Abstract
Lipolysis and lipogenesis are two opposite processes that control lipid storage in adipocytes. Impaired adipose lipolysis has been observed in both obese human subjects and animal models. This study investigated the mechanisms underlying impaired adipose lipolysis in a high-fat diet-induced obese (DIO) mouse model. DIO models were created using male C57BL/6 mice. Our results show that β3 adrenergic receptor-specific agonist BRL37344 induced adipose lipolysis was significantly blunted in DIO mice. The levels of Ser660 phosphorylation of hormone-sensitive lipase (HSL) were significantly decreased in the epididymal fat of DIO mice. However, protein levels of HSL, adipose triglyceride lipase and its coactivator comparative gene identification-58 were similar between DIO and control mice. It is known that upon lipolytic hormone stimulation, protein kinase A phosphorylates HSL Ser660 and activates HSL, whereas protein phosphatase 2A (PP2A) dephosphorylates and inactivates HSL. Interestingly, our study shows that high-fat feeding did not alter epididymal fat cAMP and protein kinase A protein levels but significantly increased the expression of the α-isoform of PP2A regulatory subunit B′ (B56α). To study the role of B56α in obesity-associated lipolytic defect, B56α was overexpressed or knocked down by adenovirus-mediated gene transduction in cultured 3T3-L1CARΔ1 adipocytes. Overexpression of B56α significantly decreased HSL Ser660 phosphorylation. In contrast, knocking down B56α increased hormone-stimulated HSL activation and lipolysis in mature 3T3-L1CARΔ1 adipocytes. These results strongly suggest that elevated B56α/PP2A inhibits HSL and lipolysis in white adipose tissue of DIO mice.
Phosphatase B56α/PP2A modulates lipid metabolism in white adipose tissue.
Obesity impairs glucose and lipid metabolism and is a major risk factor for type 2 diabetes and cardiovascular diseases. It has been proposed that the increase of adipocyte number and cellular volume contributes to the expansion of adipose tissue mass. However, a recent study demonstrated that the adipocyte population is very stable in adults, which suggests that the increase of adipocyte size should play a key role in the development of obesity in adults (1). Mature adipocytes are predominantly filled with triacylglycerol (TAG; also known as triglyceride)-rich lipid droplets. Adipocyte size or lipid droplet volume reflects the balance of TAG accumulation and breakdown or lipolysis. Although impaired adipose lipolysis has been observed in obese human subjects (2,3,4,5), the underlying mechanism for the lipolytic defect in obesity is still not completely understood.
Adipocyte lipolysis is a process where TAG are hydrolyzed by lipases, which then release fatty acids and glycerol. For more than three decades, hormone-sensitive lipase (HSL) has been considered a rate-limiting lipase for adipose lipolysis (6). Recent studies have revealed that adipose triglyceride lipase (ATGL) and its coactivator comparative gene identification-58 (CGI-58) also play an important role in this process (7). It is well documented that adipose lipolysis is tightly controlled by hormones, such as catecholamines and insulin (8). Protein kinase A (PKA) plays a central role in mediating hormone-stimulated lipolysis. Upon lipolytic hormone stimulation, several serine residues of HSL, including Ser660, are phosphorylated by PKA, which leads to HSL activation (9). In contrast, ATGL can be phosphorylated but not by PKA (7). Interestingly, PKA has no direct effect on ATGL activation but increases its TG-hydrolase activity indirectly by phosphorylating serine 517 of perilipin A in adipocytes (7,10).
In this study, we compared hormone-stimulated lipolysis between diet-induced obese (DIO) and control mice. Our results indicate that, similar to obese human objects (2,3,4,5), there is a defect of hormone-stimulated lipolysis in DIO mice. By using cultured adipocytes, our study further demonstrates that increased protein phosphatase 2A (PP2A) regulatory subunit B′ α-isoform (B56α) is responsible for blunted HSL Ser660 phosphrylation. Therefore, we proposed that functional inhibition of HSL is one underlying mechanism of lipolytic defect in DIO mice.
Materials and Methods
Materials
Insulin, 3-isobutyl-1-methylxanthine, dibutyryl cAMP (Bt2-cAMP), BRL37344 (BRL), isoproterenol, free glycerol measuring reagents, and dexamethasone were from Sigma-Aldrich (St. Louis, MO). Anti-ATGL, HSL, phospho-HSL, PP2A A and B subunits, perilipin A, phospho-PKA substrate and β-actin antibodies were from Cell Signaling (Danvers, MA). Antibody against B56α was from BD Biosciences (San Jose, CA). Antibody against PP2A catalytic subunits was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). PP2A activity measuring kit was ordered from R&D Systems (Minneapolis, MN). Anti-CGI-58 antibody was purchased from Abnova (Taiwan, China). SYBR Green, penicillin-streptomycin, DMEM, and KnockOut SR were from Invitrogen (Carlsbad, CA). The free fatty acid (FFA) assay kit was from Wako Diagnostics (Richmond, VA). Okadaic acid (OA) was from EMD Chemicals (Gibbstown, NJ).
Animals
Male C57BL/6J mice were from The Jackson Laboratory (Bar Harbor, ME). Obesity in mice was induced by feeding with high-fat diet (60% of calories from fat; Research Diet, New Brunswick, NJ). All mice were maintained under standardized conditions with 12-h light, 12-h dark cycle. The experiments using mouse models were carried out under the Association for Assessment and Accreditation of Laboratory Animal Care guidelines with approval of the Animal Care and Use Committee. Blood and epididymal tissue samples were collected for protein and mRNA measurement.
Adipocytes culture
3T3-L1 and 3T3-L1CARΔ1 cell culture and induction of adipocyte differentiation were described in a previous publication (11). 3T3-L1CARΔ1 cells are derived from 3T3-L1 and stably express coxsackie and adenovirus receptor (CAR), which facilities adenovirus-mediated gene transduction (12). For short hairpin RNA (shRNA)-mediated gene knockdown, adenoviruses targeting three sites of mouse B56α mRNA were used simultaneously to achieve maximal effect. Twenty-four or 48 h after adenovirus transduction, the cells were treated with indicated hormones or reagents (see figure legends). Total protein and mRNA were extracted from adipocytes for Western blot analysis and real-time PCR.
Lipolysis assay
For the in vitro adipose explant lipolysis assays, approximately 20 mg of epididymal adipose tissue explants were incubated in DMEM with 0.5% fatty acid-free BSA. BRL was added to medium at 50 ng/ml. Culture tubes were shaken at 400 rpm. Medium samples were collected at 30-, 60-, 120-, and 180-min intervals after adding BRL. The level of FFA or glycerol was normalized to the weight of adipose explants. For adipocyte lipolysis assay, 3T3-L1 adipocytes were cultured in six-well plates. Mature adipocytes were treated with or without 100 mm Bt2-cAMP in DMEM with 0.5% fatty acid-free BSA.
Generation of adenovirus vectors
Plasmid encoding human B56α cDNA was from PlasmID (Cambridge, MA). The cDNA was subcloned into pENTR and then pAd/CMV/V5-DEST vector. Adenovirus vector encoding B56α (Ad-B56α) was prepared using the ViraPower Adenoviral Expression System (Invitrogen). Three shRNA sequences targeting mouse B56α were designed using Invitrogen’s RNAi Designer. The sequences are 5′-GAAGAAGAAAGAACTGGAACG-3′, 5′-GAGAGAGAAGAAGAAAGAACT-3′, and 5′-GCCTCACATACAGTTGGTGTA-3′. Single-stranded DNA oligos were synthesized by Integrated DNA Technologies (Coralville, IA). Double-stranded oligos were cloned into pENTR/U6 vector. Adenoviruses encoding B56α shRNA were created using the BLOCK-iT Adenoviral RNAi Expression System (Invitrogen). An adenovirus encoding shRNA against lacZ (Ad-lacZshRNA) was used as control. Adenoviruses were grown in HEK293 cells and purified using cesium chloride gradient centrigugation.
Western blot analysis and real-time PCR assay
Protein samples were separated using NuPAGE gels (Invitrogen). Protein was blotted with indicated antibodies (see details in figure legends). Total RNA was prepared from epididymal fat, 3T3-L1 or 3T3-L1CARΔ1 adipocytes with TRIzol following the manufacturer’s protocol (Invitrogen). cDNA was synthesized using SuperScript III Reverse Transcriptase and oligo(dT)12-18 primer (Invitrogen). Real-time PCR was performed using an mx3000p Real-Time PCR system (Stratagene, La Jolla, CA) and SYBR Green dye (Invitrogen). Primer sequences are listed in Supplemental Table 1 published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org. The levels of PCR product were calculated from standard curves established from each primer pair. Expression data were normalized to the amount of 18S rRNA.
Statistical analysis
Data are expressed as mean ± sem. Statistical analysis was performed using the Student’s t test or ANOVA, followed by contrast test with Tukey or Dunnett error protection. Differences were considered significant at P < 0.05.
Results
Fasting increases HSL activation and lipolysis without altering HSL, ATGL, and CGI-58 protein levels in epididymal fat
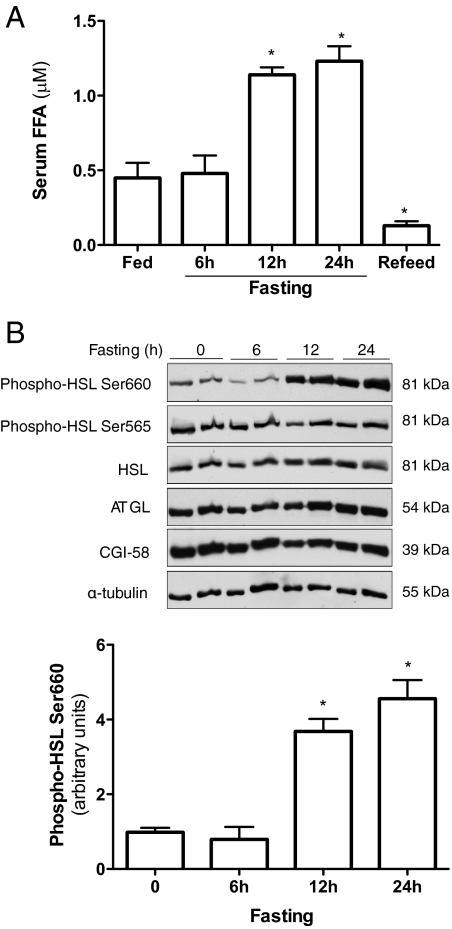
Adipose is the main energy depot storing excessive energy as TAG or releasing FFA when energy intake is deficient. To further understand how lipolysis is regulated under physiological condition, fasting and refeeding mouse models were employed. As anticipated, serum FFA concentration elevated significantly after 12 h fasting and quickly diminished after refeeding (Fig. 1A). Protein levels of HSL, ATGL, and CGI-58 in epididymal fat were measured using Western blot analysis. During 24 h of fasting, there were no significant changes of HSL and CGI-58 protein levels (Fig. 1B). ATGL protein levels were slightly increased after 24-h fasting but without statistical significance (P > 0.05; Fig. 1B).
Figure 1.
Fasting induces HSL activation and lipolysis in adipose tissue. Ten-week-old C57BL/6 male mice were fasted at indicated times with free access to water. Refed mice were fasted for 24 h and refed for 4 h, after which blood and epididymal fat samples were collected. A, Serum FFA were measured. B, Protein was prepared from epididymal fat. Total and phosphorylated HSL protein levels were measured by Western blot analysis using specific antibodies. n = 6 per group. *, P < 0.05 vs. the fed group. Error bars indicate ±sem.
Previous studies have demonstrated that in response to lypolytic hormone stimulation, PKA directly phosphorylates several serine residues in HSL. Serine phosphorylation, especially Ser660 residue, turns on HSL activity (9). Our results showed that 12- and 24-h fasting robustly increased phosphorylation of HSL Ser660 compared with samples from fed and 6-h-fasted mice (Fig. 1B). The phosphorylation of HSL Ser660 was parallel with the increased phosphorylation of other PKA substrates in epididymal fat (Supplemental Fig. 1). In contrast, there were no significant changes of phosphorylation of Ser565 (HSL Ser565) during 24-h fasting. These results indicate that HSL activation, but not total protein levels of HSL and ATGL, may play an important role in fasting-induced lipolysis. Although our study did not show any significant changes of ATGL and CGI-58 protein levels during fasting, our current study cannot rule out the involvement of these two proteins in fasting-induced lipolysis.
Impaired adipose lipolysis in DIO mice
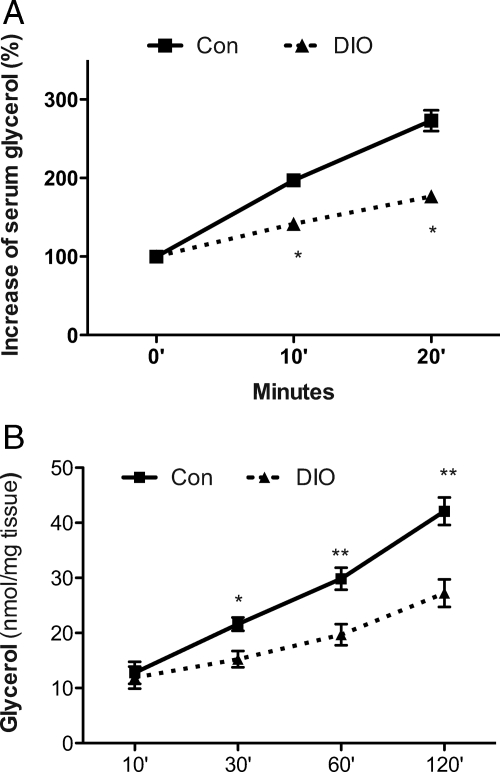
DIO mice were induced by feeding C57BL/6J male mice with high-fat diet (60% of calories from fat) for 4 months (Supplemental Table 2). Using the β3 adrenergic receptor-specific agonist BRL37344 (BRL), we examined hormone-stimulated lipolysis in these mice. The increment of serum-free glycerol and FFA represents the sensitivity of adipose tissue to hormone-stimulated lipolysis. Despite higher serum FFA concentration at the basal state in DIO mice (0.58 ± 0.10 vs. 0.41 ± 0.08 μm of control), magnitudes of BRL-stimulated increase of serum-free glycerol and FFA were significantly less in DIO mice than in control mice (Fig. 2A and Supplemental Fig. 2A).
Figure 2.
Impaired adipose lipolysis of DIO mice. DIO and control mice (Con) were fed with high-fat diet (60% of calories from fat; Research Diet) or control diet (10% of calories from fat), respectively, for 4 months. A, Three adrenergic receptor agonist BRL was ip injected into mice. Blood samples were collected at indicated time points. Serum FFA and free glycerol were measured. Basal FFA and glycerol levels were normalized for the hormone-stimulated increment calculation. n = 6. *, P < 0.05 vs. control at the same time point. B, Epididymal adipose explants were incubated in DMEM with or without BRL stimulation. FFA and glycerol in the medium were measured. n = 6. *, P < 0.05 vs. adipose explants from control mice at the same time point. Data are expressed as means ± sem.
To eliminate effects from other hormones, such as insulin, which may be quickly altered by BRL administration, an in vitro adipose explant lipolysis study was carried out. As shown in Fig. 2B, BRL-stimulated release of glycerol from DIO adipose explants at 30, 60, and 120 min was significantly less than that from control mice (P < 0.05). Together, these results from the in vivo and in vitro adipose lipolysis assays indicate that there is a defect of hormone-stimulated adipose lipolysis in DIO mice.
Impaired HSL activation in the epididymal fat of DIO mice
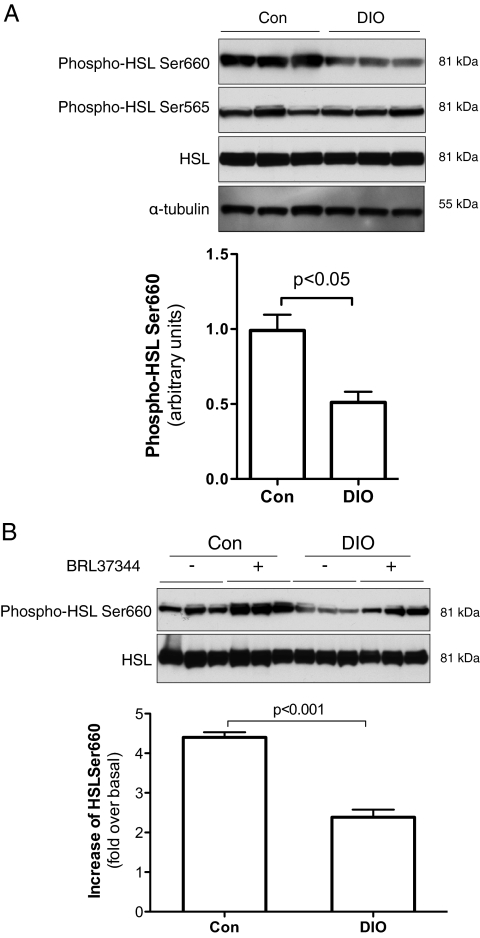
To investigate the underlying mechanism for the lipolytic defect in DIO mice, protein levels of HSL, ATGL, and CGI-58 in epididymal fat from overnight fasted mice were measured. Our results showed that total HSL and ATGL protein levels were comparable between DIO and control mice (Supplemental Table 3). There was a slight decrease of CGI-58 protein level in DIO mice but without statistical significance (P = 0.12; Supplemental Table 3). Although there was no significant change of HSL protein level in epididymal fat of DIO mice, phosphorylation of HSL Ser660 was remarkably less compared with that in control mice (P < 0.05; Fig. 3A). Phosphorylation of HSL Ser565 was similar between DIO and control mice (Fig. 3A).
Figure 3.
Reduced HSL Ser660 phosphorylation in epididymal fat of DIO mice. Epididymal fat was collected after overnight fasting. Some tissue samples were frozen immediately after collection for protein extraction (A) or immediately incubated in DMEM with or without BRL stimulation (B). The stimulation was stopped 10 min later by flash freezing. Protein samples were separated by NuPAGE, and phosphorylation of HSL and total protein levels were measured by Western blot analysis using specific antibodies. n = 10. Data are expressed as means ± sem. Con, Control mice.
Using adipose explants and the selective β3 adrenergic receptor agonist BRL, we also studied hormone-stimulated HSL phosphorylation. As expected, BRL treatment robustly increased HSL Ser660 phosphorylation in all adipose explants (Fig. 3B). Importantly, the increase of BRL-stimulated HSL Ser660 phosphorylation over the untreated basal level was significantly less in adipose explants from DIO than that from control mice (Fig. 3B). In addition, both basal and BRL-stimulated HSL Ser660 phosphorylation were significantly reduced in the adipose explants from DIO mice compared with that in control mice (Supplemental Fig. 3).
In addition to β3-adrenoceptor, activation of β1-adrenoceptor and β2-adrenoceptor also increases intracellular cAMP and results in PKA activation and lipolysis (13). However, due to the low expression level of β3-adrenoceptor in human adipose and other unknown mechanisms, the importance of β3-adrenoceptor in hormone-stimulated lipolysis in humans is uncertain (13). We repeated the adipose explant study using the nonselective β-adrenoceptor agonist isopreterenol (ISO). Consistent with the BRL study described above (Fig. 3B), ISO treatment quickly increased HSL Ser660 phosphorylation in both control and DIO adipose tissues (Supplemental Fig. 4). However, both basal and ISO-stimulated phosphorylation of HSL Ser660 were significantly less in DIO mice than that in control mice (Supplemental Fig. 4). Together, our studies show that there is not only a decrease of HSL Ser660 phosphorylation in adipose of DIO mice but also a significant reduction of responsiveness to β-adrenoceptor stimulation. Because phosphorylation of Ser660 represents the activation state of HSL (9), these results indicate that HSL activation is impaired in the adipose of DIO mice.
Increased B56α in epididymal adipose from DIO mice
It is well documented that cAMP/PKA mediates lipolytic hormonal stimulation and activates HSL by phosphorylating Ser660 and other residues in HSL (9). Our study shows that both basal and hormone-stimulated HSL Ser660 phosphorylation were significantly reduced in DIO mice. To further investigate the underlying mechanism, we measured the expression of PKA catalytic subunits and regulatory subunit RII in epididymal fat of DIO mice. Our results show that there was no significant difference of PKA expression at both the protein and mRNA levels between DIO and control mice (data not shown). In addition, cAMP levels of epididymal fat were also comparable between control and DIO mice (198 ± 36.21 vs. 201 ± 36.24 fmol/mg tissue). These results suggest that decreased HSL Ser660 phosphorylation in the white adipose tissue of DIO mice is most likely not caused by the alternation of cAMP/PKA.
Opposite to PKA, PP2A dephosphorylates and inactivates HSL (14). PP2A is a heterotrimeric protein serine/threonine phosphatase, consisting of catalytic (C), scaffolding (A), and regulatory (B) subunits (12). PP2A catalytic C subunit and structural A subunit are ubiquitously expressed. The regulatory B subunits are more diverse and have four families of genes, known as B(PB55), B′ (B56 or PR61), B″, and B‴. The regulatory B subunits play a key role in controlling phosphatase subcellular localization and substrate specificity and activity (15). Unlike C and A subunits, expression of the regulatory B subunits is tissue-specific.
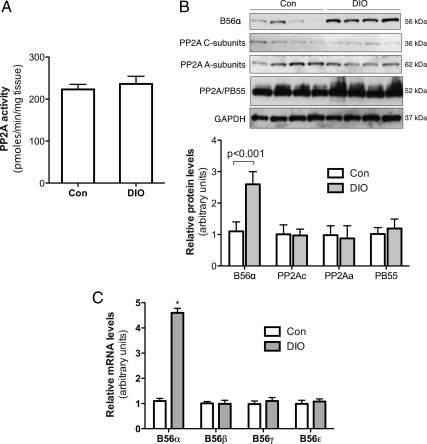
We measured total PP2A activity and expression of PP2A subunits in epididymal fat of DIO and control mice. As shown in Fig. 4, there were no significant differences of total PP2A activity and expression of C, A, and PB55 subunits of PP2A at both protein and mRNA levels between DIO and control mice. Interestingly, among the B56 family, B56α mRNA and protein levels were remarkably elevated in epididymal fat of DIO (P < 0.001; Fig. 4, B and C).
Figure 4.
Elevated B56α gene expression in epididymal fat of DIO mice. Protein and mRNA were extracted from the epididymal fat of control (Con) and DIO mice. A, PP2A catalytic subunits were captured by precoated specific antibody, and release of phosphate from a synthetic phosphopeptide substrate was measured. B, Protein levels of B56α and other indicated PP2A subunits were measured by Western blot analysis. Quantified data were from 10 mice. Error bars indicate ±sem. C, mRNA levels of B56 subunits were measured using real-time PCR. n = 10. *, P < 0.001 vs. adipose explants from control mice. GAPDH, Glyceraldehyde 3-phosphate dehydrogenase.
B56α reduces HSL Ser660 phosphorylation in adipocytes
To study the role of elevated B56α in obesity-associated lipolytic defect, three studies were carried out. Initially, we treated mature 3T3-L1 adipocytes with OA at a concentration (1 nm) that specifically inhibits PP2A (16). Phosphorylation of HSL Ser660 was robustly increased in all OA-treated cells at each time point (Fig. 5A). These results are consistent with results from a previous study (14) and indicate that PP2A reduces HSL Ser660 phosphorylation.
Figure 5.
B56α/PP2A inhibits HSL activation and lipolysis in adipocytes. A, OA increases HSL Ser660 phosphorylation in 3T3-L1 adipocytes. Mature 3T3-L1 adipocytes were treated with OA (1 nm), and protein samples were collected at indicated times. HSL Ser660 phosphorylation and total HSL protein levels were measured by Western blot analysis. n = 6. *, P < 0.05; **, P < 0.01 vs. cells without OA treatment. Data are expressed as means ± sem. B–D, 3T3-L1CARΔ1 cells were differentiated into adipocytes and transduced with adenovirus encoding B56α or siRNA against B56α. Twenty-four hours after transduction, the cells were treated with 100 mm Bt2-cAMP. Total HSL and Ser660 phosphorylation were measured by Western blot analysis (B and C). FFA and free glycerol in the medium were measured (D). cAMP-stimulated increments of FFA or glycerol were analyzed. n = 6. Data are expressed as means ± sem. GFP, Green fluorescent protein.
In the second study, we measured HSL Ser660, Ser563, and Ser565 phosphorylation in B56α overexpressed 3T3-L1CARΔ1 adipocytes. Our results showed that both basal and cAMP-stimulated HSL Ser660 and Ser563, but not Ser565, phosphorylation were significantly reduced in Ad-B56α-transduced cells (P < 0.05; Fig. 5B and Supplemental Fig. 5). These results imply that B56α/PP2A dephosphorylates Ser660 and Ser563 residues, but not Ser565, in HSL. Because phosphorylation of Ser660, but not Ser563, activates HSL (9), the effects of B56α/PP2A on Ser660 phosphorylation was further investigated in this study. Correlated with the decrease of HSL Ser660 phosphorylation, cAMP-stimulated FFA and glycerol release were also significantly inhibited in Ad-B56α-treated adipocytes (P < 0.05; Fig. 5D).
In the third study, we knocked down B56α protein using adenovirus-mediated small interfering (si) RNAs against three target sites of B56α mRNA. B56α protein was knocked 70% down in Ad-B56α siRNA-treated 3T3-L1CARΔ1 adipocytes (Fig. 5C). In contrast to the results of the overexpression study (Fig. 5B), phosphorylation of HSL Ser660 (Fig. 5C) and cAMP-stimulated FFA and glycerol release (Fig. 5D) were significantly higher in Ad-B56α siRNA-transduced adipocytes compared with the cells treated with siRNA against lacZ mRNA. Together, these results indicate that B56α increases HSL Ser660 dephosphorylation and inhibits HSL-mediated adipocyte lipolysis.
Discussion
The essential characteristic of obesity is increase of adipocyte number and TAG storage in white adipose tissue. Compelling evidence indicates that increased lipogenesis plays an important role in obesity, especially at the early phase of the development of obesity (17). However, reduced adipose lipolysis has been observed in both obese adults and children (3,4,5). It has been proposed that the lipolytic defect contributes to obesity, and it has been targeted for obesity treatment. Unfortunately, most available information, including the results from this study, cannot clarify or prove whether the lipolytic defect is the cause or consequence of obesity. In addition, adipose lipolysis was assayed using isolated primary adipocytes or explants in most of these studies, and lipolysis efficiency was expressed as glycerol or fatty acid release per amount of protein, lipids, or tissue weight. Due to the significant increase of adipose tissue mass in obesity, the absolute amounts of fatty acid and glycerol release might be still higher in obese subjects. Similar to a human study (3), our study shows that hormone-induced lipolysis was decreased by approximately 40% (120-min time point in Fig. 2B). However, total adipose tissue mass of DIO mice was 3.8 times higher than that of control mice [14.94 ± 0.68 vs. 3.92 ± 0.42 (g)]. Therefore, it is reasonable to postulate that although there is an adipocyte lipolytic defect in obese subjects and animal models, significantly enlarged adipose mass might increase total lipolysis and may be responsible for the elevated FFA concentrations in obese subjects.
Although there are 23 proteins in adipocytes that exhibit lipase activity (18), HSL and ATGL play the predominant roles in lipolysis and are responsible for more than 95% of lipase activity in murine white adipose tissue (19). Previous human studies have suggested that decreased HSL gene expression in adipose tissue may be responsible for the lipolytic defect in obese subjects (4). Interestingly, HSL gene knockout mice did not exhibit an obvious obese phenotype (20,21). In contrast, ATGL gene deletion induces obesity in mice (22). Furthermore, reduced adipose ATGL expression has also been reported in genetically obese mice (23,24). However, the role of ATGL in human obesity is not clear (2,3,25,26). Our study revealed that neither 24-h fasting nor high-fat feeding alter protein levels of HSL, ATGL, and CGI-58 in mouse epididymal fat. Our study also clearly showed that fasting induces lipolysis and that hormone-stimulated lipolysis is blunted in DIO mice. Consistent with a previous report (9), changes of adipose lipolysis were closely correlated with the HSL Ser660 phosphorylation levels. Therefore, further functional study of these key lipolytic proteins, particularly ATGL and CGI-58, will provide useful information regarding their roles in obesity.
Upon lipolytic hormone stimulation, such as with catecholamine, HSL is quickly activated by cAMP/PKA-mediated phosphorylation of several serine residues (9). In contrast, PP2A dephosphorylates some serine residues and inactivates HSL lipolytic activity (14). In this study, we used intact mature adipocytes, and PP2A inhibitor further confirmed that PP2A dephosphorylates HSL Ser660 and inhibits lipolysis. PP2A is a heterotrimeric protein phosphatase. The catalytic and scaffold subunits of PP2A are ubiquitously expressed and have remarkable sequence conservation within eukaryotes. Our study revealed that the expression of PP2A catalytic subunits and total PP2A activities in epididymal fat are similar between DIO and control mice (Fig. 4). It should be pointed out that immunoprecipitated catalytic subunits were used for PP2A activity measurement. However, the regulatory subunits of PP2A are tissue-specific and play a key role in localizing the holoenzyme to the specific substrates (15,27). Therefore, total PP2A activity measurement does not represent its phosphatase activity toward a specific substrate. Consistent with the results from a genome-scale gene expression profile study (28), our study found that B56α is remarkably highly expressed in both white and brown adipose tissue (data not shown). Interestingly, B56α protein levels were further increased in epididymal fat of DIO mice (Fig. 4B). These results led us to hypothesize that increased B56α recruits PP2A to HSL and dephosphorylates HSL in adipose tissue of DIO mice.
Perilipin is a lipid droplet-associated protein and highly expressed adipocytes. It has been reported that phosphorylation of the lipid droplet protein perilipin A is also involved in hormone-stimulated HSL and ATGL activation and lipolysis (10,29,30). Upon lipolytic hormone stimulation, perilipin A interacts with HSL and recruits HSL to the lipid droplet surface (31,32). However, phosphorylation of perilipin A is not required for transporting HSL to lipid droplets (30). Our in vitro studies indeed showed that overexpression of B56α reduced HSL Ser660 phosphorylation and cAMP-induced lipolysis in adipocytes, whereas knocking down B56α led to the opposite effect. Interestingly, similar manipulation of B56α protein levels in 3T3-L1 adipocyte failed to show any effect on both basal and hormone-stimulated perilipin phosphorylation (Supplemental Fig. 6A). In addition, PP2A inhibitor OA also did not alter perilipin A phosphorylation in adipocytes (Supplemental Fig. 6B). These results confirm a previous study, which reported that PP2A has no effect on perilipin phosphorylation in rat adipocytes (33). Therefore, our study indicates that B56α/PP2A plays an important role in regulating HSL activity without altering perilipin phosphorylation in adipocytes. However, further studies are required to determine the effects of B56α /PP2A on the interaction and translocation of perilipin A and HSL at basal state and upon hormone stimulation.
In summary, our current study reveals that under physiological conditions, fasting induces lipolysis without increasing HSL, ATGL, and CGI-58 protein levels in white adipose tissue. Our study also reveals that there is a lipolytic defect in adipose tissue of DIO mice and that elevated B56α/PP2A might inhibit HSL and play an important role in obesity-associated lipolytic defect.
Supplementary Material
Acknowledgments
We thank Drs. Jerome Schaack and David Orlicky for helpful discussion, technical support of adenovirus construction, and providing 3T3-L1CARΔ1 cells.
Footnotes
This work was supported by National Institutes of Health Grants DK-080418 and DK-077643 (to J.H.S.) and by the American Diabetes Association Grant 7-07-CD23 (to J.H.S.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 9, 2010
Abbreviations: Ad-B56α, Adenovirus vector encoding B56α; ATGL, adipose triglyceride lipase; B56α, PP2A regulatory subunit B′ α-isoform; BRL, BRL37344; Bt2-cAMP, dibutyryl cAMP; CGI-58, comparative gene identification-58; DIO, diet-induced obese; FFA, free fatty acid; HSL, hormone-sensitive lipase; ISO, isopreterenol; OA, okadaic acid; PKA, protein kinase A; PP2A, protein phosphatase 2A; shRNA, short hairpin RNA; si, small interfering; TAG, triacylglycerol.
References
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T, Concha H, Hassan M, Rydén M, Frisén J, Arner P 2008 Dynamics of fat cell turnover in humans. Nature 453:783–787 [DOI] [PubMed] [Google Scholar]
- Jocken JW, Langin D, Smit E, Saris WH, Valle C, Hul GB, Holm C, Arner P, Blaak EE 2007 Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. J Clin Endocrinol Metab 92:2292–2299 [DOI] [PubMed] [Google Scholar]
- Langin D, Dicker A, Tavernier G, Hoffstedt J, Mairal A, Rydén M, Arner E, Sicard A, Jenkins CM, Viguerie N, van Harmelen V, Gross RW, Holm C, Arner P 2005 Adipocyte lipases and defect of lipolysis in human obesity. Diabetes 54:3190–3197 [DOI] [PubMed] [Google Scholar]
- Large V, Reynisdottir S, Langin D, Fredby K, Klannemark M, Holm C, Arner P 1999 Decreased expression and function of adipocyte hormone-sensitive lipase in subcutaneous fat cells of obese subjects. J Lipid Res 40:2059–2066 [PubMed] [Google Scholar]
- Bougnères P, Stunff CL, Pecqueur C, Pinglier E, Adnot P, Ricquier D 1997 In vivo resistance of lipolysis to epinephrine. A new feature of childhood onset obesity. J Clin Invest 99:2568–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm C, Kirchgessner TG, Svenson KL, Fredrikson G, Nilsson S, Miller CG, Shively JE, Heinzmann C, Sparkes RS, Mohandas T 1988 Hormone-sensitive lipase: sequence, expression, and chromosomal localization to 19 cent-q13.3. Science 241:1503–1506 [DOI] [PubMed] [Google Scholar]
- Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R 2004 Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306:1383–1386 [DOI] [PubMed] [Google Scholar]
- Jaworski K, Sarkadi-Nagy E, Duncan RE, Ahmadian M, Sul HS 2007 Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. Am J Physiol Gastrointest Liver Physiol 293:G1–G4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthonsen MW, Rönnstrand L, Wernstedt C, Degerman E, Holm C 1998 Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem 273:215–221 [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Perfield 2nd JW, Souza SC, Shen WJ, Zhang HH, Stancheva ZS, Kraemer FB, Obin MS, Greenberg AS 2007 Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J Biol Chem 282:996–1002 [DOI] [PubMed] [Google Scholar]
- Qiao L, Maclean PS, Schaack J, Orlicky DJ, Darimont C, Pagliassotti M, Friedman JE, Shao J 2005 C/EBPα regulates human adiponectin gene transcription through an intronic enhancer. Diabetes 54:1744–1754 [DOI] [PubMed] [Google Scholar]
- Orlicky DJ, DeGregori J, Schaack J 2001 Construction of stable coxsackievirus and adenovirus receptor-expressing 3T3-L1 cells. J Lipid Res 42:910–915 [PubMed] [Google Scholar]
- Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK 2010 Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol 318:34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SL, Emmison N, Borthwick AC, Yeaman SJ 1993 The protein phosphatases responsible for dephosphorylation of hormone-sensitive lipase in isolated rat adipocytes. Biochem J 295(Pt 2):531–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virshup DM, Shenolikar S 2009 From promiscuity to precision: protein phosphatases get a makeover. Mol Cell 33:537–545 [DOI] [PubMed] [Google Scholar]
- Bialojan C, Takai A 1988 Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J 256:283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strable MS, Ntambi JM 2010 Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol 45:199–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birner-Gruenberger R, Susani-Etzerodt H, Waldhuber M, Riesenhuber G, Schmidinger H, Rechberger G, Kollroser M, Strauss JG, Lass A, Zimmermann R, Haemmerle G, Zechner R, Hermetter A 2005 The lipolytic proteome of mouse adipose tissue. Mol Cell Proteomics 4:1710–1717 [DOI] [PubMed] [Google Scholar]
- Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R 2006 Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem 281:40236–40241 [DOI] [PubMed] [Google Scholar]
- Osuga J, Ishibashi S, Oka T, Yagyu H, Tozawa R, Fujimoto A, Shionoiri F, Yahagi N, Kraemer FB, Tsutsumi O, Yamada N 2000 Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc Natl Acad Sci USA 97:787–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemmerle G, Zimmermann R, Hayn M, Theussl C, Waeg G, Wagner E, Sattler W, Magin TM, Wagner EF, Zechner R 2002 Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem 277:4806–4815 [DOI] [PubMed] [Google Scholar]
- Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R 2006 Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312:734–737 [DOI] [PubMed] [Google Scholar]
- Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul HS 2004 Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem 279:47066–47075 [DOI] [PubMed] [Google Scholar]
- Kim JY, Tillison K, Lee JH, Rearick DA, Smas CM 2006 The adipose tissue triglyceride lipase ATGL/PNPLA2 is downregulated by insulin and TNF-α in 3T3-L1 adipocytes and is a target for transactivation by PPARγ. Am J Physiol Endocrinol Metab 291:E115–E127 [DOI] [PubMed] [Google Scholar]
- Berndt J, Kralisch S, Klöting N, Ruschke K, Kern M, Fasshauer M, Schön MR, Stumvoll M, Blüher M 2008 Adipose triglyceride lipase gene expression in human visceral obesity. Exp Clin Endocrinol Diabetes 116:203–210 [DOI] [PubMed] [Google Scholar]
- Bezaire V, Mairal A, Ribet C, Lefort C, Girousse A, Jocken J, Laurencikiene J, Anesia R, Rodriguez AM, Ryden M, Stenson BM, Dani C, Ailhaud G, Arner P, Langin D 2009 Contribution of adipose triglyceride lipase and hormone-sensitive lipase to lipolysis in hMADS adipocytes. J Biol Chem 284:18282–18291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens V, Goris J 2001 Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J 353:417–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB 2004 A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA 101:6062–6067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewicz A, Gauthier D, Garcia A, Brasaemle DL 2006 The phosphorylation of serine 492 of perilipin a directs lipid droplet fragmentation and dispersion. J Biol Chem 281:11901–11909 [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Souza SC, Zhang HH, Strissel KJ, Christoffolete MA, Kovsan J, Rudich A, Kraemer FB, Bianco AC, Obin MS, Greenberg AS 2006 Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J Biol Chem 281:15837–15844 [DOI] [PubMed] [Google Scholar]
- Sztalryd C, Xu G, Dorward H, Tansey JT, Contreras JA, Kimmel AR, Londos C 2003 Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol 161:1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen WJ, Patel S, Miyoshi H, Greenberg AS, Kraemer FB 2009 Functional interaction of hormone-sensitive lipase and perilipin in lipolysis. J Lipid Res 50:2306–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford GM, McCormick DK, Londos C, Vernon RG, Yeaman SJ 1998 Dephosphorylation of perilipin by protein phosphatases present in rat adipocytes. FEBS Lett 435:125–129 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.