Abstract
Uterine arteries play a major role in regulating uteroplacental blood flow. Failure to maintain blood flow to the uteroplacental compartment during pregnancy often results in intrauterine growth retardation. Immunohistochemical staining of adrenomedullin (AM), an endogenous vasoactive peptide, in uterine artery was intense in pregnant compared to nonpregnant rats, but it is not known whether AM directly relaxes uterine artery or not. In this study, we elucidated the mechanisms of uterine artery relaxation by AM and its regulation by pregnancy and female sex steroids. AM was able to relax uterine artery, and this relaxation was influenced positively by pregnancy and estradiol as evidenced by the increased pD2 and Emax values of AM. Both pregnancy and estradiol treatment to ovariectomized rats amplified RAMP3 expression in uterine arteries while progesterone had no effect. AM-induced uterine artery relaxation is predominantly endothelium-dependent. The AM receptor antagonist CGRP8-37 is more potent than AM22-52 in inhibiting the AM relaxation, indicating the involvement of AM2 receptor subtype. Moreover, AM uses the classical nitric oxide-cyclic guanosine monophosphate pathway along with KCa channels to mediate the vasodilatory effect in uterine artery. In conclusion, sensitivity of uterine artery to AM-induced relaxation is increased with pregnancy or estradiol treatment by increasing RAMP3 expression, suggesting an important role for AM in regulating the uterine hemodynamics, probably maintaining uterine blood flow during pregnancy and in pre- and postmenopausal cardiovascular adaptation differences.
Uterine artery relaxation sensitivity to adrenomedullin is greater in pregnant compared to nonpregnant rats; this response appears to be estradiol dependent.
During normal pregnancy, uteroplacental blood flow increases tremendously to maintain uteroplacental oxygen and nutrient delivery to the developing fetus (1,2). Failure to maintain blood flow to the uteroplacental compartment during pregnancy often results in intrauterine growth retardation (IUGR), which predisposes the fetus to significant perinatal morbidity and mortality (3). It is well known that uterine arteries may play a major role in regulating uteroplacental blood flow. Reports indicate that the weight of these blood vessels increases 4-fold during pregnancy to accommodate the large increase in uterine blood flow (4). Cardiac output also increases during pregnancy, but its redistribution is not uniform. In particular, blood flow through the guinea pig uterine artery increases 3,500% by term, while flows through the mesenteric and renal arteries increase only by 90% and 10%, respectively (5). The underlying cause for this differential redistribution of cardiac output preferentially to reproductively important vessels like uterine artery is not clearly known.
Adrenomedullin (AM), a 52-amino-acid peptide, discovered from a panel of peptides extracted from a pheochromocytoma (6), was found to be produced by a wide range of cells, including vascular endothelial and smooth muscle cells. Plasma concentrations of AM are reported to be elevated during pregnancy both in humans (7) and rats (8), and AM antagonist treatment in rats causes fetoplacental growth restriction (9), suggesting a potential role for AM during pregnancy. Furthermore, immunohistochemical staining of AM in uterine artery was intense in pregnant compared with nonpregnant rats (10). However, apart from altered AM synthesis during pregnancy, either the direct effect of AM on uterine artery or altered responsiveness to AM, if any, or its possible involvement in functions other than uterine vascular adaptations are not known. Previously, several studies postulated that pregnancy increases the production of endothelium-derived relaxing factors from the uterine artery which augments endothelium-dependent relaxation and/or reduce smooth muscle responsiveness to vasoconstrictors (11,12,13,14,15) but opposite findings are also reported (16,17,18). Specifically, endothelium-dependent relaxation effects of acetylcholine in guinea pig uterine artery is not altered by pregnancy (18). But AM-induced hypotensive effect in vivo and in vitro are enhanced by pregnancy (19,20) and female sex steroids (21). However, its role in the uterine vascular adaptations is not known.
AM signaling is regulated by a unique system of receptors consisting of a seven-membrane domain G protein-coupled receptor named calcitonin receptor-like receptor (CL) associated with an accessory protein, receptor-activity-modifying protein (RAMP) (22). Three RAMP subtypes have been identified so far. In association with RAMP1, CL acquires a high affinity for calcitonin gene-related peptide (CGRP), whereas interacting with either RAMP2 or RAMP3, CL becomes an AM receptor. CL with RAMP2 is an AM selective receptor (AM1) that can be effectively antagonized by AM peptide antagonist AM22-52, whereas CL with RAMP3 form another receptor (AM2) that can respond to both CGRP and AM and is more potently antagonized by CGRP antagonist CGRP8-37 than by AM22-52 (23).
Therefore, in this study we examined whether: 1) AM is able to relax uterine artery, 2) AM-induced uterine artery relaxation, if any, and its receptor components are influenced by pregnancy or female sex steroids, and 3) the mechanisms of relaxation involve endothelium dependence, nitric oxide (NO)–cyclic guanosine monophosphate (cGMP) pathway mediation, and opening of potassium channels.
Materials and Methods
Animals
Adult female nonpregnant (age, 9–11 wk) and pregnant (d 20) Sprague Dawley rats (Rattus norvegicus; Harlan Sprague Dawley, Houston, TX) weighing between 250 and 300 g were used. All rats were maintained in the colony room with fixed photoperiod of 12-h light, 12-h dark cycle and having access to water and rat chow ad libitum. All procedures were approved by the Animal Care and Use Committee at the University of Texas Medical Branch (Galveston, TX) in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Ovariectomy
Female rats were anesthetized by ip injection of ketamine (50 mg/kg) and xylazine (8 mg/kg). Small incisions were made on each flank, and the ovaries were removed. The muscle layer was closed with suture, and the skin incisions were closed with wound clips. After 5 d of recovery, the ovariectomized (OVX) rats were implanted (through small incision in the back of the neck) sc with either estradiol [E2; 0.5 mg, 21-d release (OVX+E2 group)], progesterone [P4; 5 mg, 21-d release (OVX+P4 group)], both E2 and P4 (OVX+E2P4), or placebo pellets (OVX group) (Innovative Research of America, Sarasota, FL). These animals were used 7 d after implanting the pellets, and the efficacy of ovariectomy and hormone replacements were confirmed by measuring plasma P4 and E2 levels using DSL RIA kits (Diagnostic System Laboratories, Webster, TX).
Uterine artery isometric tension studies
Groups of rats that were either pregnant on d 20 of gestation [250–300 g body weight (BW)] or nonpregnant (175–200 g BW) at diestrus (NP-DE) were used in the present study. Rats were deeply anesthetized by an ip injection of ketamine (45 mg/kg BW; Fort Dodge Laboratory, Fort Dodge, IA) and xylazine (5 mg/kg BW), and the uterine artery was carefully dissected. Uterine artery rings (2-mm length, ∼250–400 μm) were obtained from the main uterine artery between the middle and lower third of the vascular arcade and placed in physiological salt solution (PSS) kept on ice. Uterine artery segments were mounted in the jaws of a wire myograph (Kent Scientific, Litchfield, CT) for the measurement of isometric tension (24). The unstretched vessels were allowed to equilibrate for 15 min in PSS that was gassed in 95% air/5% CO2 to maintain pH 7.4 at 37 C. The lumen of each segment was stretched to a length that would give a diameter of 250 (nonpregnant) to 400 μm (pregnant) and were allowed to equilibrate for 15 min. Finally, 5 μm norepinephrine (NE) was added into the chamber to stimulate the tissue contraction approximately three to four times. Each time, the uterine artery segment was incubated with NE for 5 min and washed with PSS for an additional 5 min. The relaxation response to AM was investigated at doses between 1 × 10−9 and 3 × 10−7 m in a cumulative manner on uterine artery rings precontracted with the ED70 (effective dose to produce 70% of maximal response) concentration of NE.
For experiments performed on endothelium-denuded arterial segments, endothelium was denuded by careful rubbing of the intimal surface of the arterial strips with 22-μm-thick tungsten wire. Successful denudation of endothelium was confirmed by the lack of acetylcholine-induced relaxation of arterial strips precontracted with submaximal concentration of NE. The viability of these strips was verified by sodium nitroprusside-induced relaxation.
Mechanisms of the AM-induced relaxation in pregnant rat uterine artery
The involvement of receptor subtypes, possible intracellular messengers, and ion channels in AM-induced vascular relaxation was investigated using selective inhibitors. In these studies, we first assessed vascular reactivity of uterine arteries of rats on d 20 of pregnancy to varying doses of AM (1 × 10−9–3 × 10−7 m) with NE preconstriction using a wire myograph. Subsequently, AM was washed out with PSS, and the uterine arterial segments were incubated for 30 min in fresh PSS with either receptor antagonists, AM22-52 (10 μm) or CGRP8-37 (10 μm), the inhibitors of guanylate cyclase [1H-(1, 2, 4) oxadizaolo (4,3-a) quinoxalin-1-one (ODQ), 10 μm], ATP-sensitive (glibenclamide, 10 μm), or calcium-dependent [tetraethylammonium (TEA), 1 mm] potassium (K+) channels, or nitric oxide synthesis [NG-nitro-l-arginine methyl ester (l-NAME), 100 μm]. After the incubation period, relaxation responses to cumulative doses of AM were repeated in NE-precontracted arterial segments. For each segment, the AM-induced vasorelaxations were calculated as the percentage of precontracted tension induced by norepinephrine (ED70).
Expression of CL and RAMPs in the rat uterine artery
Total tissue RNA was isolated from the rat uterine artery using RNeasy mini kit (Qiagen, Valencia, CA). DNase 1 treatment was performed as per the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized from 1 μg of total RNA by RT in a total volume of 20 μl using Gene Amp per system 9700 (Perkin-Elmer, NJ) with the following conditions: One cycle at 28 C for 15 min, 42 C for 50 min, 95 C for 5 min.
Quantitative real-time PCR
Real-time quantitative RT-PCR was performed with 1 μl of cDNA using TaqMan Gene Expression Assays for CL (Rn 00562334_m1), RAMP1 (Rn 01427056_ml), RAMP2 (Rn 00824652_g1), and RAMP3 (Rn 00571815_m1) from Applied Biosystems (Applied Biosystems Inc., Carlsbad, CA). Real-time PCR detection was performed on CFX96 sequence-detection system (Bio-Rad, Hercules, CA). Syber green assay was used for amplification of 18S (forward primer; 5′-gctgagaagacggtcgaact-3′, reverse primer; 5′-ttaatgatccttccgcaggt-3′) which served as an endogenous control to standardize the amount of sample RNA added to a reaction. Amplification of each cDNA sample was carried out in 25-μl reactions using Taqman PCR Reagents (Applied Biosystems Inc.) for CL and RAMPs and syber green master mix for 18S (Bio-Rad). Reactions were incubated at 50 C for 2 min (AmpErase UNG Incubation), heated to 95 C for 10 min (AmpliTaq Gold Activation), and cycled according to the following parameters: 95 C for 30s (melt) and 60 C for 1 min (anneal/extend) for a total of 40 cycles. Each group consists of four replicate samples, and each of these samples contained pooled uterine arteries from three rats each. Negative control and ‘no-RT’ control reactions were performed. Negative controls were run in which the RNA templates were replaced by nuclease-free water in the reactions. For no-RT control, nuclease-free water was used in place of the reverse transcriptase.
Data were expressed as the ratio of the DNA amplification amount of 18S against that of the samples to avoid the error from the differences in tissue weight, processing, and loading. The relative amount of every sample was calculated by use of threshold cycle (CT) 18s/CT sample; the higher value denoted the stronger mRNA expression. The CT value is the cycle at which a statistically significant increase in ΔRn (normalized reporter) is first detected. The difference between groups was determined by one-way ANOVA or unpaired t test. The significance level was set at P < 0.05.
Drugs used
Stock solutions of norepinephrine (10 mm), AM (100 μm), AM22-52 (100 mm) and CGRP8-37 (1 mm), l-NAME (100 mm), and TEA (1 mm) were prepared in triple distilled water, aliquoted, and stored in −80 C. Glibenclamide (10 mm) and ODQ (10 mm) were dissolved in dimethyl sulfoxide. AM, AM22-52, and CGRP8-37 were purchased from American Peptide Co., Inc. (Sunnyvale, CA), whereas other chemicals are purchased from Sigma-Aldrich (St. Louis, MO).
Statistical analysis
Data are presented as mean ± se. Relaxation to AM is expressed as 100% of the initial precontraction to NE. The data were analyzed using Prism GraphPad Software (GraphPad Software Inc., San Diego, CA) using appropriate statistical tools. Means of different groups were analyzed by one-way ANOVA and subjected to Newman-Keuls multiple comparison or Bonferroni’s posttests. Student’s paired t test or two-way repeated measures ANOVA were used when comparisons were made between control and drug treatment in the same preparation. P ≤ 0.05 was considered statistically significant. Individual concentration-response curves of AM were subjected to linear regression analysis to determine EC50, which was expressed as pD2 (−log EC50 molar concentration of the agonist).
Results
Effect of pregnancy and female sex steroids on AM-induced uterine artery relaxation
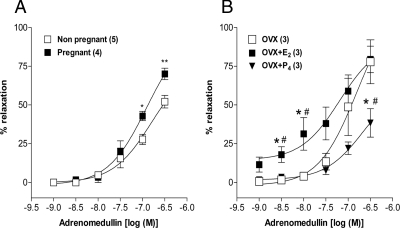
The ED70 concentration of NE produced a sustained contraction in endothelium-intact uterine artery rings from pregnant, nonpregnant at diestrus, OVX, OVX+E2, and OVX+P4 group rats. AM (1–300 nm), added cumulatively at increments of 0.5 log units, relaxed the vascular rings in a concentration-dependent manner in all the groups. The stable relaxation response induced by adrenomedullin within 5 min after administration of each concentration was used for the construction of concentration-response curve. However, the Emax of AM was significantly (P ≤ 0.01) increased in pregnant (70 ± 3.69%) compared with nonpregnant (52.05 ± 4.02%) with pD2 values of 6.9 ± 0.13 and 6.8 ± 0.18, respectively (Fig. 1A). E2 treatment to OVX animals shifted the concentration-response curve to the left with a pD2 and Emax values of 7.2 ± 0.31, 79.4 ± 8.5%, and 6.8 ± 0.32, 77.8 ± 14%, respectively. But, P4 treatment shifted the curve to the right with an Emax value of 38.33 ± 9.2% (Fig. 1B).
Figure 1.
Influence of pregnancy (A) and female sex steroids estradiol or progesterone (B) on the vasodilatory responses of rat uterine artery to AM. Cumulative addition of AM (1–300 nm) produced concentration-dependent uterine artery relaxation in all the groups. *, P ≤ 0.05 (vs. OVX); **, P ≤ 0.01; #, P ≤ 0.05 vs. E2 or P4 (unpaired t test or one-way ANOVA followed by Bonferroni posttest). Data sets in all figures represent mean ± sem, with n values (artery segments from separate animals) presented in parentheses.
Effect of pregnancy and female sex steroids on the expression of mRNA encoding CL and RAMPs
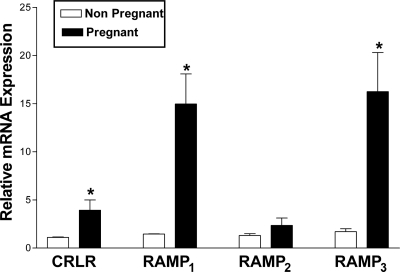
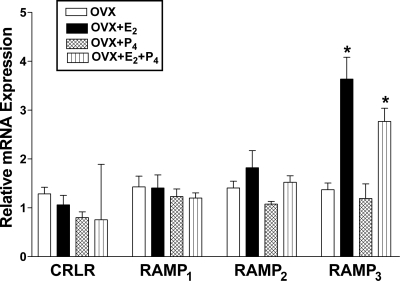
To determine any changes in the expression of the components of AM and its related peptide receptors, the levels of mRNA encoding CL, RAMP1, RAMP2, and RAMP3 in uterine arteries isolated from pregnant, nonpregnant at diestrus, OVX, OVX+E2, OVX+P4, or OVX+E2P4 group rats were measured using real-time quantitative RT-PCR. As shown in Fig. 2, the mRNA levels for CL, RAMP1, and RAMP3 are elevated in the uterine arteries in pregnant compared with nonpregnant rats and RAMP2 is unchanged. There were no significant changes in CL, RAMP1, or RAMP2 mRNA levels in the uterine arteries from OVX rats treated with either E2, P4, or E2+P4 (Fig. 3). RAMP3 mRNA levels were, however, significantly elevated upon E2 or E2+P4 treatments, and P4 administration had no effect at all on any of the receptor components in the uterine artery.
Figure 2.
Real-time quantitative RT-PCR analysis of CL and RAMPs in uterine arteries from nonpregnant rats at diestrus and pregnant rats on d 20. Changes in levels of mRNAs of CL, RAMP1, RAMP2, and RAMP3 relative to that of 18S in the uterine arteries are presented from four replicate samples, and each sample consists of pooled uterine arteries from three animals. *, P ≤ 0.05 (unpaired t test). Bars, respective mean ± sem.
Figure 3.
Real-time quantitative RT-PCR analysis of CL and RAMPs in uterine arteries from ovariectomized rats (OVX) treated with either estradiol (OVX+E2), progesterone alone (OVX+P4), or both (OVX+E2+P4). Changes in levels of mRNAs of CL, RAMP1, RAMP2, and RAMP3 relative to that of 18S in the uterine arteries are presented from four replicate samples, and each of the samples consists of pooled uterine arteries from three animals. *, P ≤ 0.05 (one-way ANOVA followed by Bonferroni posttest). F(3,12) = 14.30.
Effect of AM22-52 and CGRP8-37 on AM-induced uterine artery relaxation
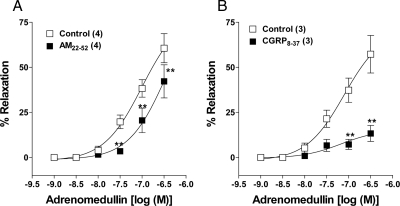
We assessed pharmacologically the receptor subtype involved in the AM-induced relaxation of uterine arteries from pregnant rats, using the receptor antagonists AM22-52 and CGRP8-37. Incubation of uterine artery rings in AM22-52 (10 μm) or CGRP8-37 (10 μm) for 30 min shifted the AM-induced concentration-dependent relaxation response curve to the right (Fig. 4, A and B). However, CGRP8-37 is more potent than AM22-52, which is the character of AM2 receptor (23). The respective pD2 and Emax values were 7.0 ± 0.16, 60.6 ± 8% in control and 6.4 ± 0.43, 42.2 ± 9% after AM22-52 treatment. CGRP8-37 treatment reduced the Emax values from 57.3 ± 10% to 13.3 ± 4.4% (P ≤ 0.01).
Figure 4.
Pharmacological characterization of AM receptors in pregnant rat uterine artery. Inhibitory effect of two kinds of AM receptor antagonists, 10 μm AM22-52 (A) and 10 μm CGRP8-37 (B) on AM-induced relaxation of uterine arterial rings, precontracted by ED70 concentration of norepinephrine. Data represent mean ± sem. *, P ≤ 0.05 (repeated measures ANOVA followed by Bonferroni posttest); **, P ≤ 0.01. F(5,18) = 26.57 (A), F(5,12) = 22.61 (B); n values (artery segments from separate animals) presented in parentheses.
Effect of endothelium on AM-induced uterine artery relaxation
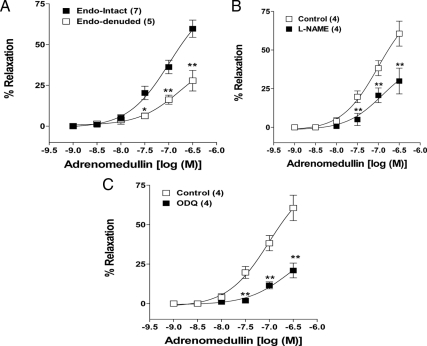
Denudation of endothelium inhibited the AM-induced concentration-dependent relaxation, reducing the Emax values of AM from 59.7 ± 5% (endothelium-intact) to 27.8 ± 6% (endothelium-denuded) (Fig. 5A). The involvement of endothelium was further confirmed by inhibiting the endothelial nitric oxide synthase (eNOS) in endothelium-intact vessel rings. Inhibition of eNOS using l-NAME (100 μm) shifted the concentration-response curve to the right, reducing the Emax of AM from 60.6 ± 8% to 29.9 ± 8% (Fig. 5B). Moreover, inhibition of soluble guanylate cyclase using ODQ (10 μm) also shifted the AM-induced concentration-response curve to the right of same magnitude as inhibition of eNOS reducing the Emax from 60.6 ± 8% to 21 ± 5% (Fig. 5C).
Figure 5.
Involvement of endothelium in AM-induced vasodilatation of pregnant rat uterine artery. Effect of denudation of endothelium (A), inhibition of nitric oxide synthesis by l-NAME (100 μm) (B) and inhibition of guanylate cyclase by ODQ (10 μm) (C) on AM-induced relaxation of uterine arterial rings, precontracted by ED70 concentration of norepinephrine. Data represent mean ± sem. *, P ≤ 0.05 [repeated measures ANOVA (B and C) and ANOVA (A) followed by Bonferroni posttest]; **, P ≤ 0.01. F(5,60) = 32.36 (A), F(5,18) = 23.66 (B), F(5,18) =27.96 (C); n values (artery segments from separate animals) presented in parentheses.
Effect of blockade of ATP-sensitive (KATP) or calcium-activated potassium (KCa) channel on AM-induced uterine artery relaxation
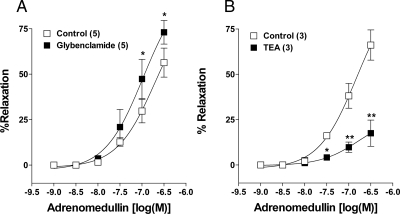
Inhibition of KATP channels by glibenclamide (10 μm) did not affect the relaxation caused by lower concentrations of AM, while higher concentration relaxations were enhanced (Fig. 6A). The pD2 and Emax values of AM in the control (6.7 ± 0.2, 56.3 ± 7%) were increased to 6.9 ± 0.2, 73.0 ± 6%, respectively after preincubation in glibenclamide. Blockade of KCa channels by TEA (1 mm) dramatically inhibited the AM-induced relaxation response in uterine artery rings. Preincubation of uterine arterial rings in TEA for 30 min reduced the Emax of AM-induced concentration-dependent response from 66.1 ± 8% to 17.5 ± 7% (Fig. 6B).
Figure 6.
Signaling mechanisms of AM-induced vasodilatation in pregnant rat uterine artery. Potentiating effect of inhibition of KATP channels by 10 μm glibenclamide (A) and inhibitory effect of blockade of KCa channels by TEA (1 mm) (B) on AM-induced relaxation of uterine arterial rings, precontracted by ED70 concentration of norepinephrine. Data represent mean ± sem. *, P ≤ 0.05 (repeated measures ANOVA followed by Bonferroni posttest); **, P ≤ 0.01. F(5,24) =28.31 (A), F(5,12) =21.72 (B); n values (artery segments from separate animals) presented in parentheses.
Discussion
The important findings in this study are: 1) AM is able to relax uterine artery, 2) the relaxation response is enhanced by pregnancy and estradiol, 3) both pregnancy and estradiol amplified RAMP3 expression in uterine arteries while progesterone had no effect, 4) AM-induced relaxation is partly endothelium-dependent, 5) the receptor antagonist CGRP8-37 is more potent than AM22-52 in inhibiting the AM relaxation, 6) AM uses the classical NO–cGMP pathway, and 7) KCa channels appear to mediate the AM-induced relaxation in uterine artery.
AM-induced relaxation of uterine artery and influence of pregnancy and sex steroids
Immunoreactivity for AM is widely distributed throughout the cardiovascular system (25). Plasma and tissue AM levels are reported to be increased in patients with cardiovascular disease, such as hypertension, renal failure, etc. (26). Importantly, plasma AM levels are elevated during pregnancy in both humans (7) and rats (8) and also in preeclamptic conditions (7). Immunohistochemical staining of AM in uterine artery was found to be intense in pregnant compared with nonpregnant rats (10), but so far it is not known whether AM relaxes uterine artery or just the synthesis is altered with pregnancy and other cardiovascular adaptive conditions. Therefore, in this study, we demonstrate that AM relaxes uterine artery and the sensitivity of the artery to AM is increased during pregnancy. Previously, we have shown that the in vivo hypotensive effect and the in vitro vasorelaxation sensitivity of mesenteric artery to AM were enhanced by pregnancy (20). Thus it is possible that pregnancy has a positive global influence on the vascular responsiveness of both resistance and uterine vessels to AM, which may play a vital role in the cardiovascular adaptation and increased uterine blood flow that occur during pregnancy. However, it has to be noted that the reported human circulating plasma AM levels were in picomolar range, while the concentrations required to produce relaxation of vessels in vitro in this study were in nanomolar to submicromolar range. There could be several possible reasons for this high concentration requirement. In vitro, we are measuring the vessels both stretched and precontracted by norepinephrine, which is in striking contrast to the in vivo setting where the small vessels are in gentle tone. It could probably be enough at picomolar concentrations in vivo to produce the required vasodilation. Also, AM can act in an autocrine/paracrine mode at the uterine artery level, which may significantly differ from the circulating plasma AM levels. The in vivo AM effects at the physiological levels are clearly supported by the hypertensive (increased vascular tone) effects of the AM antagonist, AM22-52 (27).
Mesenteric artery relaxation induced by AM is reported to be influenced by gender and female sex steroids. Both estradiol and progesterone increased the AM-induced vasodilation in rat mesenteric artery (21). It was surprising as progesterone is frequently described as opposing the actions of estradiol. In the present study, only estradiol sensitized the uterine artery to AM-induced relaxation while progesterone had no effect, if not inhibitory. Estradiol increases blood flow to a variety of tissues, however its greatest effects occur in reproductive tissues, especially the nonpregnant uterus (28). Although progesterone is considered to be the major hormone of pregnancy, it should be noted that the plasma estradiol concentrations also increase significantly during gestation (28,29,30). Also, the mRNA data of the AM receptor components in the combination of estradiol plus progesterone group show increased RAMP3 levels which correlate well with the mRNA data for pregnant groups. Thus the enhanced responsiveness of uterine artery to AM during pregnancy could be due to estradiol-induced increase in RAMP3 expression. The continuous exposure of the cardiovascular system to estradiol has been postulated to in part mediate and/or maintain these hemodynamic changes (31,32,33). We have previously reported the AM effects on mesenteric artery in the context of pregnancy (20,34). Comparing the uterine artery responsiveness to the mesenteric artery, the AM is more potent and efficacious in the mesenteric artery. This should not undermine the effect of AM in uterine artery as the role played may be different. It has to be noted that the mesenteric artery responses are critical for the arterial pressure as a resistance vessel while the uterine artery is critical for blood flow to uterus as a conduit vessel. However, it is clear that the sensitivity as well as sex steroid influence of these vessels to AM is different. Because the AM-induced relaxation is enhanced during pregnancy, sensitized by estradiol in bilaterally ovariectomized animals, and immunolocalized staining for AM in the uterine arteries is more intense in pregnant than nonpregnant rats, it is tempting to suggest that AM acts in an autocrine/paracrine manner on the vessels, more effectively in response to continuous exposure to estradiol. However, it should also be noted that the overall increase in the magnitude of uterine blood flow in vivo may not correspond exactly to the increase in sensitivity to AM seen in this study in vitro, possibly because AM may not be the sole endogenous molecule responsible for the high magnitude increase in the uterine blood flow.
Regulation of mRNA levels of AM receptor components by pregnancy, progesterone, and estradiol
Receptors for AM are heterodimeric complexes of the CL together with RAMP2 (AM1 receptor) or RAMP3 (AM2 receptor). The three potential consequences of interaction of the RAMPs with the CL are as follows: transport of the receptor (CL) to the cell surface, modification of the receptor glycosylation, and direct and indirect modification of the ligand-binding site through association with the receptor at the cell surface (35). Makino and co-workers (19) showed altered gene expression of AM receptor components in patients with pregnancy-induced hypertension. A significant negative correlation was shown between the RAMP2 mRNA levels in the umbilical artery and uterine muscle and blood pressure, suggesting an important role for the reduced expression of AM receptor components in pregnancy-induced hypertension. Earlier, we have demonstrated changes in the gene expression for CL, RAMP1, RAMP2, and RAMP3 in rat uterine tissue during pregnancy, labor, and by steroid hormone treatments (36), suggesting dynamic changes of these receptor components by respective physiological state. Additionally, in the uterine artery the CGRP-induced relaxation and the levels of mRNA, measured using semi-quantitative RT-PCR for CL and RAMP1, were also increased in pregnant rats on d 18, compared with that in nonpregnant rats (37). In the current study, we examined changes in all components of CGRP/AM family peptides at the mRNA level using real-time quantitative RT-PCR method. The elevated mRNA level of CL and RAMP1 in pregnant rat uterine artery we observed in this study are consistent with our previous report (37). In the current study, both pregnancy and estradiol amplified the RAMP3 expression in the uterine artery. These mRNA level data correlate well with the relaxation studies where both pregnancy and estradiol augmented the AM-induced relaxation. Estradiol influence on AM receptor components is also demonstrated in OVX mice exposed to estradiol for 6 h, where uterine gene expression profiles developed by DNA microarrays showed about 20.4-fold increase in RAMP3 expression (38). Because there were no changes in RAMP2 mRNA levels with E2 and in pregnant rats, it is less likely the involvement of RAMP2 in AM-induced relaxation of the uterine artery in these animals. Although there is significant elevation in RAMP1 mRNA levels in pregnant rat uterine arteries, its contribution to AM-induced relaxation is negligible as it is well accepted that AM binding and activity does not involve RAMP1. Thus, it is possible that increased expression of RAMP3 by pregnancy and estradiol may be sufficient to enhance the probability of formation of AM2 receptors, by increasing the interaction between CL and RAMP3. The increased vasodilatory response of uterine artery seen with pregnancy and estradiol treatment may be due to increased expression of AM2 receptor components.
Pharmacological characterization of AM receptors in uterine artery
The AM-selective receptor (AM1) can be antagonized by the weak AM peptide antagonist AM22-52, whereas the AM2 receptor can respond to both CGRP and AM, which can be antagonized more potently by CGRP8-37 compared with AM22-52 (23). Previously Hay et al. (39) have demonstrated the effects of the antagonist fragments of human AM and CGRP (AM22-52 and CGRP8-37) in inhibiting AM at CL/RAMP2 and CL/RAMP3 receptors transiently expressed in COS 7 cells. AM22-52 (10 μm) antagonized AM at the CL/RAMP2 complex, whereas 10 μm CGRP8-37 was an effective antagonist to AM at the CL/RAMP3 complex. The pA2 values of AM22-52 and CGRP8-37 estimated in functional studies for AM1 receptors were in the order of 7 and around 6, respectively, whereas those values for AM2 receptors were less than 5.5 and 6.18, respectively (40). In the present study, the AM-induced vasodilatation was inhibited by both 10 μm CGRP8-37 and 10 μm AM22-52, but CGRP8-37 appears to be more potent than AM22-52, which is the character of AM2 receptor subtype. The changes seen at the mRNA levels of the AM receptor components is also with RAMP3, component of AM2 receptor subtype, indicating that AM2 receptor subtype predominantly mediates the vasodilatory effect of AM in uterine artery. The pharmacological characteristics of AM receptors in the uterine artery and the lack of changes in RAMP2 mRNA levels with pregnancy and E2 treatment may indicate noninvolvement of RAMP2 in pregnancy-related changes in AM actions. It is possible that increased expression of RAMP3 is enough to increase the probability of functional AM2 receptor complex formation.
Mechanisms of AM-induced uterine artery relaxation
We examined the mechanisms by which AM caused uterine artery relaxation in pregnant rats. AM-induced uterine artery relaxation is predominantly endothelium-dependent as denudation of endothelium dramatically reduced the concentration-dependent relaxation response to AM. It is interesting to note that the mesenteric artery relaxation to AM is endothelium-independent in ovariectomized rats (21) and partially endothelium-dependent in pregnant rats (20). AM also uses the classical NO-cGMP pathway in the uterine artery as inhibition of eNOS or soluble guanylate cyclase significantly decreased its relaxation response. The difference in uterine artery (considered conduit) seen with the resistance vessel-like mesenteric artery with respect to the endothelium dependence and NO–cGMP mediation has to be pursued to determine whether the difference is due to the overall nature of the conduit vs. resistance vessels or it is the general influence of gestation on all the vessels, as receptor-mediated release of NO by uterine artery is increased both in guinea pigs (41) and humans (15). ATP-sensitive K-channels were reported to be involved in AM-induced relaxation in dog coronary artery (42), but in the rat uterine artery inhibition of KATP channels did not inhibit the relaxation caused by AM and instead enhanced the relaxation caused by the maximal concentrations of AM. In an interesting study by Chan et al. (43), glibenclamide elevated the intracellular Ca2+ levels in cultured rat aortic endothelial cells. They also suggested that glibenclamide-induced endothelium-dependent relaxation in rat isolated aortic rings involves NO release, and this effect may be related to its stimulatory effect on endothelial Ca2+ levels. Thus it is possible that the enhanced relaxation to AM observed in the presence of glibenclamide may be due to enhanced release of NO from endothelium due to the increase in [Ca2+]i after inhibition of endothelial KATP channels. But it is clear that AM by itself does not involve KATP channels of either the endothelial or smooth muscle cells of uterine artery. In contrast, KCa channels are involved in the AM-induced relaxation of uterine artery as the blockade of KCa channels by TEA, at concentrations that inhibited single arterial Maxi-K+ BK channels (44), considerably inhibited the relaxation response, although there is no direct electrophysiological evidence. These findings suggest that AM may activate KCa channels and that the resultant membrane hyperpolarization would inhibit calcium influx via voltage-gated calcium channels, causing the uterine artery relaxation.
In conclusion, the AM-induced uterine artery relaxation is predominantly endothelium-dependent and NO–cGMP-mediated, and KCa channels are involved in this process. Sensitivity of uterine artery to AM-induced relaxation is increased with pregnancy or estradiol treatment by increasing RAMP3 expression, suggesting an important role for AM in regulating the uterine hemodynamics, probably maintaining uterine blood flow during pregnancy and in pre- and postmenopausal cardiovascular adaptation differences.
Footnotes
This work was supported by National Institutes of Health Grants HL-58144 and HL-72620.
Disclosure Summary: The authors have nothing to declare.
First Published Online July 14, 2010
Abbreviations: AM, Adrenomedullin; BW, body weight; CGRP, calcitonin gene-related peptide; CL, calcitonin receptor-like receptor; E2, estradiol; eNOS, endothelial nitric oxide synthase; l-NAME, NG-nitro-l-arginine methyl ester; NE, norepinephrine; NO, nitric oxide; NO-cGMP, NO-cyclic guanosine monophosphate; OVX, ovariectomized; P4, progesterone; PSS, physiological salt solution; RAMP, receptor-activity-modifying protein; TEA, tetraethylammonium.
References
- Magness RR 1998 Maternal cardiovascular and other physiologic responses to the endocrinology of pregnancy. In: Fuller B, ed. Endocrinology of pregnancy. Totowa, NJ: Humana Press, Inc.; 507–539 [Google Scholar]
- Rosenfeld CR 1977 Distribution of cardiac output in ovine pregnancy. Am J Physiol 232:H231–H235 [DOI] [PubMed] [Google Scholar]
- Greenwald P 1963 Chronic fetal distrus and placental insufficiency. Neonate 5:215–219 [DOI] [PubMed] [Google Scholar]
- Leiberman JR, Wiznitzer A, Glezerman M, Feldman B, Levy J, Sharoni Y 1993 Estrogen and progesterone receptors in the uterine artery of rats during and after pregnancy. Eur J Obstet Gynecol Reprod Biol 51:35–40 [DOI] [PubMed] [Google Scholar]
- Peeters LL, Grutters G, Martin Jr CB 1980 Distribution of cardiac output in the unstressed pregnant guinea pig. Am J Obstet Gynecol 138:1177–1184 [DOI] [PubMed] [Google Scholar]
- Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T 1993 Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun 192:553–560 [DOI] [PubMed] [Google Scholar]
- Di Iorio R, Marinoni E, Letizia C, Alò P, Villaccio B, Cosmi EV 1998 Adrenomedullin, a new vasoactive peptide, is increased in preeclampsia. Hypertension 32:758–763 [DOI] [PubMed] [Google Scholar]
- Jerat S, Kaufman S 1998 Effect of pregnancy and steroid hormones on plasma adrenomedullin levels in the rat. Can J Physiol Pharmacol 76:463–466 [DOI] [PubMed] [Google Scholar]
- Penchalaneni J, Wimalawansa SJ, Yallampalli C 2004 Adrenomedullin antagonist treatment during early gestation in rats causes fetoplacental growth restriction through apoptosis. Biol Reprod 71:1475–1483 [DOI] [PubMed] [Google Scholar]
- van der Heijden O, Essers YP, van Eyndhoven HW, Spaanderman ME, Aardenburg R, van Eys GJ, Peeters LL 2005 Vascular expression of adrenomedullin is increased in Wistar rats during early pregnancy. Eur J Obstet Gynecol Reprod Biol 123:35–40 [DOI] [PubMed] [Google Scholar]
- Weiner CP, Lizasoain I, Baylis SA, Knowles RG, Charles IG, Moncada S 1994 Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc Natl Acad Sci USA 91:5212–5216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhimschi I, Yallampalli C, Chwalisz K, Garfield RE 1995 Pre-eclampsia-like conditions produced by nitric oxide inhibition: effects of L-arginine, D-arginine and steroid hormones. Hum Reprod 10:2723–2730 [DOI] [PubMed] [Google Scholar]
- Grbović L, Jovanović A 1996 Effect of the vascular endothelium on contractions induced by prostaglandin F2 α in isolated pregnant guinea pig uterine artery. Hum Reprod 11:2041–2047 [DOI] [PubMed] [Google Scholar]
- Grbović L, Jovanović A 1997 Indomethacin depresses prostaglandin F2 α-induced contraction in guinea-pig uterine artery with both intact and denuded endoth. Prostaglandins 53:371–379 [DOI] [PubMed] [Google Scholar]
- Nelson SH, Steinsland OS, Suresh MS, Lee NM 1998 Pregnancy augments nitric oxide-dependent dilator response to acetylcholine in the human uterine artery. Hum Reprod 13:1361–1367 [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Kanamaru K, Sugiyama Y, Murata Y 1992 Endothelium-derived relaxation of the pregnant and nonpregnant canine uterine artery. J Reprod Med 37:529–533 [PubMed] [Google Scholar]
- Jovanović A, Grbović L, Jovanović S 1995 Effect of the vascular endothelium on noradrenaline-induced contractions in non-pregnant and pregnant guinea-pig uterine arteries. Br J Pharmacol 114:805–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanović A, Jovanović S, Grbović L 1997 Endothelium-dependent relaxation in response to acetylcholine in pregnant guinea-pig uterine artery. Hum Reprod 12:1805–1809 [DOI] [PubMed] [Google Scholar]
- Makino Y, Shibata K, Makino I, Kangawa K, Kawarabayashi T 2001 Alteration of the adrenomedullin receptor components gene expression associated with the blood pressure in pregnancy-induced hypertension. J Clin Endocrinol Metab 86:5079–5082 [DOI] [PubMed] [Google Scholar]
- Ross GR, Yallampalli C 2007 Vascular hyperresponsiveness to adrenomedullin during pregnancy is associated with increased generation of cyclic nucleotides in rat mesenteric artery. Biol Reprod 76:118–123 [DOI] [PubMed] [Google Scholar]
- Ross GR, Chauhan M, Gangula PR, Reed L, Thota C, Yallampalli C 2006 Female sex steroids increase adrenomedullin-induced vasodilation by increasing the expression of adrenomedullin2 receptor components in rat mesenteric artery. Endocrinology 147:389–396 [DOI] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM 1998 RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393:333–339 [DOI] [PubMed] [Google Scholar]
- Brain SD, Grant AD 2004 Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev 84:903–934 [DOI] [PubMed] [Google Scholar]
- Wang Y, Bukoski RD 1999 Use of acute phenolic denervation to show the neuronal dependence of Ca2+-induced relaxation of isolated arteries. Life Sci 64:887–894 [DOI] [PubMed] [Google Scholar]
- Ichiki Y, Kitamura K, Kangawa K, Kawamoto M, Matsuo H, Eto T 1994 Distribution and characterization of immunoreactive adrenomedullin in human tissue and plasma. FEBS Lett 338:6–10 [DOI] [PubMed] [Google Scholar]
- Nishikimi T 2005 Circulating adrenomedullin in cardiovascular disease. New York: Springer; 105–129 [Google Scholar]
- Nishimatsu H, Suzuki E, Nagata D, Moriyama N, Satonaka H, Walsh K, Sata M, Kangawa K, Matsuo H, Goto A, Kitamura T, Hirata Y 2001 Adrenomedullin induces endothelium-dependent vasorelaxation via the phosphatidylinositol 3-kinase/Akt-dependent pathway in rat aorta. Circ Res 89:63–70 [DOI] [PubMed] [Google Scholar]
- Magness RR, Phernetton TM, Zheng J 1998 Systemic and uterine blood flow distribution during prolonged infusion of 17β-estradiol. Am J Physiol 275:H731–H743 [DOI] [PubMed] [Google Scholar]
- Challis JR, Harrison FA, Heap RB 1971 Uterine production of oestrogens and progesterone at parturition in the sheep. J Reprod Fertil 25:306–307 [DOI] [PubMed] [Google Scholar]
- Magness RR, Rosenfeld CR, Carr BR 1991 Protein kinase C in uterine and systemic arteries during the ovarian cycle and pregnancy. Am J Physiol 260:E464–E470 [DOI] [PubMed] [Google Scholar]
- Longo LD 1983 Maternal blood volume and cardiac output during pregnancy: a hypothesis of endocrinologic control. Am J Physiol 245:R720–R729 [DOI] [PubMed] [Google Scholar]
- Magness RR, Rosenfeld CR 1989 Local and systemic estradiol-17 β: effects on uterine and systemic vasodilation. Am J Physiol 256:E536–E542 [DOI] [PubMed] [Google Scholar]
- Magness RR, Phernetton TM, Zheng J 1998 Systemic and uterine blood flow distribution during prolonged infusion of 17β-estradiol. Am J Physiol 275:H731–H743 [DOI] [PubMed] [Google Scholar]
- Ross GR, Yallampalli C 2006 Endothelium-independent relaxation by adrenomedullin in pregnant rat mesenteric artery: role of cAMP-dependent protein kinase A and calcium-activated potassium channels. J Pharmacol Exp Ther 317:1269–1275 [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Nuki C, Saito A, Takasaki K 1990 Adrenergic modulation of calcitonin gene-related peptide (CGRP) containing nerve-mediated vasodilation in the rat mesenteric resistance vessel. Brain Res 506:287–290 [DOI] [PubMed] [Google Scholar]
- Thota C, Gangula PR, Dong YL, Yallampalli C 2003 Changes in the expression of CRLR, RAMP1, RAMP2 and RAMP3 in rat uterus during pregnancy, labor and by steroid hormone treatments. Biol Reprod 69:1432–1437 [DOI] [PubMed] [Google Scholar]
- Gangula PR, Thota CS, Wimalawansa SJ, Bukoski RD, Yallampalli C 2003 Mechanisms involved in calcitonin gene-related peptide-induced relaxation in pregnant rat uterine artery. Biol Reprod 69:1635–1641 [DOI] [PubMed] [Google Scholar]
- Watanabe H, Suzuki A, Mizutani T, Khono S, Lubahn DB, Handa H, Iguchi T 2002 Genome-wide analysis of changes in early gene expression induced by oestrogen. Genes Cells 7:497–507 [DOI] [PubMed] [Google Scholar]
- Hay DL, Howitt SG, Conner AC, Schindler M, Smith DM, Poyner DR 2003 CL/RAMP2 and CL/RAMP3 produce pharmacologically distinct adrenomedullin receptors: a comparison of effects of adrenomedullin22–52, CGRP8–37 and BIBN4096BS. Br J Pharmacol 140:477–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DL, Conner AC, Howitt SG, Smith DM, Poyner DR 2004 The pharmacology of adrenomedullin receptors and their relationship to CGRP receptors. J Mol Neurosci 22:105–113 [DOI] [PubMed] [Google Scholar]
- Weiner CP, Martinez E, Chestnut DH, Ghodsi A 1989 Effect of pregnancy on uterine and carotid artery response to norepinephrine, epinephrine, and phenylephrine in vessels with documented functional endothelium. Am J Obstet Gynecol 161:1605–1610 [DOI] [PubMed] [Google Scholar]
- Sabates BL, Pigott JD, Choe EU, Cruz MP, Lippton HL, Hyman AL, Flint LM, Ferrara JJ 1997 Adrenomedullin mediates coronary vasodilation through adenosine receptors and KATP channels. J Surg Res 67:163–168 [DOI] [PubMed] [Google Scholar]
- Chan W, Yao X, Ko W, Huang Y 2000 Nitric oxide mediated endothelium-dependent relaxation induced by glibenclamide in rat isolated aorta. Cardiovasc Res 46:180–187 [DOI] [PubMed] [Google Scholar]
- Langton PD, Nelson MT, Huang Y, Standen NB 1991 Block of calcium-activated potassium channels in mammalian arterial myocytes by tetraethylammonium ions. Am J Physiol 260:H927–H934 [DOI] [PubMed] [Google Scholar]