Abstract
An absolute or functional deficit in β-cell mass is a key factor in the pathogenesis of diabetes. We model obesity-driven β-cell mass expansion by studying the diabetes-resistant C57BL/6-Leptinob/ob mouse. We previously reported that cholecystokinin (Cck) was the most up-regulated gene in obese pancreatic islets. We now show that islet cholecystokinin (CCK) is up-regulated 500-fold by obesity and expressed in both α- and β-cells. We bred a null Cck allele into the C57BL/6-Leptinob/ob background and investigated β-cell mass and metabolic parameters of Cck-deficient obese mice. Loss of CCK resulted in decreased islet size and reduced β-cell mass through increased β-cell death. CCK deficiency and decreased β-cell mass exacerbated fasting hyperglycemia and reduced hyperinsulinemia. We further investigated whether CCK can directly affect β-cell death in cell culture and isolated islets. CCK was able to directly reduce cytokine- and endoplasmic reticulum stress-induced cell death. In summary, CCK is up-regulated by islet cells during obesity and functions as a paracrine or autocrine factor to increase β-cell survival and expand β-cell mass to compensate for obesity-induced insulin resistance.
Cholecystokinin is up-regulated in the islet by obesity. Islet CCK expression prevents obesity-induced hyperglycemia by increasing β-cell survival and expanding β-cell mass.
Type 1 and type 2 diabetes result from an absolute or relative deficiency in β-cell mass. In type 1 diabetes, autoimmune destruction of pancreatic β-cells results in a complete loss of β-cell mass and insulin production. In type 2 diabetes, β-cells cannot respond to the increased insulin requirement caused by insulin resistance, leading to a relative deficiency in β-cell mass and insulin production. Although obesity is a major risk factor for type 2 diabetes, most obese patients compensate for insulin resistance by expanding their β-cell mass (1,2). Thus, patients with impaired fasting glucose or type 2 diabetes have reduced β-cell mass compared with weight-matched controls (1,3). Patients with type 2 diabetes have diminished β-cell mass due to increased β-cell death (1). In fact, a β-cell mass threshold exists, wherein reductions below this level cause hyperglycemia (3). These observations have led to the investigation of mechanisms to expand β-cell mass by preventing β-cell death, to treat diabetes.
We model β-cell mass expansion using obese mice. The C57BL/6 mouse strain (B6), when made severely obese by the Leptinob mutation (ob/ob), is severely insulin resistant but not diabetic (4). The B6-ob/ob mouse avoids diabetes by increasing plasma insulin and expanding β-cell mass (5,6). We previously performed microarray analyses of islets from B6-lean and B6-ob/ob mice to identify transcripts correlated with β-cell mass expansion. We found that cholecystokinin (Cck) was the most up-regulated gene in the pancreatic islets of B6-ob/ob mice (7).
Cholecystokinin (CCK) has been extensively studied as a gastrointestinal hormone and a neuropeptide (8). In the gastrointestinal tract, CCK is secreted by duodenal I-cells to stimulate gallbladder contraction and pancreatic exocrine secretion through the CCK-A receptor (CCKAR). In the central nervous system, CCK modulates many behavioral functions including satiety, anxiety, and memory via the CCK-B receptor (CCKBR) (8).
CCK is known to play a role in glucose homeostasis. In rodents, CCK can stimulate insulin secretion in vivo or in pancreatic perfusions (9,10). In humans, CCK doses slightly above the physiological level stimulate insulin secretion (11). Because the source of plasma CCK is the duodenum and CCK is secreted in response to nutrients, it has been proposed as an incretin hormone, like glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). However, CCK receptor blockade does not diminish insulin secretion directly after a meal (12). These results led to the conclusion that CCK can stimulate insulin secretion but is not required for postprandial insulin secretion in humans. Two studies have also implicated CCK in β-cell mass regulation. CCK treatment reduces hyperglycemia and stimulates β-cell proliferation after pancreatic injury in rats (13,14).
We hypothesized that islet-derived CCK can protect an obese insulin-resistant mouse from developing hyperglycemia by aiding in β-cell mass expansion. We first confirmed the up-regulation of Cck expression by quantitative RT-PCR and determined which islet cell types express CCK. We next asked whether whole-body CCK deficiency causes a deficit in β-cell mass or disrupts glucose homeostasis. Finally, we determined whether CCK directly affects β-cell mass regulation in vitro and ex vivo.
Materials and Methods
Animals
CcklacZ mice (15) were back-crossed onto the C57BL/6 background for a minimum of 10 generations and bred with C57BL/6-Leptinob/+ mice to generate CcklacZ-ob/ob mice. Cck-eGFP transgenic mice were constructed using a bacterial artificial chromosome transgene that inserted eGFP into the endogenous Cck locus and contained 50–100 kb of 5′ and 3′ flanking intergenic DNA (16). C57BL/6-Ay/a (lethal yellow agouti) mice were fed a chow diet for 16 wk. For the diet-induced obesity study, BTBR mice were fed a high-fat diet (no. D00071501; 20% protein, 60% hydrogenated coconut oil, and 20% carbohydrate; Research Diets, Inc., New Brunswick, NJ) or a semipurified control diet (no. D12450B; 20% protein, 10% fat, and 70% carbohydrate; Research Diets) at weaning for 33 wk. All procedures were approved by University of Wisconsin Animal Care and Use Committee.
Reagents
Sulfated CCK-8 was purchased from Sigma (C2175; St. Louis, MO). Small interfering (si)-Scr and si-Cck oligonucleotides were purchased from Ambion (Austin, TX).
mRNA measurements
Pancreatic islets were isolated using collagenase digestion and hand-picked as previously described (17). RNA was extracted from islet, brain, and intestinal tissues with the RNeasy kit (QIAGEN, Valencia, CA) and synthesized into cDNA (Superscript III; Invitrogen, Carlsbad, CA). Quantitative RT-PCR with TaqMan probes (Applied Biosystems, Foster City, CA) was used to determine Cck, Cckar, and Cckbr mRNA levels, which were normalized to β-actin.
Protein measurements
CCK protein levels were measured by RIA (Alpco Diagnostics, Salem, NH). HPLC was carried out with a Waters Alliance (Milford, MA) HPLC system using a 4.6 × 250 Symmetry Shield RP 18 column to assay the forms of CCK. Islet cell extracts were sonicated in cold 0.1 N HCL, and protein concentration was determined by Bradford assay (Sigma). After loading, the column was eluted with a 60-min gradient of 27–30% acetonitrile in 0.1% trifluoroacetic acid at 1 ml/min. One-minute fractions were collected and dried before CCK RIA, which was performed as described (18). Antibodies against amidated sulfated CCK-8, glycine-extended CCK-8, and nonsulfated CCK-8 were used. Sulfated CCK-12, -22, and -33 standards were synthesized as described (19).
Histology
Pancreata used for immunofluorescence staining were fixed, sectioned, and stained as previously described (20). For localization of CCK expression using Cck-eGFP transgenic mice, pancreata from three lean and three ob/ob mice were studied. Pancreata were stained with anti-insulin antibody (20) and endogenous green fluorescent protein (GFP) was visualized. A representative image from each animal is displayed. For dual X-galactosidase (X-gal)/immunofluorescence staining of heterozygous CckLacZ/WT-ob/ob mice, sections were incubated with anti-insulin and antiglucagon antibodies as previously described (20). Immunofluorescence images were generated, and then X-gal staining was performed overnight. After X-gal staining, immunofluorescence images were generated again and overlaid with light micrographs generated at the same time. Pancreata from two CckLacZ/WT-ob/ob mice were studied; a representative islet image from each is shown.
For quantitation of 10-wk islet size and mass, four male mice from each genotype were examined. Every islet was imaged in randomly selected pancreatic sections until a minimum of 100 islets per animal were measured. Islet size was quantitated by counting β-cell nuclei. For quantitation of 14-wk islet size and islet mass, five male CckWT-ob/ob and four male CcklacZ-ob/ob pancreata were analyzed. Every islet was imaged in four pancreatic sections per animal. Individual islet areas (14 wk only) and the total pancreatic section area (10 and 14 wk) were measured with Metamorph (Molecular Devices, Sunnyvale, CA). Clusters of β-cells smaller than 2000 μm2 or seven nuclei (≤0.4% of total β-cell area) were analyzed separately.
Pancreata used for proliferating cell nuclear antigen (PCNA) and terminal deoxynucleotidyl-transferase dUPT nick end labeling (TUNEL) staining were identical to the 10-wk islet size and mass panel. TUNEL staining was performed using the DeadEnd Fluorometric TUNEL system (Promega, Madison, WI). Every islet from two to four pancreatic sections per animal was imaged for TUNEL analysis and normalized to total β-cell area. For PCNA staining (no. NA03; Calbiochem, La Jolla, CA), every islet was imaged in randomly selected pancreatic sections until a minimum of 100 islets per animal were measured; the total number of PCNA-positive β-cells was normalized to total number of β-cells.
Plasma measurements
Plasma for all measurements was collected by retroorbital sinus bleeds on nonanesthetized mice after a 4-h fast. Glucose and insulin were measured as previously described (4). Glucagon was measured by RIA (Millipore, Bedford, MA).
Glucose tolerance test (GTT) and insulin tolerance test (ITT)
For GTT, 10-wk-old male mice were fasted overnight before an ip injection of 2 g/kg dextrose in sterile saline. For ITT, nonfasting 14-wk-old male and female mice were given an ip injection of 20 U/kg Humulin (Eli Lilly & Co., Indianapolis, IN) in sterile saline. Glucose and insulin were measured as described above.
In vitro insulin secretion
Insulin secretion studies were performed on islets isolated from four CckWT-ob/ob and five CckLacZ-ob/ob 14-wk-old female mice as previously described (17). Briefly, intact islets were hand-picked, and three islets were used per static incubation condition. Secretion measurements were performed in triplicate per animal. Secreted insulin was measured as described above and normalized to insulin content.
In vivo islet proliferation measurement
The proliferation rate of islet cells was measured using the 2H2O labeling technique that has been applied to a wide range of cell types, including pancreatic islets (4,13,21). Briefly, the incorporation of 2H from 2H2O into the deoxyribose moiety of deoxyribonucleotides in replicating cells was measured by gas chromatography/mass spectrometry. To rapidly attain a stable 2H2O body water enrichment, mice were given an ip injection of 0.015 ml/g 2H2O at 3 or 8 wk of age. Mice were then given 8% 2H2O as drinking water until they were killed 2 wk later.
Propidium iodide staining
Islets were isolated from 14-wk-old mice and placed in Krebs-Ringer bicarbonate buffer with 0.5 μg/ml calcein AM (Molecular Probes, Eugene, OR; C-3100) and 2.5 μg/ml propidium iodide for 15 min at 37 C. Islets were visualized by epifluorescence microscopy; the number of dead cells was quantified by counting propidium iodide-positive nuclei and normalized to total islet area (calcein AM staining).
Cytotoxicity experiments
Mouse (MIN6-B1) insulinoma cells were a generous gift from Dr. Philippe A. Halban and were cultured as previously described (22). MIN6-B1 cells were transfected with si-Scr or si-Cck (Ambion) oligonucleotides overnight using Lipofectamine 2000 (Invitrogen). Cells were then allowed to incubate for 48 h before a cytokine cocktail was added for 24 h. The cytokine cocktail contained 10 ng/ml IL1-β (no. 1457 756; Roche Molecular Biochemicals, Indianapolis, IN) and 50 ng/ml TNF-α (no. 510-RT; R&D Systems, Minneapolis, MN). Islets from CckWT-ob/ob and CcklacZ-ob/ob mice were isolated from 10-wk-old male and female mice, immediately dispersed by cell dissociation solution (Sigma C-5789), and cultured for 24 h in RPMI 1640 medium with 1% fetal bovine serum and 1% antibiotic-antimycotic. The percentage of dead cells was measured by the CytoTox-Glo cytotoxicity assay (Promega).
Statistical analysis
Comparisons between genotypes were made by Student’s unpaired t tests unless otherwise noted. Islet size distribution comparisons were made by Kolmogorov-Smirnov tests to identify differences in histogram shape and ANOVA followed by Bonferroni-corrected Student’s t tests to look at islet size contribution to total islet area. All plasma glucose and insulin measurements were made using log10 transformed values to create normal Gaussian distributions. Plasma glucose and insulin comparisons were made on a log scale by ANOVA adjusting for sex, genotype and time nested within sample, and their interactions (see Fig. 4, A and B). GTT and ITT data were analyzed on a log scale using a linear mixed-effects model on subjects over time. Effects for genotype, time, and genotype by time were tested by adjusted (type 3) analysis. Sex-specific effects were included in experiments with males and females. Analyses were adjusted for initial levels as a covariate (Fig. 4). ITT data were also analyzed by the standard trapezoidal area-under-the-curve method. Briefly, each mouse was normalized to its initial starting glucose value, and then a trapezoidal area under the curve was determined for each mouse. Comparisons were made by Student’s t test on both combined and separated sexes. Comparisons in the β-cell line and islet cytotoxicity experiments were made by repeated-measures ANOVA followed by Bonferroni-corrected Student’s paired t tests (see Fig. 6, A and B). Sulfated CCK-8 rescue on islet cytotoxicity comparisons were made by ANOVA blocking on sample. This analysis was followed by a second ANOVA using the vehicle as a covariate to test for a CCK dose-dependent log-linear trend (Fig. 6C).
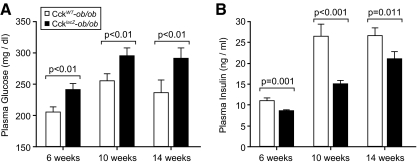
Figure 4.
Loss of CCK causes a diabetogenic phenotype. Fasting plasma glucose (A) and plasma insulin (B) levels of male and female 6-wk-old (n = 161–163), 10-wk-old (n = 155–168), and 14-wk-old (n = 75–105) CckWT-ob/ob and CcklacZ-ob/ob mice. Glucose and insulin values were log10 transformed. Comparisons were made by nested ANOVA adjusting for sex, genotype, time, and their interactions. For fasting glucose (A), no interactions of sex or time with genotype existed. For fasting insulin (B), genotype and sex interactions were not significant, but time by genotype interactions were significant (P = 0.012).
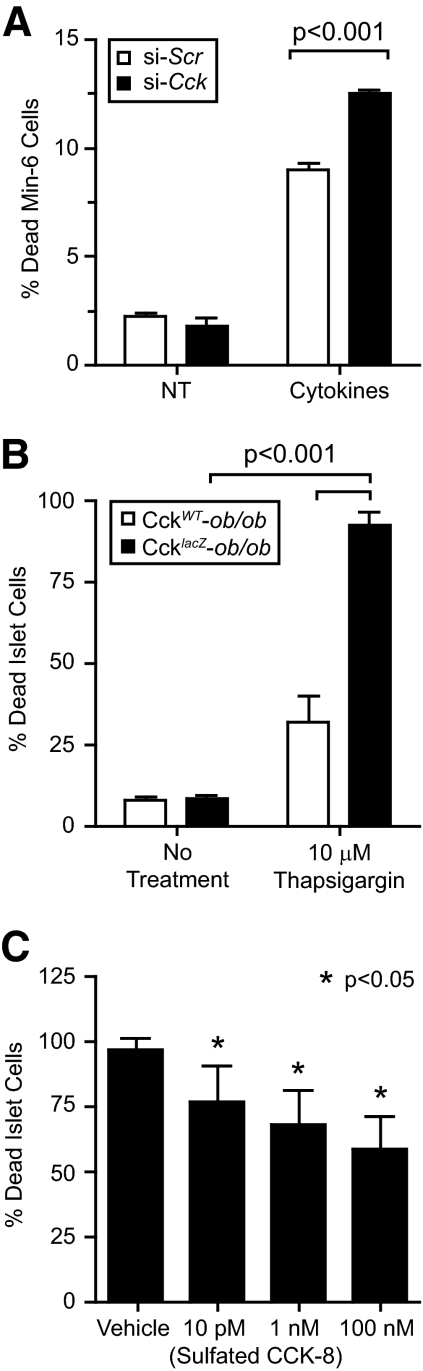
Figure 6.
CCK promotes β-cell survival. A, MIN-6 cells were reverse transfected with si-Scr negative control or si-Cck oligonucleotides (n = 3). MIN-6 were then incubated for 48 h before being treated for 24 h with IL-1β and TNF-α cytokine cocktail and cell death measured. Comparisons were made by repeated-measures ANOVA followed by Bonferroni-corrected Student’s paired t test. B, Isolated islets from CckWT-ob/ob (n = 9) and CcklacZ-ob/ob (n = 17) mice were dispersed and treated with 10 μm thapsigargin. Islet cells were incubated for 24 h, and cell death was measured. Comparisons were made by repeated-measures ANOVA followed by Bonferroni-corrected Student’s paired t test. C, Islets from CcklacZ-ob/ob (n = 8–11) mice were isolated, dispersed, and treated with 10 μm thapsigargin in the presence of increasing doses of sulfated CCK-8 peptide or vehicle control. Data were analyzed by ANOVA blocking on sample (P < 0.005). Using the vehicle as a covariate, CCK dose followed a log-linear trend to reduce islet cell death (P < 0.05).
Results
Pancreatic islets up-regulate CCK in response to obesity
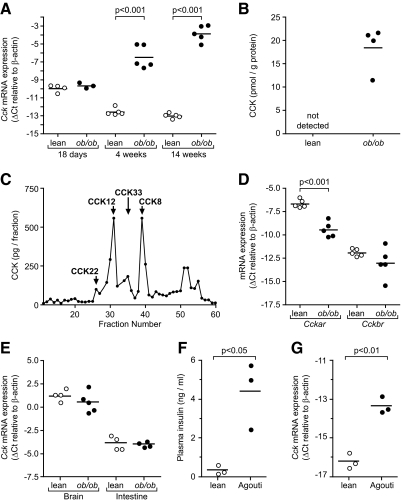
We previously identified Cck as the most up-regulated gene in ob/ob pancreatic islets (7). We measured islet Cck mRNA levels by quantitative RT-PCR in lean and ob/ob mice at various ages to understand the kinetics of Cck expression. Cck mRNA is present and equal in islets from lean and ob/ob mice at 18 d of age (Fig. 1A). At 4 wk, islet Cck abundance decreased in islets from lean mice and increased in islets from ob/ob mice, causing a 60-fold increase in Cck expression (Fig. 1A). By 14 wk, 500-fold more Cck mRNA was detected in islets from ob/ob mice (Fig. 1A). We measured total CCK protein abundance in islet samples by RIA. Islets from ob/ob mice contained approximately 15–20 pmol CCK/g total protein, whereas CCK was undetectable in islets from lean mice (Fig. 1B). We next measured CCK peptides by HPLC fractionation and subsequent RIA analysis with amidated and sulfated CCK standards to confirm that islets from ob/ob mice can process CCK into its bioactive forms. The two most abundant species detected were amidated and sulfated CCK-12 and CCK-8 (Fig. 1C). CCK-8 is known as the most bioactive peptide (23). Nonamidated, glycine-extended, or nonsulfated CCK intermediates were undetectable.
Figure 1.
CCK is up-regulated in pancreatic islets of ob/ob mice. A, Cck mRNA abundance in 18-d-, 4-wk-, and 14-wk-old islets (n = 3–5). Comparisons were made by ANOVA followed by Bonferroni-corrected Student’s unpaired t tests. B and C, CCK protein was measured by RIA analysis; B, total CCK levels were measured in 14-wk islets from lean and ob/ob islets (n = 4); C, islets from ob/ob mice were fractionated by HPLC, and RIA was performed on each fraction to determine CCK species. Antibodies for amidated and sulfated CCK are shown. Sulfated and amidated standards were used and labeled to help identify the different species. Nonamidated and nonsulfated antibodies were used, and no immunoreactivity was detected. D, Cckar and Cckbr mRNA abundance in 14-wk-old islets (n = 5 for each). E, Cck mRNA abundance in brain and intestinal tissue from 14-wk-old mice (n = 4–5 for each). F and G, Plasma insulin (F) and Cck mRNA (G) abundance in 16-wk-old agouti mice (n = 3). For all quantitative RT-PCR, TaqMan cycle threshold (Ct) values were normalized to β-actin levels to generate ΔCt values. Plasma insulin comparisons were made using log10-transformed values. All comparisons were made by Student’s unpaired t test unless otherwise stated.
We measured CCK receptor mRNA expression to determine whether a relevant CCK pathway exists in islets from ob/ob mice. We found that the CCKAR is present in islets from both lean and ob/ob mice but down-regulated by obesity (Fig. 1D). The CCKBR was present at very low abundance in islets from lean and ob/ob mice.
To determine whether obesity-dependent CCK up-regulation is ubiquitous to all CCK-expressing tissues, we assayed CCK mRNA expression in brain and intestine. CCK expression was not significantly different between lean and ob/ob mice in brain or intestinal tissue (Fig. 1E), suggesting that the obesity-induced increase of Cck expression is unique to islets.
We tested two other models of obesity and insulin resistance for up-regulation of islet Cck: agouti yellow mice and diet-induced obesity. Agouti yellow mice demonstrated hyperinsulinemia and 8-fold increased islet Cck expression by 16 wk of age (Fig. 1, F and G). We also measured islet Cck mRNA abundance in mice fed a high-fat diet for 33 wk. These mice had increased body weight, hyperinsulinemia, and 16-fold increased islet Cck mRNA expression (Supplemental Fig. 1, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals. org). These data demonstrate that obesity and/or insulin resistance, and not solely leptin deficiency, stimulate islet Cck expression.
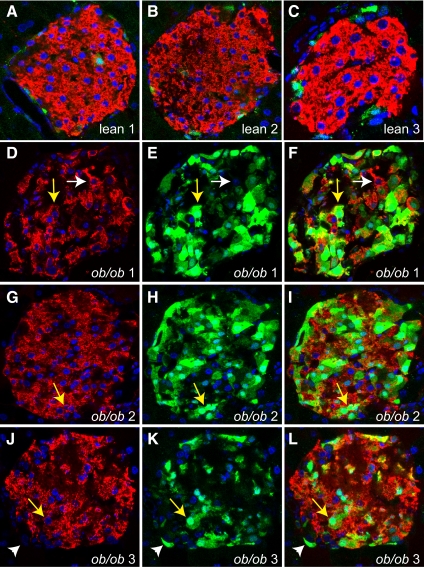
We used confocal immunofluorescence imaging to determine which islet cell types express CCK. Initial experiments using indirect immunofluorescent techniques, with antibodies raised against CCK, yielded inconclusive results due to high background or signal in CCK-deficient tissues. Therefore, we bred the ob/ob gene into transgenic mice that express eGFP driven by the endogenous Cck promoter to overcome this limitation (16). In islets from lean transgenic mice, very few cells expressing eGFP were detected (Fig. 2, A–C). In islets from ob/ob transgenic mice, eGFP expression increased dramatically and commonly costained with insulin (Fig. 2, D–L, yellow arrows). Interestingly, not all insulin-positive cells expressed GFP (Fig. 2, D–F, white arrows), and not all GFP-positive cells expressed insulin (Fig. 2, J–L, white arrowheads). To confirm and expand this result, we bred the ob/ob gene into the CckLacZ mouse (15), which is null for Cck because the LacZ gene is inserted into the Cck translational start site. These mice are whole-body CCK knockouts and express β-galactosidase in place of CCK. Because the X-gal precipitate blocks immunofluorescence signals, we first stained for insulin and glucagon (Supplemental Fig. 2, A and C). We then stained the same sections for X-gal, insulin, and glucagon the following day (Supplemental Fig. 2, B and D). We found the X-gal stain in both glucagon-positive (white arrows) and insulin-positive (yellow arrows) cells. CCK is therefore up-regulated by obesity and expressed in α-cells and β-cells of islets from ob/ob mice.
Figure 2.
CCK is up-regulated and expressed in α- and β-cells of ob/ob pancreatic islets. Immunofluorescence images of lean (A–C) and ob/ob (D–L) islets from Cck-eGFP transgenic mice. Insulin is stained red, nuclei are blue using 4′,6-diamidino-2-phenylindole (DAPI) stain, and GFP is green by autofluorescence. Each picture of a lean islet (A–C) is representative of an individual mouse (n = 3). Each ob/ob islet is separated into its insulin and DAPI (D, G, and J), GFP and DAPI (E, H, and K), and merged layers (F, I, and L) and is representative of an individual mouse (n = 3). Examples of β-cells costaining with GFP are indicated by yellow arrows. Examples of β-cells not costaining for GFP are indicated by white arrows. Examples of non-β-cells staining for GFP are indicated by white arrowheads.
CCK deficiency causes reduced islet size and β-cell mass
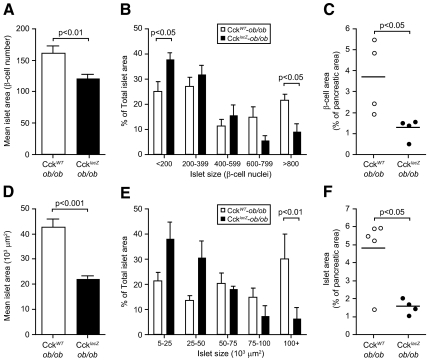
We measured islet size, fractional islet area, and fractional β-cell area by quantitative morphometry to assess the role of CCK in β-cell mass regulation, comparing CckWT-ob/ob to CckLacZ-ob/ob mice. At 10 wk, islets from CckLacZ-ob/ob mice were 25% smaller than controls (Fig. 3A). The islet size distribution demonstrated a greater number of large islets in CckWT-ob/ob pancreata (P < 0.07). We further analyzed the contribution of total islet area for differing islet sizes. This parameter was altered so that CckLacZ-ob/ob pancreata contained 12% more total area in their small islets and 12% less total area in their large islets (Fig. 3B). Decreased islet size in CckLacZ-ob/ob pancreata led to a 65% reduction in fractional β-cell area (Fig. 3C). By 14 wk, these phenotypes became more severe. Average islet size decreased by 50% in CckLacZ-ob/ob pancreata (Fig. 3D), and the largest islets comprised less than 7% of the total islet area in CckLacZ-ob/ob pancreata vs. 30% in CckWT-ob/ob pancreata (Fig. 3E). Fractional islet area decreased 67% in CckLacZ-ob/ob pancreata vs. CckWT-ob/ob controls (Fig. 3F). The total number of islets per pancreatic area, the average number of small β-cell clusters, the average β-cell size, and the average pancreatic wet weight were not different between groups (data not shown). Because fractional β-cell area was reduced and pancreatic weight was unchanged, these data suggest that CCK deficiency causes reduced β-cell mass.
Figure 3.
CCK deficiency results in reduced islet size and fractional β-cell area. Islet size and β-cell fractional area analysis from 10-wk-old (A–C) male CckWT-ob/ob (n = 4) and CcklacZ-ob/ob (n = 4) mice and 14-wk-old (D–F) male CckWT-ob/ob (n = 5) and CcklacZ-ob/ob mice (n = 4). A and D, Mean islet size of all islets analyzed (n > 225 for each genotype). Comparisons were made by Student’s unpaired t test. Histogram shape analysis was also performed by Kolmogorov-Smirnov test and found to be marginally different (P < 0.07). B and E, Total islet area contribution per mouse based upon islet size. Comparisons were made by ANOVA followed by Bonferroni-corrected Student’s unpaired t tests. C and F, Fractional β-cell (C) or islet (F) area as a percentage of total pancreatic sectional area. No difference in β-cell size or pancreatic wet weight was detected. Comparisons were made by Student’s unpaired t tests.
Loss of CCK results in a diabetogenic phenotype
We measured fasting plasma glucose and insulin to determine whether CCK deficiency affects the metabolic phenotype of CcklacZ-ob/ob mice. CcklacZ-ob/ob mice had increased fasting plasma glucose at 6, 10, and 14 wk (Fig. 4A). This increase in fasting glucose was accompanied by a reduction in fasting plasma insulin (Fig. 4B). No interactions between genotype and sex were observed. Because CCK is expressed in α-cells, we also measured fasting plasma glucagon levels at 10 wk. No differences in fasting plasma glucagon were detected (data not shown). Because CCK stimulates insulin secretion in mice (9), we measured the insulin secretory capacity of CcklacZ-ob/ob mice. We performed ip GTT at 10 wk (Supplemental Fig. 3, A and B) to assess the contribution of islet-derived CCK in vivo and avoid effects of CCK on gastric emptying (24) and as an incretin. We also measured in vitro glucose-stimulated insulin secretion in isolated islets at 14 wk (Supplemental Fig. 3C). CcklacZ-ob/ob mice had no deficit in glucose tolerance or insulin secretion after ip GTT in vivo (Supplemental Fig. 3, A and B) or glucose-stimulated insulin secretion in vitro (Supplemental Fig. 3C). In addition, we measured the glucagon and insulin content of islets from CcklacZ-ob/ob and CckWT-ob/ob mice. We detected a 52% reduction in the insulin content of CcklacZ-ob/ob islets (P < 0.05) but no difference in islet glucagon (data not shown), reflecting reduced islet size and β-cell numbers without changes in α-cells. We also measured insulin sensitivity by ITT at 14 wk (Supplemental Fig. 3D). Because CcklacZ-ob/ob and CckWT-ob/ob mice differed in their initial fed glucose values, we analyzed the shape of their glucose disposal curves using each group’s starting values as covariate adjustments. No difference in the shape of the glucose disposal curve was detected between CcklacZ-ob/ob and CckWT-ob/ob mice (Supplemental Fig. 3D). This analysis agreed with the trapezoidal area-under-the-curve method, normalizing each mouse to its initial time zero glucose value. These data suggest that reduced β-cell mass, and not insulin secretory capacity or insulin sensitivity, causes impaired glucose homeostasis in CcklacZ-ob/ob mice.
CCK deficiency causes increased β-cell death in vivo
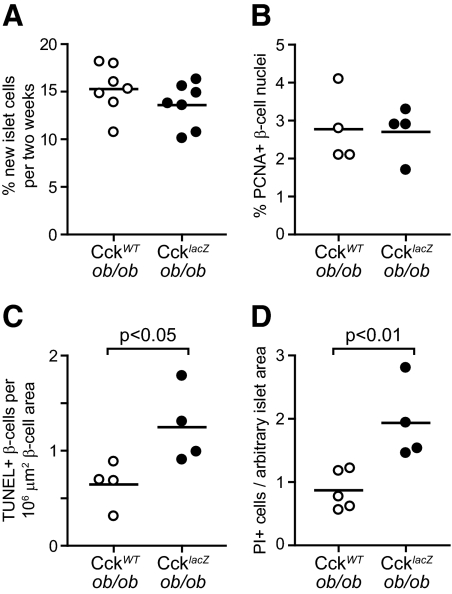
We measured the rates of β-cell proliferation and death to determine the cause of reduced β-cell mass in CcklacZ-ob/ob mice. We supplemented the drinking water with 8% 2H2O and treated CcklacZ-ob/ob and CckWT-ob/ob mice from 8–10 wk of age. We measured the enrichment of 2H in the deoxyribose moiety of deoxyribonucleotides isolated from islet DNA as an in vivo measure of islet cell proliferation (4,13,21). We found no difference in islet cell proliferation between CcklacZ-ob/ob and CckWT-ob/ob mice (Fig. 5A). We also measured β-cell proliferation by insulin and PCNA costaining in 10-wk-old mice. No change in β-cell proliferation was detected (Fig. 5B). Because Cck expression is induced between 18 d and 4 wk of age, we repeated our 2H2O-labeling study in mice from 3–5 wk of age. Again, no change in islet cell proliferation was detected (data not shown). These data suggest no β-cell proliferative difference between CcklacZ-ob/ob and CckWT-ob/ob mice, which, in the face of reduced β-cell mass, implies a difference in the β-cell death rate.
Figure 5.
CcklacZ-ob/ob mice have increased β-cell death with no change in proliferation. A, Measurement of 2H incorporation into islet DNA of 8- to 10-wk-old CckWT-ob/ob and CcklacZ-ob/ob mice (n = 7 for each). B, Percentage of PCNA-positive β-cells in 10-wk-old male CckWT-ob/ob and CcklacZ-ob/ob mice (n = 4 for each). C, Percentage of TUNEL-positive β-cells in 10-wk-old male CckWT-ob/ob and CcklacZ-ob/ob mice (n = 4 for each). D, Propidium iodide-positive cells per total islet area of freshly isolated islets from 14-wk-old male and female CckWT-ob/ob (n = 5) and CcklacZ-ob/ob (n = 4) mice. Comparisons were made by Student’s unpaired t tests.
We assessed β-cell death by TUNEL staining of pancreatic sections and propidium iodide staining of freshly isolated islets. In pancreatic sections of 10-wk-old mice, we found a 2-fold increase in the percentage of TUNEL-positive β-cells of CcklacZ-ob/ob mice (Fig. 5C). In freshly isolated islets of 14-wk-old mice, loss of CCK caused a 2-fold increase in the percentage of propidium iodide-positive islet cells (Fig. 5D). These data suggest that CCK deficiency causes reduced β-cell mass through increased β-cell death.
CCK protects β-cells from death in vitro and ex vivo
We next measured the direct effects of altering Cck expression on β-cell death in a mouse β-cell line exposed to cytokines. We chose cytokine-induced cell death because cytokines are increased by obesity and are an important regulator of β-cell survival in type 1 and type 2 diabetes (reviewed in Refs. 25 and 26). We used siRNAs to down-regulate Cck expression by 66% in Min6 cells, where Cck is highly expressed. Reduced Cck expression resulted in a 38% increase in cytokine-induced cell death (Fig. 6A).
We expanded these results by investigating the susceptibility of isolated islets from CcklacZ-ob/ob and CckWT-ob/ob mice to cytotoxic agents. Cytokine treatment stimulated very small increases in islet cell death from either genotype (data not shown). Thus, we studied thapsigargin, an endoplasmic reticulum (ER) stress-inducing agent, commonly studied in the context of β-cell survival (reviewed in Refs. 27 and 28). We investigated thapsigargin because cytokine-induced β-cell death occurs through the ER stress pathway (29), and well-documented evidence exists for ER stress in diabetes pathogenesis (reviewed in Refs. 27 and 28). Treatment of isolated CckWT-ob/ob islets with thapsigargin caused a 30% induction of cell death (Fig. 6B). However, CcklacZ-ob/ob islets were highly sensitive to ER stress, causing nearly 100% cell death. We next asked whether we could rescue CcklacZ-ob/ob islets through acute treatment with sulfated CCK-8 peptide. We found a significant reduction in islet cell death dependent upon CCK-8 peptide concentration. This caused a 39% reduction in islet cell death at 100 nm CCK-8 (Fig. 6C). These data demonstrate that CCK directly prevents β-cell death in vitro and ex vivo.
Discussion
Regulation of β-cell mass is a key factor in the pathogenesis of type 2 diabetes. In human studies, β-cell mass increases in obese patients without diabetes (1,2) but fails to increase to similar levels in patients with type 2 diabetes, due to increased β-cell death (1). We model nondiabetic obesity and expansion of β-cell mass with the B6-ob/ob mouse. Here, we report that the ob/ob mouse uses increased islet CCK expression as an adaptive mechanism that may prevent diabetes by expanding β-cell mass and increasing β-cell survival.
The existence of CCK and its receptors in islets has been previously documented. Rat pancreatic islets express CCK (30). Our data confirm a minor amount of Cck mRNA expression in lean mouse pancreatic islets. We further demonstrate an obesity-dependent up-regulation of CCK mRNA and protein expression in α- and β-cells (Figs. 1 and 2 and Supplemental Figs. 1 and 2). Our results agree with a recent report demonstrating increased Cck expression in the New Zealand mouse model of diet-induced obesity (31). Furthermore, we demonstrate that islets can posttranslationally process CCK into its most bioactive form, amidated and sulfated CCK-8 (Fig. 1C). This agrees with microarray data demonstrating that islets express the prohormone convertases, carboxypeptidase E, protein-tyrosine sulfotransferases, and peptidylglycine α-amidating monooxygenase (4), which posttranslationally process CCK into its active species (23,32). We also detected lesser amounts of intermediately processed CCK species, like CCK-22 and CCK-33 (Fig. 1C). These intermediate CCK molecules were likely generated by α-cells, which express prohormone convertase 2 only (33) and therefore could not fully process CCK. Previous immunofluorescence studies demonstrated that the CCKAR exists on both α- and β-cells (34,35,36), whereas the CCKBR is localized to α- and δ-cells (35,37,38). Our mRNA analysis for CCK receptors is consistent with this observation, demonstrating greater amounts of Cckar than Cckbr in islets (Fig. 1D).
Despite the presence of CCK and its receptors in the islet, genetic knockout studies define few pancreatic phenotypes for CCK, gastrin, or CCK receptor-deficient mice. Lean CcklacZ mice demonstrate normal pancreatic histology, but insulin secretory capacity and glucose homeostasis were not reported (15). Similarly, gastrin and CCK receptor knockout mice have normal pancreatic histology (39,40,41). These studies demonstrate that CCK, gastrin, and their receptors are not necessary for the development or maintenance of β-cell mass during unstressed conditions. Therefore, we studied obese CCK-deficient mice to test the role of increased islet CCK expression in adaptive β-cell mass expansion.
We propose a model wherein obese mice increase islet CCK expression, thereby expanding their β-cell mass by preventing β-cell death, likely through CCKAR signaling on the β-cell. CcklacZ-ob/ob mice have reduced β-cell mass (Fig. 3) and increased β-cell death (Fig. 5). We recognize that these observations occurred in a whole-body knockout animal and do not demonstrate a direct effect for CCK on β-cells. However, CCK protects cultured β-cells from cytokine-induced cell death (Fig. 6). Furthermore, isolated CcklacZ-ob/ob islets are highly sensitive to ER stress-induced cell death and are rescued by direct administration of sulfated CCK-8 (Fig. 6). These data demonstrate a direct prosurvival effect of CCK on the β-cell. These experiments do not conclusively identify the source of relevant CCK. It is possible that other cell types, like the duodenum, produce CCK to increase β-cell survival. The islet is the most likely source for CCK production because paracrine/autocrine mechanisms achieve greater CCK concentrations. Because β-cells express the CCKAR and not the CCKBR, these data suggest that signaling occurs through the CCKAR. This conclusion is supported by other studies. The OLETF rat is null for the Cckar and develops obesity-induced diabetes (42). Like CcklacZ-ob/ob mice, the OLETF rat cannot appropriately expand its β-cell mass to compensate for insulin resistance due to increased β-cell death (43,44), linking β-cell CCKAR signaling to β-cell survival. On the other hand, CCKBR signaling regulates β-cell mass by acting on non-β-cells. Gastrin, with other factors, increases β-cell mass by stimulating islet neogenesis (45,46,47,48). Additionally, gastrin-deficient mice demonstrate hypoglycemia and defective glucagon secretion (39), confirming the role of the CCKBR on non-β-cells. In summary, three lines of evidence suggest the CCKAR as the mediator of the prosurvival effect of CCK in vivo: 1) CCK can directly promote β-cell survival, 2) β-cells express only the CCKAR, and 3) the OLETF rat fails to expand its β-cell mass because of increased β-cell death due to a Cckar-null mutation.
A prosurvival effect for CCK through the CCKAR exists in nonislet tissues. Exogenous CCK treatment stimulates exocrine pancreatic growth and survival (49). Similarly, CCKAR antagonism reduces pancreatic weight (50). Outside the pancreas, CCK is overexpressed in Ewing bone tumors, and reduced CCK expression decreases tumor cell growth (51). CCKAR antagonism induces Ewing cell death and inhibits growth (52). Our data expand the prosurvival role for CCK and the CCKAR to include the β-cell.
The growth and survival effects of CCK on islet cells appear to be species specific. CCK treatment reduces hyperglycemia and stimulates β-cell proliferation after streptozotocin treatment or partial pancreatectomy in rats (13,14), demonstrating a role for CCK in rat β-cell proliferation. Similarly, OLETF rats demonstrate reduced β-cell proliferation after 70% partial pancreatectomy (53). Our data in mice demonstrate a role for CCK as an islet prosurvival factor with no effect on proliferation (Figs. 5 and 6). We previously studied the effects of adenoviral-dependent CCK overexpression on isolated islets from rats, mice, and humans (54). We found that CCK is sufficient to stimulate β-cell proliferation only in rat islets. Therefore, CCK enhances survival in rat (43,44) and mouse (Fig. 5 and 6) islets but is mitogenic only in rats (54).
The diabetogenic phenotype of CcklacZ-ob/ob mice could emanate from several sources. We observed that CcklacZ-ob/ob mice had a greater than 65% reduction in fractional β-cell area (Fig. 3). CcklacZ-ob/ob mice also demonstrated fasting hyperglycemia and reduced hyperinsulinemia (Fig. 4, A and B) without changes in insulin sensitivity or insulin secretion (Supplemental Fig. 3). This suggests that reduced β-cell mass causes the diabetogenic phenotype (Fig. 4). In agreement, human studies demonstrate that a β-cell mass threshold exists, below which fasting plasma glucose increases (3). We recognize that insulin tolerance tests are insensitive and insulin-sensing changes could still contribute to the diabetogenic phenotype. An additional regulator of glucose homeostasis is glucagon. CCK is also up-regulated in α-cells by obesity (Fig. 2 and Supplemental Fig 2). However, fasting plasma glucagon was unchanged (data not shown), suggesting that increased glucagon does not contribute to hyperglycemia. Similarly, islet glucagon content was equal, whereas islet insulin content was reduced in CcklacZ-ob/ob mice (data not shown), reflecting the reduction of β-cell numbers per islet and reduced β-cell mass. Another potential contributor to the diabetogenic phenotype of the CcklacZ-ob/ob mice is duodenal CCK, which was recently demonstrated to lower glucose production independently of insulin through a gut-brain-liver axis via the CCKAR in rats (55). Intestinal-neural networks cannot be excluded from our interpretation because of the whole-body nature of our CCK-deficient animal model. Because CcklacZ-ob/ob mice also demonstrate reduced hyperinsulinemia, a reduction in β-cell mass likely accounts for the fasting hyperglycemia.
The intestinal site of CCK production and its actions to stimulate insulin secretion and expand β-cell mass make it comparable to GLP-1 and GIP. Both hormones are incretins, and loss of their function results in reduced insulin secretion to oral glucose challenge (56,57). GLP-1 and GIP also prevent β-cell death induced by multiple cytotoxic agents including cytokines and thapsigargin in vitro (58,59,60,61,62).
The role of GIP during obesity-induced β-cell mass expansion is not clear. Lean GIP receptor knockout animals do not show a deficit in β-cell mass (56). GIP receptor knockout mice on a high-fat diet or in the ob/ob background are protected from obesity due to the effects of GIP on adipocytes (63). GIP receptor antagonism in ob/ob mice leads to improved glucose homeostasis and insulin sensitivity, accompanied by a reduction in β-cell mass due to reduced numbers of large islets (64), similar to CcklacZ-ob/ob pancreata (Fig. 3). However, this study does not discriminate between GIP receptor antagonism at the adipocyte, leading to improved insulin sensitivity, and GIP receptor antagonism at the β-cell, leading to reduced β-cell mass. These data demonstrate that GIP could be playing a role in obesity-driven β-cell mass expansion, but future studies on tissue-specific receptor knockouts may clarify this point.
The role of GLP-1 in the islet response to obesity is less important than that of GIP. Lean GLP-1 receptor-deficient mice demonstrate no change in overall β-cell mass but do demonstrate a reduction in the largest-sized islets (65). This is similar to CcklacZ-ob/ob mice (Fig. 3), demonstrating a role for both GLP-1 and CCK in the development of large islets. When the GLP-1 receptor knockout mouse was crossed into the ob/ob background, no phenotype was observed in islet mass or glucose homeostasis (66). The lack of an observed phenotype is likely because leptin stimulates GLP-1 secretion, and GLP-1 levels are therefore reduced in leptin-resistant and leptin-deficient models of obesity (67). These data imply that CCK is more important than GLP-1 in the physiological islet response to obesity-induced insulin resistance. Therefore, increased islet CCK expression could be an adaptive paracrine or autocrine mechanism in obese islets to compensate for the loss of endocrine GLP-1.
The mechanisms by which incretin hormones prevent β-cell death demonstrate significant overlap with CCK receptor-coupled pathways. Both GLP-1 and GIP receptors, like CCK receptors, are G protein-coupled receptors with similar downstream pathways like cAMP/protein kinase A, phosphatidylinositol 3-kinase/Akt, and stress-activated protein kinases (8,68). Future studies to determine which signaling pathways CCK activates, whether CCK can directly influence ER stress pathways, and whether CCK has overlapping, additive, or synergistic potential with other incretin hormones could lead to the development of new β-cell mass restorative therapeutics.
Supplementary Material
Acknowledgments
We thank Laura Vanderploeg for her artistic support on the figures.
Footnotes
This work was supported by Grants and Fellowships: J.A.L. was supported by a National Human Genome Research Institute training grant to the Genomic Sciences Training Program (5T32HG002760). P.W.R. was supported by National Institute on Aging Training Grant T32 AG20013. J.E.K. was supported by National Library of Medicine Grant T15 LM007359. J.A.D. was supported by National Institute of General Medical Sciences Training Grant T32 GM074904. M.K.H. was partially supported by a grant from the College of Natural Resources, University of California at Berkeley. A.D.A. was supported by National Institute of Diabetes and Digestive and Kidney Diseases (DK58037 and DK66369), a Hatch Grant from the University of Wisconsin College of Agriculture and Life Sciences (WIS01069), the Juvenile Diabetes Research Foundation (17-2007-1026), and Merck Research Laboratories.
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 9, 2010
Abbreviations: B6, C57BL/6 mouse strain; CCK, cholecystokinin; CCKAR, CCK-A receptor; ER, endoplasmic reticulum; GFP, green fluorescent protein; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide 1; GTT, glucose tolerance test; ITT, insulin tolerance test; PCNA, proliferating cell nuclear antigen; si, small interfering; TUNEL, terminal deoxynucleotidyl-mediated dUPT nick end labeling; X-gal, X-galactosidase.
References
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC 2003 β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52:102–110 [DOI] [PubMed] [Google Scholar]
- Klöppel G, Löhr M, Habich K, Oberholzer M, Heitz PU 1985 Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res 4:110–125 [DOI] [PubMed] [Google Scholar]
- Ritzel RA, Butler AE, Rizza RA, Veldhuis JD, Butler PC 2006 Relationship between β-cell mass and fasting blood glucose concentration in humans. Diabetes Care 29:717–718 [DOI] [PubMed] [Google Scholar]
- Keller MP, Choi Y, Wang P, Davis DB, Rabaglia ME, Oler AT, Stapleton DS, Argmann C, Schueler KL, Edwards S, Steinberg HA, Chaibub Neto E, Kleinhanz R, Turner S, Hellerstein MK, Schadt EE, Yandell BS, Kendziorski C, Attie AD 2008 A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome Res 18:706–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock T, Pakkenberg B, Buschard K 2003 Increased islet volume but unchanged islet number in ob/ob mice. Diabetes 52:1716–1722 [DOI] [PubMed] [Google Scholar]
- Tomita T, Doull V, Pollock HG, Krizsan D 1992 Pancreatic islets of obese hyperglycemic mice (ob/ob). Pancreas 7:367–375 [DOI] [PubMed] [Google Scholar]
- Lan H, Rabaglia ME, Stoehr JP, Nadler ST, Schueler KL, Zou F, Yandell BS, Attie AD 2003 Gene expression profiles of nondiabetic and diabetic obese mice suggest a role of hepatic lipogenic capacity in diabetes susceptibility. Diabetes 52:688–700 [DOI] [PubMed] [Google Scholar]
- Dufresne M, Seva C, Fourmy D 2006 Cholecystokinin and gastrin receptors. Physiol Rev 86:805–847 [DOI] [PubMed] [Google Scholar]
- Ahrén B, Hedner P, Lundquist I 1983 Interaction of gastric inhibitory polypeptide (GIP) and cholecystokinin (CCK-8) with basal and stimulated insulin secretion in mice. Acta Endocrinol 102:96–102 [DOI] [PubMed] [Google Scholar]
- Fehmann HC, Göke R, Göke B, Bächle R, Wagner B, Arnold R 1991 Priming effect of glucagon-like peptide-1 (7–36) amide, glucose-dependent insulinotropic polypeptide and cholecystokinin-8 at the isolated perfused rat pancreas. Biochim Biophys Acta 1091:356–363 [DOI] [PubMed] [Google Scholar]
- Ahrén B, Holst JJ, Efendic S 2000 Antidiabetogenic action of cholecystokinin-8 in type 2 diabetes. J Clin Endocrinol Metab 85:1043–1048 [DOI] [PubMed] [Google Scholar]
- Hildebrand P, Ensinck JW, Ketterer S, Delco F, Mossi S, Bangerter U, Beglinger C 1991 Effect of a cholecystokinin antagonist on meal-stimulated insulin and pancreatic polypeptide release in humans. J Clin Endocrinol Metab 72:1123–1129 [DOI] [PubMed] [Google Scholar]
- Chen S, Turner S, Tsang E, Stark J, Turner H, Mahsut A, Keifer K, Goldfinger M, Hellerstein MK 2007 Measurement of pancreatic islet cell proliferation by heavy water labeling. Am J Physiol Endocrinol Metab 293:E1459–E1464 [DOI] [PubMed] [Google Scholar]
- Kuntz E, Pinget M, Damgé P 2004 Cholecystokinin octapeptide: a potential growth factor for pancreatic β-cells in diabetic rats. JOP 5:464–475 [PubMed] [Google Scholar]
- Lacourse KA, Swanberg LJ, Gillespie PJ, Rehfeld JF, Saunders TL, Samuelson LC 1999 Pancreatic function in CCK-deficient mice: adaptation to dietary protein does not require CCK. Am J Physiol 276:G1302–G1309 [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N 2003 A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425:917–925 [DOI] [PubMed] [Google Scholar]
- Rabaglia ME, Gray-Keller MP, Frey BL, Shortreed MR, Smith LM, Attie AD 2005 α-Ketoisocaproate-induced hypersecretion of insulin by islets from diabetes-susceptible mice. Am J Physiol Endocrinol Metab 289:E218–E224 [DOI] [PubMed] [Google Scholar]
- Beinfeld MC, Meyer DK, Eskay RL, Jensen RT, Brownstein MJ 1981 The distribution of cholecystokinin immunoreactivity in the central nervous system of the rat as determined by radioimmunoassay. Brain Res 212:51–57 [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Aida C, Fujiwara H, Yagami T, Futaki S, Kogire M, Ida J, Inoue K 2001 Facile solid-phase synthesis of sulfated tyrosine-containing peptides: total synthesis of human big gastrin-II and cholecystokinin (CCK)-39. J Org Chem 66:1–10 [DOI] [PubMed] [Google Scholar]
- Clee SM, Yandell BS, Schueler KM, Rabaglia ME, Richards OC, Raines SM, Kabara EA, Klass DM, Mui ET, Stapleton DS, Gray-Keller MP, Young MB, Stoehr JP, Lan H, Boronenkov I, Raess PW, Flowers MT, Attie AD 2006 Positional cloning of Sorcs1, a type 2 diabetes quantitative trait locus. Nat Genet 38:688–693 [DOI] [PubMed] [Google Scholar]
- Neese RA, Misell LM, Turner S, Chu A, Kim J, Cesar D, Hoh R, Antelo F, Strawford A, McCune JM, Christiansen M, Hellerstein MK 2002 Measurement in vivo of proliferation rates of slow turnover cells by 2H2O labeling of the deoxyribose moiety of DNA. Proc Natl Acad Sci USA 99:15345–15350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilla V, Webb G, Rickenbach K, Maturana A, Steiner DF, Halban PA, Irminger JC 2003 Differential gene expression in well-regulated and dysregulated pancreatic β-cell (MIN6) sublines. Endocrinology 144:1368–1379 [DOI] [PubMed] [Google Scholar]
- Beinfeld MC 2003 Biosynthesis and processing of pro CCK: recent progress and future challenges. Life Sci 72:747–757 [DOI] [PubMed] [Google Scholar]
- Whited KL, Thao D, Lloyd KC, Kopin AS, Raybould HE 2006 Targeted disruption of the murine CCK1 receptor gene reduces intestinal lipid-induced feedback inhibition of gastric function. Am J Physiol 291:G156–G162 [DOI] [PubMed] [Google Scholar]
- Mandrup-Poulsen T 2003 Apoptotic signal transduction pathways in diabetes. Biochem Pharmacol 66:1433–1440 [DOI] [PubMed] [Google Scholar]
- Lee SC, Pervaiz S 2007 Apoptosis in the pathophysiology of diabetes mellitus. Int J Biochem Cell Biol 39:497–504 [DOI] [PubMed] [Google Scholar]
- Eizirik DL, Cardozo AK, Cnop M 2008 The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 29:42–61 [DOI] [PubMed] [Google Scholar]
- Scheuner D, Kaufman RJ 2008 The unfolded protein response: a pathway that links insulin demand with β-cell failure and diabetes. Endocr Rev 29:317–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A, Eizirik DL 2005 Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic β-cells. Diabetes 54:452–461 [DOI] [PubMed] [Google Scholar]
- Shimizu K, Kato Y, Shiratori K, Ding Y, Song Y, Furlanetto R, Chang TM, Watanabe S, Hayashi N, Kobayashi M, Chey WY 1998 Evidence for the existence of CCK-producing cells in rat pancreatic islets. Endocrinology 139:389–396 [DOI] [PubMed] [Google Scholar]
- Dreja T, Jovanovic Z, Rasche A, Kluge R, Herwig R, Tung YC, Joost HG, Yeo GS, Al-Hasani H 2010 Diet-induced gene expression of isolated pancreatic islets from a polygenic mouse model of the metabolic syndrome. Diabetologia 53:309–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld JF 2006 The endoproteolytic maturation of progastrin and procholecystokinin. J Mol Med 84:544–550 [DOI] [PubMed] [Google Scholar]
- Rouillé Y, Westermark G, Martin SK, Steiner DF 1994 Proglucagon is processed to glucagon by prohormone convertase PC2 in αTC1-6 cells. Proc Natl Acad Sci USA 91:3242–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourassa J, Lainé J, Kruse ML, Gagnon MC, Calvo E, Morisset J 1999 Ontogeny and species differences in the pancreatic expression and localization of the CCK(A) receptors. Biochem Biophys Res Commun 260:820–828 [DOI] [PubMed] [Google Scholar]
- Julien S, Lainé J, Morisset J 2004 The rat pancreatic islets: a reliable tool to study islet responses to cholecystokinin receptor occupation. Regul Pept 121:73–81 [DOI] [PubMed] [Google Scholar]
- Kageyama H, Kita T, Horie S, Takenoya F, Funahashi H, Kato S, Hirayama M, Young Lee E, Sakurai J, Inoue S, Shioda S 2005 Immunohistochemical analysis of cholecystokinin A receptor distribution in the rat pancreas. Regul Pept 126:137–143 [DOI] [PubMed] [Google Scholar]
- Morisset J, Wong H, Walsh JH, Lainé J, Bourassa J 2000 Pancreatic CCK(B) receptors: their potential roles in somatostatin release and δ-cell proliferation. Am J Physiol 279:G148–G156 [DOI] [PubMed] [Google Scholar]
- Saillan-Barreau C, Dufresne M, Clerc P, Sanchez D, Corominola H, Moriscot C, Guy-Crotte O, Escrieut C, Vaysse N, Gomis R, Tarasova N, Fourmy D 1999 Evidence for a functional role of the cholecystokinin-B/gastrin receptor in the human fetal and adult pancreas. Diabetes 48:2015–2021 [DOI] [PubMed] [Google Scholar]
- Boushey RP, Abadir A, Flamez D, Baggio LL, Li Y, Berger V, Marshall BA, Finegood D, Wang TC, Schuit F, Drucker DJ 2003 Hypoglycemia, defective islet glucagon secretion, but normal islet mass in mice with a disruption of the gastrin gene. Gastroenterology 125:1164–1174 [DOI] [PubMed] [Google Scholar]
- Kopin AS, Mathes WF, McBride EW, Nguyen M, Al-Haider W, Schmitz F, Bonner-Weir S, Kanarek R, Beinborn M 1999 The cholecystokinin-A receptor mediates inhibition of food intake yet is not essential for the maintenance of body weight. J Clin Invest 103:383–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhans N, Rindi G, Chiu M, Rehfeld JF, Ardman B, Beinborn M, Kopin AS 1997 Abnormal gastric histology and decreased acid production in cholecystokinin-B/gastrin receptor-deficient mice. Gastroenterology 112:280–286 [DOI] [PubMed] [Google Scholar]
- Takiguchi S, Takata Y, Funakoshi A, Miyasaka K, Kataoka K, Fujimura Y, Goto T, Kono A 1997 Disrupted cholecystokinin type-A receptor (CCKAR) gene in OLETF rats. Gene 197:169–175 [DOI] [PubMed] [Google Scholar]
- Huang Q, Bu S, Yu Y, Guo Z, Ghatnekar G, Bu M, Yang L, Lu B, Feng Z, Liu S, Wang F 2007 Diazoxide prevents diabetes through inhibiting pancreatic β-cells from apoptosis via Bcl-2/Bax rate and p38-β mitogen-activated protein kinase. Endocrinology 148:81–91 [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhang N, He M, Yang Z, Tong W, Wang Q, Hu R 2008 Increased β-cell apoptosis and impaired insulin signaling pathway contributes to the onset of diabetes in OLETF rats. Cell Physiol Biochem 21:445–454 [DOI] [PubMed] [Google Scholar]
- Rooman I, Bouwens L 2004 Combined gastrin and epidermal growth factor treatment induces islet regeneration and restores normoglycaemia in C57BL6/J mice treated with alloxan. Diabetologia 47:259–265 [DOI] [PubMed] [Google Scholar]
- Suarez-Pinzon WL, Lakey JR, Rabinovitch A 2008 Combination therapy with glucagon-like peptide-1 and gastrin induces β-cell neogenesis from pancreatic duct cells in human islets transplanted in immunodeficient diabetic mice. Cell Transplant 17:631–640 [DOI] [PubMed] [Google Scholar]
- Suarez-Pinzon WL, Power RF, Yan Y, Wasserfall C, Atkinson M, Rabinovitch A 2008 Combination therapy with glucagon-like peptide-1 and gastrin restores normoglycemia in diabetic NOD mice. Diabetes 57:3281–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Pinzon WL, Yan Y, Power R, Brand SJ, Rabinovitch A 2005 Combination therapy with epidermal growth factor and gastrin increases β-cell mass and reverses hyperglycemia in diabetic NOD mice. Diabetes 54:2596–2601 [DOI] [PubMed] [Google Scholar]
- Zucker KA, Adrian TE, Bilchik AJ, Modlin IM 1989 Effects of the CCK receptor antagonist L364,718 on pancreatic growth in adult and developing animals. Am J Physiol 257:G511–G516 [DOI] [PubMed] [Google Scholar]
- Wisner Jr JR, McLaughlin RE, Rich KA, Ozawa S, Renner IG 1988 Effects of L-364,718, a new cholecystokinin receptor antagonist, on camostate-induced growth of the rat pancreas. Gastroenterology 94:109–113 [DOI] [PubMed] [Google Scholar]
- Carrillo J, García-Aragoncillo E, Azorín D, Agra N, Sastre A, González-Mediero I, García-Miguel P, Pestaña A, Gallego S, Segura D, Alonso J 2007 Cholecystokinin down-regulation by RNA interference impairs Ewing tumor growth. Clin Cancer Res 13:2429–2440 [DOI] [PubMed] [Google Scholar]
- Carrillo J, Agra N, Fernández N, Pestaña A, Alonso J 2009 Devazepide, a nonpeptide antagonist of CCK receptors, induces apoptosis and inhibits Ewing tumor growth. Anticancer Drugs 20:527–533 [DOI] [PubMed] [Google Scholar]
- Shima K, Zhu M, Mizuno A 1999 Pathoetiology and prevention of NIDDM lessons from the OLETF rat. J Med Invest 46:121–129 [PubMed] [Google Scholar]
- Lavine JA, Raess PW, Davis DB, Rabaglia ME, Presley BK, Keller MP, Beinfeld MC, Kopin AS, Newgard CB, Attie AD 2010 Contamination with E1A-positive wild-type adenovirus accounts for species-specific stimulation of islet cell proliferation by CCK: a cautionary note. Mol Endocrinol 24:464–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GW, Kokorovic A, Lam CK, Chari M, Lam TK 2009 Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab 10:99–109 [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Yamada Y, Yano H, Niwa H, Ban N, Ihara Y, Kubota A, Fujimoto S, Kajikawa M, Kuroe A, Tsuda K, Hashimoto H, Yamashita T, Jomori T, Tashiro F, Miyazaki J, Seino Y 1999 Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci USA 96:14843–14847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrocchi LA, Brown TJ, MaClusky N, Brubaker PL, Auerbach AB, Joyner AL, Drucker DJ 1996 Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med 2:1254–1258 [DOI] [PubMed] [Google Scholar]
- Li L, El-Kholy W, Rhodes CJ, Brubaker PL 2005 Glucagon-like peptide-1 protects β-cells from cytokine-induced apoptosis and necrosis: role of protein kinase B. Diabetologia 48:1339–1349 [DOI] [PubMed] [Google Scholar]
- Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ 2003 Glucagon-like peptide-1 receptor signaling modulates β-cell apoptosis. J Biol Chem 278:471–478 [DOI] [PubMed] [Google Scholar]
- Widenmaier SB, Ao Z, Kim SJ, Warnock G, McIntosh CH 2009 Suppression of p38 MAPK and JNK via Akt-mediated inhibition of apoptosis signal-regulating kinase 1 constitutes a core component of the β-cell pro-survival effects of glucose-dependent insulinotropic polypeptide. J Biol Chem 284:30372–30382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wideman RD, Yu IL, Webber TD, Verchere CB, Johnson JD, Cheung AT, Kieffer TJ 2006 Improving function and survival of pancreatic islets by endogenous production of glucagon-like peptide 1 (GLP-1). Proc Natl Acad Sci USA 103:13468–13473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusta B, Baggio LL, Estall JL, Koehler JA, Holland DP, Li H, Pipeleers D, Ling Z, Drucker DJ 2006 GLP-1 receptor activation improves β-cell function and survival following induction of endoplasmic reticulum stress. Cell Metab 4:391–406 [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K, Toyokuni S, Hiai H, Mizunoya W, Fushiki T, Holst JJ, Makino M, Tashita A, Kobara Y, Tsubamoto Y, Jinnouchi T, Jomori T, Seino Y 2002 Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 8:738–742 [DOI] [PubMed] [Google Scholar]
- Gault VA, Irwin N, Green BD, McCluskey JT, Greer B, Bailey CJ, Harriott P, O'harte FP, Flatt PR 2005 Chemical ablation of gastric inhibitory polypeptide receptor action by daily (Pro3)GIP administration improves glucose tolerance and ameliorates insulin resistance and abnormalities of islet structure in obesity-related diabetes. Diabetes 54:2436–2446 [DOI] [PubMed] [Google Scholar]
- Ling Z, Wu D, Zambre Y, Flamez D, Drucker DJ, Pipeleers DG, Schuit FC 2001 Glucagon-like peptide 1 receptor signaling influences topography of islet cells in mice. Virchows Arch 438:382–387 [DOI] [PubMed] [Google Scholar]
- Scrocchi LA, Hill ME, Saleh J, Perkins B, Drucker DJ 2000 Elimination of glucagon-like peptide 1R signaling does not modify weight gain and islet adaptation in mice with combined disruption of leptin and GLP-1 action. Diabetes 49:1552–1560 [DOI] [PubMed] [Google Scholar]
- Anini Y, Brubaker PL 2003 Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes 52:252–259 [DOI] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ 2007 Biology of incretins: GLP-1 and GIP. Gastroenterology 132:2131–2157 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.