Abstract
Epidermal growth factor family plays critical roles in intestinal epithelial proliferation and differentiation. The precise function of amphiregulin (AREG), a member of the epidermal growth factor family, in intestinal biology is largely unknown. The present study attempted to address the functional roles of AREG in intestinal epithelial regeneration. Total body irradiation was performed, and intestinal regeneration was assessed in AREG knockout mice. Genetically disruption of AREG significantly impaired intestinal regeneration after radiation injury. It is known that prostaglandin E2 (PGE2) exerts radio-protective and growth-stimulatory effects on intestinal epithelium. We found that PGE2 radio-protective action did not involve AREG. However, PGE2 growth-stimulatory effects required functional AREG. Localization of AREG expression was determined by immunohistochemistry in regenerative intestine. The immunoreactivity of AREG was predominantly localized in intestinal subepithelial myofibroblasts (ISEMF). Primary ISEMF cultures were established, and growth-stimulatory actions of ISEMF-generated AREG were demonstrated in cell coculture system. In addition, we found that the cAMP/protein kinase A pathway robustly induced AREG in cultured ISEMF. These studies suggest that AREG plays critical roles in intestinal epithelial growth. Modulation of levels of AREG by targeting ISEMF represents a novel strategy for treatment of certain intestinal disorders.
Amphiregulin plays critical roles in intestinal epithelial regeneration and can be targeted for novel treatment of certain intestinal disorders.
The intestinal epithelium is one of the most rapidly proliferating tissues in the body and is able to replicate the total mass every 3–8 d (1). There is normally a dynamic equilibrium between cell proliferation at the base of the crypt and programmed cell death to preserve a functional cell steady state. The stress of major surgical procedures, i.e. irradiation, chemotherapy, nutritional deprivation, and systemic inflammation, all lead to atrophic changes in the gut epithelium, which significantly affects the outcome of these patients (2,3,4). Restricting dietary intake, a common complication in patients who have undergone severe systematic illness, such as trauma and sepsis, leads to intestinal mucosal atrophy and barrier dysfunction, which may result in the migration of intestinal microbes and toxins to the systemic circulation (5). Thus, regulation of intestinal epithelial homeostasis impacts on many problems relevant to the digestive system. Properly maintaining the integrity and function of the intestinal tract improves the outcome of these patients.
The epidermal growth factor (EGF) family, including EGF and TGF-α, and their receptor [EGF receptor (EGFR)] play critical roles in intestinal epithelial growth and transformation (6). EGF-like growth factors signal through the EGFR. Binding of the ligand to the receptor leads to activation of receptor tyrosine kinase that phosphorylates tyrosine residues of cellular signaling proteins (7,8). Administration of EGF stimulates proliferation of the intestinal epithelium and promotes healing of intestinal mucosal injury resulting from rotavirus infection, radiation, and chemotherapy (9). EGF plays an important role in intestinal adaptation (10). After massive small bowel resection, DNA and protein synthesis rates rapidly increase in the remnant intestine that acquires hyperplastic morphology in 3–7 d (11). Administration of exogenous EGF improves intestinal adaptation after small bowel resection (12). Amphiregulin (AREG) is another member within the EGF family. AREG often acts via autocrine and paracrine mechanisms (13) and is important for the growth of many cell types, including keratinocytes, mammary epithelial cells, hepatocytes, and intestinal epithelial cells (14,15,16). For example, AREG behaves as a primary mitogen for hepatocytes. AREG knockout mice display impaired proliferative response after partial liver resection (15). Studies by Inatomi et al. (17) suggest that AREG may play an important role in the mechanism underlying wound healing in damaged colonic mucosa.
Prostaglandin E2 (PGE2) exerts diverse biological functions to the gastrointestinal tract, including maintaining mucosal integrity and stimulating epithelial proliferation (18). Short-term administration of PGE2 causes significant stimulation of DNA synthesis. Prolonged PGE2 treatment markedly increases the weight and DNA and RNA contents of intestinal mucosa in rats (18,19,20). Radio-protective effects of PGE2 in the mouse intestine have been well documented (21,22,23,24). Total body irradiation results in strong apoptosis and crypt depopulation in mouse intestinal epithelium. Administration of PGE2 before irradiation reduces apoptosis index by 50% and increases the number of surviving crypts by 100% in small intestine. Molecular mechanisms governing PGE2 trophic actions in the gut are complex, which appear to involve trans-activation of the EGFR signaling system and its downstream pathways. Cyclooxygenase/PGE2 cross communicates with the EGFR signaling system, triggers ERK activation, and stimulates the growth of colonic epithelial cells through trans-activation of the EGFR in a ligand-independent pathway (25,26). We have established a ligand-dependent mechanism, whereby PGE2 rapidly induces autocrine and paracrine AREG, which, in turn, activates the EGFR signaling system and stimulates intestinal epithelial growth (14,27,28).
In the present study, we used a AREG knockout mouse model to determine the roles of AREG in intestinal epithelial regeneration. Disruption of functional AREG significantly impaired intestinal regeneration. AREG appeared to be critical for the growth-stimulatory actions of PGE2 in the gut but was not essential for its radio-protective effects. Intestinal subepithelial myofibroblasts (ISEMF) were the predominant cell type that expressed AREG in intestinal epithelium. Myofibroblast-generated AREG stimulated the growth of intestinal epithelial cells via a paracrine mechanism in vitro. The cAMP/protein kinase A (PKA) pathway robustly induced the expression of AREG specifically in myofibroblasts, suggesting that modulating AREG levels by targeting myofibroblasts provides a novel therapeutic strategy for certain intestinal disorders.
Materials and Methods
Animals
All animals were treated in a manner that complied with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Indiana University. Mice were housed in an animal-holding room under controlled light, temperature, and humidity. AREG+/− mice on 129/C57/BL6 background were kindly provided by David Lee (University of North Carolina, Chapel Hill, NC) and Tinghu Qiu (National Institutes of Health/National Cancer Institute) (29). The AREG+/− mouse was back crossed with C57BL/6J for more than 10 generations. The AREG+/− mouse on C57BL/6J background was then intercrossed to obtain AREG+/+ and AREG−/− mouse colonies. In all animal experiments, each time point included three to seven animals as indicated in the figures.
Total body irradiation and scoring of crypt regeneration
Radiation is the most extensively characterized model of intestinal injury and regeneration (30,31,32). Mice were received 12-Gy total body irradiation using a Gammacell 40 137Cs irradiator. 5-Bromodeoxyuridine (BrdU) were injected (ip) 2 h before death. The small intestine was removed and fixed in methacarn fixative. The ileal region of the small intestine was cut into 10 lengths of 1 cm each, and these were bundled in 3M surgical tape (3M, St. Paul, MN). These bundles of 10 pieces of ileum were embedded, and transverse sections were prepared. The parameters measured included 1) the percentage of regenerating crypts (similar to surviving crypts), 2) the size of regenerating crypts, and 3) the proliferating index. A regenerating crypt was determined by histological appearance (containing >10 adjacent, chromophilic, non-Paneth cells, and a lumen). The total number of crypts and the number of regenerating crypts were counted in each circumference of a transverse cross section of the ileum. Results were expressed as percentage of regenerating crypts/number of total crypts ×100%. To determine the size of regenerative crypts, three representative cross sections of the ileum from each mouse were chosen. The width and height of all regenerative crypts were measured. Relative crypt size was determined as crypt width × crypt height. Regenerative crypts were confirmed by incorporation of BrdU into five or more epithelial cells within each regenerative crypt using immunostaining. Proliferating index of the intestinal epithelium were presented by the number of crypts that containing more than 10 BrdU positive cells per cross section of the ileum.
Cell culture and reagents
18Co cells were purchased from American Type Culture Collection (Manassas, VA) and grown in MEM supplemented with 10% fetal bovine serum and nonessential amino acids. 18Co cells used for this study were passage 12 to 14. RIE cells were a generous gift from Susan Kirkland (University of London, London, UK) and grown in DMEM with 10% fetal bovine serum. PGE2 and 16,16-dimethyl PGE2 (dmPGE2) was purchased from Cayman Chemical (Ann Arbor, MI). H89 and forskolin were purchased from Calbiochem (San Diego, CA). 8-(4-Chlorophenylthio)adenosine 3′, 5′-cyclic monophosphate (8-CPT-cAMP) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Establishment of primary mouse myofibroblast isolates
Primary ISEMF cultures that contain either wild-type AREG gene (AREG+/+) or inactive AREG allele (AREG−/−) were established from AREG+/+ and AREG−/− mouse intestine using the outgrowth method described by Mahida et al. (33). Briefly, the small intestine of 1-month-old mice was opened and soaked in 0.04% bleach/PBS for 20 min. The intestine was rinsed with PBS and transferred into PBS with 3 mm EDTA and 0.5 mm DTT for 90 min then resuspended in PBS and shaken vigorously to detach and remove intestinal crypts. The intestine was cut into small pieces and cultured in DMEM containing 10% fetal bovine serum, 4 mm l-glutamin, 1% nonessential amino acids, and 100 U/ml of penicillin/streptomycin. After approximately 7 d of culture, spindle-shaped myofibroblasts appeared. After approximately 14 d of culture, established colonies of myofibroblasts were observed. Myofibroblast colonies were trypsinized and transferred to a new dish. These primary ISEMF cells exhibited spindle morphology with cytoplasmic extensions. Cell purity was confirmed by morphology and immunohistochemistry for positive α-smooth muscle actin (α-SMA) and negative desmin.
Cell coculture system and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay
The 5 × 103 RIE cells suspended in 400 μl complete medium were placed in Transwell chambers (0.4 μm; Corning Costar Co., Cambridge, MA) and then grown in serum-free medium for 24 h. Separately, confluent AREG+/+ or AREG−/− myofibroblasts were grown in a 24-well plate. Subsequently, Transwell chambers containing RIE cells were inserted into the 24-well plated and grown for 24–48 h. MTT (5 mg/ml) was added into the upper chamber and incubated for 2 h. The Transwell membrane was removed from the chamber and placed in 200 μl MTT solvent (4 mm HCl and 0.1% Nonidet P-40 in isopropanol). After the converted dye was completely dissolved, absorbance of the converted dye was measured at a wavelength of 570 nm with background subtraction at 650 nm using an aliquot of the MTT solvent (100 μl).
Quantitative real-time RT-PCR (RT-qPCR)
Relative levels of mRNA were quantified using RT-qPCR by an AB 7500 Real-Time PCR System. This method has been published in our previous studies (28,34). The sequence of the primer/probe set are based on human AREG mRNA sequence (GenBank, NM_001657) and designed using the D-LUX Designer from Invitrogen (Carlsbad, CA). The primer set includes: forward primer, cgcttATGACACCTACTCTGGGAAGcG; and reverse primer, CAAATCCATCAGCACTGTGGTC, which generates a single 65-bp band on PCR. Two-step RT-qPCR was performed with 1 μg RNA for both target gene and reference gene using the Superscript III Platinum Two-Step RT-qPCR kit (Invitrogen). Duplicate threshold cycle (CT) values were analyzed in Microsoft Excel using the comparative CT (ΔΔCT) method as described by the manufacturer (Applied Biosystems, Foster City, CA). The amount of target (2−ΔΔCT) was obtained by normalized to a reference gene, 18s, and relative to a calibrator.
Immunohistochemistry
Mouse tissue was fixed in methacarn fixative or 10% formalin, paraffin embedded, and sectioned. Endogenous peroxidase activity was quenched by incubating the sections in 0.3% hydrogen peroxide for 20 min at room temperature. After the sections were blocked in 1.5% normal serum in PBS for 1 h, anti-AREG monoclonal antibody (Ab-2, 1:200; Thermo Scientific, Barrington, IL), α-SMA antibody (mouse monoclonal 1A4, 1:200; Abcam, Cambridge, MA) or mouse preimmune IgG (negative control) was added to the sections and incubated overnight at 4 C. The sections were then incubated with biotinylated secondary antibody and ABC-AP reagent (Vectorstain ABC-AP kit; Vector Laboratories, Burlingame, CA). Peroxidase activity was demonstrated by applying 3,3′-diaminobenzidine containing 0.02% of hydrogen peroxide for 10 min. The sections were counterstained with toluidine blue O. BrdU immunostaining was carried out using BrdU staining kit from Invitrogen according to the manufacturer’s instructions.
Data analysis
All statistical analyses were performed on a personal computer with the StatView 5.0.1 software (SAS Institute, Inc., Cary, NC). Analyses between two groups were determined using the unpaired Student’s t test. Differences with a P value of less than 0.05 were considered as statistically significant.
Results
AREG was required for intestinal epithelial regeneration
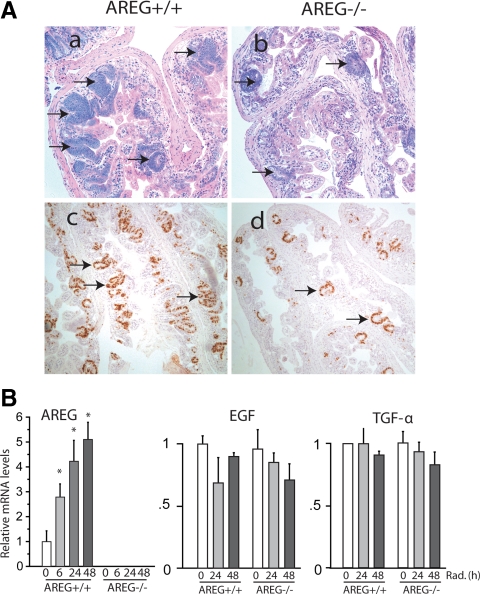
The precise roles of AREG in intestinal growth and regeneration have not been elucidated. In the present study, we compared the regeneration of radiation-injured intestine in wild-type mice (AREG+/+) and mice lacking of functional AREG (AREG−/−). The basic proliferative activity in both AREG+/+ and AREG−/− mouse ileum appeared to be similar, determined by BrdU staining (data not shown). Animals received 12-Gy total body irradiation and were killed 84 h after the irradiation. A large number of actively regenerating crypts was observed in AREG+/+ mouse ileum. In contrast, regenerative activity was barely detected in AREG−/− mouse ileum (Fig. 1A, a and b). As summarized in Table 1, although 62.8 ± 5.5% of crypts in AREG+/+ mouse ileum was actively regenerative, only 14.4 ± 9.5% regenerative crypts were counted in AREG−/− mouse ileum (P < 0.001). Relative size of regenerative crypts in AREG+/+ mouse ileum was measured 1.40 ± 0.20, the size of regenerative crypts of AREG−/− mouse ileum was reduced by approximately 54% to 0.65 ± 0.17. On average, approximately 30 proliferating crypts that contained more than10 BrdU positive cells were detected on each cross section of AREG+/+ ileum, whereas the AREG−/− ileum cross section contained less than 10 proliferating crypts (Fig. 1A, c and d). These results suggest that functional AREG stimulates intestinal epithelial cell proliferation and is critically required for the regeneration of radiation-injured intestine.
Figure 1.
AREG in intestinal epithelial regeneration after radiation injury. A, AREG+/+ and AREG−/− mice were exposed to 12-Gy total body irradiation. The ileum was fixed in methacarn fixative 84 h after the irradiation and cross sectioned. H&E staining of AREG+/+ mouse ileum (×200) (a); H&E staining of ARE−/− mouse ileum (×200) (b); BrdU staining of AREG+/+ mouse ileum (×200) (c); BrdU staining of ARE−/− mouse ileum (×200) (d). Arrows, Regenerative crypts. Results shown are representative of three separate experiments. B, AREG+/+ and AREG−/− mice were exposed to 12-Gy total body irradiation. Ileal mucosa was harvested, and RNA was extracted at indicated time points (n = 5). mRNA levels of AREG, EGF, and TGF-α were determined by RT-qPCR and expressed as fold to levels in mice without irradiation (0 h). *, P < 0.05.
Table 1.
Comparison of intestinal regeneration in AREG+/+ and AREG−/− mice
| AREG+/+ mice (n = 7) | AREG−/− mice (n = 7) | |
|---|---|---|
| Percentage of regenerative crypt | 62.8 ± 5.5 | 14.4 ± 9.5a |
| Relative size of crypt | 1.40 ± 0.20 | 0.65 ± 0.17a |
| Proliferation index | 30.3 | 9.7a |
P < 0.001.
The expression of AREG is relatively low in normal intestinal mucosa, and AREG often functions via autocrine and paracrine pathways. It was interesting to determine whether irradiation altered the expression of AREG. AREG+/+ and AREG−/− mice were exposed to 12-Gy irradiation. Intestinal mucosa was harvested at 6, 24, and 48 h after irradiation. Expression of AREG was determined by RT-qPCR. Levels of AREG mRNA in AREG+/+ intestinal mucosa increased 3- to 5-fold between 6 and 48 h after the irradiation, whereas AREG mRNA was not detected in AREG−/− mice (Fig 1B, left panel). It was critical to determine whether radiation altered the expression of other EGFR ligands. Levels of TGF-α and EGF mRNA were analyzed by RT-qPCR. The regulation of TGF-α and EGF by radiation was similar in both AREG+/+ and AREG−/− mouse intestine. Irradiation did not significantly change the expression of both TGF-α and EGF, which were expressed at similar low levels in the intestinal mucosa (Fig. 1B, middle and right panels). These findings suggest that AREG was specifically induced by radiation injury and may serve as an important growth factor in intestinal regeneration after radiation injury.
AREG was essential for PGE2 growth-stimulatory action in the gut
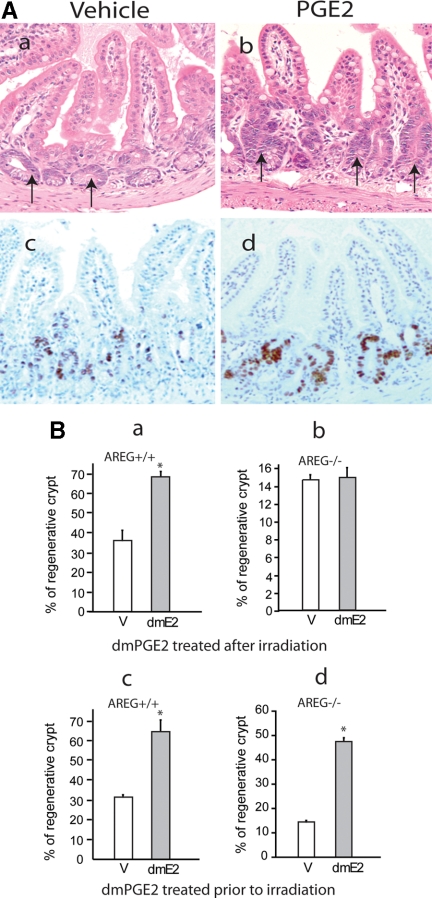
It has been well documented that PGE2 prevents gut atrophy and stimulates intestinal epithelial growth (35). PGE2 exerts protective effects on radiation-injured intestine in mouse models (24). It has not been elucidated whether administration of PGE2 after irradiation stimulates the proliferation and regeneration of radiation-injured intestine. To determine the regeneration-stimulatory effect of PGE2, AREG+/+ mice received 12-Gy total body irradiation. dmPGE2 (150 μg/kg) was administered twice daily via oral gavage. Animals were killed 78 h after the irradiation. Administration of dmPGE2 stimulated the regeneration of intestinal crypts after irradiation, which was noted in hematoxylin/eosin (H&E) stained sections, as well as in BrdU incorporation (Fig. 2A). Although approximately 37% of crypts were actively proliferating in control mouse ileum that received vehicle, 68% of regenerating crypts were determined in dmPGE2-treated mouse intestine 78 h after irradiation. Additionally, the size of regenerative crypts significantly increased in PGE2-treated mouse intestine. Relative size of regenerative crypts of vehicle-treated mouse ileum was 0.75 ± 0.15. The size of regenerative crypts in dmPGE2-treated mouse ileum was 1.15 ± 0.13, representing approximately a 53% increase.
Figure 2.
PGE2 growth-stimulatory effects in intestinal regeneration. A, AREG+/+ mice were exposed to 12-Gy total body irradiation. Vehicle (V) or dmPGE2 (dmE2; 150 μg/kg of body weight) was administered twice daily via oral gavage. Animals were killed 78 h after the irradiation. The ileum was fixed in methacarn fixative, and cross-sections were prepared. H&E staining (a) and BrdU staining (c) of vehicle-treated AREG+/+ mouse ileum (×200); H&E staining (b) and BrdU staining (d) of dmPGE2-treated AREG+/+ mouse ileum (×200). B, AREG+/+ mice (n = 5) (a) or AREG−/− mice (n = 5) (b) were exposed to 12-Gy total body irradiation. Vehicle or 16,16-dimethyl PGE2 (150 μg/kg) was administered twice daily via oral gavage. AREG+/+ mouse ileum was collected 78 h after irradiation, and AREG−/− mouse ileum was harvested 96 h after irradiation. The percentage of regenerative crypts was determined as described in Materials and Methods. Plotted is the mean ± sd of the results from five animals. *, P < 0.05. AREG+/+ mice (n = 5) (c) or AREG−/− mice (n = 5) (d) were exposed to 12-Gy total body irradiation. Vehicle or 16,16-dimethyl PGE2 (120 μg/kg) was ip injected 1 h before irradiation. AREG+/+ mouse ileum was collected 78 h after irradiation and AREG−/− mouse ileum was harvested 96 h after irradiation. The percentage of regenerative crypts was determined as described in Materials and Methods. Plotted is the mean ± sd of the results from five animals. *, P < 0.05. Results shown are representative of two independent experiments.
To determine whether AREG was involved in PGE2 proregenerative action in radiation-injured intestine, a similar experiment was conducted in AREG−/− mice. It was noted that intestinal regeneration was significantly slow and weak in AREG−/− mice than that in AREG+/+ mice. Therefore, the effects of PGE2 on intestinal epithelial regeneration was determined at 78 h after irradiation in AREG+/+ mice and at 96 h in AREG−/− mice. Although PGE2 exerted strong growth-stimulatory effects on AREG+/+ mouse intestine, interestingly, administration of dmPGE2 did not improve intestinal epithelial regeneration in AREG−/− mice (Fig. 2B, compare a with b), suggesting that AREG was a critical mediator for PGE2 growth-stimulatory actions in intestinal regeneration. Next, we elucidated the involvement of AREG in PGE2 radio-protective actions. AREG+/+ and AREG−/− mice were injected with dmPGE2 1 h before total body irradiation. Intestinal regenerative activity was analyzed 78 or 96 h after the irradiation. PGE2 significantly protected the intestinal mucosa from radiation injury in both AREG+/+ and AREG−/− mice, improving the regeneration of the intestinal mucosa at similar degree (Fig. 2B, c and d), suggesting that AREG was not necessary in PGE2 radio-protective actions.
PGE2 has been shown to induce AREG expression in vitro. To determine whether PGE2 induced AREG in vivo, dmPGE2 (120 μg/kg) was administered by ip injection to AREG+/+ mice. RNA was extracted from intestinal mucosa 2, 6, and 24 h after dmPGE2 injection. Although AREG mRNA was low in control intestinal mucosa, administration of dmPGE2 increased the expression of AREG up to approximately 45-fold (Fig. 3). Similar results were observed when dmPGE2 (150 μg/kg) was administered via oral gavage. dmPGE2 treatment increased levels of AREG mRNA 7- to 10-fold in AREG+/+ mouse intestinal mucosa.
Figure 3.
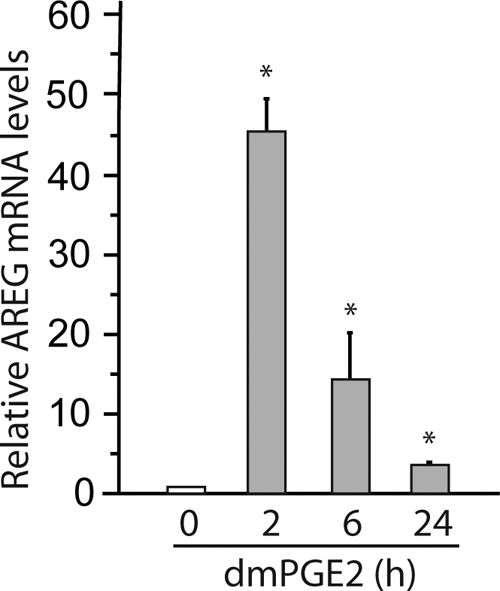
PGE2 induction of AREG expression in vivo. dmPGE2 (120 μg/kg) was administered to AREG+/+ mice via ip injection (n = 3). RNA was extracted from intestinal mucosa 2, 6, and 24 h after dmPGE2 injection. AREG mRNA levels were determined by RT-qPCR and expressed as fold to levels in mice without dmPGE2 (0 h). *, P < 0.05.
Localization of AREG in the small intestine
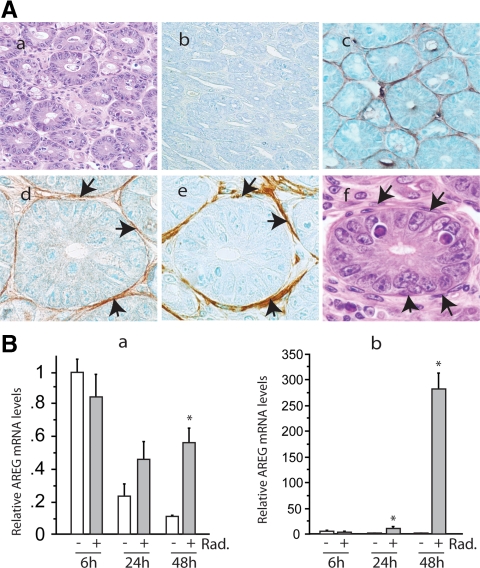
To understand the mechanism whereby AREG promoted intestinal regeneration, we next determined the localization of AREG using immunohistochemistry. Mouse ileum that was collected 48 h after irradiation was sectioned horizontally, so that cross-sections of crypts were displayed (Fig. 4A, a). The circular crypt was formed by a single layer of epithelial cells and separated by stromal compartment. As expected, the expression of AREG was not detected in AREG−/− mouse intestine (Fig. 4A, b). In AREG+/+ mouse intestine, AREG immunoreactivity was not clearly detected in crypt epithelial cells, but was concentrated on a thin layer of stromal cells that closely surround the intestinal epithelial cells of the crypt (Fig. 4A, c and d). Further studies demonstrated that the AREG-expressing cells were α-SMA positive (Fig. 4A, e) and desmin negative (data not shown). Based on their fibroblastic morphology, subepithelial location, and immunohistochemical characteristics, these AREG positive, pericryptal cells were determined as ISEMF (Fig. 4A, f).
Figure 4.
Localization of AREG in mouse intestine. A, AREG+/+ or AREG−/− mice were exposed to 12-Gy total body irradiation (Rad.). The ileum was fixed in 10% formalin 48 h after the irradiation and then sectioned horizontally. Immunostaining for AREG and α-SMA was performed as described in Materials and Methods. H&E staining of AREG+/+ mouse ileum (×400) (a); AREG staining of AREG−/− mouse ileum (×400) (b); AREG staining of AREG+/+ mouse ileum (×400) (c); AREG staining of AREG+/+ mouse ileum (×1000) (d); α-SMA staining of AREG+/+ mouse ileum (×1000) (e); H&E staining of AREG+/+ mouse ileum (×1000) (f). B, RIE (a) or ISEMF (b) cells received 6-Gy irradiation, and RNA was extracted at the indicated time points. Levels of AREG mRNA were determined by real-time RT-PCR and expressed as fold to levels in cells treated by vehicle for 6 h. *, P < 0.05.
To support our in vivo observations, we compared the expression of AREG in established intestinal epithelial cell line, RIE, and ISEMF cell line, 18Co. RIE cells were sensitive to radiation; 6-Gy irradiation resulted in strong apoptosis. In contrast, 6-Gy irradiation did not cause notable cell death in 18Co cells. When grown in serum-free medium, the expression of AREG decreased in RIE cells, and exposure to 6-Gy irradiation slightly increased the relative levels of AREG mRNA (Fig. 4B, a). However, the expression of AREG in 18Co cells increased approximately 10-fold by 24 h after the irradiation. An approximately 270-fold induction of AREG mRNA expression was observed 48 h after the irradiation (Fig. 4B, b).
Myofibroblasts stimulated the growth of intestinal epithelial cells in vitro
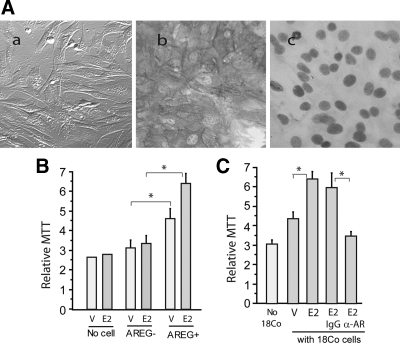
Our in vivo data have demonstrated the critical roles of AREG in intestinal regeneration. ISEMF was the predominant cell type that expressed AREG. To further elucidate the functions of ISEMF-generated AREG in intestinal epithelial growth, we established primary cultures of ISEMF from AREG+/+ and AREG−/− mouse intestine. AREG+/+ and AREG−/− ISEMF cultures were characterized on the basis of their morphology, positive immunoreactivity for α-SMA, and negative immunoreactivity for desmin (Fig. 5A). A coculture system was employed to determine the growth-stimulatory effects of ISEMF-generated AREG. In the system, nontransformed intestinal epithelial cells, RIE, were placed on the membrane of 0.4 μm Transwell (upper chamber), and ISEMF were grown on 24-well plate (bottom chamber). The Transwell with RIE cells was then inserted into the 24-well plate containing ISEMF. The growth of RIE cells was evaluated by MTT assay. Coculture with primary AREG−/− ISEMF did not appear to alter the growth of RIE cells (Fig. 5 B). Treatment with PGE2 did not stimulate RIE cell growth in the presence of AREG−/− ISEMF or without cells in the bottom well. In contrast, the presence of AREG+/+ ISEMF significantly promoted RIE cell growth. Addition of PGE2 further stimulated the growth of RIE cells. To confirm the critical roles of AREG in ISEMF growth-stimulatory action, the human ISEMF cell line 18Co was grown in the bottom well of the coculture system. The presence of 18Co cells too stimulated the growth of RIE cells, PGE2 further increased the growth-stimulatory effects of 18Co cells. However, addition of antihuman AREG neutralizing antibody almost abolished the trophic action of 18Co cells (Fig. 5C). These results suggest that ISEMF-produced AREG was able to stimulate the growth of intestinal epithelial cells in their immediate environment and that AREG was involved in PGE2 trophic effects on intestinal epithelial growth.
Figure 5.
Growth-stimulatory action of ISEMF-generated AREG. A, Establishment of primary AREG+/+ and AREG−/− mouse ISEMF cultures. Primary ISEMF cultures that contain either wild-type AREG gene (AREG+/+) or inactive AREG allele (AREG−/−) were established from AREG+/+ and AREG−/− mouse intestine using the outgrowth method described in Materials and Methods. Morphology of primary ISEMF (a); positive immunoreactivity of α-SMA (b) and negative of desmin in primary ISEMF (c). B, Trophic effects of ISEMF on intestinal epithelial cells. RIE cells were placed in Transwell chambers (0.4 μm), which were then inserted into 24-well plate containing no cells (no cell), AREG+/+ ISEMF (AREG+), or AREG−/− ISEMF (AREG+) in the presence of vehicle (V) or 1 μm PGE2 (E2). RIE/ISEMF cells were cocultured for 48 h. MTT was added into upper chamber and incubated for 2 h. Transwell membrane was removed from the chamber and placed in MTT solvent. After the converted dye was completely dissolved, absorbance of the converted dye was measured at a wavelength of 570 nm with background subtraction at 650 nm using an aliquot of the MTT solvent. Plotted is the mean ± sd of relative values of MTT conversion performed in quadruplicate. *, P < 0.05. All MTT assays were repeated at least three times. C, Trophic effects of ISEMF-generated AREG on intestinal epithelial cells. RIE cells were cocultured with 18Co cells in the presence of vehicle, PGE2, PGE2 plus control IgG (E2-IgG), or PGE2 plus 10 μg/ml AREG neutralizing antibody (E2-α-AREG) as described in B. RIE/18Co cells were cocultured for 48 h. MTT conversion was determined. Plotted is the mean ± sd of relative values of MTT conversion performed in quadruplicate. *, P < 0.05.
Induction of AREG in myofibroblasts by cAMP
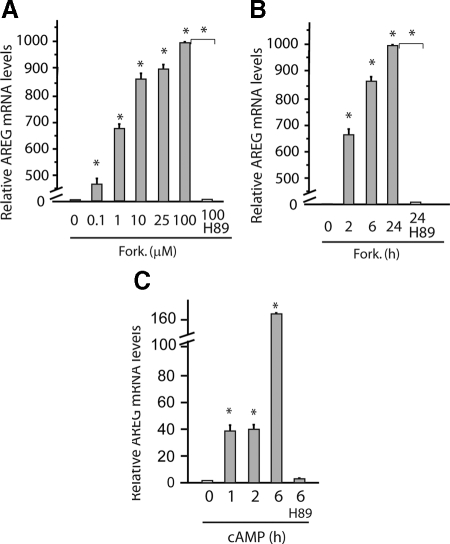
The ultimate goal of our studies is to develop therapeutic strategies by targeting ISEMF and their derived growth factors. Thus, understanding of the molecular mechanisms governing the expression of AREG in ISEMF was critical. AREG is regulated by the cAMP/PKA pathway in a number of cell types (14,36). To determine the involvement of the cAMP/PKA pathway in AREG expression in ISEMF, known cAMP inducers, forskolin, and 8-CPT-cAMP were applied to 18Co cells. Forskolin induced AREG expression at low concentration. At 100 μm, forskolin increased AREG mRNA levels by approximately 1000-fold in 18Co cells (Fig. 6A). The stimulatory effects of forskolin remained strong after 24 h. However, addition of a selective PKA inhibitor H89 completely attenuated the stimulatory effects of forskolin in 18Co cells (Fig. 6B). As expected, addition of 8-CPT-cAMP too increased the levels of AREG mRNA in 18Co cells (Fig. 6C).
Figure 6.
Regulation of AREG expression by the cAMP/PKA pathway. A, Forskolin (Fork.) induction of AREG in ISEMF. 18Co cells were grown in serum-free medium for 24 h. Forskolin at indicated concentrations was added. H89 (10 μm) was applied 15 min before 100 μm forskolin (H89). RNA was extracted 2 h after forskolin engagement. Levels of AREG were determined by RT-qPCR and expressed as fold to levels in cells without forskolin (0 μm). *, P < 0.05. B, 18Co cells were grown in serum-free medium for 24 h and then treated with 25 μm forskolin. H89 (10 μm) was applied 15 min before forskolin (H89). RNA was extracted at indicated time points after forskolin engagement. Levels of AREG were determined by RT-qPCR and expressed as fold to levels in control cells (0 h). *, P < 0.05. C, 18Co cells were grown in serum-free medium for 24 h and then treated with 300 μm 8-CPT-cAMP. H89 (10 μm) was applied 15 min before forskolin (H89). RNA was extracted at indicated time points after forskolin engagement. Levels of AREG were determined by RT-qPCR and expressed as fold to levels in control cells (0 h). *, P < 0.05.
Discussion
Properly maintaining the integrity and function of the intestinal tract is important for many patients with digestive diseases. The present report demonstrated that AREG was critical for intestinal epithelial regeneration after radiation injury. Although intestinal epithelium rapidly recovered from radiation injury in wild-type mice, genetically disruption of AREG significantly impaired intestinal epithelial regeneration, suggesting that the presence of functional AREG was essential for intestinal epithelial recovery from radiation injury. Although AREG is a member of the EGF family, functional roles of AREG in cell proliferation are controversial (37). AREG stimulates the proliferation of most cell types; however, it suppresses the growth of other cell lines. Based on the observations made in the AREG knockout mouse model, AREG deficiency is associated with impaired mammary gland development and/or function, and AREG is required during ductal morphogenesis in the mammary gland (29). Further studies have demonstrated that AREG is an early-response growth factor that contributes to the initial phase of liver regeneration (15). AREG is rapidly induced after partial hepatectomy in mice, and proliferative response of the liver is impaired in AREG knockout mice. In line with these findings, our data suggest that AREG was an essential growth factor for intestinal regeneration after irradiation. Disruption of functional AREG significantly impaired the regeneration of radiation-injured intestine. Based on the number and size of regenerative crypts, the regenerative activity was reduced by approximately 70% in AREG−/− mouse intestine compared with that in AREG+/+ mouse intestine.
Intestinal regeneration after irradiation depends on the number of surviving stem cells and proliferation rate of transit cells. PGE2 exerts both radio-protective and growth-stimulatory effects on intestinal epithelium (18,19,20,21,22,23,24). It was interesting to determine whether AREG is involved in these actions of PGE2. Our results showed that AREG was a crucial mediator for PGE2 growth-stimulatory actions in regenerative intestinal epithelium, because administration of PGE2 after irradiation significantly improved intestinal regeneration in wild-type mice. However, had no effect on AREG−/− mouse intestine. On the other hand, we demonstrated that AREG was not necessary for PGE2 radio-protective effects, because PGE2 was able to protect intestinal mucosa from radiation injury in both wild-type mice and AREG null mice. Numerous hypotheses have been presented to address the molecular mechanisms governing PGE2 trophic actions in the gut (25,38). Our animal studies strongly supported the hypothesis that PGE2 stimulates intestinal epithelial growth via induction of AREG.
The present study demonstrated the localization of AREG expression in the intestine. Immunohistochemical studies revealed that AREG immunoreactivity was concentrated on a thin layer of pericryptal cells. Based on their morphology, localization, and positive immunoreactivity to α-SMA and negative immunostaining for desmin, we concluded that ISEMF was the primary cell type that produced AREG in normal intestinal epithelium. In support of our in vivo observation, cell culture studies demonstrated that AREG was barely expressed in nontransformed intestinal epithelial cells, which was not significantly changed by irradiation or PGE2 stimulation. In contrast, irradiation and PGE2 robustly increased levels of AREG in 18Co ISEMF cells. ISEMF are located in the lamina propria throughout the gastrointestinal tract and play crucial roles in intestinal organogenesis, proliferation and differentiation of intestinal epithelial cells, mucosal protection, and wound healing (39). ISEMF act through secretion of growth factors and cytokines, regulating the microenvironment in the intestinal epithelium (40). Previous study has demonstrated that the EGFR is located on the basal-lateral membrane of intestinal epithelial cells (41). Thus, our results suggest that upon activation by extracellular stimulus, ISEMF may rapidly produce AREG into the immediate microenvironment and stimulate intestinal epithelial cell proliferation via binding to the EGFR.
The ultimate goal of our studies is to develop therapeutic strategies by targeting ISEMF and their derived growth factors. Based on our results, the cAMP/PKA pathway is a potential target. Increase in the activity of cAMP/PKA signaling may stimulate the production of AREG in ISEMF and result in growth stimulation of the intestinal epithelium. It has been known for some time that PGE2 exerts trophic effects on intestinal epithelium in a number of animal studies. PGE2 signals via specific transmembrane G protein-coupled receptors (EP1, EP2, EP3, and EP4). EP2 and EP4 receptors are coupled to stimulatory G proteins, stimulating cAMP/PKA activity and inducing AREG expression (14). A large body of studies provides the evidence that PGE2 trophic signaling involves trans-activation of the EGFR signaling system. We have presented a ligand-dependent mechanism, whereby PGE2 induces autocrine and paracrine AREG, which, in turn, activates the EGFR signaling system and stimulates intestinal epithelial growth (14,27). Although PGE2 strongly improves intestinal regeneration, PGE2 exerts a number of complex effects to the animals systematically and locally. It is known that PGE2 is related to immunosuppression. Administration of PGE2 often results in severe diarrhea in animals. These “side effects” of PGE2 limit its clinical use particularly in critically ill patients. It is of importance to identify additional compounds that stimulate AREG in ISEMF and may be suitable for clinical use. We have demonstrated that AREG was regulated by intracellular levels of cAMP (14,28). Reagents that increase cAMP levels may be tested for their ability to induce AREG in ISEMF. Forskolin has been used as dietary supplement without major side effects (42,43). Various experimental studies are underway in using forskolin as an adjuvant in treatment for nerve damage caused by trauma/accident. Our studies determined that forskolin was able to robustly induce AREG expression in ISEMF. Additional experiments are required to compare forskolin with PGE2 functionally and mechanistically in intestinal epithelial regeneration in vivo.
In summary, our studies demonstrated the important roles of ISEMF in providing the microenvironment for intestinal epithelial regeneration. Upon extracellular stimulus, ISEMF produces a variety of growth factors, including AREG, which stimulates the growth of intestinal epithelium via a paracrine pathway. Our findings in AREG null mice suggest that AREG is a critical mediator for PGE2 trophic effects in the gut. We further determined that the expression of AREG in ISEMF was strongly regulated by the cAMP/PKA pathway. These findings provide potential targets for regulating intestinal epithelial growth at levels of cell type, signaling pathway, and molecular biology. Modulating AREG levels by targeting ISEMF represents a novel therapeutic strategy for certain intestinal disorders.
Footnotes
This work was supported by the National Institutes of Health Grants DK065615 and DK086558 (to H.S.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 9, 2010
Abbreviations: AREG, Amphiregulin; BrdU, 5-bromodeoxyuridine; 8-CPT-cAMP, 8-(4-chlorophenylthio)adenosine 3’, 5’-cyclic monophosphate; CT, threshold cycle; dmPGE2, 16,16-dimethyl prostaglandin E2; EGF, epidermal growth factor; EGFR, EGF receptor; H&E, hematoxylin/eosin; ISEMF, intestinal subepithelial myofibroblasts; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; PKA, protein kinase A; RT-qPCR, quantitative real-time RT-PCR; α-SMA, α-smooth muscle actin.
References
- Babyatsky MW, Podolsky DK 1991 Growth and development in the gastrointestinal tract. In: Yamada T, Alpers DH, Owyang C, Powell DW, Silverstein FE, eds. Textbook of gastroenterology. Philadelphia: J.B. Lippincott Co.; 475–501 [Google Scholar]
- Lowry SF 1990 The route of feeding influences injury responses. J Trauma 30:S10–S15 [PubMed] [Google Scholar]
- Steinmetz OK, Meakins JL 1991 Care of the gut in the surgical intensive care unit. Fact or fashion? Can J Surg 34:207–215 [PubMed] [Google Scholar]
- Ray EC, Avissar NE, Sax HC 2002 Growth factor regulation of enterocyte nutrient transport during intestinal adaptation. A J Surg 183:361–371 [DOI] [PubMed] [Google Scholar]
- Berg RD 1980 Inhibition of Escherichia coli translocation from the gastrointestinal tract by normal cecal flora in gnotobiotic or antibiotic-decontaminated mice. Infect Immun 29:1073–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky DK 1993 Regulation of intestinal epithelial proliferation: a few answers, many questions. Am J Physiol 264:G179–G186 [DOI] [PubMed] [Google Scholar]
- Jones MK, Tomikawa M, Mohajer B, Tarnawski AS 1999 Gastrointestinal mucosal regeneration: role of growth factors. Front Biosci 4:D303–D309 [DOI] [PubMed] [Google Scholar]
- Moghal N, Sternberg PW 1999 Multiple positive and negative regulators of signaling by the EGF-receptor. Curr Opin Cell Biol 11:190–196 [DOI] [PubMed] [Google Scholar]
- McKenna KJ, Ligato S, Kauffman Jr GL, Abt AB, Stryker JA, Conter RL 1994 Epidermal growth factor enhances intestinal mitotic activity and DNA content after acute abdominal radiation. Surgery 115:626–632 [PubMed] [Google Scholar]
- Chaet MS, Arya G, Ziegler MM, Warner BW 1994 Epidermal growth factor enhances intestinal adaptation after massive small bowel resection. J Pediatr Surg 29:1035–1038; discussion 1038–1039 [DOI] [PubMed] [Google Scholar]
- Rubin DC, Levin MS 1998 Intestinal adaptation: molecular analyses of a complex process. Gastroenterology 115:1291–1294 [DOI] [PubMed] [Google Scholar]
- Shin CE, Helmrath MA, Falcone Jr RA, Fox JW, Duane KR, Erwin CR, Warner BW 1998 Epidermal growth factor augments adaptation following small bowel resection: optimal dosage, route, and timing of administration. J Surg Res 77:11–16 [DOI] [PubMed] [Google Scholar]
- Ciardiello F, Kim N, Saeki T, Dono R, Persico MG, Plowman GD, Garrigues J, Radke S, Todaro GJ, Salomon DS 1991 Differential expression of epidermal growth factor-related proteins in human colorectal tumors. Proc Natl Acad Sci USA 88:7792–7796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J, Lee SB, Guo H, Evers BM, Sheng H 2003 Prostaglandin E2 stimulates the growth of colon cancer cells via induction of amphiregulin. Cancer Res 63:5218–5123 [PubMed] [Google Scholar]
- Berasain C, García-Trevijano ER, Castillo J, Erroba E, Lee DC, Prieto J, Avila MA 2005 Amphiregulin: an early trigger of liver regeneration in mice. Gastroenterology 128:424–432 [DOI] [PubMed] [Google Scholar]
- Chang SH, Ai Y, Breyer RM, Lane TF, Hla T 2005 The prostaglandin E2 receptor EP2 is required for cyclooxygenase 2-mediated mammary hyperplasia. Cancer Res 65:4496–4499 [DOI] [PubMed] [Google Scholar]
- Inatomi O, Andoh A, Yagi Y, Bamba S, Tsujikawa T, Fujiyama Y 2006 Regulation of amphiregulin and epiregulin expression in human colonic subepithelial myofibroblasts. Int J Mol Med 18:497–503 [PubMed] [Google Scholar]
- Dembinski A, Konturek SJ 1985 Effects of E, F, and I series prostaglandins and analogues on growth of gastroduodenal mucosa and pancreas. Am J Physiol 248:G170–G175 [DOI] [PubMed] [Google Scholar]
- Johansson C, Bergström S 1982 Prostaglandin and protection of the gastroduodenal mucosa. Scand J Gastroenterol Suppl 77:21–46 [PubMed] [Google Scholar]
- Reinhart WH, Müller O, Halter F 1983 Influence of long-term 16,16-dimethyl prostaglandin E2 treatment on the rat gastrointestinal mucosa. Gastroenterology 85:1003–1010 [PubMed] [Google Scholar]
- Cohn SM, Schloemann S, Tessner T, Seibert K, Stenson WF 1997 Crypt stem cell survival in the mouse intestinal epithelium is regulated by prostaglandins synthesized through cyclooxygenase-1. J Clin Invest 99:1367–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchen CW, Stenson WF, Cohn SM 2000 Disruption of cyclooxygenase-1 gene results in an impaired response to radiation injury. Am J Physiol Gastrointest Liver Physiol 279:G858–G865 [DOI] [PubMed] [Google Scholar]
- Houchen CW, Sturmoski MA, Anant S, Breyer RM, Stenson WF 2003 Prosurvival and antiapoptotic effects of PGE2 in radiation injury are mediated by EP2 receptor in intestine. Am J Physiol Gastrointest Liver Physiol 284:G490–G498 [DOI] [PubMed] [Google Scholar]
- Tessner TG, Muhale F, Riehl TE, Anant S, Stenson WF 2004 Prostaglandin E2 reduces radiation-induced epithelial apoptosis through a mechanism involving AKT activation and bax translocation. J Clin Invest 114:1676–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS 2002 Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med 8:289–293 [DOI] [PubMed] [Google Scholar]
- Buchanan FG, Wang D, Bargiacchi F, DuBois RN 2003 Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem 278:35451–35457 [DOI] [PubMed] [Google Scholar]
- Shao J, Evers BM, Sheng H 2004 Prostaglandin E2 synergistically enhances receptor tyrosine kinase-dependent signaling system in colon cancer cells. J Biol Chem 279:14287–14293 [DOI] [PubMed] [Google Scholar]
- Shao J, Sheng GG, Mifflin RC, Powell DW, Sheng H 2006 Roles of myofibroblasts in prostaglandin E2-stimulated intestinal epithelial proliferation and angiogenesis. Cancer Res 66:846–855 [DOI] [PubMed] [Google Scholar]
- Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, Lee DC 1999 Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development 126:2739–2750 [DOI] [PubMed] [Google Scholar]
- Potten CS, Loeffler M 1990 Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 110:1001–1020 [DOI] [PubMed] [Google Scholar]
- Potten CS 1990 A comprehensive study of the radiobiological response of the murine (BDF1) small intestine. Int J Radiat Biol 58:925–973 [DOI] [PubMed] [Google Scholar]
- Riehl T, Cohn S, Tessner T, Schloemann S, Stenson WF 2000 Lipopolysaccharide is radioprotective in the mouse intestine through a prostaglandin-mediated mechanism. Gastroenterology 118:1106– 1116 [DOI] [PubMed] [Google Scholar]
- Mahida YR, Beltinger J, Makh S, Göke M, Gray T, Podolsky DK, Hawkey CJ 1997 Adult human colonic subepithelial myofibroblasts express extracellular matrix proteins and cyclooxygenase-1 and -2. Am J Physiol 273:G1341–G1348 [DOI] [PubMed] [Google Scholar]
- Shao J, Sheng H 2007 Prostaglandin E2 induces the expression of IL-1α in colon cancer cells. J Immunol 178:4097–4103 [DOI] [PubMed] [Google Scholar]
- Helton WS, Garcia R 1993 Oral prostaglandin E2 prevents gut atrophy during intravenous feeding but not bacterial translocation. Arch Surg 128:178–183; discussion 83–84 [DOI] [PubMed] [Google Scholar]
- Johansson CC, Yndestad A, Enserink JM, Ree AH, Aukrust P, Taskén K 2004 The epidermal growth factor-like growth factor amphiregulin is strongly induced by the adenosine 3′,5′-monophosphate pathway in various cell types. Endocrinology 145:5177–5184 [DOI] [PubMed] [Google Scholar]
- Shoyab M, McDonald VL, Bradley JG, Todaro GJ 1988 Amphiregulin: a bifunctional growth-modulating glycoprotein produced by the phorbol 12-myristate 13-acetate-treated human breast adenocarcinoma cell line MCF-7. Proc Natl Acad Sci USA 85:6528–6532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H 2006 Wnt/β-catenin signaling in development and disease. Cell 127:469–480 [DOI] [PubMed] [Google Scholar]
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB 1999 Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol 277:C1–C9 [DOI] [PubMed] [Google Scholar]
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB 1999 Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol 277:C183–C201 [DOI] [PubMed] [Google Scholar]
- Dempsey PJ, Coffey RJ 1994 Basolateral targeting and efficient consumption of transforming growth factor-α when expressed in Madin-Darby canine kidney cells. J Biol Chem 269:16878- 16889 [PubMed] [Google Scholar]
- Ammon HP, Müller AB 1985 Forskolin: from an ayurvedic remedy to a modern agent. Planta Med 51:473–477 [DOI] [PubMed] [Google Scholar]
- Bauer K, Dietersdorfer F, Sertl K, Kaik B, Kaik G 1993 Pharmacodynamic effects of inhaled dry powder formulations of fenoterol and colforsin in asthma. Clin Pharmacol Ther 53:76–83 [DOI] [PubMed] [Google Scholar]