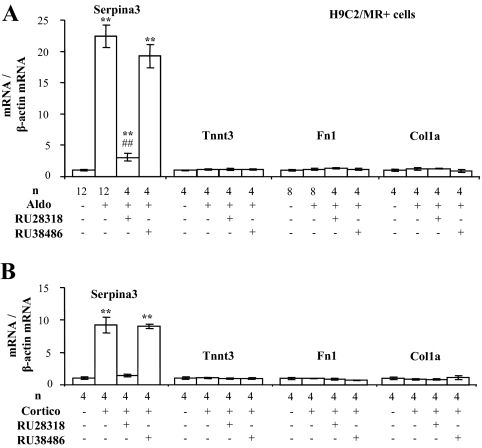
Real-time PCR validation was performed on selected MR-specific regulated genes that are involved in extracellular matrix remodeling and that may interfere with the balance between pro- and antifibrotic processes occurring during the development of cardiac diseases. Analyses were performed on hearts originating from experimental series distinct from those used for microarray analyses (Fig. 4) and on H9C2/MR+ cells treated with aldosterone or corticosterone (Fig. 5). In the heart of MRcardio mice, the serine-protease inhibitor Serpina-3 (or α1-antichymotrypsin), the fast skeletal muscle troponin T3 (Tnnt3), and two extracellular matrix-related genes fibronectin 1 (Fn1) and collagen 1a (Col1a) were increased, whereas they were not modified in the GRcardio model (Fig. 4). Specific down-regulation of genes in MRcardio mice, but not in GRcardio mice, was confirmed for pyruvate dehydrogenase, isocitrate dehydrogenase, vascular endothelial growth factor, and myeloid leukemia factor 1 (Supplemental Fig. 3).
Figure 4.
Validation of differentially expressed genes identified in microarray analysis in the heart of mice with cardiac overexpression of MR. Values of mRNA levels (real time PCR) were normalized for ubc expression. Values in control mice were set as 1 for each gene, and fold changes are shown on the figure; data are provided as mean ± sem (n = 3–8 mice per condition). *, P < 0.05, **, P < 0.01 transgenic mice compared with their own controls (Mann-Whitney U test).
Figure 5.
Differentially expressed genes identified in the heart of mice with cardiac overexpression of MR are also expressed in H9C2/MR+ cells. Cells were treated with 10−8 m aldosterone (Aldo) (A) or 10−8 m corticosterone (Cortico) (B) alone for 24 h or in association with MR antagonist RU28318 (10−6 m) or GR antagonist RU38486 (10−6 m). Values of mRNA levels (real time PCR) were normalized for β-actin expression. Values in control (nontreated) cells were set as 1 for each gene, and fold changes are shown on the figure; data are provided as mean ± sem (n = 4–12 wells per condition). *, P < 0.05, **, P < 0.01 treatment vs. control; #, P < 0.05, ##, P < 0.01 treatment vs. Aldo or Cortico alone (ANOVA analysis, Newman and Keuls test).
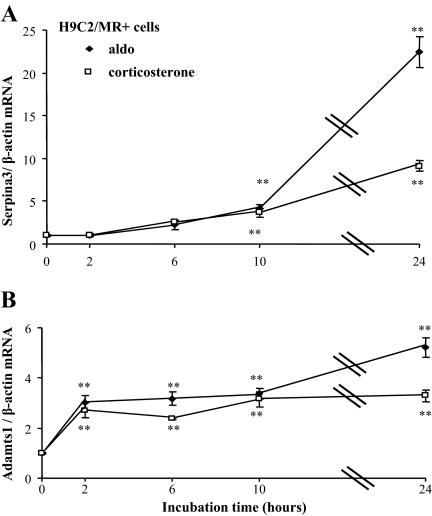
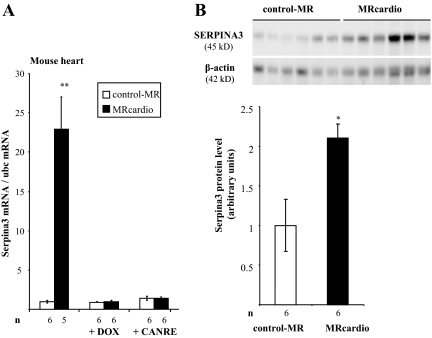
In H9C2/MR+ cells, the expression of Serpina-3 was enhanced by treatment with aldosterone, whereas Tnnt3, Fn1, and Col1a were unaffected (Fig. 5). Serpina-3 is a mineralocorticoid-specific regulated transcript because its induction by aldosterone is reduced in the presence of the specific MR antagonist RU28318 but not in the presence of the GR antagonist RU38486. Corticosterone had comparable effects, indicating that Serpina-3 may be regulated by the MR occupied by aldosterone as well as by a glucocorticoid hormone. In this cell model, Serpina-3 up-regulation by aldosterone occurred after 10 h exposure to aldosterone, i.e. not in the initial phase of hormonal response (late response gene); Adamts1 expression was enhanced much earlier (after 2 h of hormone addition), suggesting that it may be a primary MR target (Fig. 6). Corticosterone also increased Serpina-3 and Adamts1 with the same time course (Fig. 6). Thus, Serpina-3 mRNA is induced 10–24 h after MR activation by aldosterone in H9C2/MR+ cells as well as in vivo after chronic MR overexpression in cardiomyocytes. In MRcardio mice, both doxycycline treatment, which blunts MR overexpression, and canrenoate treatment, which blocks MR activity, prevented the rise in cardiac Serpina-3 expression (Fig. 7A). The up-regulation of Serpina-3 was also observed at the protein level in the heart of MRcardio mice (Fig. 7B).
Figure 6.
Time course of aldosterone and corticosterone effects on the expression of Serpina-3 and Adamts1 in H9C2/MR+ cells. Cells were incubated with 10−8 m aldosterone (Aldo; closed symbols) or corticosterone (open symbols); Serpina-3 (A) and Adamts1 (B) mRNA were measured by real-time PCR. Values of mRNA levels were normalized for β-actin expression. Values in control (nontreated) cells were set as 1 for each gene, and fold changes are shown on the figure; data are provided as mean ± sem (n = 4 wells per condition). *, P < 0.05, **, P < 0.01 aldo vs. control (ANOVA analysis, Newman and Keuls test).
Figure 7.
Serpina-3 expression in the heart of control-MR and MRcardio mice. A, Animals were treated by doxycycline (DOX) to switch off the transgene or by potassium canrenoate (CANRE), a MR antagonist. Values of Serpina-3 mRNA levels (real time PCR) were normalized for ubc expression. Values in control mice were set as 1, and fold changes are shown on the figure; data are provided as mean ± sem (n = 5–6 mice per condition). B, Western blot analysis shows increased Serpina-3 expression in the heart of 1.5-month-old MRcardio mice compared with control littermates. β-Actin was used as internal control. The signal was quantified and values are provided as mean ± sem (n = 6 mice per group). *, P < 0.05; **, P < 0.01. MRcardio vs. control-MR mice.
Discussion
This study was designed to identify cardiac genes that are specifically regulated through the MR pathway. In the literature, previous searches for cardiac aldosterone-responsive genes focused on the relatively early effects of the hormone. After in vivo injections of aldosterone to uninephrectomized salt-loaded mice, Turchin et al. (14) showed that a group of genes were transiently down-regulated, whereas no induced genes were identified. After incubation of neonatal mouse cardiomyocytes in primary culture with high doses of aldosterone (occupying both the MR and GR), about 50 up-regulated and 50 down-regulated genes were revealed (15). Modulated genes were more likely GR rather than MR dependent, as shown for the ectoribosyltransferase 3 (15). Early aldosterone-regulated genes have also been investigated using a clonal cardiomyocyte cell line stably expressing MR [H9C2/MR+ cells (16)]; 48 up-regulated and five down-regulated genes have been identified in the presence of physiological concentration of aldosterone (1 nm) for 2 h. Yoshikawa et al. (17) also used neonatal cardiomyocytes (from rat) and identified a large series of glucocorticoid- and aldosterone (100 nm)-regulated genes. These results showed limited or no overlap between studies, and this was also the case when compared with our own transcriptomic approach. This is probably due to the different hormonal conditions used in different cell or animal models, including comparison of relatively short-term responses (few hours hormone treatment) with long-term enhanced MR expression.
We used two strategies to assess MR- vs. GR-specific responses. The use of pharmacological MR/GR agonists/antagonists to analyze gene regulation and specificity in cultured cells is a classical and relatively easy approach, but it is limited by the cross-reactivity of these pharmacological agents with MR and GR. As a matter of fact, aldosterone treatment may result in both MR and GR activation when used at 10 nm; glucocorticoids can also bind to the MR (in the absence of the MR protecting enzyme HSD2). Classical MR antagonists like spironolactone may also block the GR when used at high doses (29). In vivo, the use of cardiomyocyte-specific MR vs. GR overexpression in mice has the major advantage of selectively modifying one receptor in a chosen cell type without affecting the endogenous ligands. Although the nature of the ligand that occupies cardiac MR in vivo cannot be ascertained, it is conceivable that the gene regulation observed in MRcardio mice depend on MR occupied by glucocorticoids because HSD2 is expressed at low level in cardiomyocytes. It should be noted that the level of receptor mRNA overexpression in these two mouse models is moderate. Interestingly, enhanced receptor mRNA levels were reported in some pathological situations: in animal models, and humans (30,31,32,33). It is difficult to compare directly the expression levels of MR or GR in our models with those described in the literature because the protein level or binding activity could not be assessed in our study. In addition, the translation efficiency or stability of the recombinant receptor may be different from that of the endogenous transcripts. The mostly distinct transcriptional regulation induced by MR vs. GR in the same cellular context raise the question of additional mechanisms of specificity such as differential interactions of these two receptors with nuclear coregulators such as protein inhibitor of activated signal transducer and activator of transcription (PIAS1) or eleven-nineteen lysine-rich leukemia (ELL) (6). Indeed, PIAS1 was shown to be a corepressor of MR transactivation, and ELL is a MR-specific (not GR) coactivator. Whether and how such coregulators participate in vivo to the distinct MR/GR effects remain to be established.
Two main findings arose from this study: 1) in the heart, most MR-regulated genes differ from GR-regulated ones, with a limited overlap, suggesting a specific role of MR activation in the heart, and 2) some MR/aldosterone-regulated genes are found in both the early hormonal response in the cell model and after long-term MR overexpression in the mouse model.
Table 2 compares expression patterns that are modified by aldosterone in H9C2/MR+ and the heart of mice after MR overexpression. Transcripts of the early cellular response phase to aldosterone, Sgk1 and Tnx, are not enhanced in MRcardio mouse model, indicating that these genes do not participate in the long-term adaptation to receptor activation. Sgk1 is the best-characterized aldosterone-induced protein in epithelial cells, such as the renal collecting duct, in which its expression is transiently induced by corticosteroids bound to the MR and GR (34,35,36). Tenascins are glycoproteins that modulate cell adhesion to the extracellular matrix (37). Tenascin X is expressed at high levels in the heart and other tissues and has been shown to be down-regulated by glucocorticoids in fibroblasts but not carcinoma cells (38). The in vivo increase of Tnnt3 (a fast skeletal muscle protein) and Fn1/Col1a (encoding for proteins of the extracellular matrix) may correspond to structural adaptation to enhanced cardiac MR signaling. One limitation of using the H9C2/MR+ cell line is that it exhibits features of cardiomyocytes but also skeletal muscle cells. However, in the absence of cardiac cell line with all properties of cardiomyocytes, the H9C2-MR+ model appears as a useful tool.
Table 2.
Comparison of gene expression in H9C2/MR+ cells and in the heart of mice overexpressing the MR (MRcardio)
| H9C2/MR+ cells | MRcardio mice | |
|---|---|---|
| Ex vivo | In vivo | |
| ↑ | Sgk1 | → |
| ↑ | Adamts1 | ↑ |
| ↑ | PAI-1 | ↑ |
| ↑ | Rgs2 | ↑ |
| ↑ | Fkbp5 | ↑ |
| ↑ | Tnx | → |
| ↑ | Serpina-3 | ↑ |
| → | Troponin-T3 | ↑ |
| → | Fibronectin1 | ↑ |
| → | Collagen 1a | ↑ |
Among the mineralocorticoid-specific selected transcripts, five are up-regulated in both the cell line and the MRcardio mouse model. Some of these (Rgs2, Adamts1, and PAI-1) were previously found to be up-regulated by aldosterone in H9C2/MR+ cells (16). Rgs2 is a multiple regulator and is involved in cardiovascular physiopathology (39). Fkbp5 is a protein that can bind immunosuppressive drugs used to improve efficiency of organ transplant. Fkbp5 is a cochaperone of several steroid receptors including the MR and thus may influence its functions (40). Three genes have close connections with the regulation of the extracellular matrix. Adamts1 (41) is a zinc metalloprotease involved in tissue remodeling and angiogenesis as well as a number of other pathological processes. An increase in its expression has been documented in the heart in the acute phase after coronary artery ligation, preceding the changes in matrix metalloproteinases (42). PAI-1, a member of the serpin family, is the major physiological inhibitor of tissue-type plasminogen activator and urokinase-type plasminogen activator, which activate plasminogen to its active form plasmin and lead to fibrinolysis. PAI-1 is involved in numerous pathophysiological processes, including cardiovascular injury, cell-matrix interactions, and aldosterone-related cardiovascular damage (43,44). Serpina-3 (also named α1-antichymotrypsin) is a serine protease inhibitor with multiple functions including maturation of pro-matrix metalloproteinase 9 and wound healing, regulation of apoptosis, infectious diseases, and inflammation (45). Serpina-3, as a member of acute-phase reaction, is also regulated by many stimuli including insulin and growth factors; it belongs to the genes that have been identified previously in different microarrays in the failing myocardium as reviewed by Asakura and Kitakaze (46). Because we show that Serpina-3 expression in the heart is linked to MR activation, it will be interesting to determine whether Serpina-3, which can be secreted in the blood (47), could serve as a biomarker of mineralocorticoid-related cardiac damage. Several genes linked to extracellular matrix remodeling are up-regulated in MRcardio mice. This finding contrasts with the absence of major morphological alterations in these mice (12). However, fibrosis has been evidenced when these mice are challenged with angiotensin II, suggesting that MR activation on top of a pharmacological challenge may precondition the cardiac tissue and favor cardiac remodeling and dysfunction (48).
Of note is that our transcriptomic screening did not evidence any significant change in cardiac ion channel expression between control and MRcardio mice that have been shown to display abnormal Ca transients and ion channel activities (11,12); this may be due to the low level of expression of these channels. Alternatively, channel activities may be linked to posttranscriptional events regulated by MR.
In conclusion, we have identified genes that are up-regulated in the heart after chronic MR overexpression. The signaling pathways that are affected by long-term stimulation of MR may be of particular interest to design novel therapeutic targets in cardiac diseases.
Supplementary Material
Footnotes
This work was supported by the Institut National de la Santé et de la Recherche Médicale, the Agence Nationale pour la Recherche Grants ANR 005-PCOD005 and ANR-09-BLANC0156-01, the Fondation de France, the Centre de Recherche Industrielle et Technique, the European Section of the Aldosterone Council, and National Institutes of Health Grants DK41841 and HL085024. C.L. was recipient of a PhD grant from the Ministere de la Recherche; Y.S.-M. was supported by the Convention Industrielle de Formation per la Recherche PhD program of Agence Nationale de Valorisation de la Recherche and Nucleis Société Anonyme (Lyon, France).
Disclosure Summary: The authors have nothing to declare.
First Published Online June 30, 2010
Abbreviations: Col1a, Collagen 1a; Fkbp5, FK506 binding protein; Fn1, fibronectin 1; GR, glucocorticoid receptor; h, human; HSD2, 11β-hydroxysteroid dehydrogenase-2; MHC, myosin heavy chain; MR, mineralocorticoid receptor; PAI, plasminogen activator inhibitor; Tnnt3, troponin T3; ubc, ubiquitin C.
References
- Young M, Funder JW 2000 Aldosterone and the heart. Trends Endocrinol Metab 11:224–226 [DOI] [PubMed] [Google Scholar]
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J 1999 The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 341:709–717 [DOI] [PubMed] [Google Scholar]
- Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M 2003 Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 348:1309–1321 [DOI] [PubMed] [Google Scholar]
- Fuller PJ, Young MJ 2005 Mechanisms of mineralocorticoid action. Hypertension 46:1227–1235 [DOI] [PubMed] [Google Scholar]
- Farman N, Rafestin-Oblin ME 2001 Multiple aspects of mineralocorticoid selectivity. Am J Physiol Renal Physiol 280:F181–F192 [DOI] [PubMed] [Google Scholar]
- Viengchareun S, Le Menuet D, Martinerie L, Munier M, Pascual-Le Tallec L, Lombès M 2007 The mineralocorticoid receptor: insights into its molecular and (patho)physiological biology. Nucl Recept Signal 5:e012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusvai E, Náray-Fejes-Tóth A 1993 A new isoform of 11β-hydroxysteroid dehydrogenase in aldosterone target cells. J Biol Chem 268:10717–10720 [PubMed] [Google Scholar]
- Lombès M, Alfaidy N, Eugene E, Lessana A, Farman N, Bonvalet JP 1995 Prerequisite for cardiac aldosterone action. Mineralocorticoid receptor and 11β-hydroxysteroid dehydrogenase in the human heart. Circulation 92:175–182 [DOI] [PubMed] [Google Scholar]
- Perrier R, Richard S, Sainte-Marie Y, Rossier BC, Jaisser F, Hummler E, Bénitah JP 2005 A direct relationship between plasma aldosterone and cardiac L-type Ca2+ current in mice. J Physiol 569:153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougier JS, Muller O, Berger S, Centeno G, Schütz G, Firsov D, Abriel H 2008 Mineralocorticoid receptor is essential for corticosteroid-induced up-regulation of L-type calcium currents in cultured neonatal cardiomyocytes. Pflugers Arch 456:407–412 [DOI] [PubMed] [Google Scholar]
- Gómez AM, Rueda A, Sainte-Marie Y, Pereira L, Zissimopoulos S, Zhu X, Schaub R, Perrier E, Perrier R, Latouche C, Richard S, Picot MC, Jaisser F, Lai FA, Valdivia HH, Benitah JP 2009 Mineralocorticoid modulation of cardiac ryanodine receptor activity is associated with downregulation of FK506-binding proteins. Circulation 119:2179–2187 [DOI] [PubMed] [Google Scholar]
- Ouvrard-Pascaud A, Sainte-Marie Y, Bénitah JP, Perrier R, Soukaseum C, Cat AN, Royer A, Le Quang K, Charpentier F, Demolombe S, Mechta-Grigoriou F, Beggah AT, Maison-Blanche P, Oblin ME, Delcayre C, Fishman GI, Farman N, Escoubet B, Jaisser F 2005 Conditional mineralocorticoid receptor expression in the heart leads to life-threatening arrhythmias. Circulation 111:3025–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainte-Marie Y, Nguyen Dinh Cat A, Perrier R, Mangin L, Soukaseum C, Peuchmaur M, Tronche F, Farman N, Escoubet B, Benitah JP, Jaisser F 2007 Conditional glucocorticoid receptor expression in the heart induces atrio-ventricular block. FASEB J 21:3133–3141 [DOI] [PubMed] [Google Scholar]
- Turchin A, Guo CZ, Adler GK, Ricchiuti V, Kohane IS, Williams GH 2006 Effect of acute aldosterone administration on gene expression profile in the heart. Endocrinology 147:3183–3189 [DOI] [PubMed] [Google Scholar]
- Muller O, Pradervand S, Berger S, Centeno G, Milet A, Nicod P, Pedrazzini T, Tronche F, Schütz G, Chien K, Rossier BC, Firsov D 2007 Identification of corticosteroid-regulated genes in cardiomyocytes by serial analysis of gene expression. Genomics 89:370–377 [DOI] [PubMed] [Google Scholar]
- Fejes-Tóth G, Náray-Fejes-Tóth A 2007 Early aldosterone-regulated genes in cardiomyocytes: clues to cardiac remodeling? Endocrinology 148:1502–1510 [DOI] [PubMed] [Google Scholar]
- Yoshikawa N, Nagasaki M, Sano M, Tokudome S, Ueno K, Shimizu N, Imoto S, Miyano S, Suematsu M, Fukuda K, Morimoto C, Tanaka H 2009 Ligand-based gene expression profiling reveals novel roles of glucocorticoid receptor in cardiac metabolism. Am J Physiol 296:E1363–E1373 [DOI] [PubMed] [Google Scholar]
- Gaborit N, Steenman M, Lamirault G, Le Meur N, Le Bouter S, Lande G, Léger J, Charpentier F, Christ T, Dobrev D, Escande D, Nattel S, Demolombe S 2005 Human atrial ion channel and transporter subunit gene-expression remodeling associated with valvular heart disease and atrial fibrillation. Circulation 112:471–481 [DOI] [PubMed] [Google Scholar]
- Lamirault G, Gaborit N, Le Meur N, Chevalier C, Lande G, Demolombe S, Escande D, Nattel S, Léger JJ, Steenman M 2006 Gene expression profile associated with chronic atrial fibrillation and underlying valvular heart disease in man. J Mol Cell Cardiol 40:173–184 [DOI] [PubMed] [Google Scholar]
- Pisani DF, Coldefy AS, Elabd C, Cabane C, Salles J, Le Cunff M, Derijard B, Amri EZ, Dani C, Leger JJ, Dechesne CA 2007 Involvement of BTBD1 in mesenchymal differentiation. Exp Cell Res 313:2417–2426 [DOI] [PubMed] [Google Scholar]
- Lamirault G, Le Meur N, Roussel JC, Le Cunff MF, Baron D, Bihouee A, Guisle I, Raharijaona M, Ramstein G, Teusan R, Chevalier C, Gueffet JP, Trochu JN, Leger JJ, Houlgatte R, Steenman M 9 September 2009 Molecular risk stratification in advanced heart failure patients. J Cell Mol Med 10.1111/j.1582-4934.2009.00913.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenman M, Lamirault G, Le Meur N, Le Cunff M, Escande D, Léger JJ 2005 Distinct molecular portraits of human failing hearts identified by dedicated cDNA microarrays. Eur J Heart Fail 7:157–165 [DOI] [PubMed] [Google Scholar]
- Steenman M, Chen YW, Le Cunff M, Lamirault G, Varró A, Hoffman E, Léger JJ 2003 Transcriptomal analysis of failing and nonfailing human hearts. Physiol Genomics 12:97–112 [DOI] [PubMed] [Google Scholar]
- Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, Speed TP 2002 Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res 30:e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman C, Jensen LJ, Jarmer H, Berka R, Gautier L, Nielser HB, Saxild HH, Nielsen C, Brunak S, Knudsen S 2002 A new non-linear normalization method for reducing variability in DNA microarray experiments. Genome Biol 3:research0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW 2001 A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth GK 2004 Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3:article3 [DOI] [PubMed] [Google Scholar]
- Zeeberg BR, Qin H, Narasimhan S, Sunshine M, Cao H, Kane DW, Reimers M, Stephens RM, Bryant D, Burt SK, Elnekave E, Hari DM, Wynn TA, Cunningham-Rundles C, Stewart DM, Nelson D, Weinstein JN 2005 High-Throughput GoMiner, an ‘industrial-strength’ integrative gene ontology tool for interpretation of multiple-microarray experiments, with application to studies of Common Variable Immune Deficiency (CVID). BMC Bioinformatics 6:168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couette B, Marsaud V, Baulieu EE, Richard-Foy H, Rafestin-Oblin ME 1992 Spironolactone, an aldosterone antagonist, acts as an antiglucocorticosteroid on the mouse mammary tumor virus promoter. Endocrinology 130:430–436 [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Robert V, Escoubet B, Heymes C, Oubénaïssa A, Desopper C, Swynghedauw B, Delcayre C 2000 Different regulation of cardiac and renal corticosteroid receptors in aldosterone-salt treated rats: effect of hypertension and glucocorticoids. J Mol Cell Cardiol 32:1249–1263 [DOI] [PubMed] [Google Scholar]
- Takeda M, Tatsumi T, Matsunaga S, Hayashi H, Kimata M, Honsho S, Nishikawa S, Mano A, Shiraishi J, Yamada H, Takahashi T, Matoba S, Kobara M, Matsubara H 2007 Spironolactone modulates expressions of cardiac mineralocorticoid receptor and 11β-hydroxysteroid dehydrogenase 2 and prevents ventricular remodeling in post-infarct rat hearts. Hypertens Res 30:427–437 [DOI] [PubMed] [Google Scholar]
- Ohtani T, Ohta M, Yamamoto K, Mano T, Sakata Y, Nishio M, Takeda Y, Yoshida J, Miwa T, Okamoto M, Masuyama T, Nonaka Y, Hori M 2007 Elevated cardiac tissue level of aldosterone and mineralocorticoid receptor in diastolic heart failure: beneficial effects of mineralocorticoid receptor blocker. Am J Physiol Regul Integr Comp Physiol 292:R946–R954 [DOI] [PubMed] [Google Scholar]
- Yoshida M, Ma J, Tomita T, Morikawa N, Tanaka N, Masamura K, Kawai Y, Miyamori I 2005 Mineralocorticoid receptor is overexpressed in cardiomyocytes of patients with congestive heart failure. Congest Heart Fail 11:12–16 [DOI] [PubMed] [Google Scholar]
- Muller OG, Parnova RG, Centeno G, Rossier BC, Firsov D, Horisberger JD 2003 Mineralocorticoid effects in the kidney: correlation between αENaC, GILZ, and Sgk-1 mRNA expression and urinary excretion of Na+ and K+. J Am Soc Nephrol 14:1107–1115 [DOI] [PubMed] [Google Scholar]
- Náray-Fejes-Tóth A, Canessa C, Cleaveland ES, Aldrich G, Fejes-Tóth G 1999 sgk is an aldosterone-induced kinase in the renal collecting duct. Effects on epithelial na+ channels. J Biol Chem 274:16973–16978 [DOI] [PubMed] [Google Scholar]
- Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D 1999 Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci USA 96:2514–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker RP, Chiquet-Ehrismann R 2009 The regulation of tenascin expression by tissue microenvironments. Biochim Biophys Acta 1793:888–892 [DOI] [PubMed] [Google Scholar]
- Sakai T, Furukawa Y, Chiquet-Ehrismann R, Nakamura M, Kitagawa S, Ikemura T, Matsumoto K 1996 Tenascin-X expression in tumor cells and fibroblasts: glucocorticoids as negative regulators in fibroblasts. J Cell Sci 109(Pt 8):2069–2077 [DOI] [PubMed] [Google Scholar]
- Riddle EL, Schwartzman RA, Bond M, Insel PA 2005 Multi-tasking RGS proteins in the heart: the next therapeutic target? Circ Res 96:401–411 [DOI] [PubMed] [Google Scholar]
- Deppe CE, Heering PJ, Viengchareun S, Grabensee B, Farman N, Lombès M 2002 Cyclosporine a and FK506 inhibit transcriptional activity of the human mineralocorticoid receptor: a cell-based model to investigate partial aldosterone resistance in kidney transplantation. Endocrinology 143:1932–1941 [DOI] [PubMed] [Google Scholar]
- Gerhardt S, Hassall G, Hawtin P, McCall E, Flavell L, Minshull C, Hargreaves D, Ting A, Pauptit RA, Parker AE, Abbott WM 2007 Crystal structures of human ADAMTS-1 reveal a conserved catalytic domain and a disintegrin-like domain with a fold homologous to cysteine-rich domains. J Mol Biol 373:891–902 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hirohata S, Murakami T, Miyoshi T, Demircan K, Oohashi T, Ogawa H, Koten K, Toeda K, Kusachi S, Ninomiya Y, Shiratori Y 2004 Dynamic induction of ADAMTS1 gene in the early phase of acute myocardial infarction. J Biochem 136:439–446 [DOI] [PubMed] [Google Scholar]
- Lijnen HR 2005 Pleiotropic functions of plasminogen activator inhibitor-1. J Thromb Haemost 3:35–45 [DOI] [PubMed] [Google Scholar]
- Brown NJ, Agirbasli MA, Williams GH, Litchfield WR, Vaughan DE 1998 Effect of activation and inhibition of the renin-angiotensin system on plasma PAI-1. Hypertension 32:965–971 [DOI] [PubMed] [Google Scholar]
- Gooptu B, Lomas DA 2009 Conformational pathology of the serpins: themes, variations, and therapeutic strategies. Annu Rev Biochem 78:147–176 [DOI] [PubMed] [Google Scholar]
- Asakura M, Kitakaze M 2009 Global gene expression profiling in the failing myocardium. Circ J 73:1568–1576 [DOI] [PubMed] [Google Scholar]
- Pages G, Rouayrenc JF, Le Cam G, Mariller M, Le Cam A 1990 Molecular characterization of three rat liver serine-protease inhibitors affected by inflammation and hypophysectomy. Protein and mRNA analysis and cDNA cloning. Eur J Biochem 190:385–391 [DOI] [PubMed] [Google Scholar]
- Di Zhang A, Cat AN, Soukaseum C, Escoubet B, Cherfa A, Messaoudi S, Delcayre C, Samuel JL, Jaisser F 2008 Cross-talk between mineralocorticoid and angiotensin II signaling for cardiac remodeling. Hypertension 52:1060–1067 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.