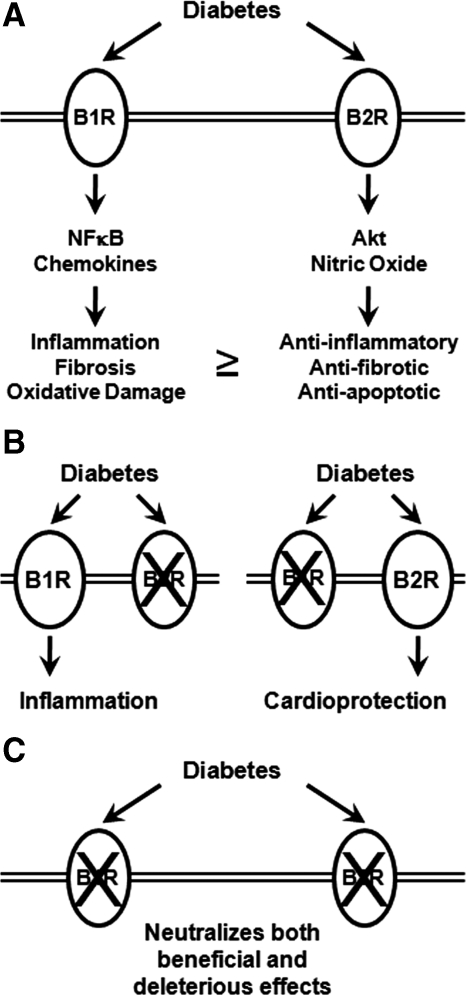
Figure 3.
Schematic diagram of the potential role for bradykinin receptors in diabetic cardiomyopathy. A, Diabetes increases the expression of both B1R and B2R (Ref. 3). Although increased B1R signaling promotes tissue injury and B2R signaling is cytoprotective, we speculate that, in diabetes, the balance might be tilted toward more B1R signaling, which contributes to diabetes-associated cardiac injury and contractile dysfunction. NFκB, Nuclear factor κB. B, Loss of B2R signaling (left) leads to unopposed B1R signaling, which may account for the increased injury and cardiac dysfunction that occurs in B2R-deficient hearts (Ref. 10). Thus, selective inhibition of B2R signaling would be predicted to exacerbate diabetic cardiomyopathy. Conversely, loss of B1R signaling (right) has been demonstrated to ameliorate oxidative stress and tissue dysfunction in diabetic hearts (Ref. 14). This is likely the result of unopposed B2R signaling, which promotes cytoprotection via AKT and endothelial nitric oxide synthase mediated pathways. C, The present study indicates that combined loss of B1R and B2R signaling in the heart is relatively neutral. However, in light of evidence that combined loss of B1R and B2R signaling might accelerate renal injury in diabetes, we believe that selective pharmacological inhibition of B1R signaling represents a more rational strategy for minimizing cardiac and renal complications of diabetes than inhibition of both receptors.