Abstract
During early pregnancy, the concerted actions of the maternal steroid hormones, estrogen and progesterone, promote a unique process known as decidualization, which involves extensive proliferation and differentiation of uterine stromal cells. The molecular pathways underlying this hormonally induced cellular transformation, an essential prerequisite for embryo implantation, remain poorly understood. We previously identified CCAAT/enhancer binding protein β (C/EBPβ) as a target of steroid regulation in the uterus. Uteri of mice lacking C/EBPβ failed to undergo decidualization. In the present study, analyses of C/EBPβ-null uteri indicated that loss of this factor leads to a block in stromal cell proliferation in response to a decidual stimulation. The mutant stromal cells entered S phase of the cell cycle and completed DNA synthesis but were unable to execute mitosis. Further analysis revealed that C/EBPβ facilitates the transition of these cells into mitosis by binding directly to the cyclin B2 promoter to regulate its expression. The expression of cdc25C, a phosphatase that maintains the active state of the cyclin B-cyclin-dependent kinase complex during mitosis, is also strongly suppressed in C/EBPβ-null stromal cells. Furthermore, the expression of the tumor suppressor p53 and the cell cycle inhibitors p21 and p27 was markedly elevated in C/EBPβ-null stromal cells before the mitotic phase, uncovering additional mechanisms by which C/EBPβ controls G2 to M transition. Collectively, these results revealed that C/EBPβ mediates the effects of steroid hormones during decidualization by modulating the expression of multiple key cell cycle regulatory factors that control the G2 to M transition of the proliferating uterine stromal cells.
The mechanisms by which the transcription factor C/EBPβ controls steroid-induced mitotic expansion of uterine stromal cells during decidualization are described.
The mouse model has been used extensively to study the molecular signaling mechanisms underlying the process of embryo implantation (1,2). During the preimplantation phase of pregnancy in this species, the maternal steroid hormones, estrogen (E) and progesterone (P), orchestrate molecular and cellular alterations in the uterine surface epithelium that make it competent to attach to the blastocyst to initiate the process of implantation (3,4,5,6). The attachment of the blastocyst on d 4.5 of pregnancy triggers the process of decidualization, which involves a remarkable transformation of the fibroblastic endometrial stromal cells underlying the surface epithelium into morphologically and functionally distinct decidual cells (7,8,9,10,11,12). This cellular transformation process occurs under the influence of E and P during d 5–8 of gestation. Initially the undifferentiated stromal cells undergo mitotic expansion, and then they enter the differentiation program that converts them into decidual cells. The formation of the decidual tissue surrounding the implanting embryo is a prerequisite for successful implantation. It serves as a source of paracrine effectors such as hormones, growth factors, and cytokines, which promote uterine angiogenesis and embryo development, mediate immunoregulatory functions during pregnancy, and regulate trophoblast invasion (7,8,9,10,11,12). The current challenge is to understand the complex process by which steroid hormones regulate the formation and function of the decidual tissue. To this end, it is critical to identify and characterize the factors induced by the maternal hormones that regulate the proliferation and differentiation of uterine stromal cells during the decidualization process.
We previously used gene expression profiling in pregnant mouse uterus to identify steroid-regulated gene networks that have functional relevance in implantation (13,14). Our studies identified CCAAT/enhancer binding protein β (C/EBPβ) as a novel mediator of the biological actions of E and P in the uterus during early pregnancy (13,14). This transcription factor belongs to a family of basic leucine zipper (bZIP) proteins, which controls numerous biological processes, including cell proliferation, differentiation, metabolic homeostasis, acute phase inflammation, and apoptosis (15,16,17). The C/EBP family members regulate transcription of target genes by binding to a consensus nucleotide sequence motif, which resides in the regulatory regions of these genes. Previous studies revealed that female mice lacking C/EBPβ are infertile, while the mutant males are fertile (18). In a previous study, we demonstrated that functional abnormalities in the uterine tissue of the mutant mouse contribute to the observed infertility (13,14). The uterine defects in the mutant mice included a reduced epithelial cell proliferation in response to E and a lack of stromal response to a deciduogenic stimulus (13). The decidualization defect was observed in the presence of exogenously administered steroid hormones, indicating that it was independent of ovarian malfunction and intrinsic to the uterus.
During the decidualization phase of pregnancy, the uterine stromal cells undergo proliferation for 24–48 h and then enter the differentiation program (19,20,21,22). The lack of decidual response in C/EBPβ-null uteri raised the possibility that this transcription factor is a potential regulator of pathways directing stromal proliferation or differentiation or both. Morphological analysis of uterine sections of wild-type (WT) and C/EBPβ-null uteri in response to decidual stimulus showed a significantly reduced stromal/decidual cell mass in the mutant mice, hinting at a defect in uterine stromal cell proliferation in these mice. In the present study, we tested the hypothesis that C/EBPβ regulates stromal cell proliferation during decidualization by controlling the expression or activity of critical cell cycle regulatory molecules. We performed a detailed analysis of the expression and function of potential cell cycle regulators in the stromal compartment of C/EBPβ-null uteri to pinpoint the molecular defect(s) underlying the lack of decidual response in the mutant tissue.
Materials and Methods
Animals and tissue collection
All experiments involving animals were conducted in accordance with the National Institutes of Health standards for the use and care of animals. The animal protocols were approved by the University of Illinois Institutional Animal Care and Use Committee. Female 129-SV mice (from Charles River, Wilmington, MA) and C/EBPβ-null mice of same genetic background (provided by Dr. Peter Johnson of National Cancer Institute, National Institutes of Health, Bethesda, MD) were subjected to experimentally induced decidualization. Mice were euthanized at various time points after the administration of the artificial decidualization stimulus and uteri were collected.
Experimentally induced decidualization
Decidualization was induced artificially as described previously (13,23). Briefly, mice were subjected to ovariectomy. Two weeks after ovariectomy, animals were injected with 100 ng of E (Sigma, St. Louis, MO) in 0.1 ml of sesame oil for three consecutive days. This was followed by the administration of daily injections of 1 mg of P (Sigma) and 10 ng E for three consecutive days. Six hours after the third P and E injection, 20 μl sesame oil was introduced into uterine horn to mimic the decidualization stimulus.
Isolation of primary stromal cells and analysis of decidualization in vitro
Mouse uteri were collected at various time points after artificial decidualization, and uterine stromal cell were isolated as described previously (24). Briefly, uterine horns were dissected and cut into 3- to 5-mm pieces. The uterine tissues were placed in Hanks’ balanced salt solution (HBSS) containing 6 g/liter dispase (Invitrogen, Carlsbad, CA) and 25 g/liter pancreatin (Sigma) for 1 h at room temperature and then 10 min at 37 C. The tissues were gently agitated, and the supernatant was discarded to remove the endometrial epithelial clumps. The partially digested tissues were washed twice in HBSS and then placed in HBSS containing 0.5 g/liter collagenase (Sigma). After incubation for 45 min at 37 C, the tubes were vortexed for 10–12 sec until the supernatant became turbid with dispersed endometrial stromal cells. The contents of the tube were then passed through a 70-μm gauze filter (Millipore, Billerica, MA). The stromal cells in the filtrate were pelleted, resuspended in DMEM-F12 medium containing 5% fetal calf serum, and plated. After plating, the stromal cells were found to be positive for vimentin stain and negative for cytokeratin stain. By our estimate, they were >95% pure. The cell culture was continued after addition of fresh medium supplemented with P (1 μm) and E (10 nm) as described previously (24).
Real-time RT-PCR
Total RNA was isolated from uterine cells by standard Trizol-based protocols and converted to cDNA. The cDNA was amplified by real-time PCR to quantify gene expression using gene-specific primers and SYBR Green (Bio-Rad Laboratories, Hercules, CA) as described in the legends to Fig. 3. The primer sequences used in real-time PCR experiments are shown in Supplemental Table 1 (published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org/).
Figure 3.
Analysis of expression of S phase-specific cell cycle regulators in uterine stromal cells of C/EBPβ-null mice. Ovariectomized WT and C/EBPβ-null mice were subjected to artificial decidual stimulation. Stromal cell were isolated at different times after application of the stimulus, and total RNA was prepared from these cells. WT or C/EBPβ-null mice (n = 3–5) were used at each time point and the uteri of each category were pooled. The expression levels of mRNAs corresponding to cyclin D3 (A), Cdk4 (B), cyclin E1 (C), Cdk2 (D), and cdc25A (E) were monitored by real-time PCR using gene-specific primers. As described previously (26,58), for each gene expression measurement, the mean threshold cycle (Ct) and sd were calculated from Ct values obtained individually from three replicates of that sample. Each sample was subjected to three independent real-time PCR trials. Normalized Ct (ΔCt) values were determined by subtracting the mean Ct values of 36B4 from the mean Ct of each gene. The ΔΔCt values were then calculated as the differences between ΔCt values of genes from treated samples and control samples. Fold change was derived by applying the formula 2−ΔΔCt to the ΔΔCt values. The error bars indicate 2−ΔΔCt ± sd. The relative fold inductions of the expression of these genes at different times were determined after setting that of 0 h WT cells at 1.0. ANOVA single factor analysis was conducted on the grouped means to determine statistical significance at a significance level of P < 0.05. Black bar, WT; grey bar, C/EBPβ-null.
Immunohistochemistry and immunocytochemistry
Paraffin-embedded endometrial sections were subjected to immunohistochemistry as described previously (25). Polyclonal antibodies against bromodeoxyuridine (BrdU, BD PharMingen, Franklin Lakes, NJ) and phospho-histone 3 (Upstate Biotechnology) were used for immunohistochemistry at dilutions of 1:500 and 1:1000, respectively. Mouse primary stromal cell were fixed in 10% formalin (Sigma) and subjected to immunocytochemistry using antibodies against C/EBPβ, cyclin-dependent kinase (Cdk)1, cdc25C (c-20), and phospho-Cdk1 (Thr14/Tyr15) (all from Santa Cruz Biotechnology, Santa Cruz, CA). The antibody against cyclin B2 was a gift from Dr. Mark Carrington of Cambridge University, UK.
Western blotting
Stromal cell were isolated from uteri collected from mice at different times after artificial decidual stimulus as described (24). The cells were lysed in lysis buffer containing 25 mm Tris/HCl (pH 7.4), 50 mm NaF, 200 mm NaCl, 1 mm sodium vanadate, 5 mm EGTA, 1 mm EDTA, 1% (vol/vol) Triton X-100, 10 mm sodium pyrophosphate, 1 mm benzamidine, 0.1 mm phenylmethylsulfonylfluoride, 0.27 m sucrose, and 0.1% (vol/vol) 2-mercaptoethanol. The cell debris was removed by centrifugation, and the protein concentration of the lysate was determined by the method of Bradford, using BSA as a standard. The cell lysates were analyzed by Western blotting using antibodies against cyclin A, Cdk1 (Santa Cruz Biotechnology), p53 (FL-393), p21 (Cell Signaling Technology, Danvers, MA), p27 (BD Transduction Laboratories), and cyclin B2.
Chromatin immunoprecipitation (ChIP)
The chromatin immunoprecipitation analysis was performed using the EZ ChIP (Upstate Biotechnology), according to the manufacturer’s protocol. Briefly, mouse uterine stromal cells were isolated from WT mice subjected to artificial decidualization protocol and cultured in the presence of E and P for 20 h. The cells (8 × 106) were cross-linked with 1% formaldehyde for 10 min. The cross-linked cells were harvested, lysed using SDS lysis buffer, and sonicated. After preclearing the lysates with salmon sperm DNA-protein A at 4 C for 2 h, the DNA-protein complexes in the supernatant were immunoprecipitated using antibodies against RNA polymerase II, mouse IgG (Upstate Biotechnology), or C/EBPβ (Santa Cruz Biotechnology). The immune complexes were recovered by adding protein A agarose. The beads were then washed repeatedly, and the bound complexes were eluted using the elution buffer. The cross-linking was reversed, and then proteins were digested using 0.5 mg/ml proteinase K. Purified DNA were used as templates for PCR using various primer sets to amplify specific regions of the cyclin B2 promoter.
Adenovirus-mediated expression of dominant-negative C/EBPβ
An adenoviral vector expressing a dominant negative C/EBP (A-C/EBP) was provided by Dr. Charles Vinson (National Cancer Institute, National Institutes of Health). This vector expresses A-C/EBP under the control of CMV promoter. The protein is expressed with a HA epitope tag. An adenoviral vector expressing GFP (Ad-GFP) was used as a control. Stromal cells were isolated from WT uteri collected immediately after artificial decidual stimulation and allowed to attach to slides for 2 h. The cells were cultured in medium supplemented with P (1 μm) and E (10 nm) and transduced with adenovirus expressing A-C/EBP or Ad-GFP at a multiplicity of infection (MOI) of 10:1. After transduction for 20 h, cells were treated with formaldehyde and ChIP assays were performed as described above.
Statistical analysis
All real-time RT-PCR experiments were repeated at least three times. Statistical significance was assessed by ANOVA at a significance level of P < 0.05 and is indicated by an asterisk in the figures.
Results
C/EBPβ-null uterine stromal cells undergo DNA replication in response to a decidual stimulation
To investigate the role of C/EBPβ in the proliferation of steroid hormone-primed uterine stromal cells, we subjected WT and C/EBPβ-null mice to experimentally induced decidualization as described in Materials and Methods. To assess DNA synthesis, we monitored the incorporation of BrdU in uterine tissue at different times after decidual stimulation. Previous studies in our laboratory indicated a marked increase in BrdU incorporation in WT uterine stroma at 12 h after decidual stimulation (Wang, W., and M. K. Bagchi, unpublished result). Therefore, to examine the S phase activity in the stromal tissue, we administered a 2-h pulse of BrdU at 12 and 14 h after the decidual stimulation and collected uteri at 14 and 16 h, respectively. In the WT animals, a significant portion of the uterine stroma, particularly the subluminal stromal cells, exhibited BrdU immunostaining by 14 h (Fig. 1A, top left). By 16 h, strong and widespread BrdU incorporation was seen in the stromal compartment of WT uteri (Fig. 1A, top right). Sections of C/EBPβ-null uteri collected at the same time points after decidual stimulation exhibited similar robust incorporation of BrdU (Fig. 1A, bottom). Consistent with these results, quantitation of the BrdU immunostaining revealed no significant difference in the number of BrdU-positive cells in the stromal compartments of the WT and C/EBPβ-null uteri (Fig. 1B). We also examined the expression of PCNA, another S phase marker, in the stromal compartment. As shown in Supplemental Fig. 1, there was no significant difference in the spatial expression of PCNA in WT and C/EBPβ-null stromal cells. The stromal cells of C/EBPβ-null uteri, therefore, were able to progress through the G1 to S checkpoint, enter the S phase, and execute DNA synthesis.
Figure 1.
Examination of S phase activity of uterine stromal cells of C/EBPβ-null mice using BrdU immunostaining. Ovariectomized WT and C/EBPβ-null mice were subjected to artificial decidual stimulation, and uteri were collected at different time points after application of the stimulus. A, Mice were given BrdU injection at 12 and 14 h after stimulation. After a 2-h pulse, the uterine tissues were collected at 14 and 16 h. The tissues were fixed, embedded in paraffin, sectioned, and subjected to immunohistochemistry using an antibody specific for BrdU. At least three WT or C/EBPβ-null mice were used at each time point, and representative data are shown. B, The number of cells in sections of C/EBPβ-null uteri that are immunoreactive to BrdU antibody was quantitated in several nonoverlapping fields at ×40 magnification and compared with those in WT uteri. Total number of cells in any given field was estimated by 4′,6-diamidino-2-phenylindole nuclear staining. Data were expressed as average ± sd of five separate measurements.
C/EBPβ-null uterine stromal cells exhibit impaired mitosis
To monitor the mitotic activity of these mutant stromal cells, we used an antibody that recognizes phosphorylated Ser 10 of histone 3 (P-His3), a unique mitotic phase marker (27). In animal cells, the Ser 10 phosphorylation of H3 begins in pericentromeric heterochromatin in late G2 interphase cells. As mitosis progresses, the P-His3 mark spreads throughout the condensing chromatin, and this modification is complete in most cells by the time prophase chromosomes are formed (27). We observed that by 18 and 20 h after decidual stimulation, a large number of the stromal cells in the WT uteri displayed specific staining for P-His3, suggesting that they undergo mitotic division (Fig. 2A, top; and Supplemental Fig. 2). In contrast, markedly reduced and only sporadic staining for P-His3 was seen in the stromal compartment of C/EBPβ-null uteri at a similar time point, indicating that the majority of the mutant cells are unable to proceed through mitosis (Fig. 2A, bottom; and Supplemental Fig. 2). Quantitation of the immunofluorescence signal indicated an approximately 5-fold reduction in P-His3-positive cells in C/EBPβ-null stromal tissue relative to the WT tissue (Fig. 2B). Examination of sections of uterine tissue obtained from the null animals at later time points, such as 22 and 24 h, did not indicate any substantial immunostaining for P-His3 in the stromal compartment (data not shown), confirming that mitosis is not delayed but truly impaired in the mutant stromal cells. The reduced mitotic activity of the C/EBPβ-null uterine stromal cells indicated that these cells, which are able to undergo DNA replication, are arrested in the cell cycle before mitosis, presumably at the G2 to M boundary.
Figure 2.
Stromal proliferation is blocked at G2 to M transition in C/EBPβ-null uteri. Ovariectomized WT and C/EBPβ-null mice were subjected to artificial decidual stimulation, and uteri were collected at different time points after application of the stimulus. A, Sections of uterine tissues, obtained at 20 h after artificial decidual stimulation, were subjected to immunofluorescence using an antibody specific for phosphorylated Ser 10 of histone 3. E and S denote surface epithelium and stroma, respectively. B, The number of phospho-histone 3-positive cells in C/EBPβ-null uteri was quantitated in several nonoverlapping fields at ×40 magnification and compared with those in WT uteri. Total number of cells in any given field was estimated by 4′,6-diamidino-2-phenylindole nuclear staining. Data were expressed as average ± sd of five separate measurements.
C/EBPβ controls the expression of B-type cyclins at G2 to M transition of the cell cycle
The cell cycle is primarily regulated by a complex interplay of cyclins, cyclin-dependent kinases (Cdks), and Cdk inhibitors (28,29,30,31). We therefore investigated whether C/EBPβ controls the proliferation of stromal cells by regulating the expression of certain of the molecules that specifically control various cell cycle checkpoints. Uteri were collected from WT and C/EBPβ-null mice at different times after experimentally induced decidualization, and the stromal cells were isolated from these tissue samples. We then monitored the expression of specific cyclins and Cdks that are hallmarks of G1 to S and G2 to M phases.
Previous studies showed that cyclin D3 and cyclin E1 are predominant D-type and E-type cyclins, respectively, in mouse stromal cells (32). When we analyzed the expression of these critical cyclins and their target Cdks during G1 to S transition, we found no statistically significant difference in their expression levels in WT and mutant stromal cells. Comparable levels of expression of cyclin D3 mRNA and protein and Cdk4 mRNA were observed in stromal cells of WT and C/EBPβ-null uteri at 12 and 15 h after decidual stimulation (Fig. 3, A and B; and Supplemental Fig. 3). We also noted that cyclin E1 and Cdk2 were expressed at similar levels in uterine stromal cells of WT and C/EBPβ-null mice at 15 h, and the expression of both factors declined as the cells completed the S phase and entered the G2 phase (Fig. 3, C and D). We also did not observe any statistically significant difference in the expression levels of cdc25A, a phosphatase that regulates Cdk4 activity, in WT and C/EBPβ-null uterine stromal cells (Fig. 3E). These results are consistent with our observation (see Fig. 1) that the lack of C/EBPβ does not affect the advancement of the stromal cells through the S phase of the cell cycle.
We next examined the expression of several factors such as Cyclin B, Cyclin A, and Cdk1, which are critical for the entry of the stromal cells into the mitotic phase. During the G2 to M transition, binding of cyclin A or cyclin B to Cdk1 induces phosphorylation and activation of this kinase, which plays a critical role in this process. As shown in Fig. 4, A and B, no significant alteration in stromal expression of cyclin A or Cdk1 mRNA was seen in the C/EBPβ-null uteri relative to the WT uteri at either 16 or 20 h after decidual stimulation. In contrast, the expression of cyclin B1 and cyclin B2 mRNAs was markedly reduced in C/EBPβ-null uterine stromal cells, particularly at 20 h after decidual stimulation, when the cells are supposed to enter the mitotic phase (Fig. 4, C and D). It is pertinent to mention here that cyclin B2 mRNA is expressed at a higher level compared with cyclin B1 mRNA in the uterine stromal cells (our unpublished data). Whereas the mRNA level of cyclin B1 decreased by approximately 50% in uterine stromal cells lacking C/EBPβ, that of cyclin B2 decreased by more than 80%.
Figure 4.
C/EBPβ controls the expression of the cell cycle regulators at G2 to M phase. Uterine stromal cells were isolated from WT and C/EBPβ-null mice at different times after application of artificial decidualization stimulus. A–D, WT or C/EBPβ-null (n = 3–5) mice were used at each time point, and the uteri of each category were pooled. The expression levels of mRNAs corresponding to cyclin A, Cdk1, cyclin B1, and cyclin B2 were monitored by real-time PCR at indicated time points using gene-specific primers. All PCR data were analyzed as described in legend to Fig. 3. The relative fold inductions of the expression of these genes at different times were determined after setting that of 0 h WT cells at 1.0. Black bar, WT; grey bar, C/EBPβ-null. *, Statistically significant change (P < 0.05). E, Stromal cells were isolated from WT or C/EBPβ-null uteri collected at 16 and 20 h after artificial decidual stimulation. The cells were lysed, analyzed by SDS-PAGE, and subjected to Western blotting using antibodies directed against cyclin B2 and cyclin A. Immunostaining of calnexin served as a loading control. Calnexin is a 90-kDa integral protein of the endoplasmic reticulum. Its expression in uterine stromal cells is not regulated by the steroid hormones or the cell cycle stages. F, Stromal cells were isolated from WT or C/EBPβ-null uteri collected immediately after artificial decidual stimulation and allowed to attach to slides for 2 h. The cells were cultured in vitro in medium supplemented with P (1 μm) and E (10 nm). At 20 h, the cells were fixed and subjected to immunocytochemical staining using a cyclin B2 antibody.
To further validate the regulation of cyclin B2 by C/EBPβ, we analyzed its expression at the protein level. Our in vivo study showed that the spatial expression of C/EBPβ and cyclin B2 overlap extensively in proliferating stromal cells (Supplemental Fig. 4). As shown in the Western blot in Fig. 4E, the signal(s) of cyclin B2 was drastically reduced in cell lysates prepared from stromal cells obtained from C/EBPβ-null uteri subjected to experimentally induced decidualization. We also monitored cyclin B2 expression using immunocytochemistry in primary uterine stromal cells undergoing decidualization in vitro. The cells were isolated after decidual stimulation and cultured after attachment to plastic surface. Under these conditions, the stromal cells undergo progressive decidualization over a 0- to 96-h period as evidenced by the expression of classical biomarkers such as prolactin-related protein (Supplemental Fig. 5). We observed a strong expression of cyclin B2 in WT stromal cells undergoing mitosis at 20 h after the initiation of the culture (Fig. 4F). This immunostaining of cyclin B2 was markedly diminished in the stromal cells isolated from C/EBPβ-null uteri and cultured under similar conditions. Collectively, these results supported the concept that C/EBPβ is a critical regulator of the expression of B-type cyclins, which control the transition of the uterine stromal cells into mitosis.
C/EBPβ manifests its transcriptional function by binding to a consensus motif ATTCGG/CCAAT at the promoter regions of its target genes to directly influence their rate of transcription. In silico analysis of the promoter of cyclin B2, using the bioinformatics software TESS, TFSearch, and Consite, revealed that it contains four candidate C/EBPβ binding sites within the 2-kb 5′-flanking region of the gene. The locations of these sites are centered at −403, −686, −930, and −1651 relative to the transcription start site (Fig. 5A, top). To test whether C/EBPβ actually binds to one or more of these sites, we used ChIP using an antibody specific for C/EBPβ. Primary cultures of uterine stromal cells were analyzed at 20 h after decidual stimulation when maximal expression of cyclin B2 is observed (Fig. 4F). As shown in Fig. 5A (middle and bottom), in stromal cells actively expressing cyclin B2, no significant binding of C/EBPβ was detected at the −403, −686, and −1651 sites of the cyclin B2 promoter. In contrast, a relatively strong binding of this transcription factor was located at −930 site of the cyclin B2 promoter.
Figure 5.
Regulation of uterine stromal cyclin B2 expression by C/EBPβ. A, The nucleotide positions of the candidate C/EBP binding sites within the four different regions in cyclin B2 promoter are indicated. For ChIP experiments, stromal cells were isolated from WT uteri collected immediately after artificial decidual stimulation and allowed to attach to slides for 2 h. The cells were cultured in vitro in medium supplemented with P (1 μm) and E (10 nm). At 20 h, the cells were treated with formaldehyde, and DNA-protein complexes were cross-linked and subjected to chromatin ChIP assay as described in the Materials and Methods. Chromatin immunoprecipitates obtained using antibodies against RNA polymerase II (RPII) and mouse IgG served as positive and negative controls, respectively. DNAs isolated from the anti-C/EBPβ immunoprecipitates were used as templates for regular PCR (middle) and real-time PCR (bottom) using various primer sets to amplify specific regions of the cyclin B2 promoter. The real-time PCR results were normalized with respect to the expression level of each fragment in input sample. The relative fold changes of the expression of these fragments were determined after setting that of RPII at 1.0. The experiment was repeated twice, and representative data are shown. B, Stromal cells were isolated from WT uteri collected immediately after artificial decidual stimulation and allowed to attach to slides for 2 h. The cells were transduced with adenovirus expressing dominant-negative A-C/EBP or control adenovirus Ad-GFP at MOI of 10:1. Twenty hours after virus addition, the cells were examined by immunofluorescence using an anti-HA antibody. C, Stromal cells were isolated from WT uteri collected immediately after artificial decidual stimulation and allowed to attach to slides for 2 h. Then the cells were cultured in medium supplemented with P (1 μm) and E (10 nm) and transduced with or without adenovirus expressing dominant-negative A-C/EBP or control virus Ad-GFP at MOI of 10:1. After 20-h transduction, cells were treated with formaldehyde and ChIP assays were performed as described in Materials and Methods. Chromatin immunoprecipitates obtained using antibodies against RNA polymerase II and mouse IgG served as positive and negative controls, respectively. DNAs isolated from the immunoprecipitates were used as templates for regular PCR (top) and real time PCR (bottom) to determine promoter occupancy by C/EBPβ at the −930 site of the Cyclin B2 promoter. The experiment was repeated twice, and representative data are shown.
To further test the functionality of the binding of C/EBPβ at the −930 region of the cyclin B2 promoter, we used a dominant-negative mutant of C/EBPβ. Previous studies showed that this mutant, A-C/EBP, which lacks the DNA binding domain but retains the dimerization domain, inhibits DNA binding and transcriptional function of endogenous C/EBPβ (33). If the binding of C/EBPβ at the −930 site represents a transcriptionally relevant interaction, one would predict that it would be blocked by A-C/EBP, which inhibits transcription of C/EBPβ-target genes. Primary stromal cells were transduced with recombinant adenovirus expressing A-C/EBP (Fig. 5B) or GFP. As shown in Fig. 5C, A-C/EBP efficiently suppressed the occupancy of the −930 site of the cyclin B2 promoter by C/EBPβ. These data suggested that the −930 region of the cyclin B2 promoter represents a bonafide C/EBPβ binding site and is likely to mediate the direct regulation of the expression of this cyclin by C/EBPβ.
Loss of cdc25C expression in C/EBPβ-null uterine stromal cells contributes to decreased Cdk1 activity leading to G2 to M arrest
cdc25C is a phosphatase, which is a critical regulator of Cdk1 activity. It maintains Cdk1 activity during mitosis via the reversal of inhibitory phosphorylations at Thr14 and Tyr15 of Cdk1. We observed that the level of cdc25C mRNA was markedly down-regulated in C/EBPβ-null uterine stromal cells relative to the WT cells at the time of G2 to M transition (Fig 6A). This result was further confirmed upon analysis of the levels of cdc25C protein in uterine stromal cells subjected to in vitro decidualization. Immunocytochemical analysis and Western blotting revealed that the expression of cdc25C was significantly reduced in the C/EBPβ-null uterine stromal cells, whereas Cdk1 was expressed at similar levels in WT and mutant stromal cells (Fig. 6, B, C, and E).
Figure 6.
C/EBPβ regulates the activity of Cdk1 during mitosis. A, Ovariectomized WT and C/EBPβ-null mice were subjected to artificial decidual stimulation. Stromal cells were isolated at different times after application of the stimulus, and total RNA was prepared from these cells. Five WT or C/EBPβ-null mice were used at each time point, and the uteri of each category were pooled. The expression levels of Cdk1 mRNAs were monitored by real-time PCR using gene-specific primers. The PCR data were analyzed as described in the legend to Fig. 3. *, Statistically significant change (P < 0.05). B–E, Stromal cells were isolated from WT or C/EBPβ-null uteri collected immediately after artificial decidual stimulation and allowed to attach to slides for 2 h. The cells were cultured in vitro in medium supplemented with P (1 μm) and E (10 nm). At 20 h, the cells were fixed and subjected to immunocytochemical staining using antibodies against Cdk1, cdc25C, and phospho-Cdk1 (B–D). Western blot analysis was also performed using antibodies against Cdk1, pCdk1, cdc25c, and calnexin (E).
To ascertain that the down-regulation of cdc25C expression in C/EBPβ-null uterine stromal cells indeed affects the inhibitory phosphorylation of Cdk1 at Thr14/Tyr15, we performed immunocytochemistry and Western blotting, using an antibody that specifically recognizes this modification. Our results confirmed that the level of p-Cdk1, phosphorylated at Thr14/Tyr15, was higher in C/EBPβ-null uterine stromal cells relative to the WT cells (Fig. 6, D and E). These results are consistent with the hypothesis that the regulation of the expression of cdc25C represents an additional mechanism by which C/EBPβ controls the activity of Cdk1 during mitotic entry.
Elevated expression of cell cycle inhibitors in C/EBβ-null uterine stromal cells
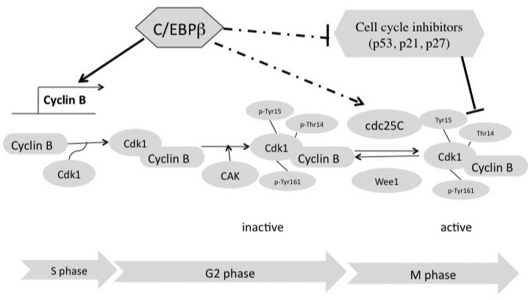
It is well documented that cyclin-dependant kinase inhibitors p21 and p27 and the cell cycle suppressor p53 inhibit the activity of various cyclin-Cdk complexes, resulting in the arrest of the cell cycle at the G1 to S and G2 to M checkpoints (34,35,36,37,38,39). To determine whether C/EBPβ controls uterine stromal proliferation by regulating the expression of these cell cycle suppressors, we analyzed the levels of their mRNAs in WT and C/EBPβ-null uterine stromal cells (Fig. 7A). We found that the expression of p53 mRNA was modestly increased in C/EBPβ-null stromal cells at 16 h after decidual stimulation, whereas the levels of mRNAs corresponding to p21 and p27 did not change significantly at this time point (Fig. 7, A–C). In contrast, the levels of p21, p27, and p53 mRNAs were markedly elevated in the mutant stromal cells at 20 h after the decidual stimulation when G2 to M phase transition is supposed to occur. Similar changes in their protein levels at 20 h were confirmed by Western blot analysis (Fig. 7D). These results indicated that C/EBPβ-mediated stromal cell proliferation during decidualization is associated with the suppression of expression of p21, p27, and p53 proteins during the G2 to M phase transition. Loss of C/EBPβ expression in C/EBPβ-null stromal cells results in the up-regulation of these inhibitors, which then repress the activity of cyclin B-Cdk1 complex and contribute to the blockade of stromal cell mitosis (Fig. 8).
Figure 7.
Increased expression of p53, p21, and p27 in C/EBPβ-null uteri. A–C, Ovariectomized WT and C/EBPβ-null mice were subjected to artificial decidual stimulation. Stromal cell were isolated at different times after application of the stimulus, and total RNA was prepared from these cells. Five WT or C/EBPβ-null mice were used at each time point, and the uteri of each category were pooled. The expression levels of mRNAs corresponding to p53, p21, and p27 were monitored by real-time PCR, using gene-specific primers. The PCR data were analyzed as described in the legend to Fig. 3. The relative fold inductions of the expression of these genes at different times were determined after setting that of 0 h WT cells at 1.0. Black bar, WT; grey bar, C/EBPβ-null. *, Statistically significant change (P < 0.05). D, Stromal cells were isolated from WT or C/EBPβ-null uteri collected at 20 h after artificial decidual stimulation. The cells were lysed, analyzed by SDS-PAGE, and subjected to Western blotting using antibodies directed against p53, p21, and p27. Calnexin immunostaining served as a loading control.
Figure 8.
A schematic of the mechanisms underlying C/EBPβ regulation of endometrial stromal proliferation during decidualization. C/EBPβ plays a central role in stromal proliferation during decidualization by controlling the expression of multiple cell cycle regulatory molecules, such as the B-type cyclins, the phosphatase cdc25C, and the inhibitory factors p53, p21, and p27, during G2 to M transition of the cell cycle. CAK is a kinase that induces activating phosphorylation at Tyr 161 of Cdk1. Wee1 and Myt1 are kinases that induce inhibitory phosphorylations at Thr 14 and Tyr 15 of Cdk1.
Discussion
Previous studies indicated that C/EBPβ is a critical regulator of cell proliferation and/or differentiation in multiple tissues including the liver, adipose tissue, immune system, skin, and mammary gland (18,40,41,42,43,44,45,46,47). C/EBPβ is implicated in both positive and negative control of the cell cycle, depending on the cellular context (42,45,46,47). This article addresses the role of C/EBPβ in uterine stromal proliferation. During the decidualization phase of pregnancy, steroid hormone-primed uterine stromal cells undergo proliferation and mitotic expansion during the first 24–48 h after decidual stimulation (19,20,21,22,48). We have previously reported that the expression of C/EBPβ is robustly induced in the decidual tissue during normal as well as experimentally induced decidualization (13). The expression of C/EBPβ in decidual cells arises from a complex interplay of E and P within the uterine compartments. Although a transient rise in the level of E in the preimplantation period induces C/EBPβ in the epithelial and stromal cells of pregnant uterus, progesterone receptor is also a critical regulator of this gene in the stromal cells (13). Here, we provide compelling evidence that C/EBPβ plays a central role in controlling the stromal cell cycle during decidualization.
It is well established that the proliferation of animal cells proceeds through various stages of the cell cycle, G1, S, G2, and M, and is controlled at distinct checkpoints (28,30,31,49). Progression through each phase of the cell cycle is under the strict control of multiple regulatory factors, such as, cyclins, Cdks, and Cdk-inhibitors (28,30,31,49). Cyclin levels fluctuate dramatically through the cell cycle as a consequence of changes in transcription and ubiquitin-mediated degradation. Different cyclins bind specifically to different Cdks, which are serine/threonine protein kinases, to form distinct regulatory complexes at specific phases of the cell cycle and thereby drive the cell from one phase to another. The association of D-type cyclins with Cdk4 or Cdk6 is important for G1 to S transition. Binding of B-type cyclins to Cdk1 allows phosphorylation of this enzyme by the Cdk-activating kinase (CAK) and its activation, which plays an essential role in G2 to M transition. Our present study showed that the C/EBPβ-null stromal cells are able to enter the cell cycle and proceed through the S phase in response to a decidual stimulation. Consistent with this finding, we did not observe any significant change in the expression of cyclin D, cyclin E, Cdk2, Cdk4, and cdc25A, which control G1 to S phase transition and S phase activity, in WT vs. mutant stromal cells.
Our results suggested that the uterine stromal cells are arrested at the G2 to M checkpoint of the cell cycle in the absence of C/EBPβ. We considered the possibility that the expression of one or more critical regulators of G2 to M transition is lost in the C/EBPβ-null uterine stromal cells. We found that the mRNAs corresponding to the B-type cyclins B1 and B2 are markedly down-regulated in the mutant stromal cells compared with the WT cells. Our studies confirmed that the level of cyclin B2 protein declined sharply in the C/EBPβ-null stromal cells. Previous reports indicated that multiple CCAAT box-like motifs are located at human or mouse cyclin B2 promoter (50,51). To explore whether the C/EBPβ directly binds to the cyclin B2 promoter and regulates its expression, we used the ChIP procedure. Our results demonstrated that C/EBPβ binds strongly to a specific region in the cyclin B2 promoter that contains a CCAAT-like motif. This finding is consistent with the hypothesis that C/EBPβ is a direct regulator of transcription of the cyclin B2 gene.
Previous studies showed that targeted deletion of cyclin B1 gene resulted in embryonic lethality in mice (52). In contrast, mutant mice lacking cyclin B2 are viable and both sexes are fertile. However, mating of homozygous cyclin B2-null mice produced markedly reduced litter size, although the reason for this sub-fertility remains unclear (52). It was proposed that cyclin B1, whose expression overlaps with that of cyclin B2 in many tissues, compensates for the loss of cyclin B2 in the mutant mice (52). We found that both cyclins B1 and B2 are expressed in the uterine stromal cells during pregnancy, although the cyclin B2 levels are significantly higher than that of cyclin B1. It is important to note that C/EBPβ controls the expression of both B-type cyclins. Therefore, the absence of this transcription factor would lead to a drastic reduction in the overall levels of B-type cyclins in the uterine stromal cells, consistent with the observed block in their mitotic activity.
A well-established mechanism to control entry into mitosis is to regulate the activity of the cyclin B/Cdk1 complex (31). The function of Cdk1 is tightly controlled via phosphorylation or dephosphorylation (30). Binding of cyclin B triggers phosphorylation of Cdk1 by CAK, which leads to its activation. In contrast, the Cdk1 activity is greatly reduced by inhibitory phosphorylation at Thr14 and Tyr15 by the Wee1 and Myt1 kinases. Cdc25C, a phosphatase, maintains the active state of Cdk1 through dephosphorylation of the Thr14 and Tyr15 sites. We investigated whether C/EBPβ regulates the Cdk1 activity during mitosis by altering the expression of any of the kinases or phosphatases that control Cdk1 activity. While no change was seen in the expression of the Wee1 and Myt1 kinases that modulate Cdk1 function (data not shown), we found that the expression of cdc25C is greatly reduced in the C/EBPβ-null uterine stromal cells. As expected, concomitant with the decrease in the level of cdc25C, there was a significant up-regulation in the level of phospho-Cdk1 (Thr 14/Tyr 15), a likely contributor to the mitosis block.
We further noted that the levels of several well-known cell cycle suppressors, p21, p27, and p53, were markedly elevated specifically at the time of G2 to M transition. This finding uncovered additional mechanisms by which C/EBPβ controls stromal proliferation during decidualization progression. p53, an important cell cycle repressor, is a transcription factor induced and activated in response to various stress signals or DNA damage events (53). Numerous studies have shown that p53 is able to trigger cell cycle arrest at G1 to S and G2 to M checkpoints via different mechanisms involving its downstream transcriptional targets such as p21. Previous reports indicated that C/EBPβ binds efficiently to the p53 promoter and suppresses its transcription (55). Yoon et al. (54) noted a striking increase of p53 expression in the keratinocytes of C/EBPβ-null mice, indicating that C/EBPβ suppresses p53 expression in normal keratinocytes. Conversely, p53 represses the transcriptional activity of C/EBPβ in human stromal cells by physically interacting with its C-terminal domain (56). This two-way cross talk between C/EBPβ and p53 is likely to influence the expression of both p53 and C/EBPβ and their downstream genes during the stromal decidualization process.
p21, the transcriptional target of p53, binds directly to a broad spectrum of cyclin-Cdk complexes and inhibits their function (37). Another well-known inhibitor, p27, also functions by binding to the G1/S and G2/M cyclins and interfering with their activities (38,39). Interestingly, the levels of p27- and p53-induced p21 did not change significantly in C/EBPβ-null uterine stromal cells at the time of G1 to S transition after a decidual stimulation. In contrast, the levels of p27 and p21 were elevated in the mutant stromal cells specifically at the time of G2/M transition, indicating that these cell cycle inhibitory molecules contribute to the G2/M block of stromal proliferation in the C/EBPβ-null mice.
A comparison of the mechanisms by which C/EBPβ controls uterine stromal proliferation with previously reported mechanisms of its action in other tissues, such as the liver, adipose tissue, and mammary gland, revealed interesting differences. In C/EBPβ-null female mice, mammary epithelial cells fail to proliferate in response to steroid hormones, leading to an impaired ductal morphogenesis (46,47). It was shown that loss of C/EBPβ in this tissue markedly reduced the expression of cyclin E, which contributed to a G1/S arrest (57). Additionally, the mutant mammary epithelial cells showed decreased cdc25A expression and increased p27 stability. Loss of C/EBPβ expression in models of liver regeneration showed a similar defect in hepatocyte cell cycle progression through the S phase, and it was mainly attributed to a lack of expression of cyclin E in the mutant cells (45). In the C/EBPβ-null stromal cells, however, we did not find any defect in the G1 to S transition as the cells were able to proceed through the S phase before their arrest at the G2 to M checkpoint. These findings suggested that C/EBPβ regulation of cell proliferation is cell context-specific, and a unique regulatory strategy involving the control of the mitosis step operates during uterine stromal proliferation during decidualization.
In summary, this study provides new insights into the mechanisms by which C/EBPβ mediates the effects of steroid hormones during decidualization. Our results show that C/EBPβ is a key regulator of the stromal proliferation, and it operates by controlling the expression and function of multiple cell cycle regulatory molecules that control the G2 to M transition of the stromal cells. This study is important because it provides a mechanistic understanding of the hormone-regulated pathways that control decidualization, which is a prerequisite for the successful establishment of pregnancy.
Supplementary Material
Acknowledgments
We thank Dr. Mark Carrington and Michal Kubelka (Cambridge, UK) who kindly provided the antibody against cyclin B2. We also thank Elizabeth Hunt, Katya Dribinski, and Jarrad Marcell for genotyping.
Footnotes
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through cooperative agreement U54 HD055787 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research. This work was also supported by the NIH Grant 3U54 HD055787-02W1.
Disclosure Summary: The authors have nothing to declare.
First Published Online May 25, 2010
Abbreviations: A-C/EBP, Adenoviral vector expressing a dominant negative C/EBP; Ad-GFP, adenoviral vector expressing GFP; BrdU, bromodeoxyuridine; C/EBPβ, CCAAT/enhancer binding protein β; CAK, Cdk-activating kinase; Cdk, cyclin-dependent kinase; E, estrogen; HBSS, Hanks’ balanced salt solution; MOI, multiplicity of infection; P, progesterone; P-His3, phosphorylated Ser 10 of histone 3; WT, wild type.
References
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H 2004 Molecular cues to implantation. Endocr Rev 25:341–373 [DOI] [PubMed] [Google Scholar]
- Lee KY, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ 2007 Mouse models of implantation. Trends Endocrinol Metab 18:234–239 [DOI] [PubMed] [Google Scholar]
- Yoshinaga K 1988 Uterine receptivity for blastocyst implantation. Ann NY Acad Sci 541:424–431 [DOI] [PubMed] [Google Scholar]
- Parr MB, Parr EL 1989 The implantation reaction. New York: Plenum Press [Google Scholar]
- Psychoyos A 1973 Endocrine control of egg implantation. Washington, DC: American Physiological Society [Google Scholar]
- Weitlauf HM 1994 Biology of implantation in the physiology of reproduction. New York: Raven Press; 391–440 [Google Scholar]
- Finn CA 1977 The implantation reaction. In: Wynn RM, ed. Biology of the uterus. New York: Plenum Press; 246–308 [Google Scholar]
- DeFeo VJ 1967 Decidualization. In: Wynn RM, ed. Cellular biology of the uterus. Amsterdam: North Holland; 191–290 [Google Scholar]
- Glasser SR, Mulholland J, Mani SK, Julian J, Munir I, Lampelo S, Soares MJ 1991 Blastocyst-endometrial relationships: reciprocal interactions between uterine epithelial and stromal cells and blastocysts. Trophoblast Res 5:229–280 [Google Scholar]
- Gu Y, Gibori G 1999 Deciduoma in encyclopedia of reproduction. San Diego: Academic Press; 836–842 [Google Scholar]
- Irwin JC, Giudice D 1999 Encyclopedia of reproduction. New York: Academic Press [Google Scholar]
- Cross JC, Werb Z, Fisher SJ 1994 Implantation and the placenta: key pieces of the developmental puzzle. Science 266:1508–1518 [DOI] [PubMed] [Google Scholar]
- Mantena SR, Kannan A, Cheon YP, Li Q, Johnson PF, Bagchi IC, Bagchi MK 2006 C/EBPβ is a critical mediator of steroid hormone-regulated cell proliferation and differentiation in the uterine epithelium and stroma. Proc Natl Acad Sci USA 103:1870–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi MK, Mantena SR, Kannan A, Bagchi IC 2006 Control of uterine cell proliferation and differentiation by C/EBPβ: functional implications for establishment of early pregnancy. Cell Cycle 5:922–925 [DOI] [PubMed] [Google Scholar]
- Wedel A, LomsZiegler-heitbrock HW 1995 The C/EBP family of transcription factors. Immuno Biol 9:171–185 [DOI] [PubMed] [Google Scholar]
- McKnight SL 2001 McBindall—a better name for CCAAT/enhancer binding proteins? Cell 107:259–261 [DOI] [PubMed] [Google Scholar]
- Ramji DP, Foka P 2002 CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J 365:561–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterneck E, Tessarollo L, Johnson PF 1997 An essential role for C/EBPβ in female reproduction. Genes Dev 11:2153–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das RM, Martin L 1978 Uterine DNA synthesis and cell proliferation during early decidualization induced by oil in mice. J Reprod Fertil 53:125–128 [DOI] [PubMed] [Google Scholar]
- Moulton BC 1979 Effect of progreterone on DNA, RNA, and protein synthesis during preimplantation procursors of stromal cell differentiation during decidualization. Biol Reprod 21:667–672 [DOI] [PubMed] [Google Scholar]
- Moulton BC, Keonig BB 1984 Uterine deoxyribonucleic acid synthesis during preimplantation procursors of stromal cell differentiation during decidualization. Endocrinology 115:1302–1307 [DOI] [PubMed] [Google Scholar]
- Dey SK 1996 Implantation. New York: Lipincott-Raven [Google Scholar]
- Cheon YP, DeMayo FJ, Bagchi MK, Bagchi IC 2004 Induction of cytotoxic T-lymphocyte antigen-2β, a cysteine protease inhibitor in decidua: a potential regulator of embryo implantation. J Biol Chem 279:10357–10363 [DOI] [PubMed] [Google Scholar]
- Li Q, Kannan A, Wang W, Demayo FJ, Taylor RN, Bagchi MK, Bagchi IC 2007 Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem 282:31725–31732 [DOI] [PubMed] [Google Scholar]
- Li Q, Cheon YP, Kannan A, Shanker S, Bagchi IC, Bagchi MK 2004 A novel pathway involving progesterone receptor, 12/15-lipoxygenase-derived eicosanoids, and peroxisome proliferator-activated receptor γ regulates implantation in mice. J Biol Chem 279:11570–11581 [DOI] [PubMed] [Google Scholar]
- Ramathal C, Bagchi IC, Bagchi MK 2010 Lack of CCAAT enhancer binding protein β (C/EBPβ) in uterine epithelial cells impairs estrogen-induced DNA replication, induces DNA damage response pathways, and promotes apoptosis. Mol Cell Biol 30:1607–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendzel M, Wei Y, Mancini MA, Hooser A, Ranalli T, Brinkley BR, Bazett-Jones, Allis CD 1997 Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106:348–360 [DOI] [PubMed] [Google Scholar]
- Alberts B 2002 Molecular biology of the cell. New York: Science [Google Scholar]
- Harper JV 2005 Synchronization of cell populations in G1/S and G2/M phases of the cell cycle. Methods Mol Biol 296:157–166 [DOI] [PubMed] [Google Scholar]
- Brooks G 2005 Cyclins, cyclin-dependent kinases and cylcin dependent kinase inhibitors. Methods Mol Biol 296:291–298 [DOI] [PubMed] [Google Scholar]
- Nurse P 2000 A long twentieth century of the cell cycle and beyond. Cell 100:71–78 [DOI] [PubMed] [Google Scholar]
- Das SK, Lim H, Paria BC, Dey SK 1999 Cyclin D3 in the mouse uterus is associated with the decidualization process during early pregnancy. J Mol Endocrinol 22:91–101 [DOI] [PubMed] [Google Scholar]
- Zhang JW, Tang QQ, Vinson C, Lane MD 2004 Dominant-negative C/EBP disrupts mitotic clonal expansion and differentiation of 3T3–L1 preadipocytes. Proc Natl Acad Sci USA 101:43–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu KA, Fang Q, Cheng JQ, Nicosia SV, Coppola D 2007 P53 enhances ascorbyl stearate-induced G2/M arrest of human ovarian cancer cells. Anticancer Res 27:3927–3934 [PubMed] [Google Scholar]
- Kaushal N, Bansal MP 2007 Inhibition of CDC2/Cyclin B1 in response to selenium-induced oxidative stress during spermatogenesis: potential role of Cdc25c and p21. Mol Cell Biochem 298:139–150 [DOI] [PubMed] [Google Scholar]
- Taylor BF, McNeely SC, Miller HL, Lehmann GM, McCabe Jr MJ, States JC 2006 p53 suppression of arsenite-induced mitotic catastrophe is mediated by p21CIP1/WAF1. J Pharmacol Exp Ther 318:142–151 [DOI] [PubMed] [Google Scholar]
- Smits VA, Klompmaker R, Vallenius T, Rijksen G, Mäkela TP, Medema RH 2000 p21 inhibits Thr161 phosphorylation of Cdc2 to enforce the G2 DNA damage checkpoint. J Biol Chem 275:30638–30643 [DOI] [PubMed] [Google Scholar]
- Pagano M 2004 Control of DNA synthesis and mitosis by the Skp2–p27-Cdk1/2 axis. Mol Cell 14:414–416 [DOI] [PubMed] [Google Scholar]
- Payne SR, Zhang S, Tsuchiya K, Moser R, Gurley KE, Longton G, deBoer J, Kemp CJ 2008 p27kip1 deficiency impairs G2/M arrest in response to DNA damage, leading to an increase in genetic instability. Mol Cell Biol 28:258–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Akira S, Yoshida K, Umemoto M, Yoneda Y, Shirafuji N, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T 1995 Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell 80:353–361 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Yoshida N, Kishimoto T, Akira S 1997 Defective adipocyte differentiation in mice lacking the C/EBPb and /or C/EBPd gene. EMBO J 16:7432–7443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Yoon K, Sterneck E, Johnson PF, Smart RC 2002 CCAAT/enhancer binding protein-β is a mediator of keratinocyte survival and skin tumorigenesis involving oncogenic Ras signaling. Proc Natl Acad Sci USA 99:207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang QQ, Otto TC, Lane MD 2003 CCAAT/enhancer-binding protein β is required for mitotic clonal expansion during adipogenesis. Proc Natl Acad Sci USA 100:850–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy LM, Sealy L 2003 CCAAT/enhancer binding protein β (C/EBPβ)-2 transforms normal mammary epithelial cells and induces epithelial to mesenchymal transition in culture. Oncogene 22:869–883 [DOI] [PubMed] [Google Scholar]
- Greenbaum LE, Li W, Cressman DE, Peng Y, Ciliberto G, Poli V, Taub R 1998 CCAAT enhancer- binding protein β is required for normal hepatocyte proliferation in mice after partial hepatectomy. J Clin Invest 102:996–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GW, Johnson PF, Hennighausen L, Sterneck E 1998 The C/EBPβ transcription factor regulates epithelial cell proliferation and differentiation in the mammary gland. Genes Dev 12:1907–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagroves TN, Krnacik S, Raught B, Gay J, Burgess-Beusse B, Darlington GJ, Rosen JM 1998 C/EBPβ, but not C/EBPα, is essential for ductal morphogenesis, lobuloalveolar proliferation, and functional differentiation in the mouse mammary gland. Genes Dev 12:1917–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser SR 1990 Biochemical and structural changes in uterine endometrial cell types following natural or artificial deciduogenic stimuli. Trophoblast Res 4:377–416 [Google Scholar]
- Harper JV, Brooks G 2005 The mammalian cell cycle. Methods Mol Biol 296:113–153 [DOI] [PubMed] [Google Scholar]
- Wasner M, Haugwitz U, Reinhard W, Tschöp K, Spiesbach K, Lorenz J, Mossner J, Engeland K 2003 Three CCAAT-boxes and a single cell cycle genes homology region (CHR) are the major regulating sites for transcription from the human cyclin B2 promoter. Gene 312:225–237 [DOI] [PubMed] [Google Scholar]
- Bolognese F, Wasner M, Dohna CL, Gurtner A, Ronchi A, Muller H, Manni I, Mossner J, Piaggio G, Mantovani R, Engeland K 1999 The cyclin B2 promoter depends on NF-Y, a trimer whose CCAAT-binding activity is cell-cycle regulated. Oncogene 18:1845–1853 [DOI] [PubMed] [Google Scholar]
- Brandeis M, Rosewell I, Carrington M, Crompton T, Jacobs MA, Kirk J, Gannon J, Hunt T 1998 Cyclin B2-null mice develop normally and are fertile whereas cyclin B1-null mice die in utero. Proc Natl Acad Sci USA 95:4344–4349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden KH, Lu X 2002 Live or let die: the cell’s response to p53. Nat Rev Cancer 2:594–604 [DOI] [PubMed] [Google Scholar]
- Yoon K, Zhu S, Ewing SJ, Smart RC 2007 Decreased survival of C/EBP β-deficient keratinocytes is due to aberrant regulation of p53 levels and function. Oncogene 26:360–367 [DOI] [PubMed] [Google Scholar]
- Boggs K, Reisman D 2007 C/EBPβ participates in regulating transcription of the p53 gene in response to mitogen stimulation. J Biol Chem 282:7982–7990 [DOI] [PubMed] [Google Scholar]
- Schneider-Merck T, Pohnke Y, Kempf R, Christian M, Brosens JJ, Gellersen B 2006 Physical interaction and mutual transrepression between CCAAT/enhancer-binding protein β and the p53 tumor suppressor. J Biol Chem 281:269–278 [DOI] [PubMed] [Google Scholar]
- Grimm SL, Contreras A, Barcellos-Hoff MH, Rosen JM 2005 Cell cycle defects contribute to a block in hormone-induced mammary gland proliferation in CCAAT/enhancer-binding protein (C/EBPβ)-null mice. J Biol Chem 280:36301–36309 [DOI] [PubMed] [Google Scholar]
- Kim J, Sato M, LiQ, Lydon JP, DeMayo FJ, Bagchi IC, Bagchi MK 2008 Peroxisome proliferator-activated receptor γ is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice. Mol Cell Biol 28:1770–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.