Abstract
Copper (Cu), iron (Fe), and iodine/thyroid hormone (TH) deficiencies lead to similar defects in late brain development, suggesting that these micronutrient deficiencies share a common mechanism contributing to the observed derangements. Previous studies in rodents (postweanling and adult) and humans (adolescent and adult) indicate that Cu and Fe deficiencies affect the hypothalamic-pituitary-thyroid axis, leading to altered TH status. Importantly, however, relationships between Fe and Cu deficiencies and thyroidal status have not been assessed in the most vulnerable population, the developing fetus/neonate. We hypothesized that Cu and Fe deficiencies reduce circulating and brain TH levels during development, contributing to the defects in brain development associated with these deficiencies. To test this hypothesis, pregnant rat dams were rendered Cu deficient (CuD), FeD, or TH deficient from early gestation through weaning. Serum thyroxine (T4) and triiodothyronine (T3), and brain T3 levels, were subsequently measured in postnatal d 12 (P12) pups. Cu deficiency reduced serum total T3 by 48%, serum total T4 by 21%, and whole-brain T3 by 10% at P12. Fe deficiency reduced serum total T3 by 43%, serum total T4 by 67%, and whole-brain T3 by 25% at P12. Brain mRNA analysis revealed that expression of several TH-responsive genes were altered in CuD or FeD neonates, suggesting that reduced TH concentrations were sensed by the FeD and CuD neonatal brain. These results indicate that at least some of the brain defects associated with neonatal Fe and Cu deficiencies are mediated through reductions in circulating and brain TH levels.
Some of the brain defects associated with neonatal iron and copper deficiencies are mediated through reductions in circulating and brain thyroid hormone levels.
Dietary micronutrient deficiencies are a significant health problem throughout the world, especially in developing countries. The 2008 Copenhagen Consensus Conference ranked micronutrient deficiency issues as two of the top three global challenges that if solved, would most benefit global welfare (1). The World Health Organization estimates that 2 billion people worldwide, including 31.5% of school-age children, have insufficient iodine intake (2). Iodine deficiency impairs thyroid hormone (TH) synthesis and results in a spectrum of disorders, including goiter, cretinism, cognitive impairment, and growth retardation. The World Health Organization also estimates that over 1.6 billion people suffer from anemia or iron (Fe) deficiency, including over 500 million women of childbearing age (3). Fe deficiency anemia (IDA) results in cognitive impairment, growth retardation, impaired immune responses, and poor temperature regulation. Copper (Cu) deficiency is less prevalent than Fe or iodine deficiencies but may contribute to the millions of people suffering from non-IDA (3). Due to a lack of nutrient diversity in diets in developing countries, millions of people are deficient in not just one but multiple micronutrients (4). In fact, an estimated 20–44% of iodine-deficient goitrous children in North and West Africa also suffer from IDA (5,6,7).
The most striking effect of imbalances in micronutrients such as Cu, Fe, and iodine/TH is compromised human intellectual outcome. Interestingly, Cu, Fe, and iodine/TH deficiencies result in similar defects in rodent brain development, including hypomyelination of axons, aberrant hippocampal structure and function, altered brain energy metabolism, and altered neuronal signaling (8,9,10,11,12,13). In addition, the behavioral and neurochemical abnormalities associated with perinatal Cu, Fe, and iodine/TH deficiencies are irreversible and persist into adulthood (14,15,16). These similarities suggest that there may be a common underlying mechanism associated with all three deficiencies contributing to the observed neurodevelopmental defects.
Several studies in postweanling rodents show that Cu and Fe deficiencies impair thyroid metabolism. Fe deficiency reduces circulating thyroxine (T4) and triiodothyronine (T3) concentrations (17,18,19,20), peripheral conversion of T4 to T3 (18,19), TSH response to TRH (19), and thyroid peroxidase (TPO) activity (20). Cu deficiency also reduces circulating T4 and T3 concentrations and peripheral conversion of T4 to T3 (21,22). In addition, Cu deficiency reduces serum and brain Fe levels, which may contribute to the Cu-dependent effect on thyroidal status (23).
Importantly, however, the effects of reduced Fe or Cu levels on the thyroidal status of developing mammals during the critical perinatal period of late brain development have not been assessed. Based on the previous human and rodent studies, we hypothesized that Cu and Fe deficiencies during perinatal development will reduce circulating and brain TH levels. We further hypothesized that the associated reductions in brain TH content will affect TH-dependent gene expression and contribute to the derangements in brain development observed in Cu-deficient (CuD) and Fe-deficient (FeD) animals. In this study, we show, for the first time, that both Cu and Fe deficiencies reduce serum total T4 and T3 concentrations in neonatal rat pups. Fe deficiency also reduced neonatal brain T3 content, with associated changes in whole-brain mRNA levels of some TH-responsive genes.
Materials and Methods
Animals and diets
Twenty sperm-positive Sprague Dawley female rats were purchased from Charles River Laboratories (Wilmington, MA). At gestational d (E)2, five sperm-positive rats were randomly assigned to one of four groups: control, CuD, FeD, and 10 mg/liter 6-propyl-2-thiouracil (PTU) (Sigma-Aldrich, St. Louis, MO) treatment. Beginning at E2, CuD and FeD dams were fed a semipurified diet (Harlan Laboratories, Madison, WI) deficient in Cu or Fe, respectively (Supplemental Tables 1 and 2, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). Control and PTU dams were fed a Cu and Fe adequate control diet (Supplemental Tables 1 and 2). The rodent diets were based on the AIN-93G formulation but modified by the investigators to increase Cu, Fe, calcium, and phosphorous levels to better support reproduction according to the 1995 National Research Council guidelines for rats (Supplemental Table 1). Dams in the control, CuD, and FeD groups drank deionized water. Beginning at E6, PTU dams were offered deionized water containing 10 mg/liter PTU. The PTU stock solution was stored in the dark and administered to dams in glass amber bottles. Day of birth was designated as postnatal d (P)0, and at P2, all litters were culled to 10 pups per dam. At P12, two male pups from each litter were killed to evaluate several Cu and Fe biomarkers and T4 and T3 concentrations. Remaining pups were kept with their dams until P24. Dams were killed at P25 to evaluate metal and TH status.
Animals were given free access to diet and drinking water throughout the study and were housed at constant temperature and humidity on a 12-h light, 12-h dark cycle. All animal studies were conducted in accordance with the principles and procedures outlined in the National Institutes of Health guide for the Care and Use of Laboratory Animals. The local Institutional Animal Care and Use Committee approved these procedures.
Sample collection
At P12, two male pups from each litter were killed (10 total per group). From each pup, trunk blood was collected after decapitation and kept on ice until centrifuged to collect serum. Serum was stored at −80 C until analyzed. A 5-μl blood sample was removed to determine hemoglobin concentrations. Hearts were removed and weighed. The whole liver was removed, rinsed with deionized water, and a portion weighed and processed for metal analysis. Brains were removed and bisected at the midline. Five half brains for metal analysis were rinsed with deionized water and weighed. Five half brains for enzyme analysis and five additional half brains for TH analysis were weighed and flash-frozen in liquid nitrogen. Finally, five half brains for mRNA analysis were placed immediately into 5 ml RNAlater RNA stabilization reagent (QIAGEN, Valencia, CA) following the manufacturer’s tissue processing instructions. Tissues in RNAlater reagent were placed at 4 C overnight and then transferred to minus 20 C for storage until RNA extraction. Half brains for metal and enzyme analyses were harvested from the same pup and half brains for TH and mRNA analyses were harvested from the same pup.
Cu, Fe, and zinc analyses
Diet, brains, and liver samples were wet digested with HNO3, and the residue was resuspended in 0.1 n HNO3 and analyzed by flame atomic absorption spectroscopy (AAS) as previously described (24). Brain Fe content was corrected for blood contamination (23). Serum Fe concentrations were determined by flame AAS after hemoglobin was removed (25).
Biochemical analyses
Hemoglobin concentrations were determined spectrophotometrically as metcyanohemoglobin (24). Serum ceruloplasmin activity was measured after oxidation of ο-dianisidine at 37 C (24). Cytochrome c oxidase (CCO) activity was measured spectrophotometrically on fresh brain homogenates by monitoring oxidation of ferrocytochrome c at 550 nm (24). Total protein levels of brain homogenates were determined using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL).
Western blot analyses
Homogenates of P12 rat brains used for CCO assay were diluted with an equal volume of lysis buffer [0.05 m Tris (pH 8.0), 0.15 m NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate] and centrifuged at 10,000 × g for 5 min. Protein content was determined on the supernatant using a modified Lowry method (26). A 25 μg aliquot of protein was subjected to SDS-PAGE on either a 15% gel [subunit IV of CCO (COX IV), Cu chaperone for superoxide dismutase (CCS), or lactate dehyrogenase, (LDH)] or a 8% gel [transferrin receptor 1 (TfR)]. Description of the transfer, reagents used for incubation, antibodies used, and techniques of detection are described elsewhere (27). LDH protein levels were assessed for both the 15 and 8% gels as loading controls. Densitometry measurements were performed for each band using a Kodak Image Station 2000M and Molecular Imaging Software (Kodak, New Haven, CT). Each value was normalized to the average of the control values.
Serum and brain TH measurements
Serum total T4 and T3 concentrations were measured using commercially available RIA kits (Siemens Medical Solutions Diagnostics, Los Angeles, CA). The manufacturer’s RIA procedure was followed except in-house T3 and T4 calibrators prepared in hormone-stripped rat serum were used. Stripped rat serum was prepared by incubating 100 ml normal rat serum (Millipore, Bedford, MA) spiked with 3 μCi 125I-T4, with 5 g Dowex 1 × 8, 50–100 mesh, ion-exchange resin (Sigma-Aldrich) overnight at 4 C. Serum was separated from the resin by filtration, and TH removal was assessed by monitoring γ count elimination.
THs were extracted from half brains using a modification of the method described by Morreale de Escobar et al. (28). Tissue was homogenized in 4 ml methanol containing 1 mm PTU (methanol-PTU) per gram tissue with a Potter-Elvehjem homogenizer at approximately 1500 rpm for 10 strokes. Homogenates were transferred to a disposable 50 ml polypropylene screw-cap tube, and 100 μl 125I-T4 tracer (0.02 pg/μl in methanol-PTU) was added to each sample to assess individual sample percent recoveries. Chloroform was added at double the volume of methanol-PTU, and samples were mixed by vortexing. The mixture was centrifuged at 2000 rpm for 15 min, and the liquid was transferred to a clean 50 ml tube with a Pasteur pipet. During this first extraction, the cell particulates generally float on top of the liquid, and thus the tube was tilted to allow penetration of the pipet to the bottom of the tube without disturbing the floating particulate layer. The remaining particulates were subjected to two additional extractions by vortexing in 5 ml chloroform:methanol (2:1) per gram tissue, centrifuging at 2000 rpm for 15 min, and removing and combining the supernatant with the first extract. In the second and third extractions, the particulates form a pellet. To the combined extracts, 1 ml 0.05% CaCl2 was added for every 5 ml of extract. The mixture was vortexed and centrifuged at 2000 rpm for 5 min to separate organic and aqueous layers. The upper aqueous layer, containing THs, was transferred to a clean 50 ml tube. The lower organic layer was reextracted two more times with a volume of pure upper layer (chloroform:methanol:0.05% CaCl2, 3:49:48) equal to the amount of upper layer removed in the previous step. The combined extracted upper layers were subjected to rotary evaporation to remove the remaining chloroform and methanol. The aqueous mixture was transferred to a clean 50 ml tube, shell-frozen using a dry ice/ethanol bath, and evaporated to complete dryness by lyophilization. Each lyophilized sample was resuspended in 500 μl stripped rat serum, and T3 content was measured using the modified serum total T3 RIA kit described above.
Brain mRNA analysis
Total RNA was extracted from half brains using the QIAGEN RNeasy Maxi kit (QIAGEN) according to the manufacturer’s protocol. The optional on-column digestion of DNA with deoxyribonuclease was performed. RNA integrity and purity was established spectrophotometrically and by RNA gels. cDNA was synthesized from 2 μg total RNA using SuperScript III First-Strand Synthesis SuperMix and random hexamers (Invitrogen, Carlsbad, CA) in a reaction volume of 40 μl. For type II deiodinase (Dio2), neurogranin (Rc3), hairless (Hr), parvalbumin (Pvalb), myelin basic protein (Mbp), myelin-associated oligodendrocyte basic protein (Mobp), ferritin heavy polypeptide 1 (Fth1), Cox IV, and glyceraldehyde 3-phosphate dehydrogenase (Gapdh), quantitative real-time PCR (qRT-PCR) was performed using a LightCycler FastStart DNA Master SYBR Green I kit and a Roche LightCycler (Roche Applied Science, Indianapolis, IN) or Corbett RotorGene RG-3000 (QIAGEN). Primer pairs for these genes are described in Supplemental Table 3. PCRs were performed on cDNA equivalent to 80 ng of total RNA according to the manufacturer’s protocol except that a final volume of 10 μl was used. Threshold cycle values were determined in the log-linear amplification phase using the qRT-PCR machine’s software. Relative mRNA levels were calculated relative to an internal standard cDNA sample. Quantitative RT-PCR for brain-derived neurotropic factor isoform IV (Bdnf IV) and early growth response factor 1 (Egr1) was performed on cDNA equivalent to 20 ng of total RNA, as previously described (29).
Statistical analysis
One-way ANOVA was used for making statistical comparisons between treatment groups. Bartlett’s test was used to assess homogeneity of variances. When variances were equal across groups, Tukey’s multiple comparison test was used. When variances were unequal, data were ln transformed, and then Tukey’s multiple comparison test was used. When ln transformation did not normalize the variances, Scheffé’s multiple comparison test was used on the untransformed data. All data are presented as mean ± sem. Statistical analyses and data graphing were carried out using Prism (GraphPad Software, La Jolla, CA) or Kaleidagraph (Synergy Software, Reading, PA) software packages. An α = 0.05 was chosen to define significant differences.
Results
Establishing Cu and Fe deficiencies in rat dams and pups
Cu deficiency was confirmed in CuD dams as measured by significant reductions in liver Cu content and serum ceruloplasmin activity (Table 1). Fe deficiency was confirmed in FeD dams as measured by significant reductions in liver Fe, serum Fe, and hemoglobin levels (Table 1). TH deficiency was confirmed in PTU-treated dams as determined by measured reductions in serum total T4 and T3 (Table 1) and increased thyroid gland weights (data not shown). Serum total T3 concentrations were reduced by 28% in FeD dams, and serum total T4 concentrations were increased by 56% in CuD dams (Table 1). Litter sizes from each treatment group were similar (data not shown).
Table 1.
Copper, iron, and thyroid hormone status of rat dams
| Characteristic | Control | CuD | FeD | PTU |
|---|---|---|---|---|
| Body weight, g | 319 ± 14.2a | 275 ± 10.4a | 317 ± 22.9a | 294 ± 5.8a |
| Hemoglobin, g/liter | 187 ± 3.15a | 174 ± 4.42a | 131 ± 4.97b | 171 ± 5.97a |
| Serum Fe, μg/ml | 4.97 ± 0.94a,c | 3.05 ± 0.06a,b | 1.49 ± 0.22b | 5.63 ± 0.25c |
| Ceruloplasmin, U/liter | 337 ± 23.9a | <0.30b | 393 ± 37.2a | 334 ± 21.8a |
| Liver Fe, μg/g | 184 ± 23.8a | 147 ± 36.2a | 1.88 ± 0.20b | 159 ± 65.5a |
| Liver Cu, μg/g | 3.59 ± 0.10a,c | 1.07 ± 0.26b | 5.41 ± 0.74a | 3.25 ± 0.25c |
| Serum TT4, ng/ml | 31.4 ± 1.64a | 49.9 ± 2.32b | 30.3 ± 3.76a | 6.19 ± 0.77c |
| Serum TT3, ng/dl | 43.5 ± 5.65a | 41.5 ± 8.67a | 31.3 ± 1.91a,b | 14.0 ± 3.31b |
Data are presented as the mean ± sem (n = 5 for control, FeD, and PTU; n = 4 for CuD). Unlike superscripts within a specific row indicate a statistical difference (P < 0.05) by one-way ANOVA and Tukey’s or Scheffe’s multiple comparison test. One CuD dam and her pups were excluded from data analysis because this dam ate several pups, and serum ceruloplasmin activity, liver Cu content, and P12 brain Cu content were significantly higher in this cohort compared to others from the CuD group.
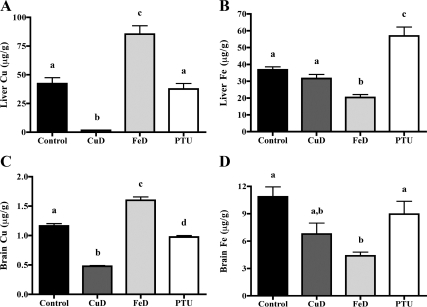
To evaluate Cu and Fe status of P12 pups, several characteristics were measured (Table 2 and Fig. 1). Blood hemoglobin and serum Fe concentrations were decreased in both CuD and FeD pups (Table 2). Serum ceruloplasmin activity, a measure of Cu status, was reduced in CuD pups compared with controls (Table 2). Liver and brain Fe and Cu levels, as expected, were reduced in CuD pups (Fig. 1, A and C), and liver and brain Fe levels were reduced in FeD pups (Fig. 1, B and D). Brain Cu content was higher in FeD pups (Fig. 1C). Together, these data demonstrate that Fe and Cu deficiencies were generated in P12 pups nursing on dams fed nutritionally restricted diets.
Table 2.
Characteristics of P12 rat pups after perinatal copper deficiency, iron deficiency, or PTU treatment
| Characteristic | Control | CuD | FeD | PTU |
|---|---|---|---|---|
| Body weight, g | 31.8 ± 0.92a | 26.9 ± 0.38b | 22.2 ± 0.65c | 26.1 ± 0.31b |
| Heart:body weight, mg/g | 5.31 ± 0.09a,c | 6.50 ± 0.35a | 11.3 ± 0.63b | 4.13 ± 0.09c |
| Brain:body weight, mg/g | 35.4 ± 1.10a | 43.4 ± 1.11b | 44.8 ± 0.80b | 43.8 ± 0.73b |
| Hemoglobin, g/liter | 112 ± 1.55a | 87.0 ± 2.28b | 51.2 ± 1.86c | 133 ± 3.02d |
| Serum Fe, μg/ml | 1.53 ± 0.13a | 0.63 ± 0.05b | 0.42 ± 0.05b | 2.22 ± 0.13c |
| Ceruloplasmin, U/liter | 55.4 ± 6.63a | <0.3b | 40.3 ± 3.57a | 52.8 ± 1.97a |
Data are presented as the mean ± sem (n = 4–10). Unlike superscripts within a specific row indicate a statistical difference (P < 0.05) by one-way ANOVA and Tukey’s or Scheffe’s multiple comparison test.
Figure 1.
Cu and Fe content of P12 pup brains and livers. A, Liver Cu content. B, Liver Fe content. C, Brain Cu content. D, Brain Fe content. Cu and Fe content was measured in livers and half brains from P12 male pups (n = 5 for control, FeD, and PTU; n = 4 for CuD; for liver Fe measurement n = 4 for PTU) by flame AAS. Data are presented as the mean ± sem. Bars with unlike letters indicate a statistical difference (P < 0.05) by one-way ANOVA and Tukey’s or Scheffé’s multiple comparison test.
Effects of Cu and Fe deficiency on thyroidal status
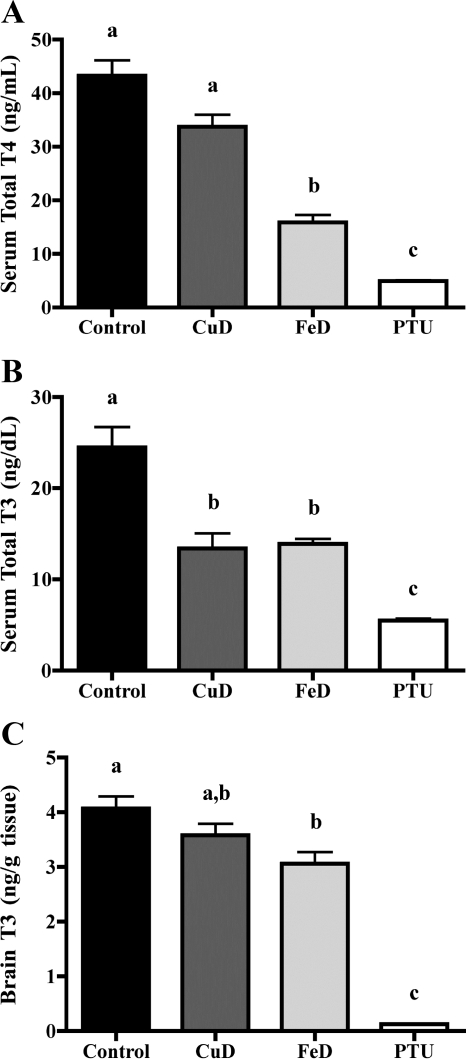
To determine whether gestational and lactational Fe or Cu deficiency affect neonatal thyroidal status, serum total T4 and T3 were measured (Fig. 2). Both serum total T4 and T3 were decreased in PTU pups, indicating that these animals are TH deficient (Fig. 2, A and B). Interestingly, Cu deficiency reduced serum total T4 by 21% (P = 0.06) and Fe deficiency reduced serum total T4 by 67% compared with controls in P12 pups (Fig. 2A). In addition, both Cu deficiency (48% reduction) and Fe deficiency (43% reduction) significantly reduced serum total T3 concentrations (Fig. 2B).
Figure 2.
Perinatal Cu and Fe deficiencies lead to altered serum and brain TH levels. A, Serum total T4. B, Serum total T3. Serum was harvested from P12 male pups (n = 10 for control, FeD, and PTU; n = 8 for CuD). C, Brain total T3. Half brains were harvested from P12 male pups (n = 5 for control, FeD, and PTU; n = 4 for CuD). Data are presented as the mean ± sem. Bars with unlike letters indicate a statistical difference (P < 0.05) by one-way ANOVA and Tukey’s or Scheffé’s multiple comparison test. Similar results were obtained from a second independent study (data not shown).
Given the significant reductions in circulating total T4 and T3 concentrations in CuD and FeD pups, we hypothesized that Cu and Fe deficiencies may also reduce T3 content in the developing brain. To test this hypothesis, we extracted THs from half brains of P12 pups and measured T3 content. Brain T3 content was reduced by 10% in CuD pups (not statistically significant), 25% in FeD pups, and 97% in PTU pups compared with controls (Fig. 2C).
Brain biochemical measures of Cu, Fe, and TH deficiencies
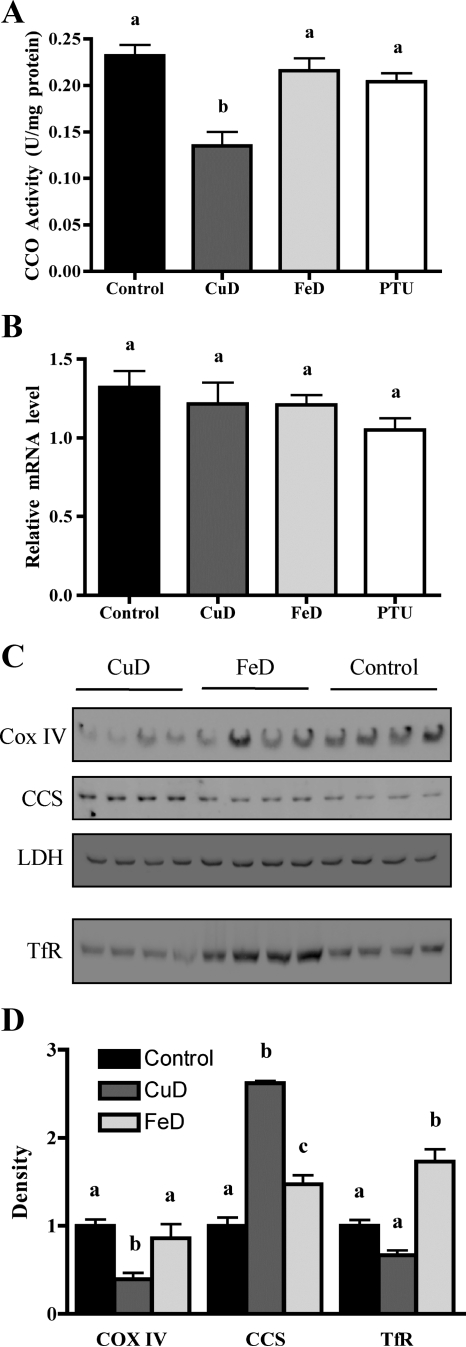
Perinatal Cu, Fe, and TH deficiencies were all previously shown to reduce brain CCO activity (10,30,31). As expected, brain CCO activity was 42% reduced in CuD pups (Fig. 3A). However, brain CCO activity was only slightly reduced in FeD and PTU pups (Fig. 3A). Brain Cox IV mRNA levels were also measured, because TH deficiency was previously shown to reduce Cox IV brain transcript levels (31). However, whole-brain Cox IV mRNA levels were not significantly altered by PTU treatment, Cu deficiency, or Fe deficiency (Fig. 3B). To further evaluate the impact on CuD and FeD brains, Western blot analyses were performed for Cox IV, CCS, and TfR proteins (Fig. 3, C and D). COX IV protein levels were reduced, and CCS protein levels were increased in CuD brains, demonstrating a functional reduction in brain Cu content. COX IV and CCS protein levels were not altered in FeD brains. TfR protein levels were increased in FeD brains, demonstrating a functional reduction in brain Fe concentration. TfR protein levels were not altered in CuD brains, despite a trend toward reduced brain Fe content (Fig. 1D). LDH protein levels, measured as a loading control, were not altered by dietary treatments.
Figure 3.
Perinatal Cu or Fe deficiencies alter P12 brain proteins. A, Brain CCO activity. Half brains were harvested from P12 male pups (n = 5 for control, FeD, and PTU; n = 4 for CuD). B, Brain CCO mRNA analysis. Half brains were harvested from P12 male pups (n = 5 for control, FeD, and PTU; n = 4 for CuD). Quantitative RT-PCR was performed for Cox IV. C, Brain COX IV, CCS, and TfR protein analysis. Western blot analyses were performed on brain homogenates for the indicated proteins. LDH protein levels were measured as a loading control for both the 15 and 8% gels. The LDH blot for the 15% gel is shown. A similar result was obtained for LDH with the 8% gel. D, Quantification of densities of COX IV, CCS, and TfR immunoblot bands in C relative to the control mean. Data are presented as the mean ± sem. Bars with unlike letters indicate a statistical difference (P < 0.05) by one-way ANOVA and Tukey’s or Scheffé’s multiple comparison test.
Effect of PTU-treatment and Cu and Fe deficiencies on brain mRNA expression of TH-responsive genes
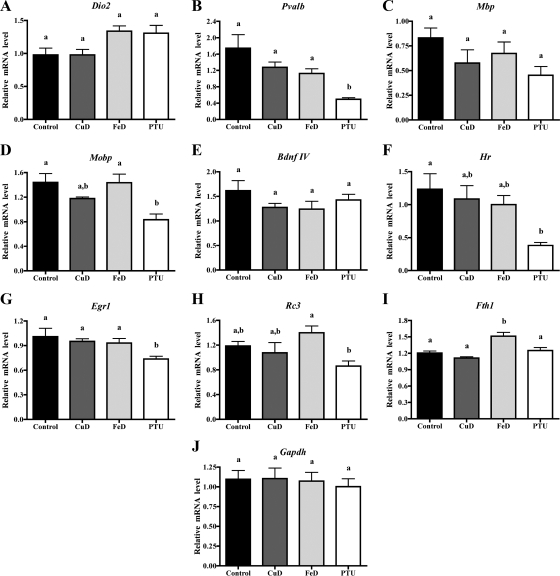
We hypothesized that the reductions in circulating and brain TH levels observed in Cu and Fe deficiencies may affect TH-responsive gene expression in the developing brain. As an initial test of this hypothesis, total RNA was extracted from half brains of P12 pups, cDNA was synthesized, and qRT-PCR was performed for several genes known to be TH-responsive in the developing brain. Some of these genes were also previously shown to be Fe responsive in the developing brain. However, expression of these genes in CuD, FeD, and TH-deficient brains has never been directly compared. The mRNA expression of many, but not all, genes was altered in P12 PTU brains, as shown in Fig. 4 and discussed below. Cu and Fe deficiencies resulted in trends toward altered mRNA expression for several genes as shown in Fig. 4. However, no changes were statistically significant.
Figure 4.
The effect of perinatal Fe and Cu deficiency on TH-responsive brain gene expression. Half brains were harvested from P12 male pups (n = 5 for control, FeD, and PTU; n = 4 for CuD), total RNA was extracted, and cDNA was synthesized from 2 μg of RNA. Quantitative RT-PCR was performed for several TH-responsive genes. A, Dio2. B, Pvalb. C, Mbp. D, Mobp. E, Bdnf IV. F, Hr. G, Egr1. H, Rc3. I, Fth1; qRT-PCR was also performed on one housekeeping gene. J, Gapdh. Relative mRNA levels are calculated relative to an internal control sample. Data are presented as the mean ± sem. Bars with unlike letters indicate a statistical difference (P < 0.05) by one-way ANOVA and Tukey’s or Scheffé’s multiple comparison test.
Dio2 mRNA expression is negatively regulated by brain T3 levels in several regions of the developing brain (32). In addition, we recently reported that Fe deficiency up-regulates Dio2 mRNA levels in the P15 hippocampus (33). In keeping with these previous findings, P12 whole-brain Dio2 mRNA levels were increased 34% in PTU pups (P = 0.12) and 37% in FeD pups (P = 0.08) compared with controls (Fig. 4A). Although individual comparisons were not statistically significant for Dio2, the overall ANOVA was significant (P = 0.03). Pvalb mRNA expression is down-regulated in the cortex of P14 hypothyroid rat brains (34) and in P21 FeD whole brains (35). In agreement with these studies, P12 whole-brain Pvalb mRNA expression levels were decreased 72% in PTU pups, 36% in FeD pups, and 27% in CuD pups (Fig. 4B). The brain Pvalb mRNA expression levels for PTU, FeD, and CuD pups correlate well with the observed reductions in brain T3 content. Mbp mRNA expression is down-regulated in the cortex, hippocampus, and whole brain of developing hypothyroid rats (36,37), in P21 FeD rat brains (35), and in the cerebral cortex of the developing human Menkes disease (CuD) brain (38). In keeping with previous work, P12 Mbp mRNA levels were reduced 46% in PTU brains, 31% in CuD brains, and 19% in FeD brains (Fig. 4C). Mobp expression is down-regulated in the cortex and hippocampus of P14 hypothyroid rat brains (36,37) and in P21 FeD rat brains (35). Mobp brain mRNA levels were reduced 42% in PTU pups and 18% in CuD pups (Fig. 4D). Bdnf IV mRNA is down-regulated in the P15 hypothyroid rat cerebellum (39) and in the P15 FeD rat hippocampus (40). P12 Bdnf IV mRNA expression levels were decreased 12% in PTU brains, 23% in FeD brains, and 21% in CuD brains (Fig. 4E). Egr1 mRNA is down-regulated in the developing hypothyroid rat cortex (34,41) and hippocampus (36) and in the P15 FeD rat hippocampus (40). As expected, Egr1 mRNA levels were decreased 27% in P12 PTU brains (P < 0.05). However, P12 whole-brain Egr1 mRNA levels were not altered by Cu or Fe deficiencies in this dietary model (Fig. 4G).
We also assessed the brain mRNA expression of several known TH-responsive genes that have not previously been examined in Fe or Cu deficiencies during brain development. Fth1 mRNA expression is transiently down-regulated in developing hypothyroid brains (42), but to our knowledge, Fth1 mRNA has not been assessed in the developing FeD or CuD brain. Neither PTU treatment or Cu deficiency affected P12 brain Fth1 mRNA levels but Fe deficiency increased P12 brain Fth1 mRNA levels by 26% (Fig. 4I). Hr mRNA expression is down-regulated in the developing hypothyroid rat cortex, hippocampus (34,43) and cerebellum (44). As expected, Hr mRNA levels were decreased 69% in P12 PTU brains (Fig. 4F). Hr mRNA levels were also 19 and 12% reduced in P12 FeD and CuD brains, respectively (Fig. 4F). Rc3 mRNA levels are down-regulated in the developing hypothyroid cortex (45) and striatum (46). In keeping with these studies, Rc3 mRNA levels were decreased 27% in P12 PTU brains (Fig. 4H). Interestingly, Rc3 mRNA levels were 18% higher in P12 FeD brains (Fig. 4H). Brain mRNA expression of Gapdh was measured as a control gene. Gapdh mRNA levels were not altered in P12 CuD, FeD, or PTU brains (Fig. 4J).
Discussion
Iodine and Fe deficiencies pose significant global health problems. The major impact of these deficiencies early in life is a failure to reach full cognitive potential in adulthood due to deleterious effects on the developing brain. Recent studies show that both Fe and Cu deficiencies impair thyroid function in adolescent and adult humans and rodents. Interestingly, perinatal Fe, Cu, and TH deficiencies exert similar molecular, cellular, and behavioral impacts on brain development. However, the effects of Fe and Cu deficiencies on thyroid function and their potential interactions have not previously been assessed in the developing neonatal brain.
We have now determined whether rat TH status is impaired during perinatal Cu and Fe deficiencies. Perinatal Fe and Cu deficiencies reduced serum total T4 and T3 concentrations in nursing neonatal rats (Fig. 2, A and B). This result is consistent with previous studies in postweanling young adult CuD or FeD rats also demonstrating reductions in circulating T4 and T3 concentrations (17,18,19,20,21,22). The significant reduction in both serum total T4 and T3 concentrations suggests that perinatal Cu and Fe deficiencies inhibit production of T4 and T3 at the thyroid gland, peripheral metabolism of T4 and T3, or both. Hess et al. (20) recently demonstrated that Fe deficiency in postweanling young adult rats reduces activity of the Fe-containing enzyme TPO. In addition, perinatal Cu deficiency reduces serum Fe concentration (Table 2) (23). Therefore, low thyroid gland Fe content resulting in reduced TPO activity may explain the low circulating T4 and T3 concentrations observed in CuD and FeD neonatal pups. Studies in postweanling young adult rats have also demonstrated that Cu and Fe deficiencies impair peripheral conversion of T4 to T3, possibly through reduced hepatic type I deiodinase or brown adipose tissue type II deiodinase activities (18,19,21,22). In addition, increased peripheral type III deiodinase activity or increased T4 and T3 clearance could also contribute to the reduced serum T3 concentrations observed in our study.
Perinatal Cu and Fe deficiencies may also disrupt central control of the hypothalamic-pituitary-thyroid axis. In postweanling FeD rats, plasma TSH concentrations are reduced and TSH response to exogenous TRH injection is blunted (19), suggesting a defect at the anterior pituitary. In CuD adult rats this is not the case, as iv injection of exogenous TRH results in normal pituitary TSH release into the serum (47). Interestingly, Cu deficiency may regulate TRH activity through the activity of the cuproenzyme peptidylglycine α-amidating monooxygenase. Peptidylglycine α-amidating monooxygenase is involved in the biosynthesis of α-amidated peptides, including TRH, and requires two Cu atoms for maximal amidating activity (48). Therefore, reduced hypothalamic Cu content may result in reduced TRH activity.
Whole-brain T3 content was also significantly reduced in FeD neonatal rats (Fig. 2C). To our knowledge, this is the first study showing that Fe deficiency alters brain TH content. This result, along with the reduced circulating TH concentrations observed in CuD and FeD neonatal rats, suggests that altered thyroidal status may contribute to the neurodevelopmental defects associated with Cu and Fe deficiencies. Importantly, a series of recent studies by Gilbert and Sui (49), Gilbert and co-workers (50), and Sui and Gilbert (51) demonstrated that even mild perturbations to the thyroid axis exert deleterious effects on the developing rat brain. A perinatal 3-parts per million (ppm) PTU treatment altered several measures of synaptic transmission and plasticity in area CA1 of the P30 rat hippocampus despite only 36% reduced serum T3 concentration (51). Additional studies found that hippocampal impairments associated with 3-ppm PTU treatment persisted into adulthood when pups were removed from PTU treatment at weaning (49,50). Although brain T3 content was not measured in these previous studies, comparison of serum T3 concentrations indicates that Cu and Fe deficiencies impair the thyroid axis to a similar level as a 3-ppm PTU treatment. Thus, reduced serum total T4 and T3 and brain T3 levels in nursing CuD and FeD neonatal pups likely contribute to developmental defects associated with these deficiencies.
The molecular basis of TH action in the developing brain is predominately at the nuclear level, where T3 regulates brain gene transcription (52). Therefore, we predicted that TH-regulated brain gene mRNA levels would be altered in FeD neonatal brains. Our previous data demonstrated that perinatal Fe deficiency increases Dio2 mRNA levels in the P15 hippocampus (33). Interestingly, although not statistically significant, whole-brain Dio2 mRNA levels were up-regulated to a similar extent in both PTU and FeD brains (Fig. 4A). This result indicates that FeD neonatal rat brains are sensing reduced T3 content and attempt to maintain adequate brain T3 by up-regulating Dio2 expression, thereby increasing local T4 to T3 conversion. These data also suggest that a 25% reduction in brain T3 content induces a maximal increase in Dio2 mRNA expression. Interestingly, Pvalb mRNA expression levels in CuD, FeD, and PTU brains correlate with brain T3 content (Figs. 2C and 4B). This suggests that expression of Pvalb mRNA during brain development may be dose dependent and sensitive to small reductions in brain T3 content. In keeping with this, Royland et al. (34) found that P14 Pvalb cortex mRNA levels were decreased in a dose-dependent manner by graded levels of PTU-treatment. Mbp and Bdnf IV mRNA levels also showed a trend toward reduction in both CuD and FeD brains (Fig. 4, C and E). Fe deficiency was previously shown to reduce Pvalb (35), Mbp (35), Mobp (35), Bdnf IV (33), and Egr1 (33) neonatal rat brain mRNA levels. TH deficiency was previously shown to reduce Mbp (36,37), Bdnf IV (39), Fth1 (42), and Rc3 (45,46) neonatal rat brain mRNA levels. However, statistically significant reductions were not observed for these genes in this study. One explanation for this discrepancy is that many of the previous studies were performed using discrete brain regions, whereas this study was performed on whole-brain RNA. For example, Bdnf IV mRNA is down-regulated in the P15 hypothyroid rat cerebellum (39) and in the P15 FeD rat hippocampus (40). However, in our study, changes in Bdnf IV mRNA expression in a particular brain region may have been overwhelmed by a lack of Fe- or TH-dependent regulation in the remainder of the brain. Future studies focusing on discrete brain regions are warranted.
This is the first study using a new rodent diet to achieve Cu and Fe deficiency, thus comparison with previous findings merits comment. Our previous work demonstrated that perinatal Cu deficiency reduces neonatal brain Fe content and concomitantly increases brain TfR protein levels (23,27). In the current study, brain Fe content in P12 CuD pups trended downward, but brain TfR protein levels were not increased (Figs. 1 and 3, C and D). This discrepancy may be explained by the increased Fe content of the new CuD diet compared with diets used in previous studies. In addition, the effect of Fe deficiency on neonatal brain Fth1 and ferritin L (Ftl) expression has not previously been studied. In this study, brain ferritin H, Fth1, mRNA levels were up-regulated in FeD pups (Fig. 4I), whereas brain Ftl mRNA levels were not altered (data not shown). In postweanling rats, Fe deficiency does not alter brain Fth1 or Ftl mRNA expression but does decrease ferritin H and L protein levels (53). Logically, ferritin protein expression should decrease in Fe deficiency, because ferritin is involved in Fe storage and is iron regulatory element regulated. However, ferritin H may also function in Fe delivery to oligodendroctytes (54). In addition, only Fe/ferritin H positive oligodendrocytes express myelin basic protein (55), indicating that ferritin H expression is important for myelination. Therefore, up-regulation of Fth1 mRNA expression in the developing FeD brain may reveal an attempt to deliver more Fe to oligodendrocytes to maintain myelination. Previous studies have demonstrated that Cu and Fe interact in multiple ways. Cu deficiency increases liver Fe content and decreases serum and brain Fe levels (this study and Refs. 23,27). Fe deficiency also affects Cu homeostasis as evidenced by increased liver and brain Cu content in FeD rat dams and neonates (this study and Refs. 56,57) and decreased liver Cu content in FeD fetuses (58).
Cu, Fe, and TH deficiencies were all previously shown to reduce brain CCO activity (10,30,31). Consistent with previous reports, Cu deficiency reduced brain CCO activity (Fig. 3A). However, Fe deficiency and PTU treatment did not significantly alter brain CCO activity. This result suggests that although Fe and TH may play a role in CCO activity in discrete brain regions (30,31), brain Cu content is the critical determinant of global brain CCO activity. We also observed that although both Cu and Fe deficiencies reduced pup hemoglobin concentrations and lead to development of heart hypertrophy, PTU treatment conversely increased pup hemoglobin concentrations and decreased heart size (Table 2). These observations demonstrate that Cu and Fe exert physiological roles independent of thyroidal effects.
The interactions between Cu, Fe, and TH may have serious implications for human health. An estimated 20–44% of iodine-deficient goitrous children in North and West Africa also suffer from IDA (5,6,7). In these children, Fe repletion improves the efficacy of iodine supplementation, resulting in decreased thyroid volume and goiter prevalence (5,7,59). These studies demonstrate the importance of Fe for normal thyroid function in school-age children. An important unanswered question is whether IDA compounds the deleterious effects of iodine/TH deficiency earlier in development. A recent study in pregnant Swiss women showed that low maternal body Fe stores are correlated with increased circulating TSH and decreased circulating T4 concentrations (60). Altered maternal thyroidal status, including hypothyroxinemia, is associated with impaired neurological development of the offspring (61,62,63). Thus, the study in pregnant Swiss women suggests IDA may compound the deleterious effects of iodine/TH deficiency during human brain development.
Due to a lack of nutrient diversity in diets in developing countries, millions of people are deficient in not just iodine and Fe but multiple other micronutrients as well (4). Deficiencies in selenium, zinc, and vitamin A also adversely affect thyroidal status (64). In addition, people living in developing countries are often exposed to dietary goitrogens, such as thiocyanate precursors in cassava, on a daily basis. During development, when micronutrients and THs are in high demand, combinations of micronutrient deficiencies and dietary goitrogens may create a more severe insult to the thyroid axis. The recent finding that maternal hypothyroxinemia adversely affects offspring brain development highlights the importance of eliminating all possible threats to normal thyroid function during all stages of development.
Supplementary Material
Acknowledgments
We thank the members of the Anderson and Prohaska labs for their invaluable assistance with tissue harvesting and selected assays and Georgieff lab members Stephanie Fretham and Phu Tran for assistance in qRT-PCR experiments. Kevin Viken provided excellent technical assistance and received financial support from the University of Minnesota Brain Barriers Research Center. The Duluth Medical Research Institute core facilities were used for some qRT-PCR experiments.
Footnotes
This work was supported by National Institutes of Health Grants 5RO3HD055423-02, R01HD039708-08, and R01 HD29421-15.
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 23, 2010
Abbreviations: AAS, Atomic absorption spectroscopy; Bdnf IV, brain-derived neurotropic factor isoform IV; CCO, cytochrome c oxidase; CCS, Cu chaperone for superoxide dismutase; COX IV, subunit IV of CCO; Cu, copper; CuD, Cu deficient; Dio2, type II deiodinase; E, gestational day; Egr1, early growth response factor 1; Fe, iron; FeD, Fe deficient; Fth1, ferritin heavy polypeptide 1; Ftl, ferritin L; Gapdh, glyceraldehyde 3-phosphate dehydrogenase; Hr, hairless; IDA, Fe deficiency anemia; LDH, lactate dehyrogenase; Mbp, myelin basic protein; Mobp, myelin-associated oligodendrocyte basic protein; methanol-PTU, methanol containing 1 mm PTU; P, postnatal day; ppm, parts per million; PTU, 6-propyl-2-thiouracil; Pvalb, parvalbumin; qRT-PCR, quantitative real-time PCR; Rc3, neurogranin; T3, triiodothyronine; T4, thyroxine; TfR, transferrin receptor 1; TH, thyroid hormone; TPO, thyroid peroxidase.
References
- Lomborg B 2009 Global crises, global solutions. 2nd ed. Cambridge, UK: Cambridge University Press [Google Scholar]
- de Benoist B, McLean E, Andersson M, Rogers L 2008 Iodine deficiency in 2007: global progress since 2003. Food Nutr Bull 29:195–202 [DOI] [PubMed] [Google Scholar]
- McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B 2009 Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr 12:444–454 [DOI] [PubMed] [Google Scholar]
- UNICEF and The Micronutrient Initiative, Vitamin and mineral deficiency: a global progress report. United Nations Publications, Blue Ridge Summit, PA, 2004 [Google Scholar]
- Hess SY, Zimmermann MB, Adou P, Torresani T, Hurrell RF 2002 Treatment of iron deficiency in goitrous children improves the efficacy of iodized salt in Cote d’Ivoire . Am J Clin Nutr 75:743–748 [DOI] [PubMed] [Google Scholar]
- Zimmermann M, Adou P, Torresani T, Zeder C, Hurrell R 2000 Persistence of goiter despite oral iodine supplementation in goitrous children with iron deficiency anemia in Cote d’Ivoire . Am J Clin Nutr 71:88–93 [DOI] [PubMed] [Google Scholar]
- Zimmermann MB, Zeder C, Chaouki N, Torresani T, Saad A, Hurrell RF 2002 Addition of microencapsulated iron to iodized salt improves the efficacy of iodine in goitrous, iron-deficient children: a randomized, double-blind, controlled trial. Eur J Endocrinol 147:747–753 [DOI] [PubMed] [Google Scholar]
- Anderson GW, Schoonover CM, Jones SA 2003 Control of thyroid hormone action in the developing rat brain. Thyroid 13:1039–1056 [DOI] [PubMed] [Google Scholar]
- Hunt CD, Idso JP 1995 Moderate copper deprivation during gestation and lactation affects dentate gyrus and hippocampal maturation in immature male rats. J Nutr 125:2700–2710 [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Wells WW 1975 Copper deficiency in the developing rat brain: evidence for abnormal mitochondria. J Neurochem 25:221–228 [DOI] [PubMed] [Google Scholar]
- Zatta P, Frank A 2007 Copper deficiency and neurological disorders in man and animals. Brain Res Rev 54:19–33 [DOI] [PubMed] [Google Scholar]
- Lozoff B, Georgieff MK 2006 Iron deficiency and brain development. Semin Pediatr Neurol 13:158–165 [DOI] [PubMed] [Google Scholar]
- Bernal J 2005 Thyroid hormones and brain development. Vitam Horm 71:95–122 [DOI] [PubMed] [Google Scholar]
- Felt BT, Beard JL, Schallert T, Shao J, Aldridge JW, Connor JR, Georgieff MK, Lozoff B 2006 Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav Brain Res 171:261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penland JG, Prohaska JR 2004 Abnormal motor function persists following recovery from perinatal copper deficiency in rats. J Nutr 134:1984–1988 [DOI] [PubMed] [Google Scholar]
- Bongers-Schokking JJ, Koot HM, Wiersma D, Verkerk PH, de Muinck Keizer-Schrama SM 2000 Influence of timing and dose of thyroid hormone replacement on development in infants with congenital hypothyroidism. J Pediatr 136:292–297 [DOI] [PubMed] [Google Scholar]
- Beard J, Green W, Miller L, Finch C 1984 Effect of iron-deficiency anemia on hormone levels and thermoregulation during cold exposure. Am J Physiol 247:R114–R119 [DOI] [PubMed] [Google Scholar]
- Beard JL, Tobin BW, Smith SM 1990 Effects of iron repletion and correction of anemia on norepinephrine turnover and thyroid metabolism in iron deficiency. Proc Soc Exp Biol Med 193:306–312 [DOI] [PubMed] [Google Scholar]
- Beard J, Tobin B, Green W 1989 Evidence for thyroid hormone deficiency in iron-deficient anemic rats. J Nutr 119:772–778 [DOI] [PubMed] [Google Scholar]
- Hess SY, Zimmermann MB, Arnold M, Langhans W, Hurrell RF 2002 Iron deficiency anemia reduces thyroid peroxidase activity in rats. J Nutr 132:1951–1955 [DOI] [PubMed] [Google Scholar]
- Lukaski HC, Hall CB, Marchello MJ 1995 Body temperature and thyroid hormone metabolism of copper-deficient rats. J Nutr Biochem 6:445–451 [Google Scholar]
- Olin KL, Walter RM, Keen CL 1994 Copper deficiency affects selenoglutathione peroxidase and selenodeiodinase activities and antioxidant defense in weanling rats. Am J Clin Nutr 59:654–658 [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Gybina AA 2005 Rat brain iron concentration is lower following perinatal copper deficiency. J Neurochem 93:698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohaska JR 1983 Changes in tissue growth, concentrations of copper, iron, cytochrome oxidase and superoxide dismutase subsequent to dietary or genetic copper deficiency in mice. J Nutr 113:2048–2058 [DOI] [PubMed] [Google Scholar]
- Pyatskowit JW, Prohaska JR 2008 Multiple mechanisms account for lower plasma iron in young copper deficient rats. Biometals 21:343–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell MA, Haas SM, Bieber LL, Tolbert NE 1978 A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem 87:206–210 [DOI] [PubMed] [Google Scholar]
- Pyatskowit JW, Prohaska JR 2008 Iron injection restores brain iron and hemoglobin deficits in perinatal copper-deficient rats. J Nutr 138:1880–1886 [DOI] [PubMed] [Google Scholar]
- Morreale de Escobar G, Pastor R, Obregon MJ, Escobar del Rey F 1985 Effects of maternal hypothyroidism on the weight and thyroid hormone content of rat embryonic tissues, before and after onset of fetal thyroid function. Endocrinology 117:1890–1900 [DOI] [PubMed] [Google Scholar]
- Tran PV, Fretham SJ, Carlson ES, Georgieff MK 2009 Long-term reduction of hippocampal brain-derived neurotrophic factor activity after fetal-neonatal iron deficiency in adult rats. Pediatr Res 65:493–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Deungria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK 2000 Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res 48:169–176 [DOI] [PubMed] [Google Scholar]
- Vega-Núñez E, Menéndez-Hurtado A, Garesse R, Santos A, Perez-Castillo A 1995 Thyroid hormone-regulated brain mitochondrial genes revealed by differential cDNA cloning. J Clin Invest 96:893–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadaño-Ferraz A, Escámez MJ, Rausell E, Bernal J 1999 Expression of type 2 iodothyronine deiodinase in hypothyroid rat brain indicates an important role of thyroid hormone in the development of specific primary sensory systems. J Neurosci 19:3430–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson ES, Stead JD, Neal CR, Petryk A, Georgieff MK 2007 Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus. Hippocampus 17:679–691 [DOI] [PubMed] [Google Scholar]
- Royland JE, Parker JS, Gilbert ME 2008 A genomic analysis of subclinical hypothyroidism in hippocampus and neocortex of the developing rat brain. J Neuroendocrinol 20:1319–1338 [DOI] [PubMed] [Google Scholar]
- Clardy SL, Wang X, Zhao W, Liu W, Chase GA, Beard JL, True Felt B, Connor JR 2006 Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. J Neural Transm Suppl:173–196 [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Akune H, Sumida K, Saito K, Yoshioka T, Tsuji R 2009 Perinatal exposure to PTU decreases expression of Arc, Homer 1, Egr 1 and Kcna 1 in the rat cerebral cortex and hippocampus. Brain Res 1264:24–32 [DOI] [PubMed] [Google Scholar]
- Schoonover CM, Seibel MM, Jolson DM, Stack MJ, Rahman RJ, Jones SA, Mariash CN, Anderson GW 2004 Thyroid hormone regulates oligodendrocyte accumulation in developing rat brain white matter tracts. Endocrinology 145:5013–5020 [DOI] [PubMed] [Google Scholar]
- Liu PC, Chen YW, Centeno JA, Quezado M, Lem K, Kaler SG 2005 Downregulation of myelination, energy, and translational genes in Menkes disease brain. Mol Genet Metab 85:291–300 [DOI] [PubMed] [Google Scholar]
- Koibuchi N, Fukuda H, Chin WW 1999 Promoter-specific regulation of the brain-derived neurotropic factor gene by thyroid hormone in the developing rat cerebellum. Endocrinology 140:3955–3961 [DOI] [PubMed] [Google Scholar]
- Tran PV, Carlson ES, Fretham SJ, Georgieff MK 2008 Early-life iron deficiency anemia alters neurotrophic factor expression and hippocampal neuron differentiation in male rats. J Nutr 138:2495–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipaon C, Santos A, Perez-Castillo A 1992 Thyroid hormone up-regulates NGFI-A gene expression in rat brain during development. J Biol Chem 267:21–23 [PubMed] [Google Scholar]
- Levenson CW, Fitch CA 2000 Effect of altered thyroid hormone status on rat brain ferritin H and ferritin L mRNA during postnatal development. Brain Res Dev Brain Res 119:105–109 [DOI] [PubMed] [Google Scholar]
- Potter GB, Zarach JM, Sisk JM, Thompson CC 2002 The thyroid hormone-regulated corepressor hairless associates with histone deacetylases in neonatal rat brain. Mol Endocrinol 16:2547–2560 [DOI] [PubMed] [Google Scholar]
- Thompson CC 1996 Thyroid hormone-responsive genes in developing cerebellum include a novel synaptotagmin and a hairless homolog. J Neurosci 16:7832–7840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez MA, De Lecea L, Guadano-Ferraz A, Morte B, Gerendasy D, Sutcliffe JG, Bernal J 1996 Cell-specific effects of thyroid hormone on RC3/neurogranin expression in rat brain. Endocrinology 137:1032–1041 [DOI] [PubMed] [Google Scholar]
- Iannacone EA, Yan AW, Gauger KJ, Dowling AL, Zoeller RT 2002 Thyroid hormone exerts site-specific effects on SRC-1 and NCoR expression selectively in the neonatal rat brain. Mol Cell Endocrinol 186:49–59 [DOI] [PubMed] [Google Scholar]
- Allen DK, Hassel CA, Lei KY 1982 Function of pituitary-thyroid axis in copper-deficient rats. J Nutr 112:2043–2046 [DOI] [PubMed] [Google Scholar]
- Kulathila R, Consalvo AP, Fitzpatrick PF, Freeman JC, Snyder LM, Villafranca JJ, Merkler DJ 1994 Bifunctional peptidylglcine α-amidating enzyme requires two copper atoms for maximum activity. Arch Biochem Biophys 311:191–195 [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Sui L 2006 Dose-dependent reductions in spatial learning and synaptic function in the dentate gyrus of adult rats following developmental thyroid hormone insufficiency. Brain Res 1069:10–22 [DOI] [PubMed] [Google Scholar]
- Sui L, Anderson WL, Gilbert ME 2005 Impairment in short-term but enhanced long-term synaptic potentiation and ERK activation in adult hippocampal area CA1 following developmental thyroid hormone insufficiency. Toxicol Sci 85:647–656 [DOI] [PubMed] [Google Scholar]
- Sui L, Gilbert ME 2003 Pre- and postnatal propylthiouracil-induced hypothyroidism impairs synaptic transmission and plasticity in area CA1 of the neonatal rat hippocampus. Endocrinology 144:4195–4203 [DOI] [PubMed] [Google Scholar]
- Oppenheimer JH, Schwartz HL 1997 Molecular basis of thyroid hormone-dependent brain development. Endocr Rev 18:462–475 [DOI] [PubMed] [Google Scholar]
- Han J, Day JR, Thomson K, Connor JR, Beard JL 2000 Iron deficiency alters H- and L-ferritin expression in rat brain. Cell Mol Biol 46:517–528 [PubMed] [Google Scholar]
- Hulet SW, Heyliger SO, Powers S, Connor JR 2000 Oligodendrocyte progenitor cells internalize ferritin via clathrin-dependent receptor mediated endocytosis. J Neurosci Res 61:52–60 [DOI] [PubMed] [Google Scholar]
- Blissman G, Menzies S, Beard J, Palmer C, Connor J 1996 The expression of ferritin subunits and iron in oligodendrocytes in neonatal porcine brains. Dev Neurosci 18:274–281 [DOI] [PubMed] [Google Scholar]
- Sherman AR, Tissue NT 1981 Tissue iron, copper and zinc levels in offspring of iron-sufficient and iron-deficient rats. J Nutr 111:266–275 [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Gellein K, Syversen T, Aschner M 2007 Iron deficient and manganese supplemented diets alter metals and transporters in the developing rat brain. Toxicol Sci 95:205–214 [DOI] [PubMed] [Google Scholar]
- Gambling L, Dunford S, McArdle HJ 2004 Iron deficiency in the pregnant rat has differential effects on maternal and fetal copper levels. J Nutr Biochem 15:366–372 [DOI] [PubMed] [Google Scholar]
- Zimmermann M, Adou P, Torresani T, Zeder C, Hurrell R 2000 Iron supplementation in goitrous, iron-deficient children improves their response to oral iodized oil. Eur J Endocrinol 142:217–223 [DOI] [PubMed] [Google Scholar]
- Zimmermann MB, Burgi H, Hurrell RF 2007 Iron deficiency predicts poor maternal thyroid status during pregnancy. J Clin Endocrinol Metab 92:3436–3440 [DOI] [PubMed] [Google Scholar]
- Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, Vulsma T, Wiersinga WM, Drexhage HA, Vader HL 1999 Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 50:149–155 [DOI] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O'Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ 1999 Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341:549–555 [DOI] [PubMed] [Google Scholar]
- Berbel P, Mestre JL, Santamaría A, Palazón I, Franco A, Graells M, González-Torga A, de Escobar GM 2009 Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: the importance of early iodine supplementation. Thyroid 19:511–519 [DOI] [PubMed] [Google Scholar]
- Hess SY, Zimmermann MB 2004 The effect of micronutrient deficiencies on iodine nutrition and thyroid metabolism. Int J Vitam Nutr Res 74:103–115 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.