Abstract
Early pregnancy loss is common and can be caused by a range of factors. The Brown Norway (BN) rat exhibits reproductive dysfunction characterized by small litter size and pregnancy failure and represents a model for investigating early pregnancy loss. In this study, we investigated the establishment of pregnancy in the BN rat and gained insight into mechanisms causing its subfertility. Early stages of BN uteroplacental organization are unique. The BN primordial placenta is restricted in its development and correlates with limited BN uterine decidual development. BN uterine decidua was shown to be both structurally and functionally distinct and correlated with decreased circulating progesterone (P4) levels. Ovarian anomalies were also apparent in BN rats and included decreased ovulation rates and decreased transcript levels for some steroidogenic enzymes. Attempts to rescue the BN uterine decidual phenotype with steroid hormone therapy were ineffective. BN uteri were shown to exhibit reduced responsiveness to P4 but not to 17β-estradiol. P4 resistance was associated with decreased transcript levels for the P4 receptor (Pgr), a P4 receptor chaperone (Fkbp4), and P4 receptor coactivators (Ncoa1 and Ncoa2). In summary, the BN rat exhibits luteal insufficiency and uterine P4 resistance, which profoundly affects its ability to reproduce.
The Brown Norway rat exhibits luteal insufficiency and uterine progesterone resistance, which profoundly affect its ability to reproduce.
Pregnancy failure is a significant cause of infertility. It can be linked to ovarian, uterine, and placental dysfunction and other pathologies associated with the mother and fetus (1,2,3,4,5,6). In species with hemochorial placentation (human, rat, mouse, etc.), successful pregnancy requires specialized uterine adaptations, which establish a functional vascular interface with trophoblast cells of the placenta (7,8,9). These uterine adaptations include the formation of a maternal structure, the decidua. Decidual cells are differentiated uterine endometrial stromal cells (10,11). During gestation, decidual cells are located at the interface between trophoblast cells and the maternal environment. The ovaries and blastocyst provide signals responsible for initiating pregnancy-dependent changes in the uterus. Differentiation of decidual cells is among the earliest uterine adaptation to pregnancy (10,11). Decidual cell differentiation is exquisitely sensitive to the regulatory actions of ovarian steroids, progesterone (P4) and 17β-estradiol (E2) (9,12,13,14). Decidual cells are then responsible for controlling a cascade of other changes in maternal and extraembryonic tissues necessary for pregnancy to continue (10,15,16). Disruptions in decidual cell development are not compatible with pregnancy (17,18,19).
The Brown Norway (BN) rat represents a unique animal model for investigating the etiology of pregnancy failure and subfertility. The BN rat has a small litter size, increased pregnancy loss, and peculiarities in its placentation (20,21,22,23). These oddities include a small placenta with limited intrauterine trophoblast invasion and retention of uterine natural killer (NK) cells within the mesometrial compartment late in gestation (23). Small placentas, disruptions in trophoblast invasion, and aberrations in NK cell behavior have each been implicated in pregnancy disorders (5,8,24,25). The BN rat is an ideal animal model for further exploration of the etiology of subfertility because of the availability of powerful genetics tools for studying this rat strain (26,27,28,29). Thus far, factors responsible for BN rat subfertility are unknown.
In this report, we identify sites of dysfunction responsible for the reproductive phenotype of the BN rat. We demonstrate that the BN rat possesses deficiencies in embryo-uterine interactions, which are associated with luteal insufficiency, uterine P4 resistance, and deficits in uterine decidua development. These observations will form the basis of future physiology-directed discovery of genes regulating fertility and may provide insights into the etiology of infertility in other species with hemochorial placentation.
Materials and Methods
Animals and tissue preparation
BN and Fischer 344 (F344) inbred rats were obtained from Charles River Laboratories (Wilmington, MA). Dahl salt-sensitive (DSS) inbred and Holtzman Sprague Dawley (HSD) outbred rats were purchased from Harlan (Indianapolis, IN). Animals were housed in an environmentally controlled facility, with lights on from 0600 to 2000 h, and allowed free access to food and water. DSS rats were maintained on a low-salt diet (5010; Purina, Richmond, IN). Under these dietary conditions, DSS rats do not exhibit significant pathologies. BN rats were provided the low-salt diet or a standard rodent diet (8604; Purina). The latter diet was used for comparisons with HSD rats. Pregnancy parameters are similar for BN rats fed either diet.
Virgin female rats 8–10 wk of age were cohabited with adult males (>3 months of age) of the same strain. Mating was assessed by inspection of vaginal lavages. The presence of sperm in the vaginal lavage was considered d 0.5 of pregnancy. Female rats were made pseudopregnant by mating with vasectomized males. Presence of seminal plugs was designated d 0.5 of pseudopregnancy. Decidualization was induced via scratching the antimesometrial endometrial lumenal surface of one uterine horn on d 4.5 of pseudopregnancy (30). Uteri were collected on d 8.5 of pseudopregnancy, weighed, and the ratio of stimulated and unstimulated uterine horns calculated. Embryo transfer was accomplished through the collection of d 0.5 embryos and transferring them into oviducts of d 0.5 pseudopregnant recipients. In some experiments, bilateral ovariectomies were performed and hormone replacement was achieved with sc implantation of P4 pellets (Innovative Research, Sarasota, FL) or injection of steroid hormones dissolved in sesame oil. Pellets devoid of hormones or sesame oil vehicle were used as controls. All survival surgeries and blood collections were performed under isofluorothane anesthesia. At the time the animals were killed, tissues used for in situ hybridization and immunocytochemistry were frozen in dry ice-cooled heptane, whereas tissues used for biochemical analyses were snap frozen in liquid nitrogen. All samples were stored at −80 C until processed. Protocols for these methods have been described (31). The University of Kansas Medical Center Animal Care and Use Committee approved procedures for rodent handling and experimentation described in this report.
Measurements of serum steroid hormones
Serum P4, E2, and androstenedione were measured with 125I-labeled RIA kits according to the manufacturer’s instructions (Siemens Diagnostics, Los Angeles, CA).
Immunocytochemistry and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) analyses
Immunocytochemical analyses were performed on 10-μm frozen tissue sections using Histostain-AEC-plus kits (Zymed, San Francisco, CA). Negative controls were performed with normal rabbit serum or isotype-specfic control mouse IgG and did not exhibit positive reactivity in the tissue sections. All immunostained tissue sections were inspected and images recorded with a Leica MZFLIII stereomicroscope equipped with a charge-coupled device camera (Leica, Welzlar, Germany).
Trophoblast cells
Trophoblast cells were detected using a mouse anti-Pan cytokeratin antibody (catalog no. C2931; Sigma-Aldrich, St. Louis, MO) at a dilution of 1:400 (31).
NK cells
Rabbit antirat perforin-1 antibodies (catalog no. TP251; Torrey Pines Biolabs, Houston, TX) were used at a concentration of 2.5 μg/ml to detect NK cells. Immunolocalization of rat perforin-1 antibodies was visualized using alkaline phosphatase-conjugated goat antirabbit immunoglobulin (catalog no. A3687; Sigma-Aldrich) and nitroblue tetrazolium/bromochloroindoyl phosphate (31).
Endothelial cells
Mouse antibodies to a rat endothelial cell-specific surface antigen (catalog no. MCA970; Serotec, Oxford, UK) (32) were used to detect endothelial cells (1:20 dilution).
Smooth muscle cells
Smooth muscle cells were monitored with a mouse antismooth muscle α-actin (α-actin-2, ACTA2) antibody (catalog no. A2547; Sigma-Aldrich; 1:400 dilution).
Proliferating cells
Rabbit antibodies to MKI67 (catalog no. SC-15402; Santa Cruz Biotechnology, Santa Cruz, CA) were used at a dilution of 1:200 to detect proliferating cells.
TUNEL assay
TUNEL assays were performed with in situ cell death detection kits (Roche, Indianapolis, IN).
P4 receptor (PGR)
A mouse antibody to the PGR (catalog no. MA1-410; Affinity Bioreagents, Rockford, IL; 1:200 dilution) was used to localize the PGR.
DNA microarray
Total RNA was prepared from d 8.5 deciduomal tissue of HSD and BN pseudopregnant rats (n = 9 for each strain) using TRIzol reagent (Invitrogen, Carlsbad, CA). RNA extractions were pooled to form three groups of three for each rat strain in nuclease-free water at a concentration of 1.0 μg/μl. RNA samples were hybridized to Affymetrix 230 2.0 DNA microarray chips using the GeneChip Hybridization Oven 640 (Affymetrix, Santa Clara, CA). Washing and staining of the hybridized chips were conducted using the GeneChip Fluidics Station 450 (Affymetrix). Chips were scanned using the Affymetrix GeneChip Scanner 3000 (Affymetrix) with autoloader by the University of Kansas Medical Center Biotechnology Support Facility. Hybridization signals were normalized to internal controls. Expression data sets were analyzed using the expression analysis software GeneSpring 7.0 and R statistics software (http://www.r-project.org/) with BioConductor software (http://www.bioconductor.org/) packages. The MAS5 method from the BioConductor software was used for background correction, normalization, and summarization of the DNA microarray data. We selected a subset of genes that exhibited robust differences among the genetic rat strains for further validation by quantitative RT-PCR (qRT-PCR).
Quantitative RT-PCR
cDNAs were synthesized from total RNA (1 μg) for each sample using Moloney murine leukemia virus reverse transcriptase (Invitrogen), diluted five times with water, and subjected to qRT-PCR to estimate mRNA levels. Primers were designed using Primer Express 2.0 (Applied Biosystems, Foster City, CA), and sequences can be found in Supplemental Tables 1–5, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org. Real-time PCR amplification of cDNAs was carried out in a reaction mixture (10 μl) containing SYBR GREEN PCR Mix (Applied Biosystems) and primers (600 nm each). Amplification and fluorescence detection were carried out using the ABI Prism 7500 real time PCR system (Applied Biosystems). Cycling conditions included an initial hold step (95 C for 10 min) and 40 cycles of a two-step PCR (92 C for 15 sec, then 60 C for 1 min), followed by a dissociation step (95 C for 15 sec, 60 C for 15 sec, and then 95 C for 15 sec). The comparative cycle threshold method was used for relative quantification of the amount of mRNA for each sample normalized to 18S RNA.
Uterine stromal cell culture
Primary uterine stromal cell cultures were prepared as described (33). On d 4.5 of gestation, uterine horns were harvested, bisected to expose the lumen, minced, and digested sequentially with Dispase [2.4 U/ml in Hanks’ balanced salt solution (HBSS; Invitrogen] and then collagenase (0.5 mg/ml in HBSS; Invitrogen). Incubations were performed at 37 C for 1 h on a rocker. Samples were briefly vortexed. The digested tissue solution was then passed through a 70-μm filter (Millipore, Billerica, MA) and cells collected by centrifugation. Cell pellets were resuspended in DMEM-F12 supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), Fungizone (1.25 μg/ml), and 2% heat-inactivated fetal bovine serum. Cells were counted and viability assessed by trypan blue staining using a hemocytometer and plated at a density of 1 × 106 cells per 25-cm2 culture plate. After 2 h unattached cells were removed by washing with HBSS and fresh culture medium added containing P4 (1 μm) and E2 (10 nm). Cultures were terminated after 72 h and RNA extracted with an RNeasy kit (QIAGEN, Valencia, CA) and transcript levels assessed by qRT-PCR.
Analysis of the Pgr coding sequence and promoter
The Pgr coding sequence was analyzed by RT-PCR followed by sequencing of the PCR products. Briefly, total RNA extracted from BN or DSS rat deciduoma tissue was reverse transcribed using Superscript II (Invitrogen). Pgr cDNA sequences were PCR amplified by using sets of specific primers to obtain overlapping amplicons spanning the complete coding region. Pgr promoter sequences (1500 bp upstream of the Pgr B transcription start site) were amplified by PCR from DNA extracted from livers of DSS, F344 and BN rat strains (DNeasy kit; QIAGEN). PCR products were electrophoresed, UV identified on 1% agarose gel containing ethidium bromide, and purified products (QIAquick kit; QIAGEN) sequenced (Northwestern University DNA Sequencing Center, Chicago, IL). Primer sequences used for the Pgr analysis are provided in Supplemental Tables 6 and 7.
Rat Pgr promoter regions spanning −1290 to +610 (34) were PCR amplified using genomic DNA templates isolated from BN, DSS, and F344 livers. PCR-amplified products were initially cloned into the TA cloning vector (Invitrogen) for sequence confirmation and then subcloned into pGL4.10 [luc2] luciferase vector (Promega, Madison, WI) for in vitro assays using U1 rat uterine stromal cells (35) and human MCF7 breast cancer cells (American Type Culture Collection, Manassas, VA). A Renilla luciferase vector with HSVTK promoter (pGL4.74hRluc/TK; Promega) was included as an internal control. U1 and MCF7 cells grown in 24-well plates (DMEM with 10% fetal bovine serum) were cotransfected with 100 ng of promoter vectors and 15 ng of control Renilla vector using Lipofectamine 2000 (Invitrogen). Twelve hours after transfection, the medium was changed and incubations continued for an additional 24 h before cell lysis and processing with a standard dual-luciferase assay (Promega).
Morphological measurements
Strain differences in ectoplacental cone development were evaluated from serial cross-sections spanning the entire structure and analyzed with National Institutes of Health Image J software (Bethesda, MD). Cross-sectional ectoplacental cone areas measured with National Institutes of Health Image J software were used to determine the impact of uterine environment on ectoplacental cone development.
Statistical analyses
Statistical analyses were performed using the R Statistical Package (http://www.r-project.org/). Nonparametric multiple comparison tests were used as described by Hothorn and Munzel (36). Specific details are elaborated in the figure legends.
Results
Ontogeny of the BN pregnancy phenotype
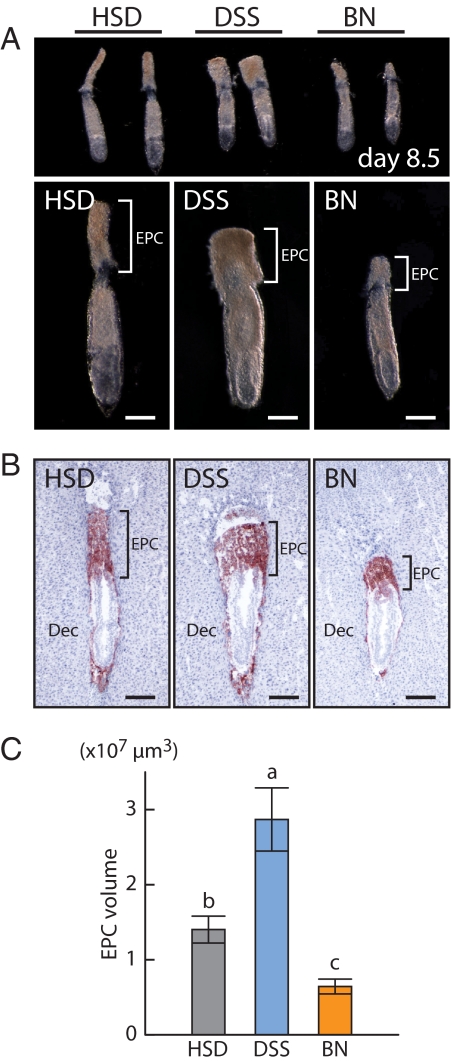
Pregnancy in the BN rat is distinct from pregnancies of other rat strains (23). These differences include fecundity and structure of the placentation site. The origin of these reproductive phenotypes was investigated by examining the structure of placental primordia (ectoplacental cone) at gestation d 8.5. Ectoplacental cone volumes of BN rat placentation sites were significantly smaller than ectoplacental cone volumes from either HSD or DSS rats (Fig. 1). The sizes of early BN rat placentation sites were also smaller than either the HSD or the DSS rat placentation sites (Fig. 2A). These strain differences were consistently observed from tissues obtained between gestation d 7.5 and d 9.5 (Supplemental Fig. 1). Strain differences in sizes of placentation sites were largely attributed to the decidual compartment. DSS rats possessed larger decidual compartments than did BN rats (Fig. 2A). At gestation d 8.5, the decidual vasculature and mesometrial NK cell accumulation were more advanced in the DSS rat than in the BN rat (Supplemental Fig. 2). Strain differences in decidual cell proliferation and cell death were not apparent at gestation d 8.5 (Supplemental Fig. 3).
Figure 1.
Ectoplacental cone development in HSD, DSS, and BN rats. A, Gross morphology of gestation d 8.5 embryos. Bars, 200 μm. B, Trophoblast cells were identified on frozen tissue sections from gestation d 8.5 placentation sites using immunostaining for cytokeratin. Bars, 200 μm. C, Quantification of ectoplacental cone volumes. Data were analyzed with the Kruskal-Wallis rank sum test followed by the Behrens-Fisher-type nonparametric multiple comparison test (a > b > c, P < 0.001, HSD, n = 6, DSS, n = 6, BN, n = 5). EPC, Ectoplacental cone; Dec, decidua.
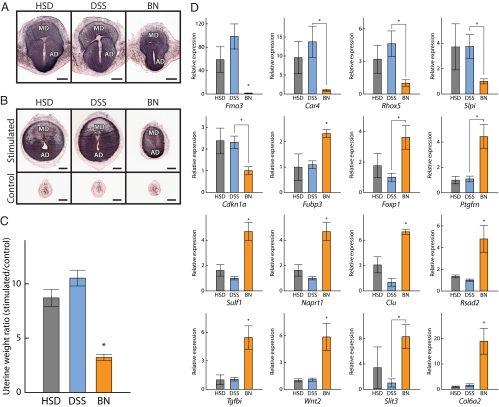
Figure 2.
Decidual and deciduomal development in HSD and BN rats. A, Gross morphology of gestation d 8.5 placentation sites of HSD (left panel), DSS (middle panel), and BN (right panel) rats. MD, Mesometrial decidua; AD, antimesometrial decidua. B, Decidualized uterine stromal cells from gestation d 8.5 placentation sites of HSD (left panel), DSS (middle panel), and BN (right panel) rat strains were visualized by alkaline phosphatase histochemical staining using nitroblue tetrazolium/bromochloroindoyl phosphate. Bars, 1 mm. MD, Mesometrial decidua; AD, antimesometrial decidua. C, Assessment of decidualization in HSD, DSS, and BN female rats. HSD, DSS, or BN female rats were mated with vasectomized males. On d 4.5 of pseudopregnancy, the antimesometrial endometrial lumenal surface of one uterine horn was scratched to induce a decidual reaction. The other uterine horn was not stimulated and served as a control. Stimulated and control uterine horns from HSD, DSS, and BN rats were collected and weighed on d 8.5 of pseudopregnancy. Ratios of stimulated vs. control uterine horn weights are shown in the panel. The data were analyzed with the Kruskal-Wallis rank sum test followed by the Behrens-Fisher-type nonparametric multiple comparison test (*, P < 0.001, HSD: n = 12, DSS: n = 11, BN: n = 10). D, qRT-PCR analyses of gene expression in HSD, DSS, and BN deciduomal tissues. Differentially expressed genes identified in the DNA microarray (Table 1) were analyzed by qRT-PCR. Total RNA samples from the deciduoma tissues of HSD, DSS, and BN rat strains were isolated, reverse transcribed, and subjected to qRT-PCR. Reactions were performed in duplicate, and the data were analyzed using the change in cycle threshold value method. 18S ribosomal RNA served as the internal control. The data were analyzed by the Kruskal-Wallis rank sum test followed by the Behrens-Fisher-type nonparametric multiple comparison test (*, P < 0.05, HSD: n = 5, DSS: n = 5, BN: n = 5).
Decidual development was also examined independently in pseudopregnant rats (Fig. 2B). Decidual reactions were observed in more than 90% of the HSD and DSS rats, whereas only 50–60% of the BN rats exhibited decidual reactions. Among the animals with decidual responses, HSD or DSS rat responses were significantly greater than BN rat responses (Fig. 2C).
In addition to the size of the decidual reactions, decidual tissue from the BN strain functioned differently when compared with decidual tissue from DSS and HSD rats. Gene expression profiles of deciduomas from the BN rat differed from HSD and DSS rats, as determined by DNA microarray and verified by qRT-PCR (Fig. 2D). Sixteen genes identified from the deciduomal DNA microarray were shown to possess distinct expression patterns in deciduomal tissues among the rat strains (Table 1 and Fig. 2D). Among the 16 genes, HSD and DSS rats exhibited similar expression patterns. The biological significance of the strain differences in decidual gene expression are unknown.
Table 1.
Differentially expressed genes in d 8.5 deciduomal tissue
| Symbol | Accession no. | Description | Chromosome | HSD/BNa |
|---|---|---|---|---|
| Fmo3 | NM_053433 | Flavin containing monooxygenase 3 | 13 | 56.28 |
| Car4 | NM_019174 | Carbonic anhydrase 4 | 10 | 12.96 |
| Fubp3 | BF282112 | EST similar to far upstream element binding protein 3 | 3 | 10.44 |
| Cdkn1a | U24174 | Cyclin-dependent kinase inhibitor 1A | 20 | 8.59 |
| Slpi | NM_053372 | Secretory leukocyte peptidase inhibitor | 3 | 7.05 |
| Rhox5 | NM_022175 | Reproductive homeobox 5 (placentae and embryos oncofetal gene) | X | 5.53 |
| Naprt1 | BF416417 | EST similar to nicotinate phosphoribosyltransferase domain 1 | 7 | 0.36 |
| Col6a2 | AI030021 | EST similar to Col6a2 collagen, type VI, α2 | 20 | 0.35 |
| Ptgfrn | NM_019243 | Prostaglandin F2 receptor negative regulator | 2 | 0.31 |
| Sulf1 | NM_134378 | Sulfatase 1 | 5 | 0.31 |
| Clu | AF314657 | Clusterin | 15 | 0.31 |
| Wnt2 | BF556985 | EST similar to wingless-type MMTV integration site family member 2 | 4 | 0.29 |
| Tgfbi | BG379319 | EST similar to transforming growth factor-β, induced | 17 | 0.22 |
| Slit3 | BF386446 | EST similar to slit homolog 3 (Drosophila) | 10 | 0.22 |
| Foxp1 | AI072641 | EST similar to forkhead box P1 | 4 | 0.13 |
| Rsad2 | AF134409 | RSAD family, member 2 | 19 | 0.12 |
EST, Expressed sequence tag.
Ratio of HSD to BN deciduomal expression as determined by the DNA microarray.
Uterine stromal cells were readily isolated from both DSS and BN rat gestation d 4.5 uteri. Cultured uterine stromal cells differentiated in vitro toward a decidual cell phenotype. A number of transcripts characteristic of decidualization were up-regulated after in vitro differentiation, including Prl6a1, Prl8a2, Prl3c1, and Gja1. Transcript levels did not differ between the strains (Supplemental Fig. 4), indicating that there were not intrinsic differences in the uterine stromal cells from DSS and BN rats.
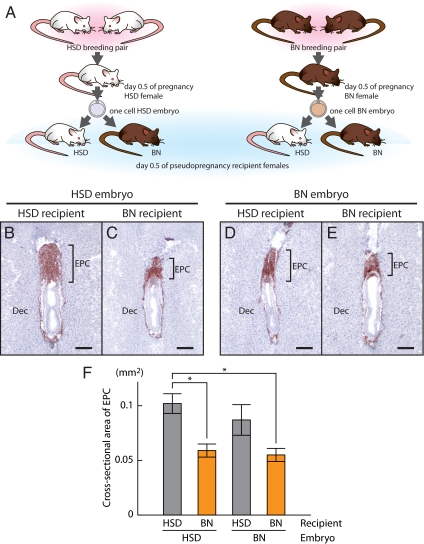
Finally, we noted functional differences in HSD vs. BN rat uterine environments (Fig. 3A). HSD rat embryos transplanted into BN rat uteri developed smaller ectoplacental cones at gestation d 8.5 than did HSD rat embryos transplanted into HSD rat uteri (Fig. 3, B, C, and F). BN embryos developed similarly after transfer into either BN or HSD rat uteri (Fig. 3, D–F).
Figure 3.
EPC development of HSD and BN embryos transferred into HSD or BN recipient uteri. A, Schematic representation of the reciprocal embryo transfer experiment. One-cell embryos were collected from gestation d 0.5 females of HSD × HSD or BN × BN breeding pairs and transferred to d 0.5 pseudopregnant HSD or BN recipient females. Combinations for embryo transfer included: HSD embryos in HSD recipients (B); HSD embryos in BN recipients (C); BN embryos in HSD recipients (D); and BN embryos in BN recipients (E). Trophoblast cells were identified by cytokeratin immunostaining of cryosections from gestation d 8.5 implantation sites. Bars, 200 μm. F, Quantification of ectoplacental cone cross-sectional area. Data were analyzed with a two-way ANOVA and Tukey’s honestly significant difference multiple comparison test (*, P < 0.05, n = 5 for each group). EPC, Ectoplacental cone; Dec, decidua.
In summary, some aspects of the unique BN rat placentation can be traced to early deficits in the growth of the ectoplacental cone and growth, differentiation, and in vivo function of the uterine decidua.
Steroid hormone milieu and ovarian function
Uterine decidual cell development is exquisitely dependent on ovarian steroid hormones (9,12,13,14). Because we observed a pronounced difference in decidual development among the rat strains, we next examined circulating levels of P4 and E2 on d 8.5 of pseudopregnancy with and without induction of decidualization. Serum P4 levels were significantly lower in BN vs. HSD or DSS decidualized or nondecidualized pseudopregnant rats (Fig. 4, A and C). Serum E2 did not differ among the strains (Fig. 4B).
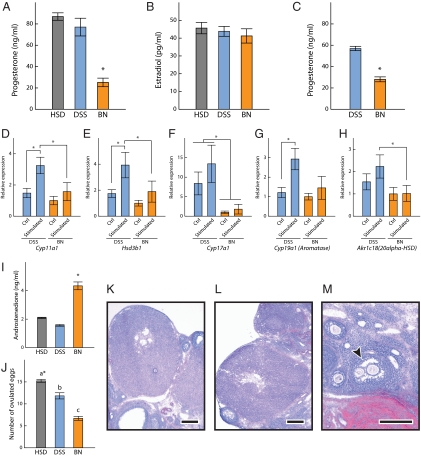
Figure 4.
Steroid hormone milieu and ovarian function. Serum P4 (A and C) and E2 (B) in HSD, DSS, and BN decidualized (A and B; Kruskal-Wallis rank sum test followed by Behrens-Fisher-type nonparametric multiple comparison test; *, P < 0.01, HSD: n = 6, DSS: n = 8, BN: n = 8) and nondecidualized (C; Wilcoxon rank sum test, *, P < 0.001, DSS: n = 10, BN: n = 10) pseudopregnant rats. Ovarian transcript levels for Cyp11a1 (D), Hsd3b1 (E), Cyp17a1 (F), Cyp19a1 (G), and Akr1c18 (H) were monitored in decidualized pseudopregnant DSS and BN rats (Wilcoxon rank sum test, *, P < 0.05, DSS: n = 5, BN: n = 5). Ovaries ipsilateral and contralateral to the decidualized uterine horn were examined. I, Serum androstenedione levels in HSD, DSS, and BN decidualized pseudopregnant rats (Kruskal-Wallis rank sum test followed by the Behrens-Fisher type nonparametric multiple comparison test, *, P < 0.01, HSD: n = 6, DSS: n = 8, BN: n = 8). J, Ovulation rates in HSD, DSS, and BN rats (Kruskal-Wallis rank sum test followed by the Behrens-Fisher type nonparametric multiple comparison test; *, a > b > c, P < 0.001, HSD: n = 17, DSS: n = 6, BN: n = 24). Histological analyses of ovaries from d 8.5 pseudopregnant DSS (K) and BN (L and M) rats are shown. Bars, 250 μm. The arrow in M indicates the location of a polyovular (multioocytic) follicle.
The lower serum P4 levels were associated with altered expression of ovarian transcripts for enzymes involved in P4 biosynthesis and metabolism (Fig. 4, D–H). Cyp11a1 and Hsd3b1 transcripts encoding enzymes critical to P4 biosynthesis were lower in BN rat ovaries than in DSS rat ovaries (Fig. 4, D and E). Both transcripts were significantly higher in the ovary ipsilateral to the decidualized uterine horn in the DSS rat but not in the BN rat (Fig. 4, D and E), suggesting a functional difference in the luteotropic activity of decidual tissue from each of the two strains. P4 can be inactivated through the actions of 20α-hydroxysteroid dehydrogenase, or alternatively it can act as a prohormone for the formation of androgens. Akr1c18 transcripts, which encode 20α-hydroxysteroid dehydrogenase, were less abundant in the BN ovary than in the DSS ovary. A profound difference was found in DSS vs. BN ovarian transcript levels for Cyp17a1, which encodes the 17α-hydroxylase enzyme directing androgen production (Fig. 4F). Decreased Cyp17a1 in BN ovaries prompted measurements of circulating androstenedione. Surprisingly, serum androstenedione was actually higher in the BN rat than the DSS rat (Fig. 4I). Cyp19a1 encodes aromatase, an enzyme responsible for the conversion of androgens to estrogens. Aromatase transcript levels were lower in the BN rat and were not responsive to the ipsilateral actions of decidual tissue (Fig. 4G).
Members of the decidual PRL family have been implicated as potential luteotropic hormones (37,38,39). Prl, Prl6a1, Prl8a2, and Prl3c1 transcript levels in decidual tissue did not differ between DSS and BN rats (Supplemental Fig. 5).
Decidual transcripts for steroidogenic and steroid metabolizing enzymes were also evaluated. The only difference noted was a prominent diminution in BN rat transcript levels for Hsd17b2 (Supplemental Fig. 6). Hsd17b2 encodes 17β-hydroxysteroid dehydrogenase type 2, which catalyzes the conversion of E2 to estrone, a less potent estrogen (40,41).
We also determined that ovulation rates were significantly less in BN than HSD or DSS rats (Fig. 4J). The histological appearance of pseudopregnant d 8.5 ovaries from BN and DSS rats were characterized by prominent corpora lutea (Fig. 4, K and L). Polyovular (multioocyte) follicles were identified in some of the BN ovarian sections but were not found in any of the DSS ovarian sections examined (Fig. 4M). Polyovular follicles are indicative of early disruptions in ovarian morphogenesis (42).
Collectively our analyses indicate prominent strain differences in ovarian function and suggest that early pregnancy in the BN rat is characterized by luteal insufficiency.
Attempts to rescue the BN decidualization phenotype
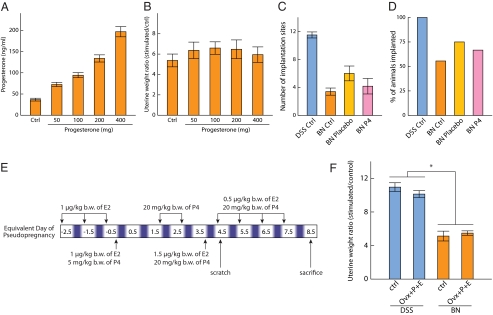
Because serum P4 levels were significantly lower in the BN rats and the decidualization reaction is dependent on P4, we attempted to rescue the BN decidualization phenotype by elevating serum P4 levels (Fig. 5). BN rats were implanted with P4 pellets ranging from 50 to 400 mg on d 0.5 of pseudopregnancy. On d 4.5 of pseudopregnancy, decidualization was induced and animals were killed on pseudopregnancy d 8.5, blood collected, and uterine horn weights determined. Serum P4 levels were increased in a dose-dependent pattern after the implantation of increasing sizes of P4 pellets ranging from subphysiological and physiological to supraphysiological levels (Fig. 5A). Elevation of circulating P4 did not have significant impact on BN decidualization responses (Fig. 5B). We next postulated that prepregnancy circulating ovarian steroid hormone exposure might be critical for the preparation of the uterus and an optimal decidual reaction. Consequently, we treated ovariectomized DSS and BN rats with an empirically determined hormone regimen known to appropriately prepare the uterus for embryo implantation and induction of the decidual reaction (Fig. 5E) (43). Ovariectomized and hormonally prepared DSS and BN rats exhibited decidual reactions resembling but not exceeding decidual reactions in gonadally intact pseudopregnant rats (Fig. 5F).
Figure 5.
Attempts to rescue the BN decidualization phenotype. Two strategies for steroid hormone therapy were evaluated. In the first protocol, BN rats were implanted sc with P4 pellets ranging from 50 to 400 mg on d 0.5 of pseudopregnancy. On d 4.5 of pseudopregnancy, decidualization was induced via scratching the antimesometrial lumenal endometrial surface of one uterine horn. Animals were killed on pseudopregnancy d 8.5, blood collected, uterine horn weights determined, and the ratio of stimulated to unstimulated uterine horns calculated. A, Serum P4 levels. B, Decidualization responses (Ctrl, control: n = 9; 50 mg: n = 5; 100 mg: n = 5; 200 mg: n = 7; 400 mg: n = 6). The effect of P4 supplementation on implantation efficiency was examined. C and D, Sperm-positive BN rats were sc implanted with placebo or 100 mg P4 pellets on d 0.5 of pregnancy and killed on gestation d 8.5. C, Viable placentation sites at d 8.5 of gestation. D, Percentage of rats with viable placentation sites. In the second protocol, ovariectomized DSS and BN rats were treated with an empirically determined hormonal regimen of P4 and E2 known to promote sensitivity to a deciduogenic stimulus (E) and decidualization responses evaluated and compared with responses of gonadally intact pseudopregnant rats (F). Data were analyzed with the Wilcoxon rank sum test (*, P < 0.001, DSS control: n = 12, DSS P4+E2 treatment: n = 8, BN control: n = 12, BN P4+E2 treatment: n = 8).
Although hormonal supplementation and replacement did not impact the size of the decidual reaction in BN rats, we postulated that these exogenous treatments might improve the efficiency of BN rats responding to an implantation stimulus. We next determined whether P4 supplementation could improve implantation efficiency in the BN rat. Sperm-positive BN rats were sc implanted with control or 100 mg P4 pellets on d 0.5 of pregnancy and killed on gestation d 8.5. P4 supplementation at d 0.5 of pregnancy did not significantly improve embryo implantation efficiency in the BN rat (Fig. 5, C and D). However, the pellet implantation procedure, including exposure to isofluorothane anesthesia, by itself resulted in some modest improvements in embryo implantation efficiency.
Although the BN rat possesses lower circulating levels of P4 than does the DSS rat, P4 supplementation does not improve implantation efficiency or the magnitude of the decidual response.
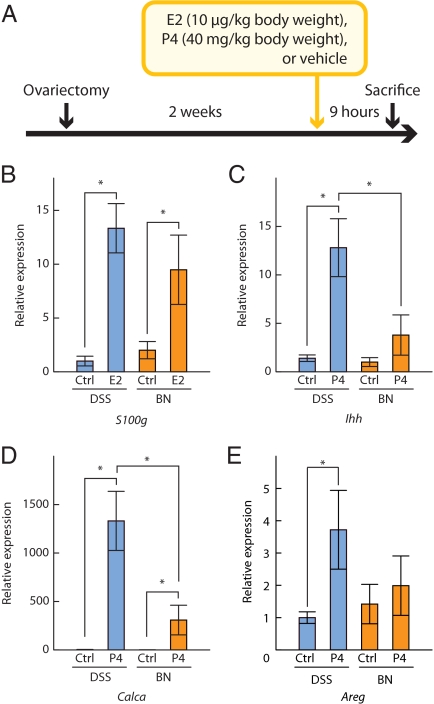
Evaluation of E2 and P4 responsiveness
The above results suggested that the limited decidual reaction observed in the BN rat might be associated with an impairment in uterine responsiveness to ovarian steroid hormones. We next evaluated classical E2 and P4 uterine actions in DSS and BN rats. S100 g was used as a marker for E2 action (44,45), whereas Ihh, Calca, and Areg were used as markers for P4 action (46,47,48). Rats from each strain were ovariectomized, rested for 2 wk, and acutely treated with a sc injection of E2 (10 μg/kg body weight), P4 (40 mg/kg body weight), or vehicle. Nine hours after injection, rats were killed, uteri collected, and gene expression assessed by qRT-PCR (Fig. 6A). Uterine responsiveness to E2 did not differ significantly between DSS and BN rats (Fig. 6B), whereas responsiveness to P4 was significantly greater in DSS than BN rats (Fig. 6, C–E). The results indicate that the uterus of the BN rat is resistant to P4.
Figure 6.
Evaluation of uterine responsiveness to E2 and P4. Rats from DSS and BN strains were ovariectomized, rested for 2 wk, and acutely treated with a sc injection of E2 (10 μg/kg body weight), P4 (40 mg/kg body weight), or vehicle. Nine hours after injection, rats were killed, uteri collected, and gene expression assessed by qRT-PCR (A). B, Uterine responsiveness to E2 (S100 g). Ctrl, Control. C–E, Uterine responsiveness to P4 (Ihh, Calca, Areg). Data were analyzed with the Wilcoxon rank sum test (*, P < 0.05, n = 8 for each group).
We next investigated whether P4 resistance was restricted to the uterus. The mammary gland is also a known target for P4 action (49). Several genes, including Areg, are known to be up-regulated in the mammary gland in response to P4 treatment (49). We repeated the above protocol except we collected mammary gland tissue 24 h after P4 or vehicle administration. The Areg transcript showed similar P4 responsiveness in mammary glands of both strains (Supplemental Fig. 7).
In summary, the evidence suggests that the BN rat exhibits features of uterine P4 resistance.
Uterine PGR and the P4 signaling pathway
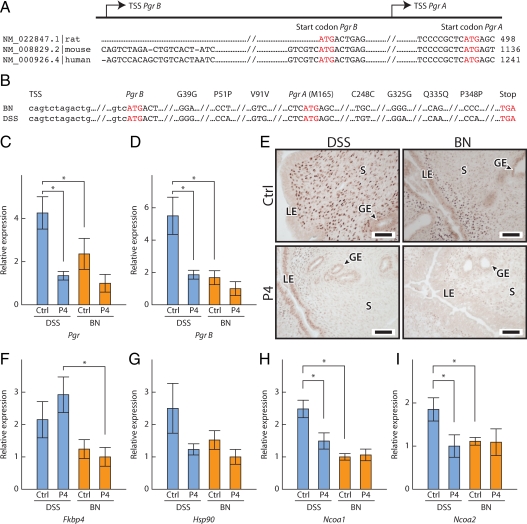
Uterine responsiveness to P4 is dependent on the PGR (50,51). Female Pgr null mice are infertile, and their uteri do not respond to P4 and fail to undergo a decidual reaction (12). Two major forms of the PGR, PGR-A and PGR-B, are expressed in the uterus (50,51). Each PGR is encoded from the same Pgr gene through the use of alternative promoters and translation start sites (34). PGR-B is approximately 164 amino acids longer than PGR-A (Fig. 7A). Pgr cDNAs for DSS and BN rat strains were sequenced (GenBank accession no. HM037361 and HM037362). Predicted amino acid sequences for PGR-A and PGR-B were identical in both strains (Fig. 7B). Next, we examined the concentrations of total Pgr and Pgr B transcripts and transcript concentrations for other components of the P4 signaling pathway, including cytoplasmic PGR chaperones (Hsp90 and Fkbp4) and nuclear receptor coactivators (Ncoa1 and Ncoa2). Heat shock protein 90 is an important chaperone for numerous client proteins, including the PGR (52). Disruption of Fkbp4, Ncoa1, and Ncoa2 can cause uterine P4 resistance (53,54,55,56,57). Transcript levels for total Pgr and Pgr B were significantly higher in the DSS vs. BN rat uteri (Fig. 7, C and D). The strain difference was more prominent for Pgr B (Fig. 7D). Strain differences in PGR expression were also evident when assessed by immunocytochemistry (Fig. 7E). PGR immunoreactivity was distributed throughout the stromal compartment of the ovariectomized DSS rat uterus with minimal epithelial expression. In contrast, PGR protein was concentrated within the uterine epithelium and subepithelial stroma of the ovariectomized BN rat uterus. P4 administration down-regulated uterine PGR expression in both strains (Fig. 7, C–E). After P4 treatment, PGR protein was confined primarily to the uterine lumenal and glandular epithelium of the DSS rat, whereas in the BN rat uterus, PGR immunoreactivity was difficult to detect. The Fkbp4, Ncoa1, and Ncoa2 transcript levels were also lower in the BN rat strain (Fig. 7, F–I). On gestation d 4.5, uterine transcript levels for Pgr B and Ncoa1 were decreased in the BN rat strain (Supplemental Fig. 8). Additionally, PGR immunoreactivity within the gestation d 4.5 uterus exhibited strain-specific patterns. In the DSS rat strain, PGR protein was robust in the uterine epithelium and stroma, whereas in the BN rat strain, uterine epithelial PGR immunoreactivity was less abundant and most PGR protein was confined to the uterine stroma (Supplemental Fig. 9, A and B). Decidual cell PGR protein expression patterns were similar in the two strains (Supplemental Fig. 9, C–I).
Figure 7.
Uterine PGR and the P4 signaling pathway. A, Structure of the Pgr gene. B, Nucleotide comparisons of the coding sequences of DSS and BN Pgr genes (GenBank accession no. HM037361, HM037362). Transcript levels for total Pgr (C) and Pgr B (D) in uteri of ovariectomized and P4-treated DSS and BN rats are shown. E, PGR immunocytochemistry. Bars, 50 μm. LE, Lumenal epithelium; GE, glandular epithelium; S, stromal cells; Ctrl, control. Transcript levels for Fkbp4 (F), Hsp90 (G), Ncoa1 (H), and Ncoa2 (I) in uteri of ovariectomized and P4-treated DSS and BN rats are shown. Wilcoxon rank sum test (*, P < 0.05, n = 8 for each group).
Strain differences in Pgr expression prompted an investigation of Pgr gene regulatory sequences (GenBank no. HM037358, HM037359, and HM037360). Several nucleotide differences and a unique feature were discovered within the sequence of the BN Pgr promoter region (Supplemental Fig. 10A). A 16-bp insertion was located between −548 and −532 of the BN rat promoter that was not present in the DSS or F344 rat Pgr promoters. The impact of this nucleotide insertion on Pgr promoter activity was investigated in P4-responsive rat UI uterine stromal and human MCF7 breast cancer cells (Supplemental Fig. 10, B–D). Pgr promoters from DSS, F344, and BN rat strains each exhibited similar activities in UI and MCF7 cells.
The experimentation indicates that attenuated expression of components of the P4 signaling pathway may be factors leading to uterine P4 resistance in the BN rat.
Discussion
The BN rat is subfertile, characterized by small litter sizes, increased pregnancy failure, and deficits in placentation (21,22,23), and represents a unique model for investigating genetic mechanisms controlling reproductive processes. In this report, we examined the establishment of pregnancy in the BN rat with the goal of identifying mechanisms that might be responsible for its high incidence of pregnancy failure. The distinctive features of BN rat placentation could be identified as early as d 8.5 of gestation and were associated with restricted and functionally compromised uterine decidual development. P4 is a key hormone in regulating uterine decidual development (9,12,13,14). Our observations are consistent with disruptions in P4 production and signaling underlying the defects in the establishment and maintenance of pregnancy in the BN rat.
Prominent strain differences were identified in the ability of the uterine environment to support embryo implantation. Only 50–60% of mated BN rats establish a successful uterine-blastocyst interaction, which contrasts with greater than 90% of mated DSS or HSD rats undergoing embryo implantation. BN rats successfully initiating pregnancy qualitatively and quantitatively differ from controls in the development and functioning of their uterine decidua. BN rat decidua is inferior in stimulating ovarian progesterone production and fostering embryo development. This can be attributed in part to a limited decidual response in BN rats. Defects did not appear intrinsic to the uterine stromal cell in that they readily decidualized in vitro independent of the rat strain of their origin. Thus, it is appears that defective differentiation of the pre- and postimplantation BN rat uterus is caused by systemic factors.
The preparation of the uterus for embryo implantation requires steroid hormones arising from the ovary (9,12,13,14). BN rats have deficits in circulating P4 levels. Low circulating P4 was associated with a decreased ovulation rate and decreased concentrations of ovarian transcripts for steroidogenic enzymes critical to the production of P4. An ineffective BN rat deciduum may also contribute to a loss in support for the corpus luteum, the main source of P4. The etiology of the deficit in systemic P4 in the BN rat could be caused by aberrations anywhere along the hypothalamic/hypophyseal/ovarian axis ultimately impacting luteal P4 production (58,59,60,61). Luteal insufficiency is a known factor leading to failures in the establishment of pregnancy (62) and a contributor to the subfertility characteristic of the BN rat.
Attempts to rescue the BN rat reproductive phenotype with steroid hormone therapy were not successful. This lack of therapeutic efficacy is unlikely related to the choice of treatment regimens because the DSS control rats responded effectively to steroid hormone treatments. Instead, it is apparent that the BN rat exhibits uterine resistance to P4. Uterine P4 resistance has been identified clinically (63) and is characteristic of a human uterine inflammatory disease, referred to as endometriosis (41,64,65,66). An additional parallel between BN rat uterine biology and endometriosis is the decreased expression of Hsd17b2 (40,41,67). Hsd17b2 encodes 17β-hydroxysteroid dehydrogenase type 2, an enzyme catalyzing the conversion of E2 to estrone, a less potent estrogen (40,41). E2 is a pivotal regulator of the preimplantation uterus, including P4 signaling (9,68). Uterine stromal cell PGR regulation is dependent on the developmental state. The PGR is regulated by E2 in undifferentiated uterine stromal cells, whereas decidual cell PGR is not dependent on E2 (68). Perturbations in the P4 signaling pathway can lead to uterine P4 resistance and subfertility or infertility (12,53,54,55,56,57). Mice with null mutations in the Pgr or in chaperones or coactivators interacting with the PGR exhibit uterine P4 resistance. P4 resistance and subfertility can also be caused by the disruption of the Bteb gene, which encodes a member of the Krüppel-like factor transcription factor family (69). The rat Pgr gene was sequenced in the current report and did not show strain differences impacting the amino acid sequence of the A or B isoforms of the PGR. Strain differences were noted in the expression of the Pgr and some other components of the P4 signaling pathway. Pgr expression was decreased in the BN rat uterus, especially the longer Pgr B transcript. The PGR-B isoform is an antagonist of uterine stromal cell proliferation (70); its down-regulation promotes proliferation and interferes with stromal cell differentiation. PGR-B expression is similarly dysregulated in endometriosis (64,65,66), which is caused, at least in part, by hypermethylation of the Pgr B promoter (71). Related epigenetic modifications are responsible for decreased PGR-B expression in sporadic breast cancer (72). TNFα and TGFβ can contribute to the regulation of PGR expression in uterine stromal cells (73,74) and are candidate regulatory factors having an impact on PGR expression in the BN rat uterus. The elevated TGFβ-induced (Tgfbi) expression observed in the decidual/uterine stromal cell compartment of the BN rat (the present study) might be indicative of a hyperactive TGFβ signaling pathway.
There are several miscellaneous features linking BN rat genetics, P4 responsiveness, and pregnancy failure. A quantitative trait locus for litter size (Inf1) was mapped in spontaneous hypertensive rat × BN recombinant rat inbred strains to chromosome 8 (21), which represents the chromosomal location of the Pgr gene. Single-nucleotide polymorphisms within the Pgr gene are associated with reproductive performance in the rabbit (75). We identified several strain differences in the nucleotide sequence of the regulatory DNA associated with the rat Pgr gene. The most prominent sequence difference was a 16-bp nucleotide insertion within the BN rat Pgr promoter that was not present in either HSD or F344 rat Pgr promoters. The insertion could potentially affect the recruitment of regulatory factors controlling transcriptional regulation of the Pgr gene. The 16-bp nucleotide insertion did not significantly affect Pgr promoter activities when in vitro reporter assays were performed using rat UI uterine stromal cells or human MCF7 breast cancer cells. The BN rat Pgr promoter insertion may be a bystander or an active participant in gene regulation. Its relevance may be dependent on cell type, developmental state, and environmental factors.
The rat has proven to be an effective model organism for investigating complex traits (26,27,29,76), including elucidating genetic mechanisms involved in diabetes, cancer, cardiovascular, neurological diseases, obesity, and metabolic disorders (77,78). Infertility and pregnancy failure are also multifactoral processes. It is evident that the rat exhibits quantitative traits associated with reproductive function. In particular, the BN rat exhibits unique features in both male and female reproductive physiology that make it an ideal centerpiece for fertility research (Refs. 23,79,80 and present study). There is a wealth of genetic tools that can facilitate dissection of reproductive phenotypes, such as a sequenced BN rat genome (81) and unique strains of congenic, consomic, and recombinant inbred rats possessing well-defined components of the BN rat genome that permit a physiology-directed search for genes implicated in fertility regulation and pregnancy failure (27,28,29). These strategies can also be complemented with an array of techniques for manipulating the rat genome and the generation of mutant animal models with gene-specific perturbations (26,29,76,78,82). Elucidation of mechanisms underlying BN rat subfertility may provide insights into infertility and pregnancy failure of other species with hemochorial placentation.
Supplementary Material
Acknowledgments
We thank Dr. Melissa Larson for assistance with the embryo transfer experiments, Diana Lambert for assistance with the uterine stromal cell cultures, and Dr. Virginia Rider (Pittsburgh State University, Pittsburgh, KS) for providing the U1 uterine stromal cells. We acknowledge Dr. Fariba Behbod for help with the mammary gland dissections. Dr. David Albertini provided insights about ovarian morphology.
Footnotes
This work was supported by Grants HD20676, HD48861, HD49503, HD055523, and HD060115 from the National Institutes of Health and the Hall Family Foundation.
Current address for T.K.: Laboratory of Animal Breeding, Graduate School of Agricultural and Life Science, The University of Tokyo, Tokyo 113-8657, Japan.
Current address for L.A.R.: U.S. Department of Agriculture, Agricultural Research Service, Clay Center, Nebraska.
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 21, 2010
Abbreviations: BN, Brown Norway; DSS, Dahl salt sensitive; E2, 17β-estradiol; F344, Fischer 344; HBSS, Hanks’ balanced salt solution; HSD, Holtzman Sprague Dawley; NK, natural killer; P4, progesterone; PGR, P4 receptor; qRT-PCR, quantitative RT-PCR; TUNEL, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling.
References
- Cooke ID 1988 Failure of implantation and its relevance to subfertility. J Reprod Fertil Suppl 36:155–159 [PubMed] [Google Scholar]
- Regan L 1992 Recurrent early pregnancy failure. Curr Opin Obstet Gynecol 4:220–228 [PubMed] [Google Scholar]
- Arck PC 2001 Stress and pregnancy loss: role of immune mediators, hormones and neurotransmitters. Am J Reprod Immunol 46:117–123 [DOI] [PubMed] [Google Scholar]
- Quenby S, Vince G, Farquharson R, Aplin J 2002 Recurrent miscarriage: a defect in nature’s quality control? Hum Reprod 17:1959–1963 [DOI] [PubMed] [Google Scholar]
- Chaddha V, Viero S, Huppertz B, Kingdom J 2004 Developmental biology of the placenta and the origins of placental insufficiency. Semin Fetal Neonatal Med 9:357–369 [DOI] [PubMed] [Google Scholar]
- Christiansen OB, Nielsen HS, Kolte AM 2006 Future directions of failed implantation and recurrent miscarriage research. Reprod Biomed Online 13:71–83 [DOI] [PubMed] [Google Scholar]
- Enders AC, Welsh AO 1993 Structural interactions of trophoblast and uterus during hemochorial placenta formation. J Exp Zool 266:578–587 [DOI] [PubMed] [Google Scholar]
- Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, Fisher SJ 2004 Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest 114:744–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Dey SK 2006 Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet 7:185–199 [DOI] [PubMed] [Google Scholar]
- DeFeo VJ 1967 Decidualization. In: Wynn RM, ed. Cellular biology of the uterus. New York: Appleton-Century-Crofts; 191–290 [Google Scholar]
- Parr MB, Parr EL 1989 The implantation reaction. In: Wynn RM, Jollie WP, eds. Biology of the uterus. New York: Plenum; 233–278 [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery Jr CA, Shyamala G, Conneely OM, O'Malley BW 1995 Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278 [DOI] [PubMed] [Google Scholar]
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H 2004 Molecular cues to implantation. Endocr Rev 25:341–373 [DOI] [PubMed] [Google Scholar]
- Lee KY, DeMayo FJ 2004 Animal models of implantation. Reproduction 128:679–695 [DOI] [PubMed] [Google Scholar]
- Bell SC 1983 Decidualization: regional differentiation and associated function. Oxford Rev Reprod Biol 5:220–271 [Google Scholar]
- Brosens JJ, Gellersen B 2006 Death or survival—progesterone-dependent cell fate decisions in the human endometrial stroma. J Mol Endocrinol 36:389–398 [DOI] [PubMed] [Google Scholar]
- Bilinski P, Roopenian D, Gossler A 1998 Maternal IL-11Rα function is required for normal decidua and fetoplacental development in mice. Genes Dev 12:2234–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb L, Li R, Hartley L, Nandurkar HH, Koentgen F, Begley CG 1998 Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat Med 4:303–308 [DOI] [PubMed] [Google Scholar]
- Aplin J 2000 Maternal influences on placental development. Semin Cell Dev Biol 11:115–125 [DOI] [PubMed] [Google Scholar]
- Gill 3rd TJ, Kunz HW, Hansen CT 1979 Litter sizes in inbred strains of rats (Rattus norvegicus). J Immunogenet 6:461–463 [DOI] [PubMed] [Google Scholar]
- Zídek V, Pintír J, Musilová A, Bílá V, Kren V, Pravenec M 1999 Mapping of quantitative trait loci for seminal vesicle mass and litter size to rat chromosome 8. J Reprod Fertil 116:329–333 [DOI] [PubMed] [Google Scholar]
- Buresova M, Zidek V, Musilova A, Simakova M, Fucikova A, Bila V, Kren V, Kazdova L, Di Nicolantonio R, Pravenec M 2006 Genetic relationship between placental and fetal weights and markers of the metabolic syndrome in rat recombinant inbred strains. Physiol Genomics 26:226–231 [DOI] [PubMed] [Google Scholar]
- Konno T, Rempel LA, Arroyo JA, Soares MJ 2007 Pregnancy in the Brown Norway rat: a model for investigating the genetics of placentation. Biol Reprod 76:709–718 [DOI] [PubMed] [Google Scholar]
- Dosiou C, Giudice LC 2005 Natural killer cells in pregnancy and recurrent pregnancy loss: endocrine and immunologic perspectives. Endocr Rev 26:44–62 [DOI] [PubMed] [Google Scholar]
- Quenby S, Farquharson R 2006 Uterine natural killer cells, implantation failure, and recurrent miscarriage. Reprod Biomed Online 13:24–28 [DOI] [PubMed] [Google Scholar]
- Jacob HJ, Kwitek AE 2002 Rat genetics: attaching physiology and pharmacology to the genome. Nat Rev Genet 3:33–42 [DOI] [PubMed] [Google Scholar]
- Cowley AW 2003 Genomics and homeostasis. Am J Physiol 284:R611–R627 [DOI] [PubMed] [Google Scholar]
- Cowley Jr AW, Roman RJ, Jacob HJ 2004 Application of chromosomal substitution techniques in gene-function discovery. J Physiol 554:46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar J, Moreno C, Jacob HJ, Kwitek AE 2005 Impact of genomics on research in the rat. Genome Res 15:1717–1728 [DOI] [PubMed] [Google Scholar]
- DeFeo VJ 1963 Temporal aspect of uterine sensitivity in the pseudopregnant or pregnant rat. Endocrinology 72:305–316 [DOI] [PubMed] [Google Scholar]
- Ain R, Konno T, Canham LN, Soares MJ 2006 Phenotypic analysis of the rat placenta. Methods Mol Med 121:295–313 [DOI] [PubMed] [Google Scholar]
- Duijvestijn AM, van Goor H, Klatter F, Majoor GD, van Bussel E, van Breda Vriesman PJ 1992 Antibodies defining rat endothelial cells: RECA-1, a pan-endothelial cell-specific monoclonal antibody. Lab Invest 66:459–466 [PubMed] [Google Scholar]
- Yee GM, Kennedy TG 1991 Role of cyclic adenosine 3′5′-monophosphate in mediating the effect of prostaglandin E2 on decidualization in vitro. Biol Reprod 45:165–171 [DOI] [PubMed] [Google Scholar]
- Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P 1990 Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 9:1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider V 2006 Isolation of hormone responsive uterine stromal cells: an in vitro model for stromal cell proliferation and differentiation. Methods Mol Med 121:57–67 [PubMed] [Google Scholar]
- Munzel U, Hothorn LA 2001 A unified approach to simultaneous rank test procedures in the unbalanced one-way layout. Biom J 43:553–569 [Google Scholar]
- Gibori G, Jayatilak PG, Khan I, Rigby B, Puryear T, Nelson S, Herz Z 1987 Decidual luteotropin secretion and action: its role in pregnancy maintenance in the rat. Adv Exp Med Biol 219:379–397 [DOI] [PubMed] [Google Scholar]
- Soares MJ 2004 The prolactin and growth hormone families: pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod Biol Endocrinol 2:51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam SM, Konno T, Sahgal N, Lu L, Soares MJ 2008 Decidual cells produce a heparin-binding prolactin family cytokine with putative intrauterine regulatory actions. J Biol Chem 283:18957–18968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Lin SX, Simard J, Labrie C 2000 Role of 17β-hydroxysteroid dehydrogenases in sex steroid formation in peripheral intracrine tissues. Trends Endocrinol Metab 11:421–427 [DOI] [PubMed] [Google Scholar]
- Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, Innes J, Julie Kim J 2006 Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol 248:94–103 [DOI] [PubMed] [Google Scholar]
- Crain DA, Janssen SJ, Edwards TM, Heindel J, Ho SM, Hunt P, Iguchi T, Juul A, McLachlan JA, Schwartz J, Skakkebaek N, Soto AM, Swan S, Walker C, Woodruff TK, Woodruff TJ, Giudice LC, Guillette Jr LJ 2008 Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril 90:911–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy TG 1986 Intrauterine infusion of prostaglandins and decidualization in rats with uteri differentially sensitized for the decidual cell reaction. Biol Reprod 34:327–335 [DOI] [PubMed] [Google Scholar]
- Bruns ME, Overpeck JG, Smith GC, Hirsch GN, Mills SE, Bruns DE 1988 Vitamin D-dependent calcium binding protein in rat uterus: differential effects of estrogen, tamoxifen, progesterone, and pregnancy on accumulation and cellular localization. Endocrinology 122:2371–2378 [DOI] [PubMed] [Google Scholar]
- Darwish H, Krisinger J, Furlow JD, Smith C, Murdoch FE, DeLuca HF 1991 An estrogen-responsive element mediates the transcriptional regulation of calbindin D-9K gene in rat uterus. J Biol Chem 266:551–558 [PubMed] [Google Scholar]
- Zhu LJ, Cullinan-Bove K, Polihronis M, Bagchi MK, Bagchi IC 1998 Calcitonin is a progesterone-regulated marker that forecasts the receptive state of endometrium during implantation. Endocrinology 139:3923–3934 [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Zhao X, Das SK, Hogan BL, Dey SK 2002 Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev Biol 245:280–290 [DOI] [PubMed] [Google Scholar]
- Takamoto N, Zhao B, Tsai SY, DeMayo FJ 2002 Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol Endocrinol 16:2338–2348 [DOI] [PubMed] [Google Scholar]
- Fernandez-Valdivia R, Mukherjee A, Creighton CJ, Buser AC, DeMayo FJ, Edwards DP, Lydon JP 2008 Transcriptional response of the murine mammary gland to acute progesterone exposure. Endocrinology 149:6236–6250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Jeong J, Tsai MJ, Tsai S, Lydon JP, DeMayo FJ 2006 Molecular mechanisms involved in progesterone receptor regulation of uterine function. J Steroid Biochem Mol Biol 102:41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ 2008 In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin Cell Dev Biol 19:178–186 [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO 1997 Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev 18:306–360 [DOI] [PubMed] [Google Scholar]
- Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O'Malley BW 1998 Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science 279:1922–1925 [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Amato P, Allred DC, DeMayo FJ, Lydon JP 2007 Steroid receptor coactivator 2 is required for female fertility and mammary morphogenesis: insights from the mouse, relevance to the human. Nucl Recept Signal 5:e011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Soyal SM, Fenandez-Valdivia R, Gehin M, Chambon P, Demayo FJ, Lydon JP, O'Malley BW 2006 Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Mol Cell Biol 26:6571–6583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Wolf IM, Chen H, Periyasamy S, Chen Z, Yong W, Shi S, Zhao W, Xu J, Srivastava A, Sánchez ER, Shou W 2006 FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor A isoforms. Mol Endocrinol 20:2682–2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranguch S, Wang H, Daikoku T, Xie H, Smith DF, Dey SK 2007 FKBP52 deficiency-conferred uterine progesterone resistance is genetic background and pregnancy stage specific. J Clin Invest 117:1824–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender GD 2002 Molecular control of luteal secretion of progesterone. Reproduction 123:333–339 [DOI] [PubMed] [Google Scholar]
- Devoto L, Kohen P, Vega M, Castro O, González RR, Retamales I, Carvallo P, Christenson LK, Strauss JF 2002 Control of human luteal steroidogenesis. Mol Cell Endocrinol 186:137–141 [DOI] [PubMed] [Google Scholar]
- Bachelot A, Binart N 2005 Corpus luteum development: lessons from genetic models in mice. Curr Top Dev Biol 68:49–84 [DOI] [PubMed] [Google Scholar]
- Stocco C, Telleria C, Gibori G 2007 The molecular control of corpus luteum formation, function, and regression. Endocr Rev 28:117–149 [DOI] [PubMed] [Google Scholar]
- Arredondo F, Noble LS 2006 Endocrinology of recurrent pregnancy loss. Semin Reprod Med 24:33–39 [DOI] [PubMed] [Google Scholar]
- Keller DW, Wiest WG, Askin FB, Johnson LW, Strickler RC 1979 Pseudocorpus luteum insufficiency: a local defect of progesterone action on endometrial stroma. J Clin Endocrinol Metab 48:127–132 [DOI] [PubMed] [Google Scholar]
- Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE 2000 Progesterone receptor isoforms A but not B is expressed in endometriosis. J Clin Endocrinol Metab 85:2897–2902 [DOI] [PubMed] [Google Scholar]
- Gurates B, Bulun SE 2003 Endometriosis: the ultimate hormonal disease. Semin Reprod Med 21:125–134 [DOI] [PubMed] [Google Scholar]
- Bulun SE 2009 Endometriosis. N Engl J Med 360:268–279 [DOI] [PubMed] [Google Scholar]
- Zeitoun K, Takayama K, Sasano H, Suzuki T, Moghrabi N, Andersson S, Johns A, Meng L, Putman M, Carr B, Bulun SE 1998 Deficient 17β-hydroxysteroid dehydrogenase type 2 expression in endometriosis: failure to metabolize 17β-estradiol. J Clin Endocrinol Metab 83:4474–4480 [DOI] [PubMed] [Google Scholar]
- Kurita T, Lee K, Saunders PT, Cooke PS, Taylor JA, Lubahn DB, Zhao C, Mäkelä S, Gustafsson JA, Dahiya R, Cunha GR 2001 Regulation of progesterone receptors and decidualization in uterine stroma of the estrogen receptor-α knockout mouse. Biol Reprod 64:272–283 [DOI] [PubMed] [Google Scholar]
- Simmen RC, Eason RR, McQuown JR, Linz AL, Kang TJ, Chatman Jr L, Till SR, Fujii-Kuriyama Y, Simmen FA, Oh SP 2004 Subfertility, uterine hypoplasia, and partial progesterone resistance in mice lacking the Kruppel-like factor 9/basic transcription element-binding protein-1 (Bteb1) gene. J Biol Chem 279:29286–29294 [DOI] [PubMed] [Google Scholar]
- Wu Y, Shi X, Guo SW 2008 The knockdown of progesterone receptor isoform B (PR-B) promotes proliferation in immortalized endometrial stromal cells. Fertil Steril 90:1320–1323 [DOI] [PubMed] [Google Scholar]
- Wu Y, Strawn E, Basir Z, Halverson G, Guo SW 2006 Promoter hypermethylation of progesterone receptor isoforms B (PR-B) in endometriosis. Epigenetics 1:106–111 [DOI] [PubMed] [Google Scholar]
- McCormack O, Chung WY, Fitzpatrick P, Cooke F, Flynn B, Harrison M, Fox E, Gallagher E, McGoldrick A, Dervan PA, McCann A, Kerin MJ 2008 Progesterone receptor B (PRB) promoter hypermethylation in sporadic breast cancer. Breast Cancer Res Treat 111:45–53 [DOI] [PubMed] [Google Scholar]
- Kane N, Jones M, Brosens JJ, Saunders PT, Kelly RW, Critchley HO 2008 Transforming growth factor-β1 attenuates expression of both the progesterone receptor and Dickkopf in differentiated human endometrial stromal cells. Mol Endocrinol 22:716–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Starzinski-Powitz A, Guo SW 2008 Prolonged stimulation with tumor necrosis factor-α induced partial methylation at PR-B promoter in immortalized epithelial-like endometriotic cells. Fertil Steril 90:234–237 [DOI] [PubMed] [Google Scholar]
- Peiró R, Mercháan M, Santacreu MA, Argente MJ, García ML, Folch JM, Blasco A 2008 Identification of a single-nucleotide polymorphism in the progesterone receptor gene and its association with reproductive traits in rabbits. Genetics 180:1699–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley KC, Weinstock GM, Gibbs RA 2008 Rats in the genomic era. Physiol Genomics 32:273–282 [DOI] [PubMed] [Google Scholar]
- Kwitek AE, Jacob HJ, Baker JE, Dwinell MR, Forster HV, Greene AS, Kunert MP, Lombard JH, Mattson DL, Pritchard Jr KA, Roman RJ, Tonellato PJ, Cowley Jr AW 2006 BN phenome: detailed characterization of the cardiovascular, renal, and pulmonary systems of the sequenced rat. Physiol Genomics 25:303–313 [DOI] [PubMed] [Google Scholar]
- Aitman TJ, Critser JK, Cuppen E, Dominiczak A, Fernandez-Suarez XM, Flint J, Gauguier D, Geurts AM, Gould M, Harris PC, Holmdahl R, Hubner N, Izsvák Z, Jacob HJ, Kuramoto T, Kwitek AE, Marrone A, Mashimo T, Moreno C, Mullins J, Mullins L, Olsson T, Pravenec M, Riley L, Saar K, Serikawa T, Shull JD, Szpirer C, Twigger SN, Voigt B, Worley K 2008 Progress and prospects in rat genetics: a community view. Nat Genet 40:516–522 [DOI] [PubMed] [Google Scholar]
- Wang C, Leung A, Sinha-Hikim AP 1993 Reproductive aging in the male Brown Norway rat: a model for the human. Endocrinology 133:2773–2781 [DOI] [PubMed] [Google Scholar]
- Zirkin BR, Chen H 2000 Regulation of Leydig cell steroidogenic function during aging. Biol Reprod 63:977–981 [DOI] [PubMed] [Google Scholar]
- Gibbs RA, Weinstock GM, Metzker ML, Muzny DM, Sodergren EJ, Scherer S, Scott G, Steffen D, Worley KC, Burch PE, Okwuonu G, Hines S, Lewis L, DeRamo C, Delgado O, Dugan-Rocha S, Miner G, Morgan M, Hawes A, Gill R, Celera, Holt RA, Adams MD, Amanatides PG, Baden-Tillson H, et al. 2004 Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature 428:493–521 [DOI] [PubMed] [Google Scholar]
- Voigt B, Serikawa T 2009 Pluripotent stem cells and other technologies will eventually open the door for straightforward gene targeting in the rat. Dis Model Mech 2:341–343 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.