Abstract
Steroid hormones are formed by the successive action of enzymes that are localized in mitochondria and the endoplasmic reticulum (ER). Compartmentalization of these enzymes in different subcellular organelles dictates the need for efficient transfer of intermediary metabolites between the mitochondrion and ER; however, the molecular determinants that regulate interorganelle substrate exchange are unknown. The objective of this study was to define the molecular mechanism by which adrenocorticotropin (ACTH) signaling regulates communication between mitochondria and the ER during steroidogenesis. Using live cell video confocal microscopy, we found that ACTH and dibutyryl cAMP rapidly increased the rate of mitochondrial movement. Inhibiting tubulin polymerization prevented both basal and ACTH/cAMP-stimulated mitochondrial trafficking and decreased cortisol secretion. This decrease in cortisol secretion evoked by microtubule inhibition was paralleled by an increase in dehydroepiandrosterone production. In contrast, treatment with paclitaxel to stabilize microtubules or latrunculin B to inhibit actin polymerization and disrupt microfilament organization increased both mitochondrial trafficking and cortisol biosynthesis. ACTH-stimulated mitochondrial movement was dependent on RhoA and the RhoA effector, diaphanous-related homolog 1 (DIAPH1). ACTH signaling temporally increased the cellular concentrations of GTP-bound and Ser-188 phosphorylated RhoA, which promoted interaction with DIAPH1. Expression of a dominant-negative RhoA mutant or silencing DIAPH1 impaired mitochondrial trafficking and cortisol biosynthesis and concomitantly increased the secretion of adrenal androgens. We conclude that ACTH regulates cortisol production by facilitating interorganelle substrate transfer via a process that is mediated by RhoA and DIAPH1, which act to coordinate the dynamic trafficking of mitochondria.
These studies define how the cytoskeleton, RhoA, and DIAPH1 control cortisol and DHEA production in the adrenal cortex.
Glucocorticoids are essential regulators of varied physiological processes including the inflammatory response, gluconeogenesis, and vascular tone. These molecules are synthesized in the adrenal cortex from cholesterol via a series of reactions that are catalyzed by members of the cytochrome P450 superfamily and hydroxysteroid dehydrogenases (1,2,3,4). Steroidogenesis is tightly regulated by the peptide hormone ACTH, which acts by varied, temporally distinct mechanisms to increase the transcription of genes required for steroidogenesis, facilitate the uptake and intracellular delivery of free cholesterol, promote electron transfer, and modulate the activity of steroidogenic enzymes (5,6,7).
Steroid hormone biosynthesis in the adrenal cortex takes place in two organelles, the mitochondrion and the endoplasmic reticulum. Both the first step and last reactions in cortisol production occur in mitochondria, with intermediary reactions taking place in the ER. Although mechanisms that control the transcription and enzymatic activity of steroidogenic genes are extensively characterized, the factors that regulate interorganelle substrate delivery are not well understood.
Studies using chemical inhibitors to modulate the polymerization of cytoskeletal proteins have implicated both microtubules and microfilaments as key regulators of steroid hormone biosynthesis (8,9,10,11,12,13,14,15,16). A relationship between steroidogenesis and the cytoskeleton, in which microtubule depolymerization agents have been found to impair steroid hormone production, has also been identified (10,11,17,18,19,20,21). Moreover, microscopic studies in Y1 mouse adrenal cells has found that lipid droplets move along microtubule tracts (22). Collectively these data point to a key role for the cytoskeletal architecture in steroid hormone production. In contrast to studies carried out in neurons, in which mitochondria have also been visualized in association with microfilaments (23), microtubules (24,25), and intermediate filaments (26,27) and in which motor proteins, such as kinesins, facilitate the movement of these organelles along cytoskeletal fibers (28,29,30,31,32), the precise molecular mechanism by which cytoskeletal proteins control steroidogenesis is unclear. Furthermore, the specific protein(s) that couple steroid hormone output to microfilaments and/or microtubules are unknown.
Rho GTPases are a family of proteins that use the hydrolysis of GTP to transduce a wide variety of extracellular and intracellular signals, including cell migration, cytokinesis, vesicular trafficking, and phagocytosis (33,34,35,36). Signal transduction cascades stimulate the activation of Rho proteins by facilitating the exchange of GDP for GTP. In the GTP-bound form, Rho proteins act to relay extracellular signals to intracellular effectors. Diaphanous-related homolog (DIAPH)-1 is a member of the diaphanous-related formin family of Rho effector proteins that coordinate cell motility and migration by regulating the organization of microtubules and microfilaments (37). DIAPH proteins contain an N-terminal Rho-binding domain, two formin-homology regions, and a C-terminal diaphanous-autoregulatory domain (DAD) that stabilizes the proteins in an inactive conformation in the absence of Rho binding. Binding of active Rho stimulates dissociation of intermolecular interactions between the N-terminal region and the DAD, thereby activating the protein (38,39,40,41,42). Thus, truncation mutants that lack the DAD or Rho-binding domain are constitutively active (40,41,43,44). Activation of signaling pathways promote the release of the intermolecular autoinhibitory interactions via a two-step mechanism that is accelerated by the presence of Rho-GTP (45). In this study, we investigated the mechanism by which ACTH signaling controls mitochondrial trafficking during steroid hormone production in human H295R adrenocortical cells.
Materials and Methods
Reagents
Dibutyryl cAMP (Bt2cAMP) was obtained from Sigma (St. Louis, MO) and MitoTracker Red from Invitrogen (Carlsbad, CA). ACTH was purchased from American Peptide, Inc. (Sunnyvale, CA) and solubilized in 5% acetic acid. Colchicine, nocodazole, latrunculin B, and paclitaxel were obtained from EMD Biosciences (La Jolla, CA).
Cell culture
H295R adrenocortical cells (46,47) were generously donated by Dr. William E. Rainey (Medical College of Georgia, Augusta, GA) and cultured in DMEM/F12 medium (Mediatech, Manassas, VA) supplemented with 10% Nu-Serum I (BD Biosciences, Palo Alto, CA), 1% ITS Plus (BD Biosciences), antibiotics, and antimycotics.
Quantification of intracellular cAMP
Cells were treated for 5–30 min with 5 nm ACTH. The concentration of intracellular cAMP formed in response to ACTH stimulation was determined by ELISA (Assay Designs, Ann Arbor, MI), according to the manufacturer’s protocol. Data obtained were normalized to the total cellular protein concentration of each sample.
Confocal microscopy and time-lapsed video imaging
Cells were plated onto coverslips and confocal images were collected in control and treated cells using an LSM510 confocal microscope (Carl Zeiss Inc., Thornwood, NY) equipped with a helium-neon coherent laser. Emissions were collected with a C-apochromat 40 1.3 NA oil immersion objective (Zeiss) using a 560-nm long-pass filter. Some cells were transfected with 50 ng pcDNA3-EGFP-RhoA, pcDNA3-EGFP-RhoA(T19N), pEGFP-DIAPH1, or pEGFP-DIAPH1(ΔN3) using Gene Juice (EMD Biosciences) and Optimem (Invitrogen). Transfection efficiency for green fluorescent protein (GFP)-tagged plasmids ranged from 64% for RhoA plasmids to 85% for DIAPH1 constructs. RhoA expression plasmids were obtained from Addgene (Cambridge, MA), and DIAPH1 expression plasmids were generously donated by Dr. Shuh Narumiya (Kyoto University Faculty of Medicine, Kyoto, Japan). The Zeiss imaging physiology platform was used to determine the velocity of movement of individual mitochondria from video-taped recordings. To quantify the rate of movement, the time of exposure of each frame was set to 1 sec, with a 3-sec interval between successive frames. The track of mitochondria was obtained by subtracting the change in position after each frame interval. Only mitochondria that changed position during a given time interval were calculated. For these mobile mitochondria, the translocation between neighbor frames was measured and the mean rate of movement was calculated. Each experiment was performed at least three times and the movement of at least 20 mitochondria was analyzed in each experimental condition.
Cortisol and dehydroepiandrosterone (DHEA) assays
Cells were cultured in 12-well plates and transfected and/or treated with 0.4 mm Bt2cAMP, 5 nm ACTH, 1 μm nocodazole, 1 μm colchicine, 1 μm latrunculin B, and 2 μm paxitaxel for 24 h. Cortisol or DHEA released into the media was determined in triplicate against standards made up in DME/F12 medium by ELISA (Diagnostic Systems Corp., Houston, TX). Results are expressed as picograms per milligram cellular protein.
Rho activation assays
Cells were treated for time periods ranging from 5 to 30 min with 50 nm ACTH or 0.4 mm Bt2cAMP. The amount of activated Rho in cell extracts was determined using a Rho activation kit (Assay Designs) according to the manufacturer’s instructions.
Coimmunoprecipitation and Western blotting
H295R cells were cultured in 100-mm dishes, treated with ACTH (50 nm), Bt2cAMP (0.4 mm), and/or H89 (10 μm) for 15, 30, or 60 min, and whole-cell extracts were prepared in radioimmunoprecipitation assay buffer (containing 1× protease inhibitors; EMD Biosciences). Precleared lysates were incubated overnight with 30 μl protein A/G Sepharose beads (Santa Cruz Biotechnology, Santa Cruz, CA) and 5 μg anti-DIAPH1 (Millipore, Bedford, MA) or anti-GFP (Sigma). Beads were washed twice in radioimmunoprecipitation assay and twice in PBS, separated by SDS-PAGE, and transferred to polyvinyl difluoride membranes (Millipore). Western blotting was carried out using antiphospho Ser-188 RhoA antibody (Santa Cruz). Blots were developed with enhanced chemifluorescence (Amersham Biosciences, Piscataway, NJ).
RNA interference
Cells plated onto coverslips or 12-well dishes and transfected with 75 nm nonspecific small interfering RNA (siRNA) oligonucleotides or siRNA oligonucleotides directed against DIAPH1 (Dharmacon, Lafayette, CO) for 72 h using HiPerfect (QIAGEN, Valencia, CA) in Optimem (Invitrogen). The amount of steroid hormones produced was determined as described above. Protein expression of DIAPH1 was assessed in lysates isolated from transfected cells by SDS-PAGE and Western blotting.
Statistical analysis
One-way ANOVA, Tukey-Kramer multiple comparison, and unpaired Student t tests were performed using GraphPad InStat software (GraphPad Software Inc., San Diego, CA). Significant differences from a compared value were defined as P < 0.05, P < 0.01, or P < 0.001 and denoted by asterisks (*) or carats (^).
Results
ACTH rapidly increases mitochondrial movement in H295R cells
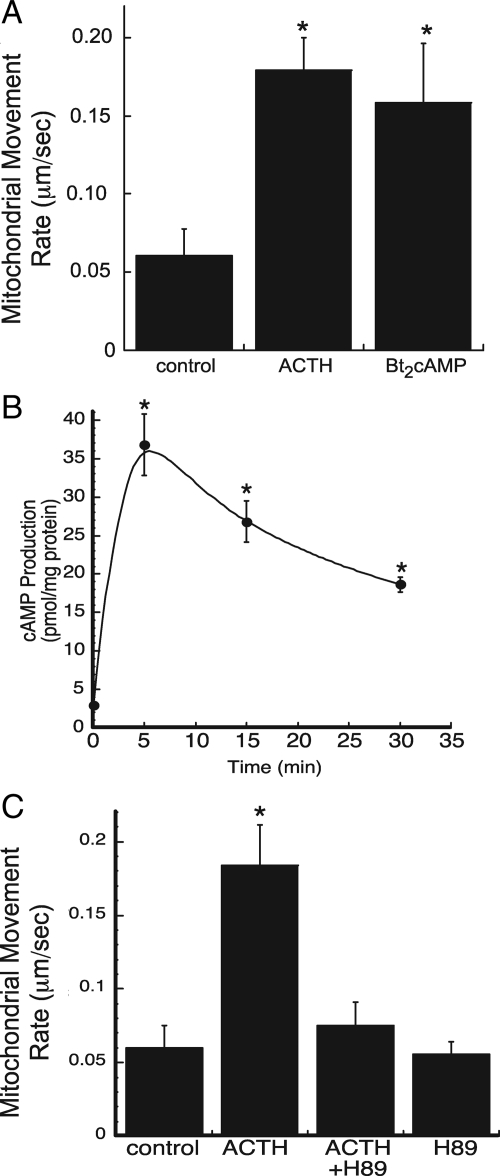
To examine the mechanism by which ACTH regulates substrate trafficking between mitochondria and ER, we carried out real-time video imaging on MitoTracker Red-labeled (Invitrogen) H295R human adrenocortical cells. The rate of mitochondrial movement was quantified in untreated, ACTH-, or Bt2cAMP-treated cells over 5-min time periods. We calculated the rate of movement of mitochondria exhibiting saltatory, unidirectional movement (48,49,50). Both ACTH and Bt2cAMP increased mitochondrial movement by approximately 2.7-fold (Fig. 1A and Supplemental Videos 1 and 2, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). Experiments using live cell imaging to track the rate of mitochondrial movement indicated that neither ACTH nor Bt2cAMP increased the rate of movement of all mitochondria. We postulate that this may be due to local concentrations of stimulus when treating the cells; however, the precise reason for the lack of increased mitochondrial movement in all cells examined is unknown.
Figure 1.
ACTH/cAMP stimulate mitochondrial movement. A, H295R human adrenocortical cells were plated on coverslips and mitochondria labeled with MitoTracker Red (Invitrogen). Mitochondrial movement was observed by time-lapsed fluorescence video microscopy in control, ACTH- (5 nm), and Bt2cAMP (0.4 mm)-treated cells. Imaging was carried out on at least five separate occasions and the rates of at least 20 mitochondria quantified on each occasion. Error bars, sd. *, Statistically significant, P < 0.05. B, Cells were treated for 5–30 min with 5 nm ACTH and the levels of intracellular cAMP quantified by ELISA. cAMP concentrations are normalized to total cellular protein content and data graphed represent the mean ± sem of three experiments, each carried out in triplicate. *, Statistical significance, P < 0.05. C, Cells were plated onto glass coverslips, labeled with MitoTracker Red, and treated with 10 μm H89 and/or 5 nm ACTH. Imaging was performed on three separate cell preparations and the rates of approximately 25 mitochondria quantified on each occasion. *, Statistically significant difference compared with untreated control, P < 0.001.
Although recent work by Janes et al. (51) demonstrated that H295R cells express the melanocortin 2 receptor and respond to ACTH by increasing intracellular cAMP and activating ERK (51), the production of cAMP in the H295R cell line has been shown to be modest in comparison with the Y1 murine adrenal cell line (52,53). To determine whether the stimulatory effects of ACTH are mediated by cAMP, we quantified the concentrations of intracellular cAMP released from H295R cells treated for time periods ranging from 5 to 30 min with 5 nm ACTH. As shown in Fig. 1B, within 5 min, ACTH maximally increases intracellular cAMP. Although the concentrations of the second-messenger decline at the 15- and 30-min time points, intracellular cAMP levels remain significantly higher than the untreated control group. The ability of ACTH to increase the rate of mitochondrial movement requires protein kinase A (PKA) activity, as evidenced by the ability of H89 to attenuate ACTH-stimulated mitochondrial movement (Fig. 1C).
cAMP-stimulated mitochondrial trafficking requires microtubule polymerization
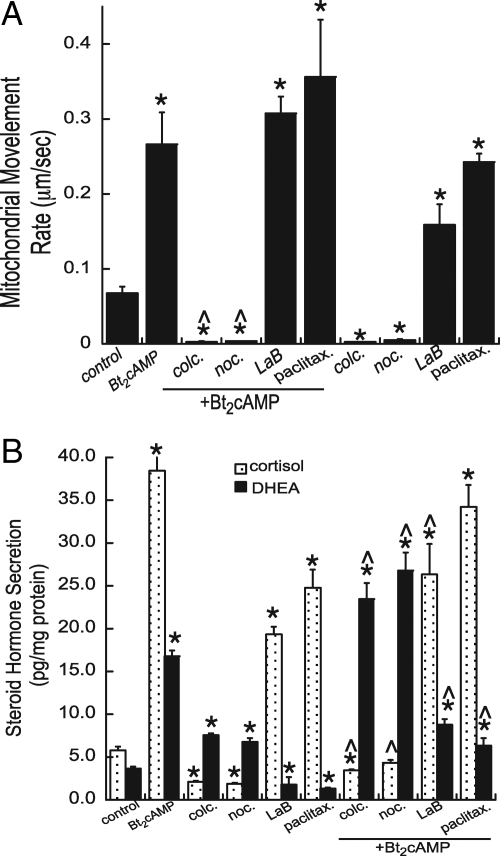
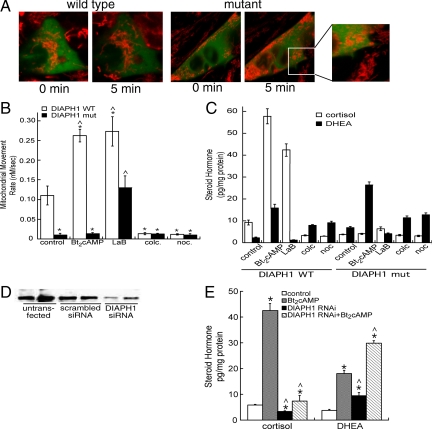
We next sought to define the role of the cytoskeleton in mediating increased mitochondrial movement in response to cAMP. Time-lapsed video imaging was performed on MitoTracker-labeled cells (Invitrogen) treated with Bt2cAMP and chemicals that alter the polymerization of tubulin and actin. Colchicine and nocodazole promote microtubule depolymerization, latrunculin B inhibits actin polymerization, and paclitaxel promotes microtubule stabilization. Figure 2A shows that both colchicine and nocodazole retard mitochondrial movement, in the absence and presence of Bt2cAMP (Supplemental Video 3). This effect of tubulin polymerization inhibitors is in direct contrast to the stimulatory effect of the microtubule stabilizer paclitaxel, which increased the rate of mitochondrial movement by 3.5-fold. Latrunculin B also increased the rate of mitochondrial movement, and this stimulatory effect was further increased in the presence of Bt2cAMP (Fig. 1B and Supplemental Video 4).
Figure 2.
A, Video microscopy was carried out on cells MitoTracker-stained cells (Invitrogen) that were treated with 0.4 mm Bt2cAMP in the presence and absence of 1 μm colchicine (colc.), 1 μm latrunculin B (LaB), 1 μm nocodazole (noc.), or 2 μm paclitaxel (paclitax.). Imaging was carried out on three cell preparations and the rates of approximately 20 mitochondria quantified each time. Error bars, sd. *, Treatments that are statistically different from the control group, P < 0.001; ^, statistically significant difference (P < 0.001) when compared with Bt2cAMP-treated group. B, The amount of cortisol and DHEA secreted into the media was determined by ELISA and normalized to total cellular protein. Data represent the average of three experiments, each carried out in duplicate. * and ^, Statistically significant difference compared with untreated controls and Bt2cAMP-treated cells, respectively (P < 0.001).
cAMP-stimulated steroid hormone biosynthesis requires microtubule polymerization
To define the physiological significance of perturbing the rate of mitochondrial movement, we collected media from cells treated with agents that alter microtubule and actin polymerization and determined the effect on cortisol and the adrenal androgen DHEA secretion. Both colchicine and nocodazole attenuated basal and Bt2cAMP-stimulated cortisol secretion and promoted the production of DHEA (Fig. 2B). In contrast, latrunculin B and paclitaxel increased basal secretion of cortisol but decreased basal and Bt2cAMP-stimulated DHEA biosynthesis.
cAMP-stimulated mitochondrial trafficking requires RhoA activation
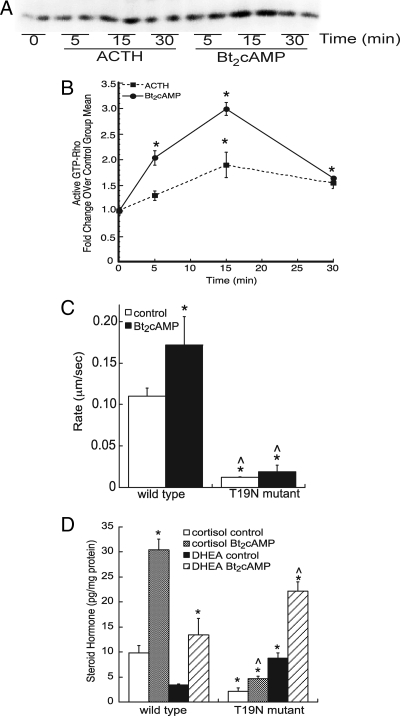
Based on the data shown above demonstrating that ACTH promotes microtubule-dependent mitochondrial trafficking, we next sought to identify proteins involved in this process. Mitochondrial transport, particularly in neurons, has been linked to several cytoplasmic factors, including kinesins, dynamine, and the Rho family of GTPases (23,31,54,55,56,57). To identify components of the signaling pathway that couple ACTH to microtubule-dependent mitochondrial trafficking, we determined the effect of ACTH signaling on the activation of RhoA. As shown in Fig. 3A, the amount of active Rho (GTP bound) increased in cells treated with ACTH or Bt2cAMP within 5 min, with maximal activation for both stimuli at 15 min. Normalization of GTP-bound Rho to total RhoA revealed that Bt2cAMP elicited a 2- and 2.9-fold increase in active Rho at the 5- and 15-min time points, respectively (Fig. 3B). ACTH significantly increased active Rho after 15 min; however, the magnitude of the effects of ACTH were less than observed in response to Bt2cAMP. Neither ACTH nor Bt2cAMP increased cell division cycle 42, GTP-binding protein /Rac activity (data not shown).
Figure 3.
RhoA mediates ACTH-stimulated mitochondrial trafficking and cortisol production. A, Cells were treated with 0.4 mm Bt2cAMP (or 50 nm ACTH, not shown) for time periods ranging from 5 to 30 min and the amount of active Rho determined by rhoteklin binding as shown in the representative blot. B, Densities obtained from Rho activation Western blots were graphed as fold change over control group mean and represent the mean ± sem of three experiments, each carried out in triplicate. *, Statistically different from untreated 0-min control group mean, P < 0.05. C, Time-lapsed video imaging was performed on cells transfected with wild-type or dominant-negative (T19N) GFP-tagged RhoA and stained with MitoTracker Red (Invitrogen). The rate of mitochondrial movement in control and Bt2cAMP-treated cells and the average rates of movement graphed. Rates were quantified only for cells expressing wild-type or mutant GFP-tagged RhoA. Data represent the mean ± sd of three separate experiments, each quantifying the rate of mitochondrial movement in at least 20 transfected cells. *, Statistically different from wild-type control group mean, P < 0.05; ^, statistically different from Bt2cAMP-treated wild-type cells, P < 0.001. D, Cells were transfected with wild-type or mutant RhoA and then treated with 0.4 mm Bt2cAMP. The amount of cortisol and DHEA secreted into the cell culture media was quantified by ELISA and normalized to total cellular protein content. Data represent the mean ± sd of three experiments, each carried out in triplicate. * and ^, Statistical significant differences (P < 0.01) from wild-type cortisol or DHEA control and wild-type Bt2cAMP-stimulated cortisol or DHEA samples, respectively.
To further explore the role of Rho in mediating mitochondrial trafficking and steroidogenesis, we assessed the effect of a dominant-negative RhoA T19N mutant (58) on Bt2cAMP-stimulated mitochondrial movement. Transfection of the dominant-negative mutant attenuated both basal and Bt2cAMP-dependent mitochondrial trafficking (Fig. 3C). Notably, although cells expressing the RhoA T19N mutant secreted lower amounts of cortisol, unstimulated cells produced 2.9-fold more DHEA (Fig. 3D).
cAMP signaling induces the interaction between RhoA and DIAPH1
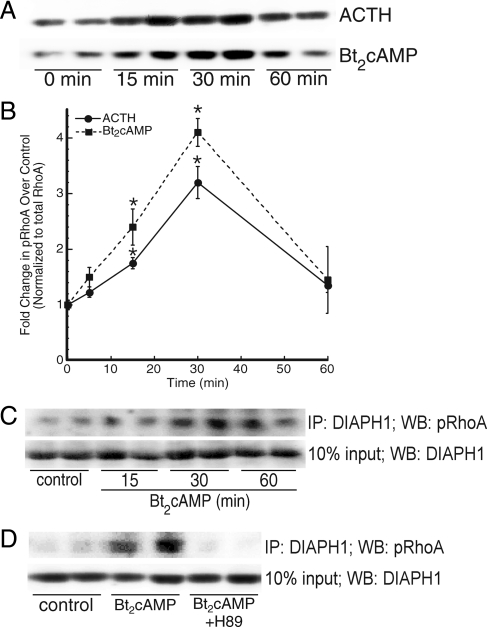
Because cAMP-dependent PKA phosphorylates RhoA at Ser-188 (59), we assessed the effect of ACTH/Bt2cAMP on the cellular levels of Ser-188 phosphorylated RhoA. As shown in Fig. 4, A and B, both ACTH and Bt2cAMP also increased the amount of phosphorylated RhoA in H295R lysates, with maximal effects occurring at the 30-min time point. Next we examined the contribution of RhoA effector proteins in this process. Activated Rho promotes changes in varied cellular functions, including cell migration, organelle trafficking, and cytoplasmic streaming, by interacting with effector proteins. Coimmunoprecipitation studies revealed that cAMP stimulates the interaction between RhoA and DIAPH1 in a time-dependent manner (Fig. 4C) and that the PKA inhibitor H89 prevents this interaction (Fig. 4D).
Figure 4.
ACTH signaling promotes the interaction of RhoA and DIAPH1 and RhoA phosphorylation. A, Cells were treated with 50 nm ACTH or 0.4 mm Bt2cAMP for 30 min and isolated lysates separated by SDS-PAGE. Western blotting was carried out using an antiphospho Ser-188 RhoA antibody (top panel) and total RhoA (bottom panel). B, Graphical representation of phospho-RhoA amounts quantified by densitometric scanning. Data represent the mean ± sem of three experiments, each carried out in triplicate. *, Statistically different from control group mean, P < 0.05. C, Lysates isolated from cells treated for 15, 30, or 60 min with 0.4 mm Bt2cAMP were precleared and immunoprecipitated (IP) with anti-DIAPH1 antibody. The immobilized proteins resolved by SDS-PAGE and Western blotting (WB). Membranes were probed with antiphospho Ser-188 RhoA antibody (top panel) or anti-DIAPH1 (bottom panel) and imaged using ECF and fluorometric scanning. D, Cells were treated for 30 min with 0.4 mm Bt2cAMP and 10 μm H89 and lysates subjected to immunoprecipitation (anti-DIAPH1 antibody), SDS-PAGE and Western blotting (antiphospho Ser-188 RhoA antibody for output and anti-DIAPH1 for input).
The DIAPH1 effector mediates cAMP-stimulated mitochondrial movement and cortisol production
Next, we determined whether DIAPH1 affected cAMP-stimulated mitochondrial movement by transfecting cells with WT or mutant effector. The constitutively active DIAPH1 mutant (ΔN3) lacks the RhoA interaction domain and has been shown to align microtubules in parallel to F-actin bundles (44). As shown in Fig. 5A, overexpression of a GFP-tagged mutant DIAPH1 significantly altered the morphology of mitochondria whereby mitochondria appeared rounded and aggregated (compare mitochondria in transfected cells to mitochondria in adjacent untransfected cells). Consistent with previous findings (44), the DIAPH1 mutant also promoted longitudinal elongation of the H295R cells (Fig. 5A). Significantly, transfection with the constitutively active mutant DIAPH1 rendered mitochondria virtually immobile in both control and Bt2cAMP-stimulated cells (Fig. 5B and Supplemental Video 5). The loss of mitochondrial trafficking in cells transfected with the DIAPH1 mutant was restored by treatment with the actin polymerization inhibitor latrunculin B, but not with inhibitors of microtubule polymerization (Fig. 5B). Untreated cells overexpressing the constitutively active DIAPH1 mutant secreted approximately 50% less cortisol, compared with cells overexpressing wild-type DIAPH1 (Fig. 5C). Treatment of cells expressing mutant DIAPH1 with Bt2cAMP exhibited increased DHEA secretion but did not secrete cortisol. Finally, as shown in Fig. 5B, although latrunculin B increased cortisol production in cells expressing wild-type DIAPH1, the microfilament disruptor was unable to promote cortisol secretion in cells transfected with the constitutively active DIAPH1 mutant.
Figure 5.
DIAPH1 regulates mitochondrial movement and steroid hormone biosynthesis. A, MitoTracker-labeled (Invitrogen) wild-type or mutant GFP-tagged, DIAPH1-expressing cells were treated with Bt2cAMP and the movement of mitochondria assessed by time-lapsed video microscopy. Frames of 0-min time point and 5-min Bt2cAMP-treated cells expressing wild-type or mutant DIAPH1 correspond to movies provided in supplemental data. B, H295R cells were plated onto coverslips and transfected with pEGFP-DIAPH1. Forty-eight hours after transfection, cells were labeled with MitoTracker Red and treated with 1 μm colchicine (colc.), 1 μm latrunculin B (LaB), 1 μm nocodazole (noc.), or 0.4 mm Bt2cAMP. The rate of mitochondrial movement was quantified and the average rates from three experiments graphed. *, Statistically different from wild-type (WT) control group mean, P < 0.05; ^, statistically different from mutant (mut) control cells, P < 0.001. C, H295R cells were transfected with pEGFP-DIAPH1 and then and treated with 1 μm colchicine (colc.), 1 μm latrunculin B (LaB), 1 μm nocodazole (noc.), or 0.4 mm Bt2cAMP for 48 h. Cortisol and DHEA secreted into the media were quantified by ELISA and normalized to the total cellular protein content in each well. The data graphed represent the mean ± sem of two experiments, each performed in triplicate. D, Cells were transfected with scrambled siRNA or siRNA oligonucleotides directed against DIAPH1 and the expression of DIAPH1 determined Western blotting using an anti-DIAPH1 antibody. E, The amount of cortisol and DHEA secreted into the cell culture media was quantified in cells transfected with DIAPH1 siRNA oligonucleotides. The amount of steroid hormone was normalized to total protein content and the data graphed represent the average of two separate experiments, each performed in triplicate. * and ^, Statistical difference from untransfected control (P < 0.05) and Bt2cAMP-treated (P < 0.01) groups for each steroid, respectively.
DIAPH1 is required for mitochondrial movement and cortisol biosynthesis
To further explore the role of DIAPH1 in cortisol production, we transfected cells with siRNA oligonucleotides targeted against DIAPH1. Suppressing DIAPH1 translation reduced expression of the protein by 88% (Fig. 5D) and decreased both basal and Bt2cAMP-stimulated cortisol production, with a concomitant increase in DHEA secretion (Fig. 5E).
Discussion
In this study, we identified key roles for RhoA and DIAPH1 in regulating optimal production of cortisol in human adrenocortical cells. We also uncovered a novel mechanism through which ACTH promotes hormone production, by controlling the rate of mitochondrial movement in steroidogenic cells. We found that stabilizing microtubules or inhibiting actin polymerization promoted both mitochondrial movement and cortisol production, whereas preventing the assembly of microtubules resulted in impaired mitochondrial trafficking and suppressed cortisol secretion (Fig. 2). Our initial hypothesis was that impairing mitochondrial movement would decrease both DHEA and cortisol secretion. However, our data indicate that the delivery of pregnenolone is not dependent on cytoskeletal proteins or DIAPH1. It would be of interest to comprehensively quantify the effect of chemicals that perturb the assembly of microtubules and microfilaments on organellar concentrations of all steroid metabolites. Studies are underway to optimize mass spectrometric conditions to simultaneously quantify both cellular (in specific organelles) and secreted steroid metabolites. Interestingly, the loss of mitochondrial movement increased the amount of DHEA produced in the cells. As mentioned earlier, roles for cytoskeletal protein steroidogenesis have been previously demonstrated, whereby chemical agents that perturb microtubule and actin polymerization alter steroid hormone production in gonadal and adrenal cells (10,11,17,18,19,20,21). However, these studies have yielded conflicting data with regard to the importance of microtubules and microfilaments. For example, colchicine has been shown to impair progesterone production in ovine follicular cells (60) and stimulate progesterone secretion from porcine luteal cells (61). Although species differences may explain some of the discrepancies between these studies, similar conflicting results have been found in adrenocortical cells (15,16,20,21,62).
Our data showing that the dominant-negative mutant of a Rho family protein prevents the movement of mitochondria (Fig. 3C) are consistent with the findings of Gasman et al. (63), who found that GTPase-deficient RhoD impairs the movement of endosomes in HeLa cells. Interestingly, these researchers found that a constitutively active DIAPH2 mutant that lacked the Rho-binding domain mimicked the loss of endosome trafficking evoked by the GTPase-dead RhoD mutant. Similar results were obtained in the current study in which transfecting H295R adrenocortical cells with a dominant-negative RhoA mutant rendered mitochondrial largely immobile (Fig. 3C). Given that DIAPH proteins play pivotal roles in organizing the alignment of both actin filaments and microtubules (44), it is possible that expression of the constitutively active mutant alters the ratio and/or organization of microtubules and F-actin. Indeed, the movement of mitochondria is regulated by the dynamic interplay between actin filaments and microtubules (23,31,54,55,56,57). In contrast to the inhibitory effect of constitutively active DIAPH1 in endosome (63) and mitochondrial movement (this study), a stimulatory role for constitutively active DIAPH1 has been found in the induction transcripts targeted by the serum response factor-dependent pathway (64,65,66). Collectively these studies implicate different functional domains of DIAPH proteins in mediating distinct cellular processes, probably by interacting with unique effector proteins in a stimulus-dependent manner.
We found that ACTH and Bt2cAMP simultaneously increase the cellular levels of active GTP-bound RhoA (Fig. 3, A and B) and phosphorylated RhoA (Fig. 4, A and B). These findings are in contrast to the reported inhibitory effects of PKA on several Rho-mediated cellular processes. Phosphorylation of RhoA by PKA at Ser-188 decreases the binding of RhoA to Rho kinase Rho-associated serine/threonine kinase (67) and inhibits thrombin-induced RhoA activation and translocation (68). cAMP has also been shown to increase Rho phosphorylation at Ser-188 and inhibit lysophosphatidic acid-stimulated RhoA activation and F-actin formation in SGC-7901 human gastric cancer cells (69). In human natural killer cells, PKA-catalyzed phosphorylation of RhoA does not affect the ability of the protein to bind GTP or alter GTPAse activity but promotes RhoA translocation from the plasma membrane to the cytosol (59). Because nerve growth factor has been found to stimulate PKA-mediated phosphorylation of RhoA on Ser-188 and inhibit its binding to ROK but not to DIAPH1 or rhotekin in PC12 adrenal medullary cells (70), it is possible that ACTH signaling and PKA-catalyzed phosphorylation of RhoA may differentially affect partnering to effector proteins in H295R adrenocortical cells. Notably, phosphorylation of RhoA by PKA protects the GTPase, particularly GTP-bound RhoA, from ubiquitin-mediated proteasomal degradation (71). The simultaneous increase in active RhoA (Fig. 3A) and Ser-188 phosphorylated RhoA (Fig. 4A) in response to Bt2cAMP suggests that ACTH signaling may regulate cortisol biosynthesis by controlling the cellular levels of endogenous RhoA in H295R cells. However, further study is required to determine whether ACTH alters the stability of active RhoA.
Our data provide evidence for a role for DIAPH1 in conferring mitochondrial movement and cortisol secretion in response to ACTH/cAMP stimulation by coordinating microtubule organization. Interestingly, breakpoint mutations in DIAPH1 have been identified in a family of patients with infertility due to premature ovarian failure (72). This breakpoint was mapped to the last intron of the gene and has been postulated to lead to an unstable truncated transcript that lacks the last coding exon and 3′ untranslated region. Lartey et al. (73) found that elevated myometrial DIAPH1 results in spontaneous preterm labor. Elevated levels of adrenal androgens have been implicated in infertility and polycystic ovarian syndrome (74,75). Moreover, prenatal exposure to androgens is a critical factor in fetal programming (76,77,78). Given that suppressing DIAPH1 increases adrenal androgen secretion (Fig. 5E), future studies are warranted in examining the effects of mutations in DIAPH1 on circulating levels of adrenal androgens, particularly during ovulation and pregnancy. A role for Rho-associated, coiled-coil-containing protein kinase in regulating adrenal androgen production has recently been suggested in studies examining the effects of phosphorylating P450c17, in which inhibition of Rho-associated, coiled-coil-containing protein kinase activity decreased the lyase activity of P450c17 (79). Because P450c17 plays a critical role in determining the ratio of glucocorticoids to androgens produced, these findings along with our data presented herein identify novel roles for the RhoA pathway in regulating multiple steps in adrenocortical steroid hormone biosynthesis.
One important caveat to take into account with our findings is the cell culture system that was used to carry out the studies. Although these conditions (Nu-Serum I and ITS+ premix; BD Biosciences) have been established as optimal for investigating steroidogenesis that occurs in the zona fasciculata and zona reticularis (47,53), it is likely that the presence of cortisol and other steroid hormones in Nu-Serum I affects the rate of mitochondrial trafficking in response to ACTH. Indeed, we found that the rate of mitochondrial movement in serum-starved cells (48 h serum withdrawal) is approximately 1.3-fold higher than in cells grown in the presence of Nu-Serum I (Li, D., and M. B. Sewer, unpublished observations). However, because we also found that 48 h of serum starvation also markedly induces the expression of multiple steroidogenic genes [CYP17, CYP11A1, and CYP11B (Li, D., and M. B. Sewer, unpublished observations)], it is possible that increased gene expression may also contribute to an increase in hormone production that is independent of interorganelle substrate delivery. Perhaps more significant is the potential role for steroid hormones (particularly cortisol) present in Nu-Serum I in differentially regulating the efflux of cortisol and DHEA from adrenocortical cells. Organic anionic transporters have been shown to regulate cortisol secretion from H295R cells (80). Moreover, the expression of these transporters is regulated by forskolin and DHEA sulfate (81). Finally, we have preliminary mass spectrometric data that identify oxysterol-related-binding proteins as DIAPH1 binding partners (Li, D., and M. B. Sewer, unpublished observation). Given that oxysterol-related-binding proteins are implicated in the interorganelle transport of multiple classes of lipids (82), it is possible that the steroid hormones in Nu-Serum I may contribute to the rate of mitochondrial movement observed in the current study by controlling the expression and/or activity of lipid transporters and binding proteins.
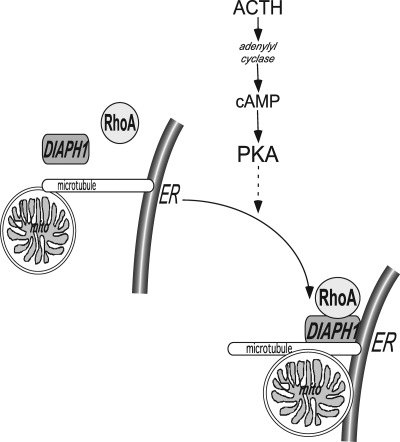
In summary, we have identified RhoA and DIAPH1 as key regulators in mediating ACTH/cAMP-stimulated mitochondrial trafficking in H295R adrenocortical cells. We postulate that this movement is integral for assuring optimal cortisol secretion and maintaining appropriate levels of adrenal androgen production. Given that aldosterone biosynthesis requires interorganelle substrate delivery, it is possible that angiotensin II may regulate mitochondrial trafficking. However, future studies are required to determine the role of mitochondrial movement in angiotensin II-stimulated aldosterone secretion. Based on the data presented herein, we propose a model whereby ACTH signaling promotes RhoA activation and subsequent interaction with DIAPH1 (Fig. 6). The assembly of the RhoA/DIAPH1 complex may facilitate interorganelle substrate delivery by stimulating the microtubule-dependent movement of mitochondria. Finally, given the stimulatory effect of impairing mitochondrial trafficking on the secretion of adrenal androgens, our data herein coupled with previous studies (72) provide evidence for altered DIAPH1 function in pathologies such as nonclassical congenital adrenal hyperplasia and polycystic ovary syndrome.
Figure 6.
Model for ACTH regulation of microtubule-dependent, interorganelle substrate delivery. ACTH increases intracellular cAMP. PKA stimulates RhoA activation and phosphorylation and promotes RhoA interaction with DIAPH1. In response to ACTH, DIAPH1 mediates microtubule-dependent mitochondrial repositioning. ER, Endoplasmic reticulum.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health/National Institute of General Medical Sciences Grant GM073241 and National Science Foundation Grant MCB1005815.
Disclosure Summary: D.L. and M.B.S. have nothing to declare.
First Published Online June 30, 2010
Abbreviations: Bt2cAMP, Dibutyryl cAMP; DAD, diaphanous-autoregulatory domain; DHEA, dehydroepiandrosterone; DIAPH, diaphanous-related homolog; GFP, green fluorescent protein; PKA, protein kinase A; siRNA, small interfering RNA.
References
- Arlt W, Stewart PM 2005 Adrenal corticosteroid biosynthesis, metabolism, and action. Endocrinol Metab Clin North Am 34:293–313, viii [DOI] [PubMed] [Google Scholar]
- Miller WL 2002 Androgen biosynthesis from cholesterol to DHEA. Mol Cell Endocrinol 198:7–14 [DOI] [PubMed] [Google Scholar]
- Waterman MR, Keeney DS 1996 Diverse molecular mechanisms regulate the expression of steroid hydroxylase genes required for production of ligands for nuclear receptors. In: Jefcoate CR, ed. Physiological functions of cytochrome P450 in relation to structure and regulation. Greenwich, CT: JAI Press, Inc.; 81–102 [Google Scholar]
- Sewer MB, Waterman MR 2003 ACTH modulation of transcription factors responsible for steroid hydroxylase gene expression in the adrenal cortex. Microsc Res Tech 61:300–307 [DOI] [PubMed] [Google Scholar]
- Sewer MB, Dammer EB, Jagarlapudi S 2007 Transcriptional regulation of adrenocortical steroidogenic gene expression. Drug Metab Rev 39:371–388 [DOI] [PubMed] [Google Scholar]
- Miller WL 2007 StAR search—what we know about how the steroidogenic acute regulatory protein mediates cholesterol import. Mol Endocrinol 21:589–601 [DOI] [PubMed] [Google Scholar]
- Miller WL 2005 Minireview: regulation of steroidogenesis by electron transfer. Endocrinology 146:2544–2550 [DOI] [PubMed] [Google Scholar]
- Whitehouse BJ, Gyles SL, Squires PE, Sayed SB, Burns CJ, Persaud SJ, Jones PM 2002 Interdependence of steroidogenesis and shape changes in Y1 adrenocortical cells: studies with inhibitors of phosphoprotein phosphatases. J Endocrinol 172:583–593 [DOI] [PubMed] [Google Scholar]
- Shiver TM, Sackett DL, Knipling L, Wolff J 1992 Intermediate filaments and steroidogenesis in adrenal Y-1 cells: acrylamide stimulation of steroid production. Endocrinology 131:201–207 [DOI] [PubMed] [Google Scholar]
- Sackett DL, Wolff J 1986 Cyclic AMP-independent stimulation of steroidogenesis in Y-1 adrenal tumor cells by antimitotic agents. Biochim Biophys Acta 888:163–170 [DOI] [PubMed] [Google Scholar]
- Rajan VP, Menon KM 1985 Involvement of microtubules in lipoprotein degradation and utilization for steroidogenesis in cultured rat luteal cells. Endocrinology 117:2408–2416 [DOI] [PubMed] [Google Scholar]
- Lee LJ, Chen JS, Ko TL, Wang SM 2001 Mechanism of colchicine-induced steroidogenesis in rat adrenocortical cells. J Cell Biochem 81:162–171 [DOI] [PubMed] [Google Scholar]
- Hall PF, Almahbobi G 1997 Roles of microfilaments and intermediate filaments in adrenal steroidogenesis. Microsc Res Tech 36:463–479 [DOI] [PubMed] [Google Scholar]
- Cortese F, Wolf J 1978 Cytochalasin-stimulated steroidogenesis from high density lipoproteins. J Cell Biol 77:507–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey WE, Shay JW, Mason JI 1984 The effect of cytochalasin D on steroid production and stress fiber organization in cultured bovine adrenocortical cells. Mol Cell Endocrinol 35:189–197 [DOI] [PubMed] [Google Scholar]
- Rainey WE, Kramer RE, Mason JI, Shay JW 1985 The effects of Taxol, a microtubule-stabilizing drug, on steroidogenic cells. J Cell Physiol 123:17–24 [DOI] [PubMed] [Google Scholar]
- Denkova R, Ivanov I, Dimitrova M 1992 Microtubules and regulation of granulosa cell steroidogenesis by porcine granulosa cell conditioned medium. Endocr Regul 26:195–199 [PubMed] [Google Scholar]
- Carnegie JA, Tsang BK 1987 Microtubules and the calcium-dependent regulation of rat granulosa cell steroidogenesis. Biol Reprod 36:1007–1015 [DOI] [PubMed] [Google Scholar]
- Carnegie JA, Dardick I, Tsang BK 1987 Microtubules and the gonadotropic regulation of granulosa cell steroidogenesis. Endocrinology 120:819–828 [DOI] [PubMed] [Google Scholar]
- Benis R, Mattson P 1989 Microtubules, organelle transport, and steroidogenesis in cultured adrenocortical tumor cells. 1. An ultrastructural analysis of cells in which basal and ACTH-induced steroidogenesis was inhibited by Taxol. Tissue Cell 21:479–494 [DOI] [PubMed] [Google Scholar]
- Benis R, Mattson P 1989 Microtubules, organelle transport, and steroidogenesis in cultured adrenocortical tumor cells. 2. Reversibility of Taxol’s inhibition of basal and ACTH-induced steroidogenesis is unaccompanied by reversibility of Taxol-induced changes in cell ultrastructure. Tissue Cell 21:687–698 [DOI] [PubMed] [Google Scholar]
- Nan X, Potma EO, Xie XS 2006 Nonperturbative chemical imaging of organelle transport in living cells with coherent anti-strokes Raman scattering microscopy. Biophys J 91:728–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RL, Hollenbeck PJ 1995 Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. J Cell Biol 131:1315–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball EH, Singer SJ 1982 Mitochondria are associated with microtubules and not with intermediate filaments in cultured fibroblasts. Proc Natl Acad Sci USA 79:123–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Weaver D, Hajnóczky G 2004 Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol 167:661–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerhayes IC, Wong D, Chen LB 1983 Effect of microtubules and intermediate filaments on mitochondrial distribution. J Cell Sci 61:87–105 [DOI] [PubMed] [Google Scholar]
- Stromer MH, Bendayan M 1990 Immunocytochemical identification of cytoskeletal linkages to smooth muscle cell nuclei and mitochondria. Cell Motil Cytoskeleton 17:11–18 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kanai Y, Okada Y, Nonaka S, Takeda S, Harada A, Hirokawa N 1998 Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell 93:1147–1158 [DOI] [PubMed] [Google Scholar]
- Miki H, Okada Y, Hirokawa N 2005 Analysis of the kinesin superfamily: insights into the structure and function. Trends Cell Biol 15:467–476 [DOI] [PubMed] [Google Scholar]
- Kwok BH, Kapoor TM 2007 Microtubule flux: drivers wanted. Curr Opin Cell Biol 19:36–42 [DOI] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WM 2005 The axonal transport of mitochondria. J Cell Sci 118:5411–5419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross SP 2004 Hither and yon: a review of bi-directional microtubule-based transport. Phys Biol 1:R1–R11 [DOI] [PubMed] [Google Scholar]
- Ridley AJ 2006 Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol 16:522–529 [DOI] [PubMed] [Google Scholar]
- Raftopoulou M, Hall A 2004 Cell migration: Rho GTPases lead the way. Dev Biol 265:23–32 [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A 2005 Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 21:247–269 [DOI] [PubMed] [Google Scholar]
- Hall A 2005 Rho GTPases and the control of cell behaviour. Biochem Soc Trans 33:891–895 [DOI] [PubMed] [Google Scholar]
- Watanabe N, Madaule P, Reid T, Ishizaki T, Watanabe G, Kakizuka A, Saito Y, Nakao K, Jockusch BM, Narumiya S 1997 p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J 16:3044–3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R, Weyand M, Lammers M, Ishizaki T, Ahmadian MR, Wittinghofer A 2005 Structural and mechanistic insights into the interaction between Rho and mammalian Dia. Nature 435:513–518 [DOI] [PubMed] [Google Scholar]
- Otomo T, Otomo C, Tomchick DR, Machius M, Rosen MK 2005 Structural basis of Rho GTPase-mediated activation of the formin mDia1. Mol Cell 18:273–281 [DOI] [PubMed] [Google Scholar]
- Li F, Higgs HN 2005 Dissecting requirements for auto-inhibition of actin nucleation by the formin, mDia1. J Biol Chem 280:6986–6992 [DOI] [PubMed] [Google Scholar]
- Li F, Higgs HN 2003 The mouse formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr Biol 13:1335–1340 [DOI] [PubMed] [Google Scholar]
- Alberts AS 2001 Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J Biol Chem 276:2824–2830 [DOI] [PubMed] [Google Scholar]
- Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S 1999 Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol 1:136–143 [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Morishima Y, Okamoto M, Furuyashiki T, Kato T, Narumiya S 2001 Coordination of microtubules and the actin cytoskeleton by the Rho effector mDia1. Nat Cell Biol 3:8–14 [DOI] [PubMed] [Google Scholar]
- Lammers M, Rose R, Scrima A, Wittinghofer A 2005 The regulation of mDia1 by autoinhibition and its release by Rho*GTP. EMBO J 24:4176–4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey WE, Bird IM, Mason JI 1994 The NCI-H295 cell line: a pluripotent model for human adrenocortical studies. Mol Cell Endocrinol 100:45–50 [DOI] [PubMed] [Google Scholar]
- Staels B, Hum DW, Miller WL 1993 Regulation of steroidogenesis in NCI-H295 cells: a cellular model of the human fetal adrenal. Mol Endocrinol 7:423–433 [DOI] [PubMed] [Google Scholar]
- Fehrenbacher KL, Yang HC, Gay AC, Huckaba TM, Pon LA 2004 Live cell imaging of mitochondrial movement along actin cables in budding yeast. Curr Biol 14:1996–2004 [DOI] [PubMed] [Google Scholar]
- Knowles MK, Guenza MG, Capaldi RA, Marcus AH 2002 Cytoskeletal-assisted dynamics of the mitochondrial reticulum in living cells. Proc Natl Acad Sci USA 99:14772–14777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shprung T, Gozes I 2009 A novel method for analyzing mitochondrial movement: inhibition by paclitaxel in a pheochromocytoma cell model. J Mol Neurosci 37:254–262 [DOI] [PubMed] [Google Scholar]
- Janes ME, Chu KM, Clark AJ, King PJ 2008 Mechanisms of adrenocorticotropin-induced activation of extracellularly regulated kinase 1/2 mitogen-activated protein kinase in the human H295R adrenal cell line. Endocrinology 149:1898–1905 [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Bird IM, Rainey WE, Cone RD 1994 ACTH induces up-regulation of ACTH receptor mRNA in mouse and human adrenocortical cell lines. Mol Cell Endocrinol 99:R17–R20 [DOI] [PubMed] [Google Scholar]
- Bird IM, Hanley NA, Word RA, Mathis JM, McCarthy JL, Mason JI, Rainey WE 1993 Human NCI-H295 adrenocortical carcinoma cells; a model for angiotensin-II-responsive aldosterone secretion. Endocrinology 133:1555–1561 [DOI] [PubMed] [Google Scholar]
- Glater EE, Megeath LJ, Stowers RS, Schwarz TL 2006 Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol 173:545–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y 2005 Regulation of mitochondrial dynamics: another process modulated by Ca2+ signals? Sci STKE 280:pe18 [DOI] [PubMed] [Google Scholar]
- Fransson S, Ruusala A, Aspenström P 2006 The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem Biophys Res Commun 344:500–510 [DOI] [PubMed] [Google Scholar]
- Boldogh IR, Pon LA 2007 Mitochondria on the move. Trends Cell Biol 17:502–510 [DOI] [PubMed] [Google Scholar]
- Qiu RG, Chen J, McCormick F, Symons M 1995 A role for Rho in Ras transformation. Proc Natl Acad Sci USA 92:11781–11785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J 1996 Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J 15:510–519 [PMC free article] [PubMed] [Google Scholar]
- Murdoch WJ 1996 Microtubular dynamics in granulosa cells of periovulatory follicles and granulosa-derived (large) lutein cells of sheep: relationships to the steroidogenic folliculo-luteal shift and functional luteolysis. Biol Reprod 54:1135–1140 [DOI] [PubMed] [Google Scholar]
- Gregoraszczuk EL, Stlomczynska M 1996 The cytoskeleton proteins and LH-regulated steroidogenesis of porcine luteal cells. Folia Histochem Cytobiol 34:35–39 [PubMed] [Google Scholar]
- Feuilloley M, Contesse V, Lefebvre H, Delarue C, Vaudry H 1994 Effects of selective disruption of cytoskeletal elements on steroid secretion by human adrenocortical slices. Am J Physiol 266:E202–E210 [DOI] [PubMed] [Google Scholar]
- Gasman S, Kalaidzidis Y, Zerial M 2003 RhoD regulates endosome dynamics through Diaphanous-related Formin and Src tyrosine kinase. Nat Cell Biol 5:195–204 [DOI] [PubMed] [Google Scholar]
- Grosse R, Copeland JW, Newsome TP, Way M, Treisman R 2003 A role for VASP in RhoA-Diaphanous signalling to actin dynamics and SRF activity. EMBO J 22:3050–3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath SD, Narumiya S, Dhawan J 2007 The RhoA effector mDiaphanous regulates MyoD expression and cell cycle progression via SRF-dependent and SRF-independent pathways. J Cell Sci 120:3086–3098 [DOI] [PubMed] [Google Scholar]
- Copeland JW, Treisman R 2002 The diaphanous-related formin mDia1 controls serum response factor activity through its effects on actin polymerization. Mol Biol Cell 13:4088–4099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong JM, Leung T, Manser E, Lim L 1998 cAMP-induced morphological changes are counteracted by the activated RhoA small GTPase and the Rho kinase ROKα. J Biol Chem 273:22554–22562 [DOI] [PubMed] [Google Scholar]
- Qiao J, Huang F, Lum H 2003 PKA inhibits RhoA: a protection mechanism against endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 284:L972–L980 [DOI] [PubMed] [Google Scholar]
- Chen Y, Wang Y, Yu H, Wang F, Xu W 2005 The cross talk between protein kinase A- and RhoA-mediated signaling in cancer cells. Exp Biol Med 230:731–741 [DOI] [PubMed] [Google Scholar]
- Nusser N, Gosmanova E, Makarova N, Fujiwara Y, Yang L, Guo F, Luo Y, Zheng Y, Tigyi G 2006 Serine phosphorylation differentially affects RhoA binding to effectors: implications to NGF-induced neurite outgrowth. Cell Signal 18:704–714 [DOI] [PubMed] [Google Scholar]
- Rolli-Derkinderen M, Sauzeau V, Boyer L, Lemichez E, Baron C, Henrion D, Loirand G, Pacaud P 2005 Phosphorylation of serine 188 protects RhoA from ubiquitin/proteasome-mediated degradation in vascular smooth muscle cells. Circ Res 96:1152–1160 [DOI] [PubMed] [Google Scholar]
- Bione S, Sala C, Manzini C, Arrigo G, Zuffardi O, Banfi S, Borsani G, Jonveaux P, Philippe C, Zuccotti M, Ballabio A, Toniolo D 1998 A human homologue of the Drosophila melanogaster diaphanous gene is disrupted in a patient with premature ovarian failure: evidence for conserved function in oogenesis and implications for human sterility. Am J Hum Genet 62:533–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lartey J, Smith M, Pawade J, Strachan B, Mellor H, López Bernal A 2007 Up-regulation of myometrial RHO effector proteins (PKN1 and DIAPH1) and CPI-17 (PPP1R14A) phosphorylation in human pregnancy is associated with increased GTP-RHOA in spontaneous preterm labor. Biol Reprod 76:971–982 [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Pérez-Bravo F, Recabarren SE 2002 Maternal serum androgens in pregnant women with polycystic ovary syndrome: possible implications in prenatal androgenization. Hum Reprod 17:2573–2579 [DOI] [PubMed] [Google Scholar]
- Yildiz BO, Azziz R 2007 The adrenal and polycystic ovary syndrome. Rev Endocr Metab Disord 8:331–342 [DOI] [PubMed] [Google Scholar]
- Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V 2005 Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology 146:3185–3193 [DOI] [PubMed] [Google Scholar]
- Sarma HN, Manikkam M, Herkimer C, Dell'Orco J, Welch KB, Foster DL, Padmanabhan V 2005 Fetal programming: excess prenatal testosterone reduces postnatal luteinizing hormone, but not follicle-stimulating hormone responsiveness, to estradiol negative feedback in the female. Endocrinology 146:4281–4291 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Manikkam M, Recabarren S, Foster D 2006 Prenatal testosterone programs reproductive and metabolic dysfunction in the female. Mol Cell Endocrinol 246:165–174 [DOI] [PubMed] [Google Scholar]
- Tee MK, Dong Q, Miller WL 2008 Pathways leading to phosphorylation of P450c17 and to the posttranslational regulation of androgen biosynthesis. Endocrinology 149:2667–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asif AR, Steffgen J, Metten M, Grunewald RW, Müller GA, Bahn A, Burckhardt G, Hagos Y 2005 Presence of organic anion transporters 3 (OAT3) and 4 (OAT4) in human adrenocortical cells. Pflugers Arch 450:88–95 [DOI] [PubMed] [Google Scholar]
- Asif AR, Ljubojevic M, Sabolic I, Shnitsar V, Metten M, Anzai N, Müller GA, Burckhardt G, Hagos Y 2006 Regulation of steroid hormone biosynthesis enzymes and organic anion transporters by forskolin and DHEA-S treatment in adrenocortical cells. Am J Physiol Endocrinol Metab 291:E1351–E1359 [DOI] [PubMed] [Google Scholar]
- Olkkonen VM, Levine TP 2004 Oxysterol binding proteins: in more than one place at one time? Biochem Cell Biol 82:87–98 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.