Abstract
Bradykinin signaling has been proposed to play either protective or deleterious roles in the development of cardiac dysfunction in response to various pathological stimuli. To further define the role of bradykinin signaling in the diabetic heart, we examined cardiac function in mice with genetic ablation of both bradykinin B1 and B2 receptors (B1RB2R−/−) in the context of the Akita model of insulin-deficient type 1 diabetes (Ins2Akita/+). In 5-month-old diabetic and nondiabetic, wild-type and B1RB2R−/− mice, in vivo cardiac contractile function was determined by left-ventricular (LV) catheterization and echocardiography. Reactive oxygen species levels were measured by 2′-7′-dichlorofluorescein diacetate fluorescence. Mitochondrial function and ATP synthesis were determined in saponin-permeabilized cardiac fibers. LV systolic pressure and the peak rate of LV pressure rise and decline were decreased with diabetes but did not deteriorate further with loss of bradykinin signaling. Wall thinning and reduced ejection fractions in Akita mouse hearts were partially attenuated by B1RB2R deficiency, although other parameters of LV function were unaffected. Loss of bradykinin signaling did not increase fibrosis in Ins2Akita/+ diabetic mouse hearts. Mitochondrial dysfunction was not exacerbated by B1RB2R deficiency, nor was there any additional increase in tissue levels of reactive oxygen species. Thus, loss of bradykinin B2 receptor signaling does not abrogate the previously reported beneficial effect of inhibition of B1 receptor signaling. In conclusion, complete loss of bradykinin expression does not worsen cardiac function or increase myocardial fibrosis in diabetes.
In contrast to the deleterious consequences of loss of bradykinin B2 receptors, combined loss of signaling via bradykinin B1 and B2 receptors does not increase cardiac injury or impair cardiac function in type 1 diabetes.
The kallikrein-kinin system (KKS) produces potent vasoactive kinin peptides, including bradykinin. Bradykinin mediates its effects through the G protein-coupled bradykinin 1 receptor [B1R (Bdkrb1)] and bradykinin 2 receptor [B2R (Bdkrb2)]. B2R is constitutively expressed in most tissues and is thought to regulate the majority of the known effects of kinins on the heart. For example, B2R signaling facilitates insulin-mediated regulation of glucose disposal, because loss of B2R results in reduced insulin sensitivity, despite compensatory induction of B1R (1). In contrast, the B1R is expressed at low levels under physiological conditions, but its expression levels are induced by pathological stimuli that are associated with increased inflammation and tissue injury (reviewed in Ref. 2).
In response to pathological stimuli, such as myocardial infarction (MI), aortic banding, or streptozotocin (STZ)-induced diabetes, there is increased cardiac expression of both bradykinin receptor isoforms (3). Activation of the kallikrein-kinin system attenuates cardiac dysfunction in models of ischemic injury (4,5,6) and STZ-induced diabetes (7,8,9), implying a protective role for bradykinin signaling. Specifically, bradykinin signaling has been proposed to ameliorate diabetes-associated oxidative stress in the heart and other tissues (7,9). It is likely that most of this protection may be mediated through the B2R, because loss of the B2R results in increased cardiac fibrosis, chamber dilation, and elevated end-diastolic pressure in nondiabetic mice (10) and increased sclerosis of kidneys in Ins2Akita/+ insulin-dependent type 1 diabetic mice (11). Furthermore, genetic ablation of the B1R was not associated with adverse cardiac remodeling after ischemic injury until pharmacologic blockade of B2R was superimposed (12). In contrast, overexpression of B1R enhances inflammatory responses and agonist-mediated hypertension (13), and a recent study reported that B1R deletion reduced fibrosis and inflammation in the hearts of mice with STZ-induced diabetes (14). Conversely, loss of B1R receptors impaired the angiogenic response in ischemic skeletal muscle (15), suggesting that, in some contexts, B1R receptor signaling is required for a regenerative response after tissue injury.
Loss of both bradykinin receptors protected mice from endotoxin-induced hypotension (16). However, deletion of both bradykinin receptors exacerbated the development of renal dysfunction after ischemic injury compared with either receptor alone (17). Thus, the relative contributions of B1R vs. B2R signaling in tissue protection may display tissue-specific differences. Although single gene knockout studies suggest that B2R signaling is cardioprotective and B1R signaling may promote cardiac injury, conclusions can be confounded by compensatory changes in the remaining receptor as exemplified by induction of B1R expression in models of B2R loss of function. In the heart, the net effect of complete bradykinin receptor blockade is unknown, and it is not possible to predict the direction of any changes from studies of single knockout mice in nonstressed hearts or after pathophysiological stresses such as diabetes. We have shown recently that hearts from insulin-deficient Ins2Akita/+ (Akita) mice have mild mitochondrial dysfunction in the presence of pyruvate, but not palmitoyl-carnitine, exhibit no evidence of oxidative stress, and exhibit no contractile dysfunction in nonstressed hearts (18). Therefore, to further define the role of B1R and B2R in diabetic cardiomyopathy, we tested the hypothesis that complete loss of bradykinin signaling would increase oxidative stress and accelerate cardiac dysfunction in the hearts of Akita diabetic mice. We investigated cardiac structure and contractile function, mitochondrial energetics, and tissue reactive oxygen species (ROS) generation in insulin-deficient Ins2Akita/+ mice with genetic ablation of both B1R and B2R. The primary findings of this study are that loss of B1R and B2R does not accelerate development of diabetic cardiomyopathy.
Materials and Methods
Animals
Insulin-deficient Akita (Ins2Akita/+) mice on the C57BL/6J background (19) were bred with the previously generated B1R and B2R null (B1RB2R−/−) mice on the C57BL/6NJ background (17). Because of the close genomic proximity of the B1R and B2R genes, the region coding both receptors was simultaneously deleted. Additionally, because of poor breeding of Ins2Akita/+ mice, the following breeding strategy was used. Female Ins2+/+ B1RB2R−/− were bred to male Ins2Akita/+ B1RB2R+/− mice. Five-month old male mice were used with Ins2+/+ B1RB2R+/− littermates as controls. A subset of the studies included age-matched wild-type mice (Ins2+/+ B1RB2R+/+). Despite a 50% reduction in B1R and B2R mRNA (data not shown), B1RB2R+/− mice were identical in all measured parameters to B1RB2R+/+, which is the rationale for using Ins2Akita/+ B1RB2R+/− as controls. All studies were performed in random-fed animals. Animals were studied in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Utah.
In vivo cardiac function
Echocardiography or left-ventricular (LV) catheterization was performed in a subset of the mice before respiration studies as described previously (20). Mice were lightly anesthetized with isoflurane and imaged in the left lateral decubitus position with a linear 13-MHz probe (Vivid V echocardiograph; GE Healthcare, Tampa, FL). Cardiac dimensions and function were calculated from these digital images (21). Invasive LV hemodynamic measurements were performed with a temperature-calibrated 1.4-Fr micromanometer-tipped catheter (Millar Instruments, Houston, TX) inserted through the right carotid artery in mice anesthetized with choral hydrate (400 mg/kg) and analyzed as described by us previously (21).
Respiration and ATP measurements in saponin-permeabilized cardiac fibers
The respiration rates of saponin-permeabilized fibers were determined using a fiber-optic oxygen sensor (Ocean Optics, Orlando, FL) as described previously (22). Studies were performed with 0.02 mm palmitoyl-carnitine and 5 mm malate. Respiration rates are defined as State 3 (VADP) maximally stimulated respiration after addition of 1 mm ADP. ATP generation was determined by a bioluminescence assay based on the luciferin/luciferase reaction with the Enliten ATP Assay System (Promega, Madison, WI). ATP/O was calculated as the ratio of ATP synthesis rates and VADP.
Oxidative stress
Tissue ROS levels were measured by the conversion of nonfluorescent 2′,7′-dichlorodihydrofluorescein diacetate to the fluorescent 2′,7′-dichlorofluorescein in the presence of cellular esterases and endogenous ROS (23) with modifications as described recently by us (18).
Western blot analysis
Whole-cell protein extracts were resolved by SDS-PAGE, and, after transfer to polyvinylidene difluoride, membranes were probed with antibodies against UCP3 (Affinity BioReagents, Golden, CO) and glyceraldehyde-3-phosphate dehydrogenase (Cell Signaling Technology, Danvers, MA). Detection and quantification were performed by measuring fluorescently labeled secondary antibodies using the Odyssey Infrared Imaging System and accompanying software (version 3.0; LI-COR Biosciences, Lincoln, NE). Protein concentrations were determined using the Micro BCA Protein Assay kit (Pierce, Rockford, IL).
Histology and stereology
Myocardial sections were fixed by immersion in neutral-buffered zinc formalin (10%; catalog no. 313-095; Thermo Fisher Scientific, Waltham, MA) for 1 h, embedded in Paraplast, cut into 5 μm sections, and stained by hematoxylin-eosin and Masson’s trichrome stains. Light microscopy was performed using an Olympus (New York, NY) LX81 inverted microscope attached to an Olympus Microfire Digital Camera, and volume density was estimated for cardiomyocytes and interstitial tissue (24,25).
Statistical analysis
Data are presented as mean ± sem. Unpaired Student’s t test was used to analyze comparisons between two groups. Datasets with more than two groups were analyzed by one-way ANOVA, and significance was assessed using Fisher’s protected least significant difference post hoc analysis when appropriate. Statistical calculations were performed using the JMP 7.0 software package (SAS Institute Inc., Cary, NC). P < 0.05 was accepted as indicating a significant difference.
Results
Animal characteristics and cardiac histology
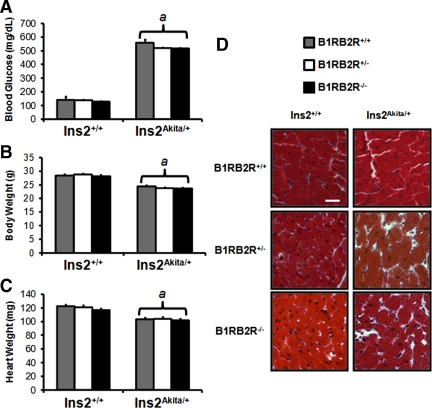
Loss of bradykinin signaling had no effect on the development of hyperglycemia in Ins2Akita/+ mice (Fig. 1A). The expected decrease in body weight and heart weight in Ins2Akita/+ mice was not altered in B1RB2R−/− mice (Fig. 1, B and C). Relative to nondiabetic controls, the hearts of Ins2Akita/+ mice did not exhibit any evidence of cellular disorganization or any evidence of increased fibrosis. Loss of bradykinin signaling did not lead to any additional histological changes and did not induce cardiac fibrosis as assessed by Masson’s trichrome staining (Fig. 1D).
Figure 1.
Animal characteristics, heart weights, and histology. Blood glucose (A), body weight (B), and heart weight (C) determined at time of study, aged 21.4 ± 0.1 wk. D, Formalin-fixed myocardial sections were stained using Masson’s trichrome stain for assessment of cellular organization and for determination of fibrosis. Scale bar, 30 μm.
Cardiac function
In vivo blood pressure and cardiac contractility was evaluated by LV catheterization. In nondiabetic mice, loss of both isoforms of the bradykinin receptor had no adverse effect on cardiac function (Ins2+/+ B1RB2R+/+ and Ins2+/+ B1RB2R+/− vs. Ins2+/+ B1RB2R−/−). LV systolic pressure and developed pressure were reduced by diabetes, and the absence of bradykinin signaling did not additionally reduce systolic function in Ins2Akita/+ mice (Table 1). Similarly, the peak rates of LV pressure increase (+dP/dt) and decrease (−dP/dt) were reduced by diabetes and were not further impaired after loss of bradykinin signaling (Table 1).
Table 1.
LV hemodynamic measurements obtained after LV catheterization
| Parameter | Ins2+/+B1RB2R+/+ | Ins2+/+B1RB2R+/− | Ins2+/+B1RB2R−/− | Ins2Akita/+B1RB2R+/− | Ins2Akita/+B1RB2R−/− |
|---|---|---|---|---|---|
| No. of mice | 7 | 7 | 7 | 7 | 7 |
| aSP (mmHg) | 91.0 ± 4.0 | 94.5 ± 3.8 | 91.4 ± 4.9 | 85.2 ± 3.1 | 91.2 ± 3.7 |
| aDP (mmHg) | 55.6 ± 4.1 | 60.1 ± 4.8 | 57.6 ± 3.8 | 58.4 ± 3.0 | 64.2 ± 3.7 |
| LVSP (mmHg) | 94.9 ± 2.5 | 99.0 ± 3.0 | 98.7 ± 4.2 | 88.7 ± 1.6a | 90.0 ± 2.3a |
| LVEDP (mmHg) | 5.1 ± 1.6 | 11.8 ± 2.9 | 6.4 ± 1.6 | 13.2 ± 2.5 | 9.6 ± 2.8 |
| LV Dev P (mmHg) | 97.2 ± 2.1 | 98.7 ± 2.6 | 98.8 ± 3.4 | 85.1 ± 1.8a | 89.3 ± 2.1a |
| +dP/dt (mmHg/sec) | 7458 ± 384 | 7424 ± 355 | 8228 ± 420 | 6089 ± 232a | 6440 ± 412a |
| −dP/dt (mmHg/sec) | −7442 ± 520 | −7393 ± 614 | −8059 ± 617 | −5765 ± 330a | −5808 ± 371a |
| Heart rate (beats/min) | 452 ± 24 | 454 ± 21 | 481 ± 22 | 450 ± 18 | 429 ± 26 |
Data shown are from studies in which aortic valve function was normal. If aortic regurgitation was present, then data were excluded from analysis. aSP, Aortic systolic pressure; aDP, aortic diastolic pressure; LVSP, left-ventricular systolic pressure; LVEDP, left-ventricular end-diastolic pressure; LV Dev P, left-ventricular developed pressure; +dP/dt, peak rate of LV pressure rise; −dP/dt, peak rate of LV pressure decline. Data are mean ± sem.
P < 0.03 vs. nondiabetic mice with the same bradykinin receptor genotype.
Loss of bradykinin signaling alone did not significantly alter any echocardiographic parameter of cardiac structure or function. Diabetic controls (Ins2Akita/+ B1RB2R+/−) had decreased wall thicknesses compared with nondiabetic mice (relative wall thickness, interventricular septum) (Table 2). These changes were attenuated with loss of bradykinin signaling. Additionally, Ins2Akita/+ B1RB2R−/− had increased fractional shortening and ejection fraction vs. Ins2+/+ B1RB2R−/− and Ins2Akita/+ B1RB2R+/− mice (Table 2).
Table 2.
Echocardiographic evaluation of cardiac function
| Parameter | Ins2+/+B1RB2R+/+ | Ins2+/+B1RB2R+/− | Ins2+/+B1RB2R−/− | Ins2Akita/+B1RB2R+/− | Ins2Akita/+B1RB2R−/− |
|---|---|---|---|---|---|
| No. of mice | 15 | 16 | 17 | 12 | 16 |
| LVDd (mm) | 3.80 ± 0.01 | 3.75 ± 0.05 | 3.73 ± 0.08 | 3.84 ± 0.08 | 3.61 ± 0.07 |
| LVDs (mm) | 2.75 ± 0.01 | 2.77 ± 0.06 | 2.81 ± 0.08 | 2.88 ± 0.10 | 2.54 ± 0.08 |
| IVSd (mm) | 0.72 ± 0.03 | 0.72 ± 0.03 | 0.74 ± 0.03 | 0.54 ± 0.02a | 0.69 ± 0.04 |
| IVSs (mm) | 1.15 ± 0.04 | 1.10 ± 0.04 | 1.12 ± 0.05 | 0.86 ± 0.05a | 1.09 ± 0.05 |
| LVPWd (mm) | 0.70 ± 0.03 | 0.72 ± 0.03 | 0.74 ± 0.02 | 0.64 ± 0.03 | 0.73 ± 0.03 |
| LVPWs (mm) | 0.89 ± 0.04 | 0.89 ± 0.04 | 0.89 ± 0.03 | 0.83 ± 0.04 | 0.97 ± 0.05 |
| Relative wall thickness | 0.38 ± 0.02 | 0.39 ± 0.02 | 0.40 ± 0.02 | 0.31 ± 0.02a | 0.40 ± 0.01 |
| Fractional shortening (%) | 28.0 ± 1.2 | 26.3 ± 0.8 | 24.9 ± 0.9 | 25.1 ± 1.9 | 29.8 ± 1.6b,c |
| Ejection fraction (%) | 62.2 ± 1.8 | 59.8 ± 1.3 | 57.4 ± 1.5 | 57.1 ± 3.1 | 64.5 ± 2.2b,c |
| Stroke volume (μl) | 37.7 ± 1.3 | 33.3 ± 1.3 | 32.7 ± 1.6 | 32.9 ± 2.2 | 33.4 ± 1.7 |
| Heart rate (beats/min) | 496 ± 18 | 475 ± 21 | 447 ± 16 | 392 ± 20d,e | 441 ± 22 |
LVDd, Left-ventricular cavity diameter at diastole; LVDs, left-ventricular cavity diameter at systole; IVSd, interventricular septum thickness at diastole; IVSs, interventricular septum thickness at systole; LVPWd, left-ventricular posterior wall thickness at diastole; LVPWs, left-ventricular posterior wall thickness at systole. Data are means ± sem.
P < 0.01 vs. all other groups.
P < 0.01 vs. Ins2+/+ B1RB2R−/−.
P < 0.02 vs. Ins2Akita/+ B1RB2R+/−.
P < 0.02 vs. Ins2+/+ B1RB2R+/+.
P < 0.01 vs. Ins2+/+ B1RB2R+/−.
Mitochondrial function and oxidative stress
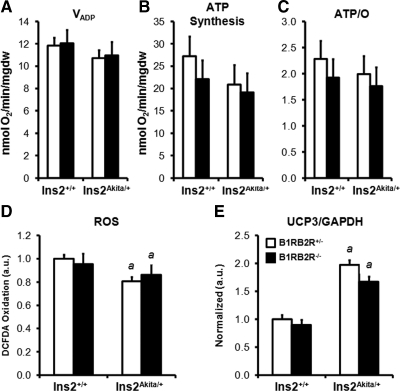
Mitochondrial function was determined using palmitoyl-carnitine as substrate (Fig. 2A–C). Consistent with our previous examination of Akita mice (18), there was no change in maximal ADP-stimulated mitochondrial respiration rates (Fig. 2A), ATP synthesis (Fig. 2B), or respiratory coupling (Fig. 2C) when palmitoyl-carnitine was used as a substrate. Loss of bradykinin signaling had no additional effect on these parameters. Previous studies have supported a role for bradykinin signaling in reducing oxidative damage (7,9,26) but, in Akita diabetic mice, ROS are reduced (18). We therefore measured oxidation of 2′-7′-dichlorofluorescein diacetate (DCFDA), a measure of cellular ROS levels, to determine whether complete loss of bradykinin signaling would induce oxidative stress in this model of diabetes. Contrary to our hypothesis, levels of oxidized DCFDA were unchanged in Ins2+/+ B1RB2R−/− mice. Relative to nondiabetic mice, levels of oxidized DCFDA were lower in Akita mice (Fig. 2D). Importantly, complete loss of bradykinin signaling did not increase cellular ROS levels in Akita mice. Mitochondrial uncoupling protein (UCP3) levels are increased in Akita mouse hearts (18), which was confirmed in the present study and was not further altered by loss of bradykinin signaling (Fig. 2E).
Figure 2.
Mitochondrial function, ROS levels, and UCP3 content. Mitochondrial respiration rates (A), ATP synthesis (B), and ATP/O ratios (C) of saponin-permeabilized cardiac fibers using palmitoyl-carnitine as substrate (n = 7–8). Data are mean ± sem. D, Oxidation of DCFDA measured in cardiac whole-tissue extracts (n = 7). Data are mean ± sem. a, P < 0.02 vs. Ins2+/+ B1RB2R+/−. E, Western blot analysis of the uncoupling protein UCP3 (n = 5–6). Graph represents fluorescent values determined as outlined in Materials and Methods corrected to glyceraldehyde-3-phosphate dehydrogenase and normalized to Ins2+/+ B1RB2R+/− controls (n = 1.0). Data are mean ± sem. a, P < 0.001 vs. Ins2+/+.
Discussion
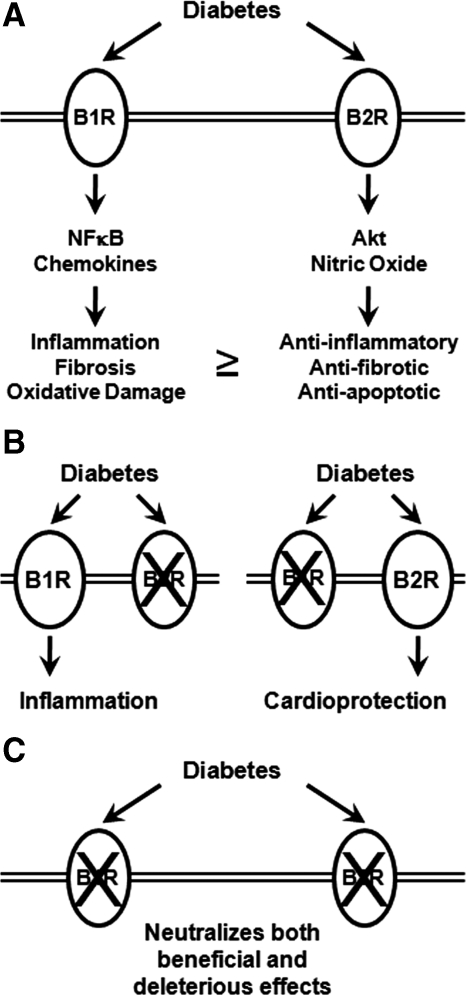
In the present study, we tested the hypothesis that bradykinin signaling may modulate cardiac contractile and mitochondrial function in the type 1 diabetic heart. In contrast to the significant deleterious impact of B1R and B2R deficiency on renal injury (17), no striking changes in cardiac structure or function were observed in the hearts of nondiabetic B1RB2R-deficient animals or after diabetes was induced by crossing these mice with Ins2Akita/+ type 1 diabetic mice. Indeed, some parameters of cardiac dysfunction were attenuated in diabetic B1RB2R-deficient mice. Although the present findings represent a negative report, our study provides important insight into the balance of B1R and B2R signaling in mediating injury vs. protection in the heart in the context of diabetic cardiomyopathy. Current evidence suggests that the bradykinin receptors may exhibit isoform-specific differences in modulating the response of the heart to ischemic injury (27). A recent report demonstrated that, in the STZ-induced model of diabetes, B1R deletion reduced cardiac inflammation, fibrosis, and oxidative stress (14). Until now, the role of B2R signaling in diabetic cardiomyopathy has not been specifically evaluated. Specifically, it remained to be determined in the context of diabetes whether the antiinflammatory effect associated with B1R deletion reflects loss of proinflammatory signaling that is downstream of B1R or antiinflammatory signaling that is downstream of B2R (Fig. 3).
Figure 3.
Schematic diagram of the potential role for bradykinin receptors in diabetic cardiomyopathy. A, Diabetes increases the expression of both B1R and B2R (Ref. 3). Although increased B1R signaling promotes tissue injury and B2R signaling is cytoprotective, we speculate that, in diabetes, the balance might be tilted toward more B1R signaling, which contributes to diabetes-associated cardiac injury and contractile dysfunction. NFκB, Nuclear factor κB. B, Loss of B2R signaling (left) leads to unopposed B1R signaling, which may account for the increased injury and cardiac dysfunction that occurs in B2R-deficient hearts (Ref. 10). Thus, selective inhibition of B2R signaling would be predicted to exacerbate diabetic cardiomyopathy. Conversely, loss of B1R signaling (right) has been demonstrated to ameliorate oxidative stress and tissue dysfunction in diabetic hearts (Ref. 14). This is likely the result of unopposed B2R signaling, which promotes cytoprotection via AKT and endothelial nitric oxide synthase mediated pathways. C, The present study indicates that combined loss of B1R and B2R signaling in the heart is relatively neutral. However, in light of evidence that combined loss of B1R and B2R signaling might accelerate renal injury in diabetes, we believe that selective pharmacological inhibition of B1R signaling represents a more rational strategy for minimizing cardiac and renal complications of diabetes than inhibition of both receptors.
In nondiabetic models, signaling through the B1R may mediate cardiac inflammation (reviewed in Ref. 28) (Fig. 3). Pharmacologic inhibition of B1R or loss of B1R (B1R−/−) reduced cardiac damage after ischemic injury (29). Loss of B1R but not B2R resulted in reduced infarct size in mouse hearts after ischemia reperfusion (27). Moreover, deletion of B2R was reported to precipitate cardiac dysfunction implying that unopposed B1R signaling might be deleterious (10) (Fig. 3). Additional support for a deleterious role of B1R came from studies examining loss of either B1R or B2R, respectively, in a model of MI (30) in which ACE inhibition in B2R−/− but not B1R−/− mice further decreased cardiac function after MI. These data implied that enhanced signaling via the upregulated B1R, in B2R−/− mice, may augment tissue injury. It is therefore possible that B2R signaling may counteract the proinflammatory effects of B1R signaling and that loss of both isoforms could recapitulate similar antiinflammatory effects as was observed when the B1R alone was inactivated or at least be associated with a neutral effect. Cayla et al. (16) recently developed an independent model of complete bradykinin receptor deficiency. These B1RB2R-null mice are healthy and fertile and have no sign of cardiac dysfunction. These mice are normotensive, exhibit a lower heart rate, and are protected from endotoxin-induced hypotension, although overall mortality was unchanged (16). The impact of total loss of bradykinin signaling on diabetes-associated tissue injury was not evaluated in this model.
In conclusion and in contrast to the previously reported requirement for both B1R and B2R signaling in ameliorating the renal response to injury, B1R and B2R appear to be dispensable for maintaining normal cardiac cellular structure and contractile function in nonstressed control and diabetic hearts (Fig. 3). These findings underscore distinct tissue-specific roles for these receptors in the pathogenesis of diabetic complications. They also support the hypothesis that B1R and B2R may mediate distinct effects (B1R being proinflammatory and B2R promoting cardioprotection) and that, in balance, the impact of loss of both isoforms may be relatively neutral. Unlike previous studies that have examined the individual receptor mutants, this is the first study to our knowledge to examine genetic ablation of both receptors in the diabetic heart. Although our studies suggest that global inhibition of bradykinin signaling in diabetes might not be detrimental in diabetic cardiomyopathy, the possibility that inhibition of both isoforms may accelerate ischemic renal injury (17) and that inhibition of B2R accelerates diabetic nephropathy (11) supports the position that isoform-specific inhibition of the B1R might represent the most rational use of bradykinin receptor inhibition in the treatment of diabetic renal or cardiovascular complications.
Footnotes
A.R.W. was supported by National Institutes of Health Grant 5T32 HL007576 and postdoctoral fellowships from the American Heart Association Western States Affiliate and the Juvenile Diabetes Research Foundation. S.E.L. has received a Veterans Administration Merit Award. This work was supported by National Institutes of Health Grants UO1 HL087947 and UO1 DK076131 (Animal Models of Diabetes Complications Consortium).
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 25, 2010
Abbreviations: B1R, Bradykinin 1 receptor; B2R, bradykinin 2 receptor; DCFDA, 2′-7′-dichlorofluorescein diacetate; LV, left-ventricular; MI, myocardial infarction; ROS, reactive oxygen species; STZ, streptozotocin.
References
- Duka I, Shenouda S, Johns C, Kintsurashvili E, Gavras I, Gavras H 2001 Role of the B2 receptor of bradykinin in insulin sensitivity. Hypertension 38:1355–1360 [DOI] [PubMed] [Google Scholar]
- Spillmann F, Van Linthout S, Schultheiss HP, Tschöpe C 2006 Cardioprotective mechanisms of the kallikrein-kinin system in diabetic cardiopathy. Curr Opin Nephrol Hypertens 15:22–29 [DOI] [PubMed] [Google Scholar]
- Spillmann F, Altmann C, Scheeler M, Barbosa M, Westermann D, Schultheiss HP, Walther T, Tschöpe C 2002 Regulation of cardiac bradykinin B1- and B2-receptor mRNA in experimental ischemic, diabetic, and pressure-overload-induced cardiomyopathy. Int Immunopharmacol 2:1823–1832 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Zhang JJ, Chao L, Chao J 2000 Kallikrein gene delivery attenuates myocardial infarction and apoptosis after myocardial ischemia and reperfusion. Hypertension 35:25–31 [DOI] [PubMed] [Google Scholar]
- Chao J, Yin H, Gao L, Hagiwara M, Shen B, Yang ZR, Chao L 2008 Tissue kallikrein elicits cardioprotection by direct kinin B2 receptor activation independent of kinin formation. Hypertension 52:715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao YY, Yin H, Shen B, Chao L, Chao J 2007 Tissue kallikrein infusion prevents cardiomyocyte apoptosis, inflammation and ventricular remodeling after myocardial infarction. Regul Pept 140:12–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikrut K, Paluszak J, Kozlik J, Sosnowski P, Krauss H, Grześkowiak E 2001 The effect of bradykinin on the oxidative state of rats with acute hyperglycaemia. Diabetes Res Clin Pract 51:79–85 [DOI] [PubMed] [Google Scholar]
- Montanari D, Yin H, Dobrzynski E, Agata J, Yoshida H, Chao J, Chao L 2005 Kallikrein gene delivery improves serum glucose and lipid profiles and cardiac function in streptozotocin-induced diabetic rats. Diabetes 54:1573–1580 [DOI] [PubMed] [Google Scholar]
- Tschöpe C, Walther T, Escher F, Spillmann F, Du J, Altmann C, Schimke I, Bader M, Sanchez-Ferrer CF, Schultheiss HP, Noutsias M 2005 Transgenic activation of the kallikrein-kinin system inhibits intramyocardial inflammation, endothelial dysfunction, and oxidative stress in experimental diabetic cardiomyopathy. FASEB J 19:2057–2059 [DOI] [PubMed] [Google Scholar]
- Emanueli C, Maestri R, Corradi D, Marchione R, Minasi A, Tozzi MG, Salis MB, Straino S, Capogrossi MC, Olivetti G, Madeddu P 1999 Dilated and failing cardiomyopathy in bradykinin B2 receptor knockout mice. Circulation 100:2359–2365 [DOI] [PubMed] [Google Scholar]
- Kakoki M, Takahashi N, Jennette JC, Smithies O 2004 Diabetic nephropathy is markedly enhanced in mice lacking the bradykinin B2 receptor. Proc Natl Acad Sci USA 101:13302–13305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Carretero OA, Sun Y, Shesely EG, Rhaleb NE, Liu YH, Liao TD, Yang JJ, Bader M, Yang XP 2005 Role of the B1 kinin receptor in the regulation of cardiac function and remodeling after myocardial infarction. Hypertension 45:747–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni A, Yin H, Agata J, Yang Z, Chao L, Chao J 2003 Overexpression of kinin B1 receptors induces hypertensive response to Des-Arg9-bradykinin and susceptibility to inflammation. J Biol Chem 278:219–225 [DOI] [PubMed] [Google Scholar]
- Westermann D, Walther T, Savvatis K, Escher F, Sobirey M, Riad A, Bader M, Schultheiss HP, Tschöpe C 2009 Gene deletion of the kinin receptor B1 attenuates cardiac inflammation and fibrosis during the development of experimental diabetic cardiomyopathy. Diabetes 58:1373–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanueli C, Bonaria Salis M, Stacca T, Pintus G, Kirchmair R, Isner JM, Pinna A, Gaspa L, Regoli D, Cayla C, Pesquero JB, Bader M, Madeddu P 2002 Targeting kinin B1 receptor for therapeutic neovascularization. Circulation 105:360–366 [DOI] [PubMed] [Google Scholar]
- Cayla C, Todiras M, Iliescu R, Saul VV, Gross V, Pilz B, Chai G, Merino VF, Pesquero JB, Baltatu OC, Bader M 2007 Mice deficient for both kinin receptors are normotensive and protected from endotoxin-induced hypotension. FASEB J 21:1689–1698 [DOI] [PubMed] [Google Scholar]
- Kakoki M, McGarrah RW, Kim HS, Smithies O 2007 Bradykinin B1 and B2 receptors both have protective roles in renal ischemia/reperfusion injury. Proc Natl Acad Sci USA 104:7576–7581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugger H, Boudina S, Hu XX, Tuinei J, Zaha VG, Theobald HA, Yun UJ, McQueen AP, Wayment B, Litwin SE, Abel ED 2008 Type 1 diabetic Akita mouse hearts are insulin sensitive but manifest structurally abnormal mitochondria that remain coupled despite increased uncoupling protein 3. Diabetes 57:2924–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka M, Kayo T, Ikeda T, Koizumi A 1997 A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 46:887–894 [DOI] [PubMed] [Google Scholar]
- Sena S, Rasmussen IR, Wende AR, McQueen AP, Theobald HA, Wilde N, Pereira RO, Litwin SE, Berger JP, Abel ED 2007 Cardiac hypertrophy caused by peroxisome proliferator-activated receptor-γ agonist treatment occurs independently of changes in myocardial insulin signaling. Endocrinology 148:6047–6053 [DOI] [PubMed] [Google Scholar]
- McQueen AP, Zhang D, Hu P, Swenson L, Yang Y, Zaha VG, Hoffman JL, Yun UJ, Chakrabarti G, Wang Z, Albertine KH, Abel ED, Litwin SE 2005 Contractile dysfunction in hypertrophied hearts with deficient insulin receptor signaling: possible role of reduced capillary density. J Mol Cell Cardiol 39:882–892 [DOI] [PubMed] [Google Scholar]
- Boudina S, Sena S, O'Neill BT, Tathireddy P, Young ME, Abel ED 2005 Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation 112:2686–2695 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Yu BP, Chung HY 2002 Molecular exploration of age-related NF-κB/IKK downregulation by calorie restriction in rat kidney. Free Radic Biol Med 32:991–1005 [DOI] [PubMed] [Google Scholar]
- Gundersen HJ 1977 Notes on the estimation of the numerical density of arbitrary profiles, the edge effect. J Microsc 111:219–223 [Google Scholar]
- Mandarim-de-Lacerda CA 2003 Stereological tools in biomedical research. An Acad Bras Cienc 75:469–486 [DOI] [PubMed] [Google Scholar]
- Kakoki M, Kizer CM, Yi X, Takahashi N, Kim HS, Bagnell CR, Edgell CJ, Maeda N, Jennette JC, Smithies O 2006 Senescence-associated phenotypes in Akita diabetic mice are enhanced by absence of bradykinin B2 receptors. J Clin Invest 116:1302–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Chao J, Bader M, Chao L 2007 Differential role of kinin B1 and B2 receptors in ischemia-induced apoptosis and ventricular remodeling. Peptides 28:1383–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschöpe C, Westermann D 2008 Development of diabetic cardiomyopathy and the kallikrein-kinin system: new insights from B1 and B2 receptor signaling. Biol Chem 389:707–711 [DOI] [PubMed] [Google Scholar]
- Lagneux C, Bader M, Pesquero JB, Demenge P, Ribuot C 2002 Detrimental implication of B1 receptors in myocardial ischemia: evidence from pharmacological blockade and gene knockout mice. Int Immunopharmacol 2:815–822 [DOI] [PubMed] [Google Scholar]
- Duka A, Kintsurashvili E, Duka I, Ona D, Hopkins TA, Bader M, Gavras I, Gavras H 2008 Angiotensin-converting enzyme inhibition after experimental myocardial infarct: role of the kinin B1 and B2 receptors. Hypertension 51:1352–1357 [DOI] [PubMed] [Google Scholar]