Abstract
Recent data have demonstrated that mutations in the receptor for neurokinin B (NKB), the NK-3 receptor (NK3R), produce hypogonadotropic hypogonadism in humans. These data, together with reports that NKB expression increases after ovariectomy and in postmenopausal women, have led to the hypothesis that this tachykinin is an important stimulator of GnRH secretion. However, the NK3R agonist, senktide, inhibited LH secretion in rats and mice. In this study, we report that senktide stimulates LH secretion in ewes. A dramatic increase in LH concentrations to levels close to those observed during the preovulatory LH surge was observed after injection of 1 nmol senktide into the third ventricle during the follicular, but not in the luteal, phase. Similar increases in LH secretion occurred after insertion of microimplants containing this agonist into the retrochiasmatic area (RCh) in anestrous or follicular phase ewes. A low-dose microinjection (3 pmol) of senktide into the RCh produced a smaller but significant increase in LH concentrations in anestrous ewes. Moreover, NK3R immunoreactivity was clearly evident in the RCh, although it was not found in A15 dopaminergic cell bodies in this region. These data provide evidence that NKB stimulates LH (and presumably GnRH) secretion in ewes and point to the RCh as one important site of action. Based on these data, and the effects of NK3R mutations in humans, we hypothesize that NKB plays an important stimulatory role in the control of GnRH and LH secretion in nonrodent species.
NK3R neurons are identified in the retrochiasmatic area, and NK3R agonist in the retrochiasmatic area stimulates surge-like LH secretion in anestrous and follicular phase ewes.
Although the negative and positive feedback actions of ovarian steroids have been well characterized, the mechanism by which these are produced remains controversial. Recent work on the feedback control of GnRH has focused on kisspeptin-containing neurons in the arcuate nucleus (ARC) and preoptic area (POA). A reproductive role for kisspeptin was first inferred from genetic studies demonstrating that mutations in its receptor resulted in hypogonadotropic hypogonadism in humans (1,2). Subsequent work demonstrated that this peptide is a potent stimulator of GnRH release in many species and implicated it in both the positive and negative feedback actions of estradiol (E2) (3,4,5).
In sheep, approximately 90% of kisspeptin neurons are in the ARC, and almost all of them also contain the tachykinin, neurokinin B (NKB), and the endogenous opioid peptide, dynorphin (6). This colocalization is reciprocal, indicating that these neurons are a single subpopulation, which we have termed kisspeptin/NKB/dynorphin (KNDy) neurons (7), that produces all three neuropeptides. There is accumulating evidence for the presence and function in reproduction of KNDy neurons in other species (8,9), including humans (10,11). In sheep, virtually all KNDy neurons contain both estrogen receptor-α (12) and progesterone receptors (13), leading to the hypothesis that they mediate the feedback actions of ovarian steroids (4). Indeed, there is evidence that dynorphin from these neurons mediates the negative feedback actions of progesterone on GnRH in sheep (13,14,15) and rats (16).
Although kisspeptin and dynorphin have been implicated in the feedback actions of ovarian steroids, the role of NKB is less clear. NKB is the only known product of the TAC3 gene and acts via its own receptor, the neurokinin-3 receptor (TACR3 in humans, NK3R in other species). The first evidence that NKB might be involved in steroid feedback was a report in 1991 that NKB expression increased in the infundibular (ARC) nucleus of postmenopausal women (17). Subsequent work in monkeys (18,19), rodents (20,21), and sheep (22) has confirmed that NKB expression in the ARC increases after ovariectomy (OVX) and is inhibited by E2. These observations led to the hypothesis that NKB stimulates GnRH release, but intracerebroventricular (icv) administration of the NK3R agonist, senktide, to rats (23) and mice (9) inhibited LH secretion. Recently, human genetic studies identified loss-of-function mutations in the TAC3 or TACR3 genes that produced hypogonadotropic hypogonadism (24). These findings indicate that NKB, like kisspeptin, is a stimulator of GnRH secretion necessary for reproductive function in humans. Furthermore, because similar mutations in mice have no obvious effect on reproduction (24,25), they raise the possibility of species differences in the actions and role of this neuropeptide.
In this study, we first tested the hypothesis that NKB, acting via the NK3R, stimulates LH secretion using sheep as an experimental model. Stimulatory effects of NK3R agonists were observed in the first experiment, but these are unlikely to be due to direct actions on GnRH neurons, because NK3R does not colocalize with GnRH in the ewe (26). However, abundant NK3R is found in several areas implicated in the control of GnRH secretion in sheep, including the POA, ARC, and retrochiasmatic area (RCh) (26). We chose the RCh for our initial studies, because NK3R are found here in ewes (26), but not in rats (27), a difference that might account for species differences in effects of senktide, and because this region has been implicated in the control of seasonal reproduction in ewes (28,29).
Materials and Methods
Animals
All experiments used adult ewes of predominantly Suffolk breed, housed indoors under controlled photoperiod simulating natural outdoor day length. Ewes were fed a pelleted diet and provided free access to water and supplemental minerals. Breeding season experiments were performed from October to mid-February and anestrus experiments from May through July. All procedures involving animals were approved by the West Virginia University Animal Care and Use Committee.
Surgeries
All surgical procedures were carried out under sterile conditions, using halothane (1.5–5%, as needed) supplemented with nitrous oxide (delivered 1:3 in oxygen) as anesthesia. OVXs were performed via midventral laparotomy (15). Chronic guide cannulae into either the third ventricle (30) or RCh (31) were implanted using stereotaxic procedures previously described in detail elsewhere. The animals were treated with dexamethasone and penicillin pre- and postoperatively (30,31), and with analgesic (200 mg, im fluinixin meglumine; Phoenix Pharmaceutical, Inc., St. Joesph, MO) at the time of anesthesia induction and for 3 d after surgery.
Tissue collection for histological and immunocytochemical processing
For all histological and immunocytochemical processing, ewes were injected with two doses of heparin 10 min apart (25,000 U, iv; Abraxiz Pharmaceutical Products, Schaumburg, IL), then immediately killed with an overdose of sodium pentobarbitol (∼2 g, iv). The heads were removed and perfused via the carotid arteries with 6 liters of 4% paraformaldehyde in PBS (pH 7.3–7.4) with 0.1% sodium nitrite and 10 U/ml heparin. After perfusion, a block of tissue containing the POA and hypothalamus was dissected and stored in paraformaldehyde overnight at 4 C. The tissue was transferred to a solution containing 20% sucrose in phosphate buffer (pH 7.3–7.4) at 4 C until fully infiltrated, then cut frozen on a microtome into 50-μm sections. Tissue from all but one ewe (see experiment 4) was cut in coronal sections and either mounted on microscope slides and stained with cresyl violet (experiments 1–3) or stored in series of every fifth section at −20 C in cryopreservative solution for later immunocytochemistry (experiment 4).
Experiment 1: effects of administration of NK3R agonists into third ventricle
In a preliminary experiment, we tested the effects of administration of NKB or the NK3R agonist, senktide, into the third ventricle using ovary-intact ewes at a random time during the estrous cycle. NKB and senktide were purchased from Tocris Bioscience (Ellisville, MO). Stock solutions of 300 μm senktide and 40 μm NKB (NKB was difficult to get into solution) were stored at −20 C and then diluted to 5 μm in sterile saline the day of treatment. Eight ewes were given single icv injections of either 200 μl sterile saline, 1 nmol NKB, or 1 nmol senktide (dose based on rodent data, see Refs. 9 and 23). Blood samples were collected by venipuncture every 12 min from 36 min before to 4 h after injection. The treatment protocol was repeated 4 d later in seven of these ewes with no ewe receiving the same two treatments.
Because some of the variability in response in the preliminary experiment might have been due to stage of cycle, we next tested the effects of icv injection of senktide on LH secretion in the luteal and follicular phases. The ovarian cycles of five ewes with chronic guide tubes in the third ventricle were synchronized with prostaglandin (PGF)2α injections (5 mg Lutalyse, im, 3 h apart; Pharmacia & Upjohn Co., Kalamazoo, MI). Seven days later, a single blood sample was collected for progesterone measurements, luteolysis was again induced with PGF2α injections, and two intravaginal progesterone release implants (Eazi-Breed CIDR; InterAg, Hamilton, New Zealand) were inserted to maintain luteal-phase progesterone concentrations. Both CIDRs were removed 3 d later and blood samples collected starting 20 h later during the early follicular phase. Samples were collected every 12 min from 36 min before to 4 h after icv injection of either 100 μl sterile saline or 1 nmol senktide/100 μl sterile saline. Eleven days later, frequent blood sampling and treatments were repeated during the midluteal phase (d 9 of next cycle). Senktide treatments in both phases of the cycle were randomized across ewes. At the end of blood sampling, luteolysis was again induced and two CIDRs inserted. Three days later, the CIDRs were removed, and the treatment protocol replicated in both stages of the cycle, using a crossover design for senktide treatments within stage of the cycle so that all ewes received both control and senktide treatments in a random order. Treatment effects were determined by comparing LH concentrations in the 4 h after senktide with those in the 4 h after saline (levels in the three samples before treatments were only used to establish an appropriate baseline, e.g. ewes were not in the middle of an LH surge). Animals were killed at the end of the experiment, fixed tissue was collected, and correct placement into the third ventricle confirmed by histological examination of the tract left by the guide tubes (30).
Experiment 2: effects of local administration of a NK3R agonist into the RCh in follicular phase ewes
Based on the results of experiment 1, and evidence that the ovine RCh contains NK3R (26), whereas the rodent RCh does not (27), we next determined the effect of the NK3R agonist, senktide, administered via microimplants into the RCh on LH secretion in follicular phase ewes. Microimplants (31) consisted of sterile 22-gauge stainless steel tubing, cut to extend 2 mm beyond the guide tubes, and filled by tamping at least 40 times in crystalline senktide. Bilateral guide cannulae aimed at the RCh were implanted in five ovary-intact ewes early in the breeding season. Estrous cycles of the ewes were synchronized with PGF2α, and 7 d later luteolysis was again induced with PGF2α and two CIDRs inserted in each ewe. On d 8 after CIDR insertion, another PGF2α injection was given to ensure no corpora lutea persisted, and CIDRs were removed. Microimplants containing senktide, or empty for controls, were inserted bilaterally via the guide cannulae into the RCh 18 h after CIDR removal. Blood was collected from the jugular vein every 12 min from 36 min before until 4 h after microimplant insertion. Microimplants were removed at the end of this frequent blood collection period, and hourly blood sampling continued for another 4 h. Two days after blood collection, estrous cycles were again synchronized with a combination of PGF2α and CIDRs, and the experimental protocol repeated in a crossover design for senktide or control treatments.
Experiment 3: effects of local administration of a NK3R agonist into the RCh in anestrous ewes
Three separate experiments were performed during anestrus using eight ewes with bilateral guide tubes aimed at the RCh. Experiment 3a examined the effects of microimplants of senktide on LH secretion in ovary-intact ewes. Blood samples were collected at 12-min intervals from 36 min before to 4 h after insertion of empty or senktide-filled microimplants. Microimplants were then removed and the protocol repeated 1 wk later, using a crossover design.
Experiment 3b tested a lower dose of agonist. To do so, we used the same protocol, except that senktide was administered as a single microinjection of 3 pmol/300 nl sterile water on each side. Controls consisted of vehicle (300 nl of sterile water), and each ewe received both treatments, using a crossover design.
Experiment 3c was conducted to test whether senktide is inhibitory when endogenous LH secretion is elevated. At the conclusion of experiment 3b, five of these ewes were OVX. Starting 2 wk after surgery, the effects of microimplants of senktide (to get a maximal dose) were tested using the same microimplant and blood collection protocol as for experiment 3a. Ewes were then killed, heads perfused with 4% paraformaldehyde solution, fixed hypothalamic tissue collected, and sites of administration determined histologically.
Experiment 4: relationship of NK3R to dopaminergic (DA) neurons in the RCh
The RCh of ewes contains the A15 population of DA neurons, which mediates suppression of GnRH and LH secretion in anestrus (28,29). To test whether A15 neurons were also a potential mediator of the effects of senktide, we determined whether they contained NK3R using dual immunofluorescent labeling of NK3R and tyrosine hydroxylase, as a marker for DA neurons. Hypothalamic tissue from six OVX ewes treated with a sc 3-cm E2-filled silicone implant in the axillary region (three anestrus, three breeding season) were used to examine the relationship between NK3R and A15 DA neurons. In addition, tissue was collected from one OVX, anestrous ewe 1 h after unilateral injection of senktide (3 pmol in 300 nl sterile water) into the RCh to assess whether binding of agonist induces internalization of NK3R (26). A series of every fifth coronal section through the RCh from each ewe was processed for dual immunocytochemistry. Lastly, parasagittal sections from one ovary-intact breeding season ewe were processed immediately after cutting as a complete series through one side of the hypothalamus.
For all ewes, immunocytochemical processing was conducted with free-floating sections at room temperature, and all reagents were diluted in PBS (pH 7.3–7.4), unless otherwise specified. Four 5-min washes with PBS were performed between each incubation step. Tissue was washed overnight in PBS to remove any residual cryoprotectant. Sections were then washed with 1% H2O2 for 10 min to block endogenous peroxidase activity. Tissue was incubated in a blocking solution containing 0.1% Triton X-100 and 4% normal goat serum (NGS) (Vector Laboratories, Burlingame, CA) for 1 h, then incubated for 20 h in rabbit anti-NK3R (Novus Biologicals, Littleton, CO) 1:10,000 with 1% Triton X-100 and 4% NGS. Subsequently, tissue was incubated in biotin-conjugated goat antirabbit IgG 1:400 with 4% NGS for 1 h. The reaction was amplified with an avidin-biotin complex (Vector ABC Elite; Vector Laboratories) 1:600 for 1 h and then for 10 min with biotinylated tyramide (PerkinElmer, Waltham, MA) 1:250 with 0.0003% H2O2 (26). Then tissue was incubated for 30 min with Alexa 488-conjugated streptavidin (1:200; Molecular Probes, Eugene, OR) to complete the immunolabeling of NK3R. Tissue was then incubated for 20 h with mouse antityrosine hydroxylase (1:5000; Chemicon, Bedford, MA) with 4% NGS and 0.1% Triton X-100. Finally, immunolabeling of tyrosine hydroxylase was completed by incubation for 30 min with Alexa 555-conjugated goat antimouse IgG (1:400; Molecular Probes). After a final wash (three times, 5 min each) in phosphate buffer, sections were mounted onto glass slides, allowed to air dry before coverslipping with gelvatol mounting medium, and stored in the dark at 4 C. Both the NK3R (26) and tyrosine hydroxylase antibodies (29) have been validated for use in sheep. Contemporaneous controls included omission of primary antisera, which eliminated all staining of the appropriate antigen. All sections through the RCh of each ewe were initially observed at ×200 magnification for evidence of colocalization of NK3R in tyrosine hydroxylase-immunolabeled cells using fluorescence microscopy (Olympus AX70; Provis Microscope, Center Valley, PA). For comparison between anestrus and breeding season, z-series of images were captured using confocal miscroscopy (Zeiss LSM 510; ×63 objective, ×0.7 optical zoom, 1.5-μm z-slices; Zeiss, Oberkochen, Germany) for two fields through the A15 cell population for each ewe. Close contacts of NK3R-immunoreactive (NK3R-ir) fibers on each tyrosine hydroxylase-containing neuron were counted. For the remaining ewes, confocal images were captured over several fields of a single tissue section using a ×10 objective and 15-μm z-thickness for illustrations of the distribution of NK3R neurons in relation to DA cell populations. Evidence of internalized and membrane-bound NK3R in cells in the RCh were obtained from the ewe with unilateral senktide injection by capturing confocal images through individual cells using a ×63 objective, ×1 optical zoom, and 1.5-μm z-slices.
Analyses
Assays
LH concentrations were measured in duplicate 50–200 μl of plasma using reagents provided by the National Hormone and Peptide Program as described previously (14). Assay sensitivity averaged 0.10 ng/tube (NIH S24) and intra- and interassay coefficients of variation of were 10.2 and 12.9%, respectively. Progesterone concentrations were measured in 150 μl of plasma using a commercial RIA that has been validated for use in sheep (14) to confirm stage of cycle (experiments 1 and 2) in a single assay. Sensitivity and intraassay variability were 2.5 pg/tube and 9.3%, respectively.
Statistical analyses
LH pulses were identified using previously described criteria (32). For experiment 1, LH pulse frequencies and amplitudes in the 4 h after senktide were compared with those in the 4 h after vehicle by Wilcoxon-Mann-Whitney (parametric statistics are not appropriate for noncontinuous variables) and paired t test, respectively. Mean LH concentrations in all experiments were analyzed by two-way ANOVA with repeated measures (main effects of treatment and time), followed by the Tukey test for multiple comparisons between control and senktide treatments at each time point. For immunocytochemistry, the percentage of A15 DA neurons with close contacts from NK3R-ir fibers was compared between breeding season and anestrous ewes by an unpaired t test.
Results
Experiment 1: effects of administration of NK3R agonists into third ventricle
The most consistent effect of both icv injection of NKB and senktide in the preliminary experiment was a prolonged increase in LH concentrations that lasted for 2–3 h before starting to decline (Fig. 1). This pattern was observed in three of five ewes given NKB and four of five ewes given senktide. In the other three treated ewes, there was no obvious effect of these NK3R agonists. In three of the ewes, senktide treatments produced a dramatic increase in LH levels to peaks (20–40 ng/ml) close to those seen during the preovulatory LH surge (Fig. 1C). The response to NKB injections was more modest, with peak levels (4–6 ng/ml) similar to late follicular phase concentrations of LH (Fig. 1B). As expected, ewes receiving vehicle injections showed infrequent LH pulse patterns typical of the midluteal phase (four of five ewes) (Fig. 1A) or more frequent pulses seen in the follicular and early luteal phase. There were no obvious behavioral effects of either treatment.
Figure 1.
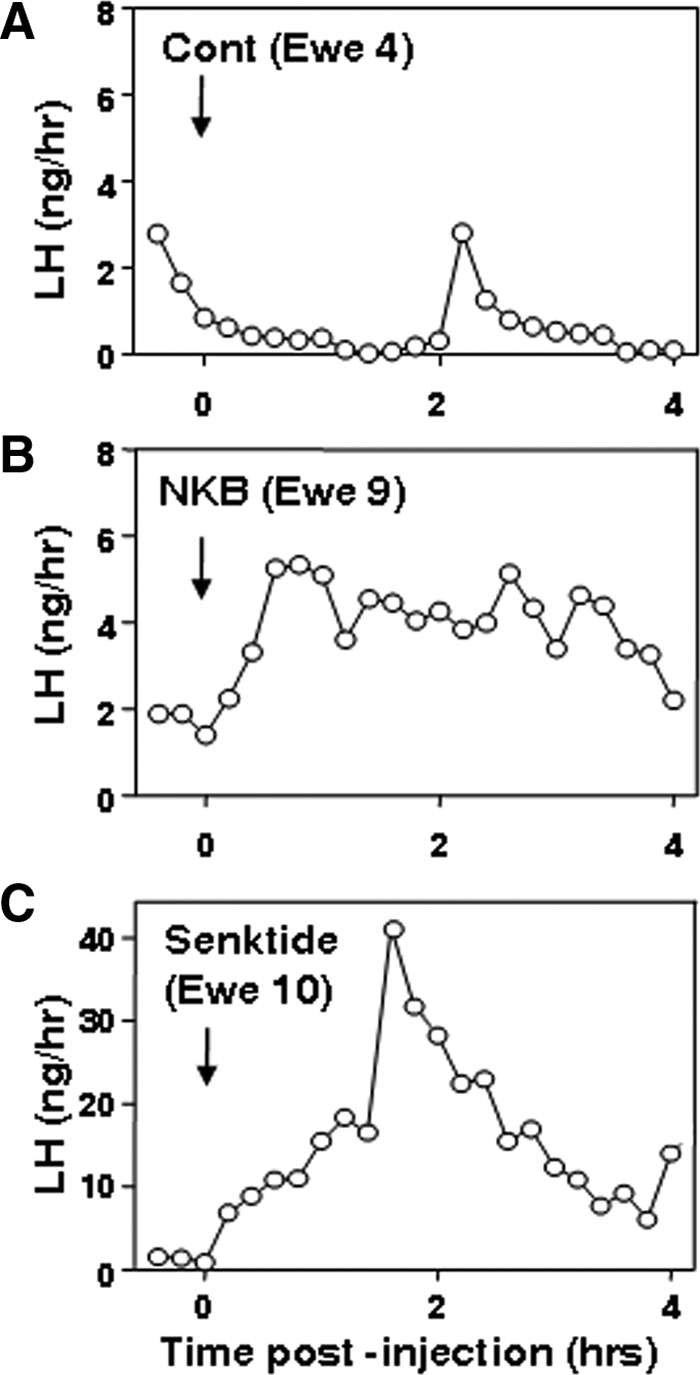
LH concentrations after a single icv injection of vehicle (Cont; A), NKB (B), or the NK3R agonist, senktide (C) in individual ovary-intact ewes during the breeding season. Note difference in y-axis in C.
Because of the variability observed in the preliminary experiment, we next tested the effects of senktide during the follicular and luteal phases, confirmed by low (0.023 ± 0.004 ng/ml) and elevated (2.62 ± 0.52 ng/ml) progesterone concentrations, respectively. In this experiment, most (80%) icv injections of senktide were followed by mild hyperventilation lasting for 20–30 min. One ewe died unexpectedly between treatments; data from this animal were excluded from analysis. There was no obvious effect of icv injection of senktide in luteal phase ewes (Fig. 2). During the follicular phase, senktide injection produced a sustained increase in LH concentrations in all animals. Peak LH reached surge-like concentrations (23–80 ng/ml) in three of the four ewes; in the last ewe, LH levels plateaued at 6–10 ng/ml. There was a statistically significant effect of treatment and time × treatment interaction in the follicular phase but no significant differences during the luteal phase. LH pulses were evident in both treated and control ewes during the luteal phase, and senktide had no effect on LH pulse frequency (senktide, 1.6 ± 0.5 pulses/4 h; controls, 1.8 ± 0.9 pulses/4 h) or amplitude (senktide, 2.0 ± 0.5 ng/ml; controls, 1.9 ± 0.3 ng/ml). LH pulses were not detected using our criteria when ewes were given senktide in the follicular phase, so follicular phase episodic LH patterns were not analyzed in this data set.
Figure 2.
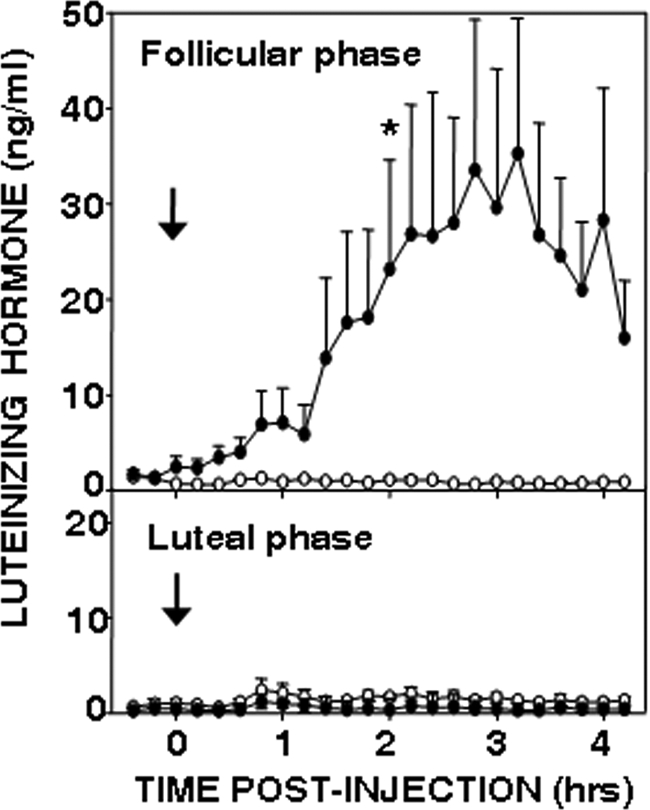
Effect of vehicle (open circles) and senktide (closed circles) injection into the third ventricle in follicular (top panel) and luteal phase (bottom panel) ewes (n = 4). Mean ± sem are shown. There was a significant treatment effect (P < 0.05) and a time × treatment interaction (P < 0.001) in the follicular phase, but not in the luteal phase, based on two-way ANOVA with repeated measures. An asterisk indicates the first time at which there was a statistically significant difference between LH concentrations after senktide and control treatments based on the Tukey test for multiple comparisons. All subsequent comparisons were significant except for the very last time point. There was no effect of senktide on LH pulse frequency (Wilcoxon-Mann-Whitney test) or amplitude (paired t test) in the luteal phase.
Experiment 2: effects of local administration of a NK3R agonist into the RCh in follicular phase ewes
Four of the five ewes had appropriately placed microimplant sites in the RCh. They were within 0.5–1.5 mm of the base of the brain at the posterior end of the optic chiasm (Fig. 3, A–C). The microimplant tracks in the fifth animal extended through the base of the brain; data from this ewe were excluded from analysis. The other four ewes all responded to senktide microimplants with a significant sustained increase in LH secretion (Fig. 3) similar to that in ewes given this NK3R agonist icv. Moreover, LH secretion continued for at least 4 h after microimplants were removed (microimplant tubing was empty at time of removal).
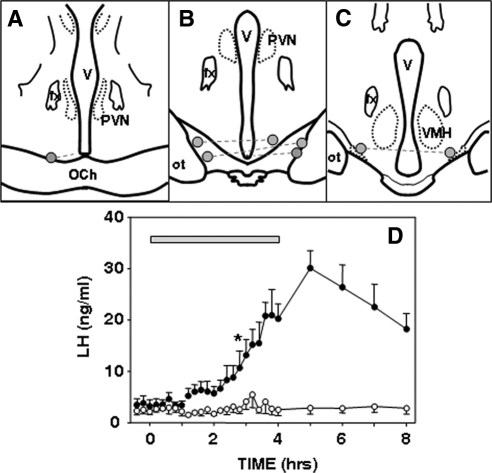
Figure 3.
Effects of local administration of senktide into the RCh of follicular phase ewes. A–C, Positions of microimplants in schematic coronal sections through the RCh. Bilateral microimplants in each ewe are connected by dashed lines. Note that in one ewe, the microimplant on one side was at the edge of the optic chiasm (OCh), whereas the other side was slightly posterior to it. In the fifth animal, microimplant tracts extended beyond the base of the brain (data not shown). D, Mean LH (±sem, n = 4) concentrations before, during, and after insertion of empty (open circles) and senktide-containing (solid circles) microimplants (horizontal bar) into the RCh. Two-way ANOVA with repeated measures indicated there was a significant treatment effect (P < 0.05) and a time × treatment interaction (P < 0.001). Asterisk indicates the first time at which there was a statistically significant difference between LH concentrations after senktide and control treatments, based on the Tukey test for multiple comparisons. All subsequent comparisons were significant. fx, Fornix; ot, optic tract; PVN, paraventricular nucleus; V, third ventricle; VMH, ventromedial hypothalamus.
Experiment 3: effects of local administration of a NK3R agonist into the RCh in anestrous ewes
Six of the eight ewes had implant placements in the RCh near the base of the brain (Fig. 4, A–C). Insertion of empty tubing had no obvious effect on LH secretion, whereas all six ewes responded to insertion of senktide microimplants with a sustained increase in LH secretion (Fig. 4D). There was a significant treatment effect and time × treatment interaction. The response to senktide microimplants was first significant at 1 h after start of treatment and lasted for the duration of the sampling period (Fig. 4), although microimplants were empty when removed after the last sample was collected.
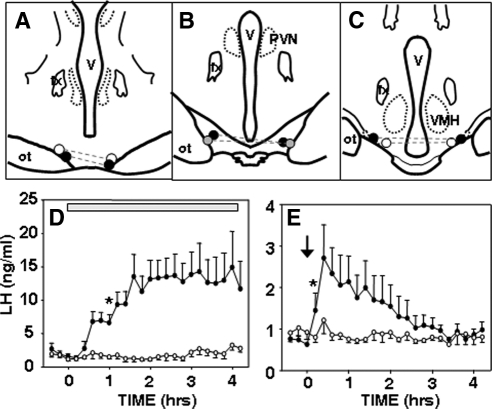
Figure 4.
Effect of local administration of senktide to the RCh of ovary-intact anestrous ewes. A–C, Positions of microimplants and microinjections in schematic coronal sections through the RCh, with bilateral sites in the same ewe connected by dashed lines. Solid circles denote ewes with a robust response to senktide microinjection (peak LH > 4 ng/ml); shaded circles denote ewe with modest response (peak LH < 2 ng/ml); and open circles denote ewes with no response to senktide microinjection. All six ewes responded to the microimplants. D, Mean (±sem, n = 6) LH concentrations before and after insertion of either empty (open circles) or senktide-containing (closed circles) microimplants (horizontal bar). There was a significant treatment effect (P < 0.01) and a time × treatment interaction (P < 0.001) (two-way ANOVA with repeated measures). E, Mean (±sem, n = 6) LH concentrations before and after bilateral microinjection (arrow) of vehicle (open circles) or 3 pmol senktide (closed circles) into the RCh. There was a significant (P < 0.05) time × treatment interaction (two-way ANOVA with repeated measures). Asterisks in D and E depict the first time at which there was a statistically significant difference between LH concentrations after senktide and control treatments, based on the Tukey test for multiple comparisons. All subsequent comparisons were significantly different in D, but only the next five time points were different after the microinjection (E).
Microinjection of a low dose (3 pmol) of senktide into the RCh of ovary-intact anestrous (Fig. 4E) ewes had a stimulatory effect on LH secretion in four of the six ewes (significant time × treatment interaction). The effect of senktide microinjections occurred rapidly (significant increase at 12 min), but peak LH concentrations (range of 1.8 to 6 ng/ml) were lower, and duration of increase was shorter (no significant differences after 72 min) than with senktide microimplants.
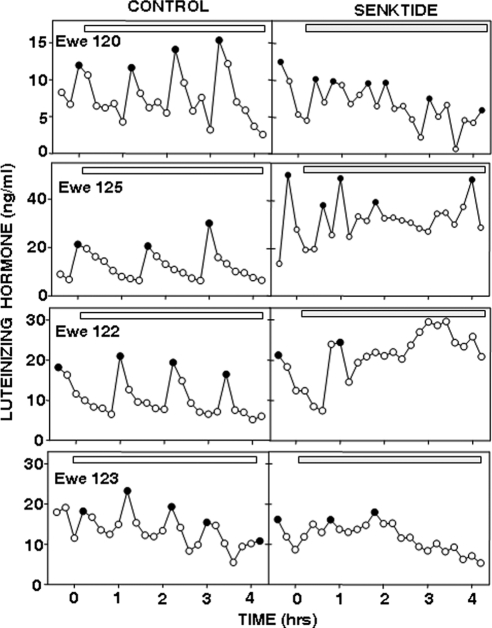
Four of the five OVX ewes in which the effects of senktide microimplants were tested had appropriately placed microimplant sites, but the effects of senktide in these ewes were inconsistent (Fig. 5). There were no statistically significant effects of senktide, but this agonist altered the regular episodic LH pattern observed during control treatments in all four ewes (Fig. 5).
Figure 5.
LH pulse patterns in four OVX ewes during anestrus before and after insertion of empty (left panels) and senktide-containing (right panels) microimplants (horizontal bars) into the RCh. Solid circles depict the peak of LH pulses. Note differences in y-axes. Microimplant sites are indicated in Fig. 4, A–C. Ewe 120, shaded circles (B); ewe 122, open circles (C); ewe 123, open circles (A); and ewe 125, solid circles (C).
Experiment 4: relationship of NK3R to DA neurons in the RCh
There were abundant NK3R-positive fibers scattered among the A15 DA neurons in tissue collected from anestrous ewes (Fig. 6A). None of the NK3R-ir was observed in tyrosine hydroxylase-positive cells, but many of these were contacted by boutons containing NK3R. There was considerably less NK3R-ir evident in tissue from breeding season ewes, and again, none was observed in DA cells (Fig. 6B). A lower percentage of A15 neurons received NK3R-positive boutons in the breeding season than in anestrus (Fig. 6D), in parallel with the overall seasonal differences in NK3R-ir in the RCh.
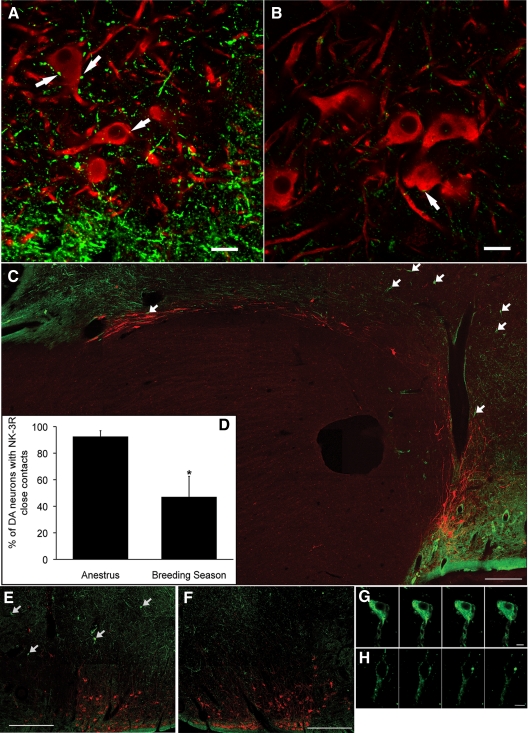
Figure 6.
NK3R and tyrosine hydroxylase dual immunofluorescence labeling in the ovine hypothalamus. A and B, Confocal images of coronal sections through the RCh from OVX+E2 anestrous (A) and breeding season (B) ewes showing NK3R-ir fibers (green) and tyrosine hydroxylase-ir cell bodies (red). Arrows indicate some NK3R-containing varicosities in close contact to A15 cells. Images captured using ×63 objective, ×0.7 optical zoom, and 1.5-μm z-section. Scale bar, 20 μm. C, Montage of confocal images of a parasagittal section illustrating a continuum of NK3R-ir fibers and tyrosine hydroxylase-positive cells along the border of the optic chiasm. Arrows indicate NK3R-positive cell bodies. Images captured using ×10 objective and 15-μm z-thickness. Scale bar, 500 μm. D, Seasonal comparison of the percentage of A15 DA neurons receiving NK3R-containing close contacts. *, P < 0.05. E–H, Confocal images of NK3R-ir neurons (arrows in E) at low (×10 objective and 15-μm z-thickness; E and F) and high (×63 objective and 1-μm z-thickness; G and H) magnification ipsilateral (E and G) and contralateral (F and H) to local injection of senktide. Panels in G and H are a series of z-sections (1 μm) through a single neuron. Scale bars, 500 μm (E and F) and 10 μm (G and H).
Examination of dual immunofluorescence in parasagittal sections again revealed extensive NK3R-positive fibers adjacent to and intermixed with both the caudal A15 DA cells (just posterior to the optic chiasm) and the more rostral A14 group (Fig. 6C). Scattered NK3R-ir cell bodies were also evident (Fig. 6C, arrows), but most of these were observed between the two DA cell groups. Finally, because we had previously observed regional differences in the extent of NK3R internalization, with RCh cells showing membrane-associated receptors (26), we tested the effects of unilateral injection of senktide on NK3R-ir in the RCh. At low magnification (Fig. 6, E and F), a number of NK3R-positive cell bodies were observed on the side receiving the senktide injection (Fig. 6E), whereas few were evident on the contralateral side (Fig. 6F). At higher magnification, NK3R-immunostaining appeared internalized throughout the cells on the ipsilateral side, whereas it was confined to the periphery of cells on the contralateral side (Fig. 6, G and H).
Discussion
These results provide strong evidence that activation of NK3R, the endogenous receptor for NKB, stimulates LH and presumably GnRH secretion in the ewe. Although stimulatory effects were not observed in all endocrine conditions, in no case were inhibitory effects observed. This contrasts with data in rodents in which icv administration of the same NK3R agonist used in these studies inhibited LH secretion (9,23). Moreover, the pattern of LH secretion in response to senktide raises the possibility that this system plays a role in generation of the preovulatory LH surge. These experiments also provide anatomical and pharmacological evidence that the RCh is one site of the stimulatory actions of NK3R agonists.
Because ovine GnRH neurons do not contain NK3R (26), the possible sites of action of senktide are not limited to those areas containing GnRH neurons. Among the likely sites of action (e.g. middle/caudal ARC, POA, and RCh), we chose the RCh for these initial experiments largely because species differences in the expression of NK3R (26,27) raised the possibility that it might be a site of stimulatory actions of senktide in ewes. The ability of senktide microimplants in the RCh to produce an increase in LH secretion similar to that seen with icv administration suggests that this area is one site of action. Based on previous work (33), diffusion is limited to approximately 1 mm from these microimplants so it is unlikely that senktide reached either the POA or middle/caudal ARC (which are >2 mm from the RCh in sheep) in effective concentrations. Moreover, local microinjection of senktide into the RCh produced an immediate increase in LH secretion. Both the low dose (3 pmol) and time course of the response support the hypothesis that this agonist acts locally at the site of administration. It should be noted that although these results demonstrate that the RCh is one site of action of senktide, they do not rule out the possibility that NKB also acts at other sites to stimulate GnRH and LH release.
Although stimulatory effects of senktide were observed in several experiments, this action appeared to vary depending on the endocrine milieu. The most striking differences were observed between stages of the ovarian cycle (experiment 1), with senktide producing maximal stimulation in the follicular phase but no stimulation in the luteal phase. These results raise the possibility that progesterone may block the stimulatory effects of NKB, like it blocks the E2-induced surge (34). However, further work is clearly needed to more directly test the effects of progesterone before any firm conclusions can be reached on the inhibitory actions of this steroid. Interestingly, quantitatively similar stimulatory effects of senktide microimplants in the RCh were observed in ovary-intact anestrous (experiment 3) and follicular phase (experiment 2) ewes. Thus, the follicular-phase rise in E2 secretion is not required for the stimulatory actions of NKB receptor activation. Microimplants of senktide did not consistently stimulate LH secretion in OVX ewes during anestrus, which could reflect the absence of E2 or that GnRH and LH secretion are already close to maximal in the absence of any steroid negative feedback.
Although these results raise the possibility that NKB is stimulatory to LH secretion in ewes, it is less clear whether this neuropeptide is involved in the control of tonic or surge secretion. Data from primates and rodents that NKB mRNA increases after OVX (5,17,19,20) and is suppressed by E2 (5,9,18,21) have led to the hypothesis that it mediates the negative feedback actions of E2. There is no information on the effects of OVX on NKB expression in sheep, and the effects of E2 are conflicting. One report observed a decrease in NKB mRNA expression 4 h after insertion of E2 implants (22), but a second observed no effect after 8 h of a similar treatment (35). The prolonged LH secretion, high peak LH concentrations, and absence of obvious LH pulses after icv injection or microimplants of the NKB agonist are all consistent with a role for NKB in the LH surge. NKB mRNA expression did not increase at the time of an estrogen-induced surge in OVX ewes (35), but we have recently observed a dramatic increase in Fos expression in the ARC KNDy neurons that contain NKB during the preovulatory LH surge at the end of the follicular phase (36). There is evidence that the ovine RCh receives afferent projections from the ventromedial hypothalamus (37), where E2 acts to induce the LH surge in ewes (38), and that neurons in this area project to the medial POA (39).
There are some striking similarities between the possible roles of NKB and kisspeptin in control of GnRH secretion. First, they are both found in the same set of ARC neurons in sheep (6), humans (5), rhesus monkeys (40), and rodents (9). Second, the expression of both peptides (or mRNA) in these neurons appears to be under the negative feedback control of E2 in all these species (3,4,5,17,18,19,20,21,22,41,42,43,44). Third, loss-of-function mutations of receptors for both kisspeptin (1,2) and NKB (24) results in hypogonadotropic hypogonadisms in humans. Finally, they are both potent stimulators of GnRH and LH secretion, at least in sheep (current data and 45,46,47,48). It is interesting to note, however, that the stimulatory effects of each appear to have different dynamics. Kisspeptin administration produces immediate release of LH, and secretion of this peptide declines shortly after the end of kisspeptin administration (42,45,46,48). In contrast, the stimulatory effects of the NK3R agonist in follicular phase ewes were delayed for at least 1 h and lasted for several hours after the end of administration (Figs. 1–3). These temporal differences, and the presence of GPR54 (49), but not NK3R (26), in ovine GnRH neurons suggest that kisspeptin may act as a simple GnRH secretogogue, whereas NKB produces its stimulatory effects more indirectly. In this regard, many KNDy neurons contain NK3R (26,50) so it is possible that senktide could stimulate LH secretion via kisspeptin release.
The limited data currently available also raises the possibility of one other difference between kisspeptin and NKB. In contrast to kisspeptin, there appear to be species differences in the actions and role of NKB. Specifically, data in sheep reported here and human genetic studies (24) support the hypothesis that NKB plays an important stimulatory role in the normal control of GnRH secretion. In contrast, administration of NKB agonists to rats and mice either had no effect (51,52) or inhibited LH secretion (9,23). The discrepancy between the response of rodents and sheep to icv senktide is probably not due to dose (0.6 nmol in rodents and 1 nmol in sheep) or the endocrine environment. Senktide was tested in E2-treated OVX rats (23), OVX and OVX+E2 mice (9), whereas we observed stimulatory effects of senktide in the presence of low E2 (experiment 3, anestrous ewes) and during the follicular phase (experiment 1 and 2). It should be noted that NKB did have stimulatory effects when given in combination with kisspeptin to male mice (52), so more work is clearly needed to determine whether there are species differences in NKB actions. Nevertheless, the contrast in effects of loss-of-function mutations in the TAC3R gene in humans (infertility) and mice (normal fertility) is consistent with this hypothesis (24,25).
One anatomical correlation with the stimulatory actions of NKB is the presence of a population of DA neurons in RCh (53). DA neurons are found in the RCh of humans (54) and sheep (55), but not in rodents (56,57), whose ventral A15 neurons are found largely in the anterior hypothalamic area (53,56,57). In sheep, A15 neurons have been implicated in the seasonal variation in E2 negative feedback (28,29,34), but their potential role in the E2-induced LH surge has not been investigated in any species. Although NKB acts in the RCh area, it is unlikely that the stimulatory effects of this neuropeptide are directly mediated by A15 DA neurons, because they do not contain NK3R. NK3R-ir in A15 cells might have been missed because the NK3R was membrane bound (26), but no NK3R was observed in DA cells even after internalization of the receptor was induced by local injection of senktide (Fig. 6E). These data thus raise the question of which neurons in the RCh mediate the stimulatory actions of NKB and point to the need for a more careful neuroanatomical characterization of this region across species.
In summary, these studies provide strong evidence that activation of the NK3R stimulates LH, and most likely GnRH, secretion in ewes and that one important site for this stimulatory effect is the RCh. These stimulatory effects appeared to vary depending on the endocrine status, but in no case were inhibitory effects similar to those seen in rodents observed. These results, together with differences in the effects of mutations in the receptor for NKB in humans and mice, raise the possibility of important species differences in the role of NKB in control of GnRH secretion.
Acknowledgments
We thank Karie Hardy, Heather Clemmer, and Jennifer Lydon at the West Virginia University Food Animal Research Facility for care of animals; Dr. Miro Valent for his assistance with assays and surgical procedures; Paul Harton for sectioning hypothalamic tissue; Dr. Al Parlow and the National Hormone and Peptide Program for reagents used to measure LH; and the West Virginia University Image Analysis Center for use of their confocal microscope.
Footnotes
This work was supported by National Institutes of Health Grants R01 HD39916 and HD17864. Microscope experiments and image analysis were performed in the West Virginia University Imaging Facility, which is supported in part by the Mary Babb Randolph Cancer Center and National Institutes of Health Grant P20 RR016440.
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 2, 2010
Abbreviations: ARC, Arcuate nucleus; DA, dopaminergic; E2, estradiol; icv, intracerebroventricular; KNDy, kisspeptin/NKB/dynorphin; TACR3, neurokinin-3 receptor; NGS, normal goat serum; NKB, neurokinin B; NK3R, NK-3 receptor; NK3R-ir, NK3R-immunoreactive; OVX, ovariectomy; PGF, prostaglandin; POA, preoptic area; RCh, retrochiasmatic area.
References
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E 2003 Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno Jr JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley Jr WF, Aparicio SA, Colledge WH 2003 The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA 2009 Kisspeptin signaling in the brain. Endocr Rev 30:713–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT 2009 Sex steroid control of hypothalamic Kiss 1 expression in sheep and rodents: comparative aspects. Peptides 30:94–102 [DOI] [PubMed] [Google Scholar]
- Rance NE 2009 Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides 30:111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ 2007 Kisspeptin neurons in the arcuate nucleus of the ewe also express dynorphin A and neurokinin B. Endocrinology 148:5752–5760 [DOI] [PubMed] [Google Scholar]
- Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN 2010 The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in the sheep. Endocrinology 141:301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke MC, Letts PA, Krajewski SJ, Rance NE 2006 Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol 498:712–726 [DOI] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA 2009 Regulation of gonadotropin-releasing hormone secretion by kisspeptin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rometo AM, Krajewski SJ, Voytko ML, Rance NE 2007 Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 92:2744–2750 [DOI] [PubMed] [Google Scholar]
- Rometo AM, Rance NE 2008 Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol 12:1376–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A 2006 Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor α. Neurosci Lett 401:225–230 [DOI] [PubMed] [Google Scholar]
- Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN 2002 Colocalization of progesterone receptors in the parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology 143:4366–4374 [DOI] [PubMed] [Google Scholar]
- Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN 2004 Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology 145:2959–2967 [DOI] [PubMed] [Google Scholar]
- Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN 2005 Progesterone increases dynorphin A concentrations in cerebrospinal fluid and preprodynorphin mRNA levels in a subset of dynorphin neurons in the sheep. Endocrinology 146:1835–1842 [DOI] [PubMed] [Google Scholar]
- Gallo RV 1990 κ-Opioid receptor involvement in the regulation of pulsatile luteinizing hormone release during early pregnancy in the rat. J Neuroendocrinol 2:685–691 [DOI] [PubMed] [Google Scholar]
- Rance NE, Young 3rd WS 1991 Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology 128:2239–2247 [DOI] [PubMed] [Google Scholar]
- Abel TW, Voytko ML, Rance NE 1999 The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J Clin Endocrinol Metab 84:2111–2118 [DOI] [PubMed] [Google Scholar]
- Sandoval-Guzmán T, Stalcup ST, Krajewski SJ, Voytko ML, Rance NE 2004 Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. J Neuroendocrinol 16:146–153 [DOI] [PubMed] [Google Scholar]
- Rance NE, Bruce TR 1994 Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology 60:337–345 [DOI] [PubMed] [Google Scholar]
- Dellovade TL, Merchenthaler I 2004 Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor α. Endocrinology 145:736–742 [DOI] [PubMed] [Google Scholar]
- Pillon D, Caraty A, Fabre-Nys C, Bruneau G 2003 Short-term effects of oestradiol on neurokinin B mRNA expression in the infundibular nucleus of ewes. J Neuroendocrinol 15:749–753 [DOI] [PubMed] [Google Scholar]
- Sandoval-Guzmán T, Rance NE 2004 Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res 1026:307–312 [DOI] [PubMed] [Google Scholar]
- Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK 2009 TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, McCarthy SA, Martin AN, Chapin DS, Stock J, Nadeau DM, Kantesaria S, Bryce-Pritt D, McLean S 2007 Disruption of the neurokinin-3 receptor (NK3) in mice leads to cognitive deficits. Psychopharmacology 194:185–195 [DOI] [PubMed] [Google Scholar]
- Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN 2010 Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalization in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol 22:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileusnic D, Lee JM, Magnuson DJ, Hejna MJ, Krause JE, Lorens JB, Lorens SA 1999 Neurokinin-3 receptor distribution in rat and human brain: an immunohistochemical study. Neuroscience 89: 1269–1290 [DOI] [PubMed] [Google Scholar]
- Thiéry JC, Gayrard V, Le Corre S, Viguié C, Martin GB, Chemineau P, Malpaux B 1995 Dopaminergic control of LH secretion by the A15 nucleus in anoestrous ewes. J Reprod Fertil Suppl 49:285–296 [PubMed] [Google Scholar]
- Lehman MN, Durham DM, Jansen HT, Adrian B, Goodman RL 1996 Dopaminergic A14/A15 neurons are activated during estradiol negative feedback in anestrous, but not breeding season, ewes. Endocrinology 137:4443–4450 [DOI] [PubMed] [Google Scholar]
- Foradori CD, Amstalden M, Coolen LM, Singh SR, McManus CJ, Handa RJ, Goodman RL, Lehman MN 2007 Orphanin FQ: evidence for a role in the control of the reproductive neuroendocrine axis. Endocrinology 148:4993–5001 [DOI] [PubMed] [Google Scholar]
- Anderson GM, Connors JM, Hardy SL, Valent M, Goodman RL 2001 Oestradiol microimplants in the ventromedial preoptic area inhibit secretion of luteinising hormone via dopaminergic neurons in anoestrous ewes. J Neuroendocrinol 1051–1058 [DOI] [PubMed] [Google Scholar]
- Goodman RL, Karsch FJ 1980 Pulsatile secretion of luteinizing hormone: differential suppression by ovarian steroids. Endocrinology 107:1286–1290 [DOI] [PubMed] [Google Scholar]
- Anderson GM, Hardy SL, Valent M, Billings HJ, Connors JM, Goodman RL 2003 Evidence that thyroid hormones act in the ventromedial preoptic area and the premamillary region of the brain to allow the termination of the breeding season in the ewe. Endocrinology 144:2892–2901 [DOI] [PubMed] [Google Scholar]
- Goodman RL, Inskeep EI 2006 Neuroendocrine control of the ovarian cycle of the sheep. In: Neill JD, ed. Knobil and Neill’s physiology of reproduction. 3rd ed. Amsterdam: Elsevier; 2389–2447 [Google Scholar]
- Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE 2000 Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology 141:4218–4225 [DOI] [PubMed] [Google Scholar]
- Merkley C, Coolen LM, Padmanabhan V, Jackson L, Goodman RL, Lehman MN, Evidence for transcriptional activation of arcuate kisspeptin neurons, and glutamatergic input to kisspeptin during the preovulatory GnRH surge of the sheep. Program of the 91st Annual Meeting of The Endocrine Society, Washington, DC, 2009 (Abstract P3-220) [Google Scholar]
- Tillet Y, Batailler M, Thiéry JC, Thibault J 2000 Neuronal projections to the lateral retrochiasmatic area of sheep with special referenc to catecholamine afferents: immunohistochemical and retrograde tract-tracing studies. J Chem Neuroanat 19:47–67 [DOI] [PubMed] [Google Scholar]
- Caraty A, Fabre-Nys C, Delaleu B, Locatelli A, Bruneau G, Karsch FJ, Herbison A 1998 Evidence that the mediobasal hypothalamus is the primary site of the action of estradiol in inducing the preovulatory gonadotropin releasing hormone surge in the ewe. Endocrinology 139:1752–1760 [DOI] [PubMed] [Google Scholar]
- Tillet Y, Batailler M, Thibault J 1993 Neuronal projections to the medial preoptic area of the sheep, with special reference to monoaminergic afferents: immunohistochemical and retrograde tract tracing studies. J Comp Neurol 330:195–220 [DOI] [PubMed] [Google Scholar]
- Plant TM 2009 Postnatal regulation of pulsatile GnRH release in the monkey. The Endocrine Society, Chevy Chase, MD (Abstract S2-2) [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA 2005 Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernández-Fernández R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M 2005 Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology 146:156–163 [DOI] [PubMed] [Google Scholar]
- Smith JT, Clay CM, Caraty A, Clarke IJ 2007 KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 148:1150–1157 [DOI] [PubMed] [Google Scholar]
- Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN 2008 Variation in kisspeptin and gonadotropin-inhibitory hormone expression and terminal connections to GnRH neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology 149:5770–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA 2005 Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraty A, Smith JT, Lomet D, Ben Saïd S, Morrissey A, Cognie J, Doughton B, Baril G, Briant D, Clarke IJ 2007 Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology 148:5258–5267 [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Nakada T, Murata K, Ohkura S, Navarro VM, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H 2009 Neurokinin B as a cotransmitter in kisspeptin neurons in the regulation of pulsatile GnRH secretion. 2009 Neuroscience Meeting Planner, Chicago, IL, Online (Abstract 665.10) [Google Scholar]
- Plant TM, Ramaswamy S, Dipietro MJ 2006 Repetitive activation of hypothalamic G protein-couple receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotrophin-releasing hormone discharges. Endocrinology 147:1007–1013 [DOI] [PubMed] [Google Scholar]
- Smith JT, Li Q, Pereira A, Clarke IJ 2009 Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology 150:5530–5538 [DOI] [PubMed] [Google Scholar]
- Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE 2005 Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via NK3 receptors in the rat median eminence. J Comp Neurol 489:372–386 [DOI] [PubMed] [Google Scholar]
- Sahu A, Kalra SP 1992 Effects of tachykinins on luteinizing hormone release in female rats: potent inhibitory action of neuropeptide K. Endocrinology 130:1571–1577 [DOI] [PubMed] [Google Scholar]
- Corander MP, Challis BG, Thompson EL, Jovanovic Z, Loraine Tung YC, Rimmington D, Huhtanieme IT, Murphy KG, Topaloglu AK, Yeo GS, O'Rahilly S, Dhillo WS,Semple RK, Coll AP 2010 The effects of neurokinin B (NKB) upon gonadotrophin release in male rodents. J Neuroendocrinol 22:181–187 [DOI] [PubMed] [Google Scholar]
- Tillet Y, Kitahama K 1998 Distribution of catecholaminergic neurons: a comparison between ungulates, humans, and other species. Histol Histopathol 13:1162–1177 [DOI] [PubMed] [Google Scholar]
- Li YW, Halliday GM, Joh TH, Geffen LB, Blessing WW 1988 Tyrosine hydroxylase-containing neurons in the supraoptic and paraventricular nuclei of the adult human brain. Brain Res 461:75–86 [DOI] [PubMed] [Google Scholar]
- Tillet Y, Batailler M, Krieger-Poullet M, Thibault J 1990 Presence of dopamine-immunoreactive cell bodies in the catecholaminergic group A15 of the sheep brain. Histochemistry 93:327–333 [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Martenson R, Bjorklund A, Kleinau S, Goldstein M 1984 Distribution maps of tyrosine-hydroxylase-immunoreactive neurons in the rat brain. In: Bjorklund A, Hokfelt T, eds. Handbook of chemical neuroanatomy. Vol. 2. Classical neurotransmitters in the CNS. Part I. Amsterdam: Elsevier; 277–379 [Google Scholar]
- Mons N, Tison F, Geffard M 1990 Existence of L-DOPA immunoreactive neurons in the rat preoptic and anterior hypothalamic area. Neuroendocrinology 51:425–438 [DOI] [PubMed] [Google Scholar]