Abstract
Lipid-induced insulin resistance is associated with intracellular accumulation of inhibitory intermediates depending on the prevalent fatty acid (FA) species. In cultured myotubes, ceramide and phosphatidic acid (PA) mediate the effects of the saturated FA palmitate and the unsaturated FA linoleate, respectively. We hypothesized that myriocin (MYR), an inhibitor of de novo ceramide synthesis, would protect against glucose intolerance in saturated fat-fed mice, while lisofylline (LSF), a functional inhibitor of PA synthesis, would protect unsaturated fat-fed mice. Mice were fed diets enriched in saturated fat, n-6 polyunsaturated fat, or chow for 6 wk. Saline, LSF (25 mg/kg · d), or MYR (0.3 mg/kg · d) were administered by mini-pumps in the final 4 wk. Glucose homeostasis was examined by glucose tolerance test. Muscle ceramide and PA were analyzed by mass spectrometry. Expression of LASS isoforms (ceramide synthases) was evaluated by immunoblotting. Both saturated and polyunsaturated fat diets increased muscle ceramide and induced glucose intolerance. MYR and LSF reduced ceramide levels in saturated and unsaturated fat-fed mice. Both inhibitors also improved glucose tolerance in unsaturated fat-fed mice, but only LSF was effective in saturated fat-fed mice. The discrepancy between ceramide and glucose tolerance suggests these improvements may not be related directly to changes in muscle ceramide and may involve other insulin-responsive tissues. Changes in the expression of LASS1 were, however, inversely correlated with alterations in glucose tolerance. The demonstration that LSF can ameliorate glucose intolerance in vivo independent of the dietary FA type indicates it may be a novel intervention for the treatment of insulin resistance.
Ceramide accumulation occurs in muscle of fat-fed mice, independent of the predominant fat source; modulation of ceramide synthase enzyme expression is associated with improved glucose tolerance by myriocin and lisofylline.
Insulin resistance is a major metabolic defect in type 2 diabetes. Dietary lipid oversupply contributes to the development of insulin resistance and is associated with ectopic lipid deposition in tissues important for glucose homeostasis, such as skeletal muscle and liver. The accrual of lipid in these tissues has been coupled to increased amounts of inhibitory lipid second messengers, such as ceramide and diacylglycerol (reviewed in Ref. 1).
In cultured muscle cell models, the prevailing fatty acid (FA) source determines the lipid intermediates that accumulate. Specifically, we (2) and others (3) have shown that oversupply of the saturated FA palmitate in myotubes results in insulin resistance associated with accumulation of ceramide. More recently we have shown that oversupply of the polyunsaturated FA linoleate in L6 myotubes also results in insulin resistance, however this was associated with dilinoleoyl-phosphatidic acid (PA) accretion (4). The involvement of ceramide and PA in the development of lipid-induced insulin resistance in vivo remains equivocal. Some clinical studies report increased skeletal muscle or plasma ceramide in obese insulin-resistant states (5,6), while others report that skeletal muscle ceramide is not correlated with obesity or insulin sensitivity (7). The more recently described PA was increased in skeletal muscle of glucose intolerant mice, fed a diet rich in linoleate (4), warranting further investigation of this novel lipid species.
Targeting the synthesis of ceramide and PA is a useful approach to examine the role of these lipid intermediates in the context of insulin resistance. Myriocin (MYR) blocks de novo ceramide synthesis by inhibition of the enzyme serine-palmitoyltransferase (8). It has been used in acute lipid infusion models of insulin resistance, where it was effective in preventing impaired insulin action in rats infused with a lard-oil (i.e. saturated fat) emulsion but not in rats infused with a soybean-oil (i.e. polyunsaturated fat) emulsion; the protective mechanism was associated with reduced ceramide content in skeletal muscle (9). The effect of MYR in chronic high fat-fed models of insulin resistance has recently been examined in conjunction with high-fat diets composed predominantly of saturated but not polyunsaturated fats (10,11). Moreover, the involvement of other aspects of sphingolipid metabolism, such as the role of ceramide synthases, has not been examined. Different ceramide synthases [encoded by longevity assurance (LASS) homolog genes] incorporate distinct long-chain acyl CoA species as acyl sidechains during ceramide generation and are also involved in intracellular remodeling of ceramide species, making a major contribution to the ceramide pool (12,13). For example, LASS1, the major ceramide synthase in skeletal muscle, is involved primarily in the synthesis of C18:0 ceramides while LASS2 is involved in the synthesis of C18:0–C26:0 ceramides (14).
Lisofylline (LSF) is a functional inhibitor of the enzyme lysophosphatidic acid acyl transferase (LPAAT) and shows preference for inhibition of unsaturated PA species generation (15). LSF has been shown to be beneficial in the context of type 1 diabetes, by reducing the onset of β-cell destruction in NOD mice and low-dose STZ-treated mice through antiinflammatory and immune-mediated mechanisms (16,17). In an in vitro model of lipid-induced insulin resistance, we have previously shown that LSF reverses the insulin receptor substrate-1 tyrosine phosphorylation defect associated with linoleate-oversupply (4). The efficacy of LSF in an in vivo model of lipid-induced insulin resistance is not known.
We sought to examine the effect of diets enriched in particular FA subtypes (saturated vs. n-6 polyunsaturated) on glucose tolerance in vivo and the accumulation of inhibitory lipid intermediates in skeletal muscle. We hypothesized that MYR would protect against the effects of a high-saturated fat diet, whereas LSF would protect against the effects of a high-polyunsaturated fat diet due to the inhibition of ceramide and PA synthesis in skeletal muscle, respectively.
Materials and Methods
Experimental animals
Animal procedures were approved by the Garvan Institute/St. Vincent’s Hospital Animal Ethics Committee and were in accordance with National Health and Medical Research Council guidelines. Male C57/BL6 mice (6 wk of age) were purchased from the Animal Resources Centre (Perth, WA, Australia). They were housed in a temperature-controlled environment (22 ± 1 C) on a 12-h light, 12-h dark cycle (lights on 0700–1900) and had ad libitum access to chow diet (Rat and Mouse Breeder Diet; Gordon’s Specialty Stockfeeds, Yanderra, NSW, Australia) and water.
Dietary and compound treatment
After one-wk acclimatization, mice were randomized to continue on the chow diet (CHOW; 8% calories as fat) or receive a high-fat (60% calories as fat) n-6 polyunsaturated (SAFF) or saturated (LARD) diet for 6 wk (see Supplemental Table 1, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org, for detailed FA composition). Diets were provided ad libitum and are well characterized to induce whole body insulin resistance in rodents, without gross hyperinsulinemia or hyperglycemia (18,19).
After 2 wk on the diets, mice were again randomized to receive saline (VEH), 25 mg/kg · d LSF (kind gift from DiaKine Therapeutics Inc, Charlottesville, VA), or 0.3 mg/kg · d MYR (Sigma, St. Louis, MO). These were administered subcutaneously for 4 wk via osmotic mini-pumps (Alzet Micro-Osmotic Pump, Model 1004; Durect Corporation, Cupertino, CA), which were surgically implanted in a subcutaneous pocket on the mouse dorsum, under isoflurane inhalation anesthesia (Provet VMS, Sydney, NSW, Australia).
Metabolic studies and metabolite measurements
Glucose tolerance tests were performed in conscious mice in the final week of the treatment period, after 6 h of fasting. A basal (0 min) blood sample was taken via tail prick, an ip injection of glucose [2 g/kg lean body mass; Phebra, Lane Cove, NSW, Australia] administered, and blood samples taken at 7.5, 15, 22.5, 30, 45, 60, and 90 min for assessment of glucose and insulin concentrations. Glucose was measured using an Accu-Chek Performa glucose monitor (Roche, Castle Hill, NSW, Australia), and insulin was assayed using blood collected with 5 μl Accu-Cap heparinized capillaries (Bilbate, Daventry, Northamptonshire, UK). Blood was dispensed directly into the insulin ELISA plate (Crystal Chem Inc., Downers Grove, IL) already containing sample diluent at 4 C, and the assay subsequently performed according to the manufacturer’s instructions for 5-μl sample volumes. Mice were anesthetized using isoflurane 24–48 h following the ip glucose tolerance test and after approximately 6 h of fasting. Basal tissues (left quadriceps and left epididymal fat pad) were quickly dissected and freeze-clamped with aluminum tongs precooled in liquid nitrogen. The same mice were subsequently injected with a bolus of insulin (4 U/kg lean body mass; Actrapid, Novo Nordisk Pharmaceuticals, Baulkham Hills, NSW, Australia) in the inferior vena cava, and insulin-stimulated tissues (right quadriceps, right epididymal fat pad, and liver) were collected after approximately 3 min. Tissues were stored at −80 C for subsequent analysis. Quadriceps skeletal muscle lipids were extracted using the method of Folch (20). Triglyceride (TG) content was quantified using an enzymatic colorimetric method (TG GPO-PAP reagent, Roche Diagnostics). Diacylglycerol (DAG) content was assessed by thin layer chromatography, using a method adapted from Nakamura and Handa (21).
RNA extraction and quantitative real-time RT-PCR
RNA was isolated from epididymal white adipose tissue using TRI-Reagent (Sigma Aldrich), according to the manufacturer’s instructions. RNA yield and quality were assessed using the NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies Inc., Wilmington DE). RT was performed using TaqMan Reverse Transcription Reagents (Roche Molecular Systems Inc, Branchburg, NJ). The 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA) was used in conjunction with TaqMan Gene Expression Assays (Applied Biosystems) and TaqMan Gene Expression Master Mix (Applied Biosystems) to assess the following genes: Il6 (interleukin 6), Tnfα (tumor necrosis factor α), Ccl2 [chemokine (C-C motif) ligand 2, also known as macrophage chemoattractant protein (MCP)-1], Cd3 (CD3), and the housekeeping gene Eef2 (eukaryotic translation elongation factor 2). Standard curves were constructed for each gene to determine amplification efficiency, and quantification was performed using ΔΔCt or absolute methods.
Lipid extraction and mass spectrometry
Quadriceps skeletal muscle was homogenized in 2 ml 2:1 vol/vol chloroform:methanol containing 0.01% vol/vol butylated hydroxytoluene (Sigma Aldrich), with 4 μl/mg of 5 μm PA (17:0/17:0) and 700 μl/mg of 75 μm ceramide (17:0) as internal standards (Avanti Polar Lipids Inc, Alabaster, AL). The homogenate was solubilized overnight at 4 C and total lipids extracted by the method of Folch (20), with minor modifications as described previously (22).
PA analysis
Electrospray ionisation mass spectrometric analysis of PA was performed essentially as described in Cazzolli et al. (4). See Supplemental Materials and Methods for details.
Ceramide analysis
Electrospray ionisation mass spectrometric analysis of ceramides was performed on a QTRAP 2000 triple quadrupole linear ion trap mass spectrometer (AB Sciex, Concord, Ontario, Canada). Molecular ceramides were detected in positive ion mode with an ion spray voltage of 5.5 kV and source heat turned off. Samples were infused at a rate of 3 μl/min using the instrument’s syringe pump. Scans for precursors of sphingosine (m/z 264) (i.e. molecular ceramides) were obtained over the mass-to-charge ratio (m/z) range of 500 to 660 using nitrogen as the collision gas, with a collision energy offset of 40 eV. Quantification of molecular ceramides was performed by comparison to the 17:0 internal ceramide standard after correction for isotope contributions (23) using a prototype of LipidProfiler software (AB Sciex).
Protein extraction and Western blotting
Western blotting was performed essentially as described in Frangioudakis et al. (24). See Supplemental Materials and Methods for details.
L6 cell culture, lipid preincubation, and glycogen synthesis assay
Experiments using L6 myotubes were performed essentially as described in Taylor et al. (25). See Supplemental Materials and Methods for details.
Statistical analysis
Results are presented as the mean ± se. Data were analyzed by ANOVA with Bonferroni post hoc tests. Differences were considered significant at P < 0.05.
Results
Muscle triglyceride accumulation and body weight was not influenced by LSF or MYR treatment of fat-fed mice
We used two high-fat diets, containing a high proportion of either n-6 unsaturated fat (SAFF diet) or saturated fat (LARD diet). These differed from commonly-used obesogenic diets in that they did not have a high sucrose content, because we wished to examine the effects of LSF and MYR treatment on the direct effects of dietary fat oversupply, in the absence of confounding factors such as lipogenesis, gross hyperinsulinemia, or hyperglycemia. Body weight was not significantly different between the various treatment groups at any stage throughout the study (terminal body weights shown in Table 1). Importantly, however, a significant 2-fold increase in epididymal fat pad mass was observed in all LARD-fed mice, independent of drug treatment (Table 1), representing an increased fat composition in these mice. Skeletal muscle TG content was also significantly increased with both SAFF (1.6- to 2.0-fold higher than CHOW) and LARD (2.5- to 2.8-fold higher than CHOW) diets. Neither LSF nor MYR affected this accumulation (Table 1). DAG content in skeletal muscle was not increased with either high-fat diet compared with CHOW and was not modulated by LSF or MYR (Table 1).
Table 1.
Body weight, fat pad mass, and quadriceps lipid content
| CHOW
|
SAFF
|
LARD
|
|||||
|---|---|---|---|---|---|---|---|
| VEH | VEH | LSF | MYR | VEH | LSF | MYR | |
| Body weight (g) | 27.9 ± 0.52 | 26.1 ± 0.41 | 26.1 ± 1.07 | 26.6 ± 0.57 | 27.5 ± 0.56 | 28.1 ± 0.53 | 27.8 ± 0.82 |
| Epididymal fat pad weight (g) | 0.146 ± 0.01 | 0.130 ± 0.01 | 0.166 ± 0.01 | 0.170 ± 0.01 | 0.305 ± 0.03a | 0.298 ± 0.04a | 0.271 ± 0.03a |
| Quadriceps TG content (μmol/g tissue) | 4.0 ± 0.59 | 6.4 ± 0.44a | 7.8 ± 1.81a | 7.8 ± 0.69a | 10.0 ± 1.51a | 11.3 ± 0.63a | 10.4 ± 1.17a |
| Quadriceps DAG content (μg/mg tissue) | 0.141 ± 0.013 | 0.126 ± 0.011 | 0.139 ± 0.014 | 0.131 ± 0.011 | 0.142 ± 0.014 | 0.151 ± 0.013 | 0.122 ± 0.015 |
Results are mean ± se of n = 6–10 mice per group.
, P < 0.001 for effect of diet.
The glucose intolerance observed in SAFF- and LARD-fed mice was differentially ameliorated by LSF and MYR
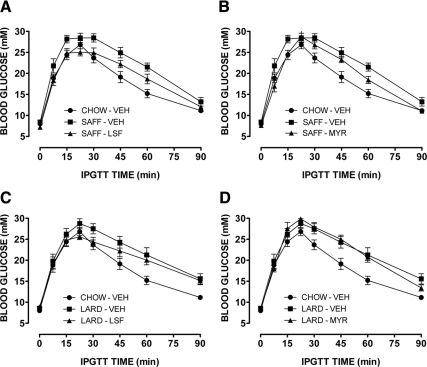
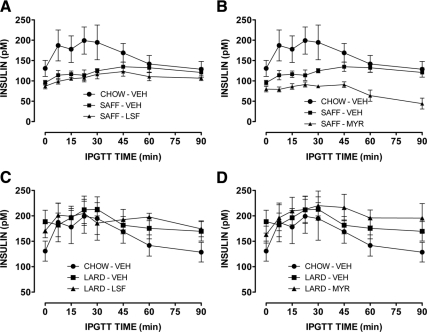
Both SAFF and LARD diets resulted in significant glucose intolerance compared with CHOW-fed mice (Fig. 1). In the SAFF-fed mice LSF, and to a lesser extent MYR, significantly improved the impaired glucose tolerance induced by the diet (Fig. 1, A and B, respectively). In the LARD-fed mice, only LSF was effective in significantly enhancing glucose tolerance (Fig. 1C), whereas there was no effect of MYR (Fig. 1D). Incremental area under the glucose curve, an indicator of glucose clearance, was increased by more than 30% in fat-fed animals, while drug treatments reduced this to approximately 15%, no longer significantly different to CHOW, except in the case of the LARD-MYR group (Supplemental Fig. 1). Enhanced basal or glucose-stimulated insulin secretion did not contribute to the improved glucose tolerance observed (Fig. 2). Indeed, all SAFF-fed mice exhibited low basal insulin levels and attenuated insulin responses, while all LARD-fed mice exhibited higher basal levels but again limited responses to the glucose challenge, in general agreement with previous studies of the effects of saturated and unsaturated fats on glucose-stimulated insulin secretion (26,27). Neither LSF nor MYR affected glucose tolerance in chow-fed mice (not shown). Furthermore, we did not detect any effects on maximal insulin signaling, either due to the fat diets or to the treatments (Supplemental Fig. 2).
Figure 1.
Blood glucose profiles from glucose tolerance tests in mice. Effect of LSF (A) and MYR (B) on glucose intolerance in SAFF mice. Effect of LSF (C) and MYR (D) on glucose tolerance in LARD mice. Results from CHOW mice are shown in each panel for ease of comparison. Results are mean ± se of n = 6–10 mice per group. Three-way ANOVA [diet (chow or fat) × time × drug (treated or untreated)]: P < 0.001 for effect of diet [SAFF (A and B) or LARD (C and D)]; P < 0.001 for effect of LSF (A) and MYR (B) in SAFF mice only; and P < 0.01 for effect of LSF in LARD mice (C).
Figure 2.
Insulin profiles from glucose tolerance tests in mice. Effect of LSF (A) and MYR (B) on glucose-stimulated insulin secretion in SAFF mice. Effect of LSF (C) and MYR (D) on glucose-stimulated insulin secretion in LARD mice. Results from CHOW mice are shown in each panel for ease of comparison. Results are mean ± se of n = 6–9 mice per group. Three-way ANOVA (as in Fig. 1): P < 0.001 for effect of SAFF diet (A and B); P < 0.05 for effect of LARD diet (C and D); P < 0.001 for effect of MYR in SAFF mice (B).
No evidence of a role for inflammation in adipose tissue
Because both LSF and MYR have been implicated as modulators of inflammation (16,17,28), and given the important role of adipose-derived mediators in the development of insulin resistance (reviewed in Ref. 29), we assessed mRNA expression of proinflammatory cytokines (IL-6 and TNF-α) and immune cell markers (MCP-1 and CD3) in epididymal white adipose tissue. However, no correlation was observed between the improvements in glucose tolerance caused by the inhibitors and these markers of inflammation. (Supplemental Fig. 3).
Increased abundance of specific FAs esterified to phosphatidic acid in skeletal muscle of SAFF- and LARD-fed mice
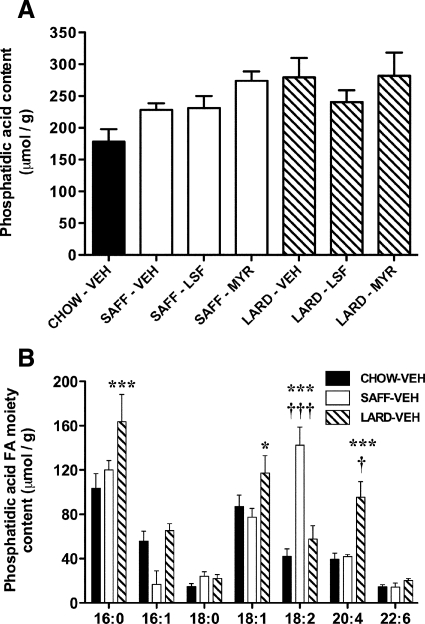
There was a trend for increased total PA abundance in quadriceps skeletal muscle with both high-fat diet interventions (P = 0.097; SAFF: 28–54% higher than CHOW-VEH; LARD: 35–58% higher than CHOW-VEH), but no effect of LSF (or MYR) to reduce this accumulation (Fig. 3A). The presence of different FAs esterified in the PA molecules primarily reflected the FA content of the diets (Supplemental Table 1). Specifically, total 18:2 FA moieties on the PA molecule were significantly increased in the SAFF-VEH mice compared with CHOW-VEH and LARD-VEH (Fig. 3B). In skeletal muscle from LARD-VEH mice, 16:0, 18:1, and 20:4 FA moieties on the PA molecule were increased compared with CHOW-VEH mice (Fig. 3B). Neither drug treatment affected FA incorporation into PA (data not shown).
Figure 3.
Phosphatidic acid content in skeletal muscle of mice. Total phosphatidic acid accumulation in all groups (A) and specific FA moiety content of phosphatidic acid molecules in VEH-treated mice only (B), by lipidomics analysis. Results are mean ± se of n = 3–7 mice per group. *, P < 0.05; ***, P < 0.001 vs. CHOW-VEH; †, P < 0.05; and †††, P < 0.001 vs. SAFF-VEH or LARD-VEH.
Diet-induced ceramide accumulation was reduced by both LSF and MYR
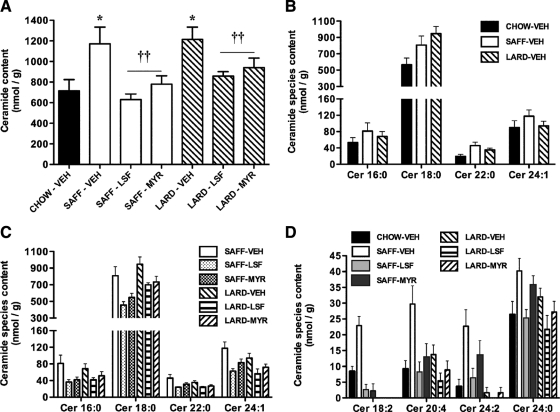
Total ceramide content in quadriceps skeletal muscle was significantly increased 1.6 to 1.7-fold with both high-fat diets (P < 0.05), and surprisingly there was a significant effect of both LSF and MYR to prevent the accrual of ceramide in SAFF- and LARD-fed mice (Fig. 4A). This was reflected in the abundance of the major ceramide species detected (i.e. 16:0, 18:0, 22:0, and 24:1 ceramides), which were similarly increased with both high-fat diets (Fig. 4B). Furthermore, the capacity of LSF and MYR to inhibit the accumulation of the major ceramide species detected was consistent with their effects on total ceramide content, with LSF and MYR exhibiting similar efficacy (Fig. 4C). Differential diet and drug effects were observed in less abundant ceramide species. Specifically, certain species (18:2, 20:4, and 24:2 ceramides) were increased to a greater extent in the SAFF-VEH mice (Fig. 4D). LSF and MYR were equally effective in suppressing some species, especially 18:2 ceramide, while LSF was more effective for others, especially the 24:2 and 24:0 species, which were decreased by this inhibitor in both dietary models (Fig. 4D).
Figure 4.
Ceramide content in skeletal muscle of mice. Total ceramide accumulation in all groups (*, P < 0.05 for effect of diet; ††, P < 0.01 for effect of drug treatment) (A), effect of diet on accrual of major ceramide species (B), effect of drug on major ceramide species abundance in SAFF and LARD mice (C), and effect of diet and drug treatment on minor ceramide species content (D) (18:2, 20:4, 24:2, and 24:0 species: P < 0.05, P < 0.001, P < 0.001, and P < 0.05 SAFF-VEH vs. CHOW-VEH, respectively; 18:2 and 20:4 species: P < 0.01 SAFF-LSF and SAFF-MYR vs. SAFF-VEH; 24:2 and 24:0 species: P < 0.01 SAFF-LSF vs. SAFF-VEH). Results are mean ± se of n = 4–7 mice per group.
LASS enzyme expression in skeletal muscle from mice is differentially modulated by diet and drug treatment
We also measured the expression of ceramide synthase (LASS) enzymes, to investigate whether they were affected in skeletal muscle of our models. LASS1 was measured as it is the most abundant isoform in skeletal muscle, and LASS2 was measured because it can use a broad range of FA-CoA substrates (13). LASS1 was significantly increased by both SAFF and LARD diets compared with CHOW; LSF reduced this expression in both high fat-fed groups, and MYR was only effective in the SAFF-fed mice (Fig. 5A), which correlates with the effects of these inhibitors on whole body glucose homeostasis (Fig. 1). LASS2 protein expression was not affected by diet alone but was significantly increased in the presence of LSF and MYR (Fig. 5B).
Figure 5.
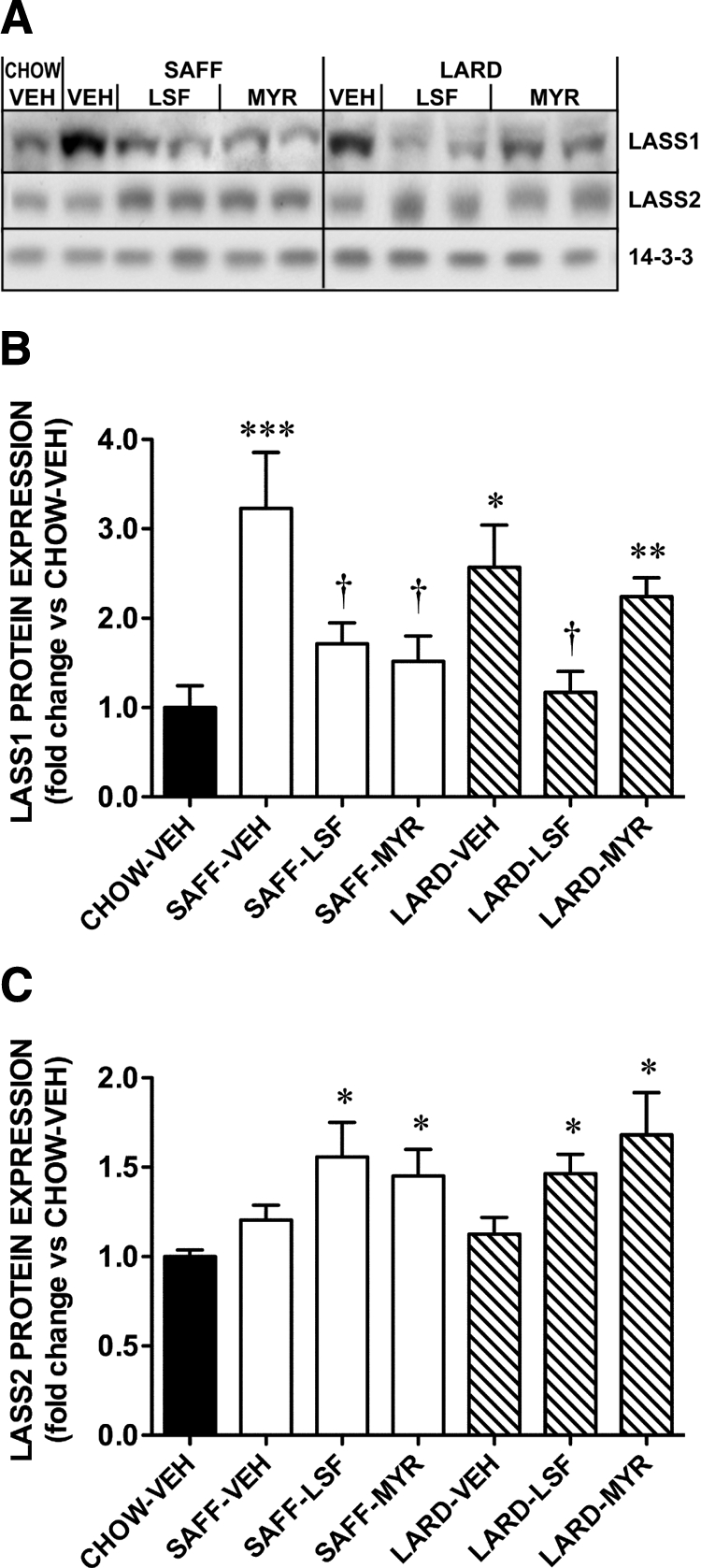
LASS isoform protein expression in skeletal muscle of mice. A, Representative blots of LASS1 and LASS 2. 14-3-3 protein expression was used as a loading control. Quantification of (B) LASS1 and (C) LASS2 protein expression by Western blotting. Results are mean ± se of n = 6–8 mice per group. ***, P < 0.001; **, P < 0.01; *, P < 0.05 vs. CHOW-VEH; †, P < 0.05 vs. FAT DIET-VEH.
Discussion
We have examined two high-fat diets, enriched in either n-6 polyunsaturated fat (SAFF) or saturated fat (LARD), and demonstrated that LSF, a functional inhibitor of PA metabolism, can ameliorate impaired glucose tolerance in 6-wk high fat-fed mice, irrespective of the major FAs supplied in the diet. The efficacy of LSF is of interest, being the first demonstration of the utility of this compound in the treatment of insulin resistance in vivo. Impaired glucose tolerance was ameliorated by both LSF and MYR in SAFF-fed mice, whereas only LSF was effective in LARD-fed mice. A second major finding is that the SAFF and LARD diets resulted in similar increases in major ceramide species in skeletal muscle and that distinct inhibitors of lipid metabolism could reverse this. Furthermore, alterations in the expression of the major ceramide synthase in skeletal muscle, LASS1, correlated well with the effect of LSF and MYR on glucose tolerance.
Given the increased skeletal muscle triglyceride content observed in the fat-fed mice which was unaffected by treatment with LSF or MYR, we focused on lipid intermediates thought to play a more direct role in the development of insulin resistance in this tissue. The in vivo role of PA was assessed further, because our earlier findings using cultured muscle cells had indicated that dilinoleoyl PA might represent a novel mediator of insulin resistance in situations of polyunsaturated FA oversupply (4). In the current study, we confirmed that total PA tended to be enriched in skeletal muscle with lipid oversupply in vivo, but this occurred similarly with both the SAFF and LARD diets, and PA content did not correlate with glucose tolerance. LSF did not have an impact on PA abundance in the fat-fed mice, in contrast to our findings in lipid-treated L6 myotubes in vitro (data not shown) and other studies in which LSF has been shown to reduce PA levels (15). This suggests that skeletal muscle PA is not a key target of this compound in vivo.
Ceramide has also been implicated in the development of insulin resistance in some (5,6,30) but not all studies (7,31). Recent work involving acute lipid infusions in rats suggests that at least in situations of acute lipid oversupply, ceramide levels only increase if the FA source provided is saturated (9), most likely because this promotes de novo ceramide synthesis through the incorporation of palmitate at an early rate-limiting step (32). Several studies in rats and humans, using lipid infusions consisting primarily of the unsaturated FA linoleate, demonstrate that insulin resistance can occur in the absence of ceramide accumulation, indicating that other mechanisms can also be responsible, involving for example protein kinase C activation upon accumulation of DAG (9,31,33,35). One such study in human subjects did however result in an increase in muscle ceramide content together with a decrease in insulin sensitivity (30). Conversely, a study that examined rat muscle lipid accumulation in response to different high-fat diets found that DAG rather than ceramide accumulated in response to both saturated and n-6 polyunsaturated fat oversupply (27). This was associated with insulin resistance only in the case of saturated fat, while n-6 polyunsaturated fatty acids modestly enhanced insulin sensitivity, in contrast to the findings made with lipid infusions but also those from other diet studies (36,37). Using mass spectrometry, we have now shown that both a SAFF and a LARD diet can elevate ceramide levels in skeletal muscle of mice, causing accumulation of ceramide containing 16:0, 18:0, 22:0 and 24:1 fatty amides. That overall ceramide accumulation in the longer term appears to be less dependent on the abundance of particular dietary FA species is interesting given the current view derived using infusions (9). Furthermore, our findings in relation to the particular ceramide species that accumulate with high fat-feeding are in agreement with clinical studies that have reported increased abundance of these species in obese insulin-resistant individuals (5,6). These ceramide species may therefore represent novel lipid biomarkers for disease progression, because they were elevated both in our relatively mild dietary models of insulin resistance and also in humans with obesity and frank type 2 diabetes. This also suggests that manipulation of sphingolipid metabolism at levels other than the incorporation of palmitate by serine-palmitoyltransferase during de novo synthesis may represent a more constructive therapeutic intervention for the treatment of insulin resistance.
As we hypothesized, MYR treatment prevented the accumulation of several ceramide species, but unexpectedly, LSF was at least equally effective. These changes were associated with improved glucose homeostasis in all mice except the LARD-MYR group, suggesting a disparity between changes in glucose homeostasis and the reduction in overall ceramide levels in this model. Our results therefore contrast with other studies which suggest that blocking ceramide accumulation can prevent lipid-induced insulin resistance, at least upon saturated FA oversupply (9,10,11,38,39). One potential explanation is that increased skeletal muscle ceramide and glucose intolerance are not always directly linked. Indeed, the lack of a beneficial effect of LSF and MYR on impaired insulin-stimulated glycogen synthesis (Supplemental Fig. 4, A and B) or LASS1 expression (Supplemental Fig. 4C) in lipid-treated L6 myotubes suggests that these inhibitors do not act directly on skeletal muscle to elicit improvements in glucose tolerance. In addition, our data do not exclude the possibility that glucose homeostasis was also improved at least in part by beneficial effects of the inhibitors on the liver. An alternative explanation is suggested by the observations that MYR treatment in fact appeared less effective in improving glucose tolerance in the SAFF diet model (Fig. 1, B vs. A) as well as in the LARD diet model (Fig. 1, D vs. C), and also less effective in reducing the levels of particular ceramide species such as Cer20:4 and Cer24:2 (Fig. 4D). Thus in contrast to models studied previously (2,3,9), the longer duration of FA oversupply here led to the accumulation of specific ceramide species less affected by MYR. This inhibitor blocks the incorporation of palmitate into ceramide by serine palmitoyl-transferase. In the longer term such de novo synthesis will be accompanied by the incorporation of other FAs by ceramide synthases during sphingolipid remodeling (40). Whether some (MYR-resistant) ceramides are more potent in generating defects in muscle glucose disposal than others requires further investigation. Diversion of lipid synthesis to other intermediates such as DAG by longer term MYR treatment, which has been reported in L6 muscle cells together with impaired insulin action despite reduced ceramide content (41), did not occur in our study because we did not observe increased skeletal muscle DAG content.
The reduction in ceramide accumulation caused by LSF treatment is unlikely to involve direct inhibition of ceramide synthesis. This compound has pleiotropic effects that act in part to reduce inflammatory responses (16,17,42). One possibility we have investigated is that LSF may reduce the production of inflammatory cytokines such as TNFα, which in turn may prevent the chronic generation of ceramide through sphingomyelinase activity (43) (Supplemental Fig. 5). Indeed, a cycle exists between ceramide and inflammatory mediators (44) which may underlie the complex nature of our findings, especially as effects on inflammatory responses have been implicated in the mechanism of action of both LSF and MYR (10,28). We did not, however, observe significant changes in the mRNA expression of cytokines or immune cell markers in adipose tissue, which correlated with alterations in glucose tolerance. Studies reporting the induction of an inflammatory state in adipose tissue in response to high-fat diet feeding use diets with higher sucrose levels which promote more rapid changes in obesity (45,46). The diets we used here were enriched only in fat and were capable of inducing glucose intolerance without causing significant weight gain. Our dietary models may therefore represent an early disease state in which inflammatory responses are not yet fully established. Similarly, we did not observe effects of the diets or of MYR and LSF on the activation of JNK or NFκB stress pathways in the liver (data not shown), an additional site of inflammation leading to whole body glucose intolerance (34).
The improvement in glucose tolerance in SAFF- and LARD-fed mice caused by LSF and/or MYR treatment appears to be linked to remodeling of skeletal muscle ceramide species (Supplemental Fig. 5). The accumulation of specific ceramide species depends on the action of different ceramide synthase (LASS) isoforms. Another interesting finding of this study is that high-fat feeding increases the expression of LASS1 and that LSF and MYR reverse this in a manner that is consistent with their differing abilities to improve glucose tolerance in the two dietary models used. Given that C18:0 ceramide was the most abundant ceramide species detected, regulation of the LASS1 enzyme may contribute to improved glucose tolerance. However, because MYR reduces total ceramide but does not reduce LASS1 or improve glucose tolerance in the LARD diet, it is also possible that LASS1 levels change in response to altered glucose disposal rather than contributing to it. Alterations in LASS2 followed a different pattern, in that this isoform appeared insensitive to the fat diets but was elevated by both inhibitors used. The regulation of LASS gene expression is poorly understood, but our data from fat-fed mice suggests this depends, at least for LASS1, on the supply of lipid substrates. Other sphingolipid species may also be affected by LASS modulation, but the measurement of these was not within the scope of the current study.
In conclusion, we have shown that high-fat feeding results in extensive ceramide accumulation in mouse skeletal muscle, irrespective of the dietary FA composition, and that LSF and MYR have less restricted effects on ceramide levels and glucose tolerance than originally hypothesized. While the improvements we have seen in glucose tolerance may not be due to direct changes in muscle, and may involve other tissues such as liver and fat, our studies suggest that ceramide remodeling, as well as de novo synthesis, could be influential in the development of chronic lipid-induced insulin resistance. While ceramide reduction alone may not be sufficient to improve glucose homeostasis in LARD-fed animals, regulation of LASS enzymes correlate with the efficacy of LSF in our models of insulin resistance. Our data therefore provide promising insight into the therapeutic potential of LSF, although more detailed research on the tissue-specific effects and actions of LSF is warranted.
Supplementary Material
Acknowledgments
We thank Mana Liao for assistance with the L6 cell experiments and the staff of the Biological Testing Facility at the Garvan Institute for assistance with animal care.
Footnotes
This work was supported by funding from the National Health and Medical Research Council of Australia (to C.S.P., T.W.M.), Eli Lilly Australia (to C.S.P.), and National Institutes of Health National Heart, Lung, and Blood Institute Grant P01 HL55798 (to J.L.N.).
Disclosure Summary: J.L.N. is Chief Science Officer and Chairman of the Board of DiaKine Therapeutics Inc., a biopharmaceutical company developing novel immune modulators into therapies for diabetes and related complications. The remaining authors have nothing to declare.
First Published Online July 21, 2010
Abbreviations: DAG, Diacylglycerol; FA, fatty acid; LASS, longevity assurance; LSF, lisofylline; MYR, myriocin; PA, phosphatidic acid; TG, triglyceride.
References
- Kraegen EW, Cooney GJ 2008 Free fatty acids and skeletal muscle insulin resistance. Curr Opin Lipidol 19:235–241 [DOI] [PubMed] [Google Scholar]
- Schmitz-Peiffer C, Craig DL, Biden TJ 1999 Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem 274:24202–24210 [DOI] [PubMed] [Google Scholar]
- Hajduch E, Balendran A, Batty IH, Litherland GJ, Blair AS, Downes CP, Hundal HS 2001 Ceramide impairs the insulin-dependent membrane recruitment of protein kinase B leading to a loss in downstream signalling in L6 skeletal muscle cells. Diabetologia 44:173–183 [DOI] [PubMed] [Google Scholar]
- Cazzolli R, Mitchell TW, Burchfield JG, Pedersen DJ, Turner N, Biden TJ, Schmitz-Peiffer C 2007 Dilinoleoyl-phosphatidic acid mediates reduced IRS-1 tyrosine phosphorylation in rat skeletal muscle cells and mouse muscle. Diabetologia 50:1732–1742 [DOI] [PubMed] [Google Scholar]
- Adams 2nd JM, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ 2004 Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53:25–31 [DOI] [PubMed] [Google Scholar]
- Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, Kirwan JP 2009 Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 58:337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovbro M, Baranowski M, Skov-Jensen C, Flint A, Dela F, Gorski J, Helge JW 2008 Human skeletal muscle ceramide content is not a major factor in muscle insulin sensitivity. Diabetologia 51:1253–1260 [DOI] [PubMed] [Google Scholar]
- Miyake Y, Kozutsumi Y, Nakamura S, Fujita T, Kawasaki T 1995 Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochem Biophys Res Comm 211:396–403 [DOI] [PubMed] [Google Scholar]
- Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA 2007 Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5:167–179 [DOI] [PubMed] [Google Scholar]
- Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F 2009 Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol 297:E211–E224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussher JR, Koves TR, Cadete VJJ, Zhang L, Jaswal JS, Swyrd SJ, Lopaschuk DG, Proctor SD, Keung W, Muoio DM, Lopaschuk GD 3 June 2010 Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole body oxygen consumption. Diabetes doi: 10.2337/db09-1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM 2008 Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9:139–150 [DOI] [PubMed] [Google Scholar]
- Pewzner-Jung Y, Ben-Dor S, Futerman AH 2006 When do Lasses (longevity assurance genes) become CerS (ceramide synthases)? Insights into the regulation of ceramide synthesis. J Biol Chem 281:25001–25005 [DOI] [PubMed] [Google Scholar]
- Mizutani Y, Kihara A, Igarashi Y 2005 Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem J 390:263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidot DM, Bursten SL, Rice GC, Chaney RB, Singer JW, Repine AJ, Hybertson BM, Repine JE 1997 Modulating phosphatidic acid metabolism decreases oxidative injury in rat lungs. Am J Physiol 273:L957–L966 [DOI] [PubMed] [Google Scholar]
- Yang ZD, Chen M, Wu R, McDuffie M, Nadler JL 2002 The anti-inflammatory compound lisofylline prevents type I diabetes in non-obese diabetic mice. Diabetologia 45:1307–1314 [DOI] [PubMed] [Google Scholar]
- Yang Z, Chen M, Fialkow LB, Ellett JD, Wu R, Nadler JL 2003 The novel anti-inflammatory compound, lisofylline, prevents diabetes in multiple low-dose streptozotocin-treated mice. Pancreas 26:e99–104 [DOI] [PubMed] [Google Scholar]
- Kraegen EW, James DE, Storlien LH, Burleigh KM, Chisholm DJ 1986 In vivo insulin resistance in individual peripheral tissues of the high fat fed rat: assessment by euglycaemic clamp plus deoxyglucose administration. Diabetologia 29:192–198 [DOI] [PubMed] [Google Scholar]
- Turner N, Li JY, Gosby A, To SW, Cheng Z, Miyoshi H, Taketo MM, Cooney GJ, Kraegen EW, James DE, Hu LH, Li J, Ye JM 2008 Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes 57:1414–1418 [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH 1957 A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509 [PubMed] [Google Scholar]
- Nakamura K, Handa S 1984 Coomassie brilliant blue staining of lipids on thin-layer plates. Anal Biochem 142:406–410 [DOI] [PubMed] [Google Scholar]
- Deeley JM, Mitchell TW, Wei X, Korth J, Nealon JR, Blanksby SJ, Truscott RJ 2008 Human lens lipids differ markedly from those of commonly used experimental animals. Biochim Biophys Acta 1781:288–298 [DOI] [PubMed] [Google Scholar]
- Ejsing CS, Duchoslav E, Sampaio J, Simons K, Bonner R, Thiele C, Ekroos K, Shevchenko A 2006 Automated identification and quantification of glycerophospholipid molecular species by multiple precursor ion scanning. Anal Chem 78:6202–6214 [DOI] [PubMed] [Google Scholar]
- Frangioudakis G, Ye JM, Cooney GJ 2005 Both saturated and n-6 polyunsaturated fat diets reduce phosphorylation of insulin receptor substrate-1 and protein kinase B in muscle during the initial stages of in vivo insulin stimulation. Endocrinology 146:5596–5603 [DOI] [PubMed] [Google Scholar]
- Taylor AJ, Ye JM, Schmitz-Peiffer C 2006 Inhibition of glycogen synthesis by increased lipid availability is associated with subcellular redistribution of glycogen synthase. J Endocrinol 188:11–23 [DOI] [PubMed] [Google Scholar]
- Dobbins RL, Szczepaniak LS, Myhill J, Tamura Y, Uchino H, Giacca A, McGarry JD 2002 The composition of dietary fat directly influences glucose-stimulated insulin secretion in rats. Diabetes 51:1825–1833 [DOI] [PubMed] [Google Scholar]
- Lee JS, Pinnamaneni SK, Eo SJ, Cho IH, Pyo JH, Kim CK, Sinclair AJ, Febbraio MA, Watt MJ 2006 Saturated, but not n-6 polyunsaturated, fatty acids induce insulin resistance: role of intramuscular accumulation of lipid metabolites. J Appl Physiol 100:1467–1474 [DOI] [PubMed] [Google Scholar]
- Daniel C, Sartory N, Zahn N, Geisslinger G, Radeke HH, Stein JM 2007 FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J Immunol 178:2458–2468 [DOI] [PubMed] [Google Scholar]
- Tilg H, Moschen AR 2006 Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6:772–783 [DOI] [PubMed] [Google Scholar]
- Straczkowski M, Kowalska I, Nikolajuk A, Dzienis-Straczkowska S, Kinalska I, Baranowski M, Zendzian-Piotrowska M, Brzezinska Z, Gorski J 2004 Relationship between insulin sensitivity and sphingomyelin signaling pathway in human skeletal muscle. Diabetes 53:1215–1221 [DOI] [PubMed] [Google Scholar]
- Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI 2002 Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277:50230–50236 [DOI] [PubMed] [Google Scholar]
- Tettamanti G 2004 Ganglioside/glycosphingolipid turnover: new concepts. Glycoconj J 20:301–317 [DOI] [PubMed] [Google Scholar]
- Itani SI, Ruderman NB, Schmieder F, Boden G 2002 Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkB-α. Diabetes 51:2005–2011 [DOI] [PubMed] [Google Scholar]
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE 2005 Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κ B. Nat Med 11:183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy AJ, Brandon AE, Turner N, Watt MJ, Bruce CR, Cooney GJ, Kraegen EW 2009 Lipid and insulin infusion-induced skeletal muscle insulin resistance is likely due to metabolic feedback and not changes in IRS-1, Akt, or AS160 phosphorylation. Am J Physiol 297:E67–E75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storlien LH, Kraegen EW, Chisholm DJ, Ford GL, Bruce DG, Pascoe WS 1987 Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science (New York, NY) 237:885–888 [DOI] [PubMed] [Google Scholar]
- Kraegen EW, Clark PW, Jenkins AB, Daley EA, Chisholm DJ, Storlien LH 1991 Development of muscle insulin resistance after liver insulin resistance in high-fat fed rats. Diabetes 40:1397–1403 [DOI] [PubMed] [Google Scholar]
- Powell DJ, Turban S, Gray A, Hajduch E, Hundal HS 2004 Intracellular ceramide synthesis and protein kinase Czeta activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem J 382:619–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez JA, Holland WL, Bär J, Sandhoff K, Summers SA 2005 Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J Biol Chem 280:20148– 20153 [DOI] [PubMed] [Google Scholar]
- Kitatani K, Idkowiak-Baldys J, Hannun YA 2008 The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal 20:1010–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson ML, Coghlan M, Hundal HS 2009 Modulating serine palmitoyl transferase (SPT) expression and activity unveils a crucial role in lipid-induced insulin resistance in rat skeletal muscle cells. Biochem J 417:791–801 [DOI] [PubMed] [Google Scholar]
- Pei H, Gu J, Thimmalapura PR, Mison A, Nadler JL 2006 Activation of the 12-lipoxygenase and signal transducer and activator of transcription pathway during neointima formation in a model of the metabolic syndrome. Am J Physiol 290:E92–E102 [DOI] [PubMed] [Google Scholar]
- Kolesnick RN, Krönke M 1998 Regulation of ceramide production and apoptosis. Annu Rev Physiol 60:643–665 [DOI] [PubMed] [Google Scholar]
- Bismuth J, Lin P, Yao Q, Chen C 2008 Ceramide: a common pathway for atherosclerosis? Atherosclerosis 196:497–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunemaker CS, Chen M, Pei H, Kimble SD, Keller SR, Carter JD, Yang Z, Smith KM, Wu R, Bevard MH, Garmey JC, Nadler JL 2008 12-Lipoxygenase-knockout mice are resistant to inflammatory effects of obesity induced by Western diet. Am J Physiol 295:E1065–E1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears DD, Miles PD, Chapman J, Ofrecio JM, Almazan F, Thapar D, Miller YI 2009 12/15-lipoxygenase is required for the early onset of high fat diet-induced adipose tissue inflammation and insulin resistance in mice. PLoS ONE 4:e7250 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.