Abstract
The hypothalamic-pituitary-gonadal (HPG) axis is involved in both the regulation of growth of the developing testis and in controlling spermatogenic and steroidogenic activity in the adult testis. Here, we develop a novel testicular xenografting model to examine to which degree testicular growth and function are controlled by intra- and extratesticular factors. Two or eight halves of neonatal Djungarian hamster testes were implanted into intact, hemicastrated, or castrated nude mouse recipients, and the development of the grafts under reduced or increased competition of testicular tissue was monitored and analyzed. We hypothesized that the outgrowth of the testicular grafts is influenced by the total amount of testicular tissue present in a host and that less testicular tissue in a host would result in more extended outgrowth of the grafts. Our results reveal that the hypothesis is wrong, because implanted hamster testis tissue irrespectively of the grafting condition grows to a similar size revealing an intrinsic mechanism for testicular growth. In contrast, similar size of seminal vesicle as bio-indicator of androgen levels in all hosts revealed that the steroidogenic activity is independent from the mass of testicular tissue and that steroid levels are extrinsically regulated by the recipient’s HPG axis. We propose that the model of testicular xenografting provides highly valuable options to explore testicular growth and endocrine regulation of the HPG axis.
The growth of testis tissue follows an intrinsic default pathway, whereas the endocrine function of this tissue is tightly regulated by the hypothalamic-pituitary-gonadal axis.
In the past decades, many studies have addressed the physiological context of testicular growth and development, as well as the control of the endocrine function of the testis during ontogenetic development. It is known that the development of the testis is regulated by gonadotropins released from the pituitary via hypothalamic input. Puberty is initiated by activation of the GnRH-pulse generator evoking a release of gonadotropins, which act on the testis to stimulate growth and differentiation of its somatic components. Although LH is more relevant for Leydig cell expansion and functional maturation, FSH acts on Sertoli cells and initiates their final proliferation and subsequent differentiation. However, the cellular mechanisms controlling the growth of the testis are poorly understood. After removal of one testis early in life, the contralateral testis showed intensified Sertoli cell proliferation, resulting in larger testes due to extended elongation of seminiferous tubules, an increase in Sertoli cell number (1,2), and an increased number of germ cells per Sertoli cells after puberty (3). Hemicastration leads to adjustment of steroidogenic activity to the loss of one testis as evidenced by Leydig cell hyperplasia (4,5,6,7). Hemicastration leads to an imbalance of the gonadal-pituitary feedback loop, thereby causing increasing FSH and LH levels. Accordingly, the effects of hemicastration can be mimicked by high-dose FSH treatment (8). Numerous studies have employed hemicastration and hypothyroidism in immature mammals, both treatments resulting in significant but independent stimulation of testis growth (2). It appears that hemicastration or FSH treatment acts primarily on the extent of tubular outgrowth, whereas hypothyroidism induces intensified mitotic expansion of immature Sertoli cells.
Here, we apply the model of xenografting to explore mechanisms of testicular growth and function. In contrast to the hemicastration model, xenografting not only allows to eliminate testis tissue but also to create scenarios to deplete or increase the load of testicular tissue within the host animal. Xenografting of testicular tissues has been explored to study testicular physiology (9,10,11,12,13,14). It has been shown in several donor species that xenografted immature testicular tissue grows and differentiates to full function in the host (15,16,17). For example, xenografted halves of newborn Djungarian hamster testes weighing approximately 2–5 mg at the time of grafting expanded to more than 40 mg after 8 wk of development (9). Xenografted halves of 5-d-old GFP+ rats increased in size from 5–10 mg to 120–150 mg 8 wk after xenografting (18). The grafted tissue discontinues growth after several weeks when functional maturation of the seminiferous epithelium occurs, mimicking the situation of testis development during ontogenesis. Grafted testicular tissue gains its full endocrine potential. Castrated hosts of testicular grafts show normal growth of seminal vesicles, indicating normal androgen levels being restored form the grafted tissues (9,15).
The aim of our study is to develop a novel model that can be used to determine whether tissue growth and/or androgen production in xenografts is controlled intrinsically or extrinsically. If maturation of xenografts is determined intrinsically, the final size of grafts and their spermatogenic activity should be independent of the number of grafts and the presence of one or both testes in the host. An intrinsic control of androgen production would be seen by higher androgen levels evoking an enlargement of seminal vesicles in intact hosts carrying many grafts and lower androgen levels in castrated hosts carrying few grafts. In contrast, if growth and endocrine activity of the grafted tissue is controlled extrinsically via the hypothalamus of the host, a more extended growth of grafts would be expected in animals carrying limited amounts of testicular tissue, and a decline of grafts size should be present in hosts carrying a high load of testicular tissue. In contrast, androgen levels should be independent from the differing amounts of testis tissue and should be comparable in all experimental groups, because androgen levels are controlled by feedback mechanisms between grafts and hypothamus/pituitary of the recipient mouse.
Materials and Methods
Animals
Breeding colonies of Djungarian hamsters (Phodopus sungorus) were maintained at the Rodent Housing Facilities of the University of Pittsburgh School of Medicine and at the Rodent Housing Facility of the Magee-Womens Research Institute. Animals were maintained in breeding pairs under a 12-h light, 12-h dark cycle regime, with food and water ad libitum. Newborn pups were collected on the day of birth and used as donors for neonatal hamster testicular tissue. Five- to 7-wk-old male immunodeficient nude mice (Crl:ν/ν-nuBR) were obtained from Charles River Laboratories (Wilmington, MA) and maintained at the Rodent Housing Facility until they reached 12 wk of age, when they were employed as graft recipients. Mice were assigned to one of six treatment groups (see below).
Animal husbandry and all experimental procedures involving the animals were performed in compliance with the University of Pittsburgh and the Magee-Womens Research Institute Guidelines for the Care and Use of Laboratory Animals.
Testis tissue preparation and cell culture
Newborn hamster pups were killed by decapitation, and the testes were excised. The epididymis was removed, and each testis was longitudinally cut in two halves. During preparation and before grafting, testis tissue was maintained for less than 2 h in ice-cold cell culture medium [L-15 Leibovitz (Mediatech, Inc., Herndon, VA), with added nonessential amino acids and antibiotics and 5% fetal calf serum].
Grafting
At 12 wk of age, recipient nude mice were assigned to one of six treatment groups (Table 1). Each treatment group was labeled using an identifier code composed of a number (2 or 8), which indicates the number of grafts the recipients in this group have received, and letters indicating whether the recipients in this group were castrated (C), hemicastrated (HC), or intact (I). Thus, mice in group “8C” were castrated and received eight neonatal hamster testis grafts (one graft = half a testis). Mice in group “8HC” were hemicastrated and received eight grafts. Mice in group “8I” were left intact and received eight grafts. Mice in group “2C” were castrated and received two grafts. Mice in group “2HC” were hemicastrated and received two grafts. Mice in group “2I” were left intact and received two grafts. Castrations were performed under anesthesia (ketamine, 80 mg/kg bodyweight, and Xylazine, 6 mg/kg bodyweight, in saline, via ip injection) through scrotal incisions. Either eight or two halves of newborn hamster testes per recipient were placed under the dorsal skin using cancer implant needles (G13; Popper and Sons, Staunton, VA).
Table 1.
Experimental groups
| No. of grafted halves of neonatal hamster testis | Intact recipients no. (ID) | Hemicastrated recipients no. (ID) | Castrated recipients no. (ID) |
|---|---|---|---|
| 2 | 4 (2I) | 4 (2HC) | 4 (2C) |
| 8 | 4 (8I) | 4 (8HC) | 4 (8C) |
2I, Intact recipients with two newborn hamster testis grafts; 8I, intact recipients with eight newborn hamster testis grafts; 2HC, hemicastrated recipients with two newborn hamster testis grafts; 8HC, hemicastrated recipients with eight newborn hamster testis grafts; 2C, castrated recipients with two newborn hamster testis grafts; 8C, castrated recipients with eight newborn hamster testis grafts. ID, identifier.
Sample retrieval and preparation
The grafts were harvested at 6 wk after implantation. For graft retrieval, nude mouse recipients were killed by exsanguination under deep anesthesia. The dorsal skin of each mouse was removed, and grafts were located on the interior surface of the skin. For all animals, body weight, weight of the unemptied seminal vesicles (as an indirect indicator for androgenic activity of the grafted cells and tissues in the castrate recipients), testis weight (if applicable), and the number of encountered grafts were determined. The weight of each graft was also recorded. Grafts were fixed in Bouin’s fixative overnight at room temperature and stored in 70% ethanol.
Sectioning and immunohistochemistry
The fixed samples were transferred and stored in 70% ethanol and embedded in paraffin; 5-μm sections were prepared on a motorized mictrotome (Microm HM360, Microm International GmbH, Walldorf, Germany). Slides were stained using the periodic acid-Schiff’s reagent followed by hematoxylin counterstaining.
Histological sample evaluations and image acquisition
Samples were analyzed using an Olympus BX61 microscope (Olympus, Melville, NY) with an attached Retiga 4000R camera (QImaging, Burnaby, British Columbia, Canada). Images were acquired digitally using Northern Eclipse 7.0 imaging software (Empix Imaging, Inc., Cheektowaga, NY) and were processed using Adobe Photoshop CS2 9.0 (Adobe Systems, Inc., San Jose, CA).
One slide carrying representative sections of each graft was evaluated by one observer (B.W.) in a blinded manner. The most advanced germ cell type present in the seminiferous epithelium of each tubular cross-section in this slide was evaluated to determine the extent of the maturation of the seminiferous epithelium.
Data evaluation
All data are presented ± sd. Statistical analysis was performed using SigmaStat 3.5 (Systat Software, Inc., San Jose, CA). Average body-, seminal vesicle-, testis-, and graft-weight were compared between the treatment groups. Statistical analysis was performed using the one-way ANOVA and the t test. The following histological data derived from xenografts were collected as relative data per graft: relative number (%) of (former) seminiferous tubules containing: 1) no seminiferous epithelium (“ghosts”), 2) a seminiferous epithelium with no germ cells (Sertoli cell only), 3) or with spermatogonia, 4) preleptotene or zygotene spermatocytes, 5) pachytene spermatocytes (P), 6) round or elongated spermatids as the most advanced germ cell type. Differences with P < 0.05 were considered statistically significant.
Results
Recipient status
Body weight
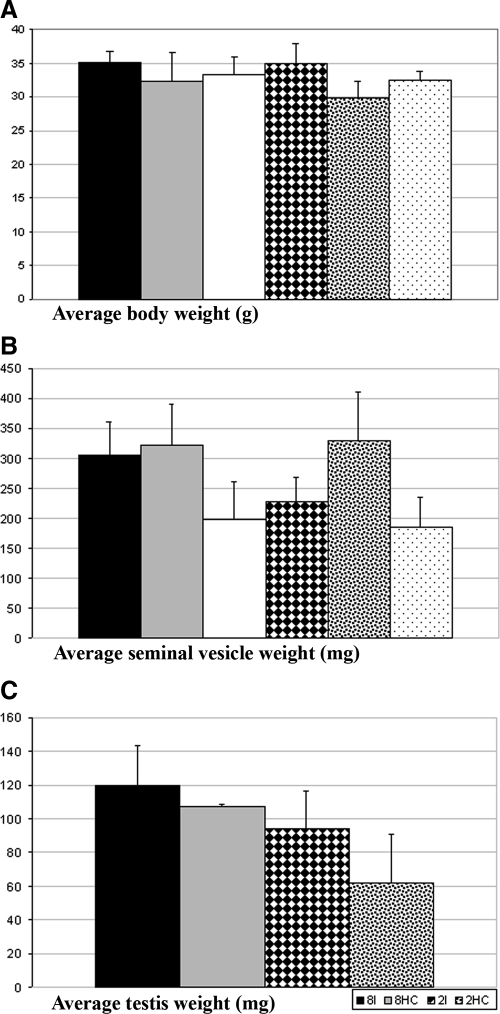
Recipient nude mice weighed between 27.6 and 38.1 g at the time of graft retrieval. Differences in average bodyweights between the six groups were statistically not significant (Fig. 1A).
Figure 1.
Graph showing the anatomical parameters (A, Average body weight; B, Average seminal vesicle weight; C, Average testis weight) of the recipient treatment groups. 8I, Intact recipients with eight newborn hamster testis grafts; 8HC, hemicastrated recipients with eight newborn hamster testis grafts; 8C, castrated recipients with eight newborn hamster testis grafts; 2I, intact recipients with two newborn hamster testis grafts; 2HC, hemicastrated recipients with two newborn hamster testis grafts; and 2C, castrated recipients with two newborn hamster testis grafts.
Seminal vesicle weight
The average weight of the seminal vesicles in the six groups ranged from 184.5 mg (±50.9) (2C) to 329.4 (±81.7) mg (2HC) at the time of graft retrieval. The seminal vesicle weights of the castrated recipients did not differ significantly from the hemicastrated or intact recipient, which had received the same number of grafts, respectively (Fig. 1B).
Testis weight
The individual testis weight ranged from 50.0 to 157.3 mg at the time of graft removal. The average testis weight of the four groups from which testes could be collected (2I, 2 HC, 8I, and 8HC) ranged from 61.88 (±28.91) to 119.7 (±23.91) mg (Fig. 1C).
Graft status: anatomy
Graft weight
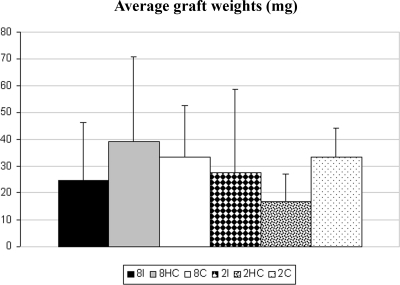
The individual graft weights ranged from 2 to 133 mg. The average graft weight of the six groups ranged from 16.57 (±10.69) to 39.10 (±31.74) mg (Fig. 2). The differences in the average graft weights between the groups were not statistically significant.
Figure 2.
Graph showing the graft weights (mg) in the different treatment groups. 8I, Intact recipients with eight newborn hamster testis grafts; 8HC, hemicastrated recipients with eight newborn hamster testis grafts; 8C, castrated recipients with eight newborn hamster testis grafts; 2I, intact recipients with two newborn hamster testis grafts; 2HC, hemicastrated recipients with two newborn hamster testis grafts; and 2C, castrated recipients with two newborn hamster testis grafts.
Grafts status: cellular composition
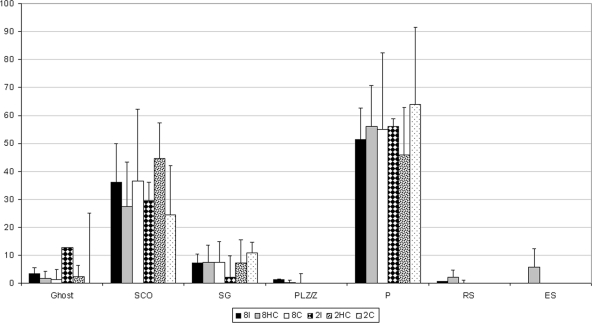
Within the grafts of the six groups, 0.00 (2C) to 12.79% (2I) did not contain a seminiferous epithelium, 24.43 (2C) to 44.71% (2HC) contained Sertoli cells but no germ cells, 2.04 (2I) to 10.69% (2C) contained spermatogonia, 0.00 (2C, 2HC, 2I) to 1.26% (8HC) preleptotene or zygotene spermatocytes, 45.88 (2HC) to 64.12% (2C) P, 0.00 to 2.18% (8HC) round spermatids, and 0.00 to 5.72% (8HC) contained elongated spermatids as the most advanced germ cell type (Fig. 3). The differences between the six groups were statistically not significant.
Figure 3.
Graph showing the status of the seminiferous epithelium (most advanced germ cell type) as percentage of all tubular structures in the grafts. 8I, Intact recipients with eight newborn hamster testis grafts; 8HC, hemicastrated recipients with eight newborn hamster testis grafts; 8C, castrated recipients with eight newborn hamster testis grafts; 2I, intact recipients with two newborn hamster testis grafts; 2HC, hemicastrated recipients with two newborn hamster testis grafts; and 2C, castrated recipients with two newborn hamster testis grafts; Ghosts, remnant of a seminiferous tubule containing no seminiferious epithelium; SCO, Sertoli cell only; SG, spermatogonia; PL/Z, preleptotene/zygotene spermatocytes; RS, rounds spermatids; ES, elongated spermatids.
Discussion
In our present study, we have used ectopic xenografting of newborn Djungarian hamster (P. sungorus) testes to assess mechanisms of growth, endocrine function, and maturation of the seminiferous epithelium in xenografts. Our novel strategy enabled us to generate exciting and new evidence that the growth of the seminiferous tubule compartment is primarily controlled intrinsically, whereas the endocrine activity of the testis is controlled by extrinsic mechanisms.
In our study, we have chosen the newborn hamster as testis tissue donors because our own previous studies have demonstrated that the newborn Djungarian hamster testis tissue provides probably the most reliable and efficient testis xenografting model, guaranteeing a high graft survival, a measurable graft growth, and a reliable maturation (appearance of postmeiotic germ cells) in most of the grafts within only very limited time (9,15).
As indirect readout of bio-available androgens, we determined the seminal vesicle weights. Castrated nude mice show seminal vesicle weights less than 50 mg (19,20), fully androgenized males show seminal vesicles between 150 and 450 mg. The determination of seminal vesicle weight thus serves as a highly accurate indicator of bio-available androgen in a mouse model.
Our results demonstrate that grafts established from halves of newborn Djungarian hamster testes grew in all six treatment groups from a few milligram at the time of grafting to an average of 17–39 mg 6 wk after grafting, the differences not being statistically significant between experimental groups. This indicates that the growth of newborn Djungarian hamster grafts was independent from the amount of testicular tissue in the host. The same outcome applies to the maturation and differentiation of the seminiferous epithelium and initiation of spermatogenesis. In all recipient groups, the seminiferous epithelium showed the same degree of development; 0–13% of the former seminiferous tubules did not contain a functional epithelium, 25–45% showed a Sertoli Cell only status, but 45–65% contained P. Differences between the treatment groups regarding the cellular status of the seminiferous epithelium of the grafts were not significant. We determined that within 6 wk, the spermatogenic epithelium had advanced into yielding postmeiotic germ cells in all experimental groups irrespective of the amount of testis tissue, indicating that the maturation of the epithelium was similar in all experimental groups. Thus, our results are in accordance with earlier studies (21,22), which demonstrated in a mouse-to-rat testis xenografting model that major components for the regulation of testicular graft growth and epithelial differentiation are intrinsic.
On the other hand, our study reveals that the control of the endocrine function of the grafted tissue is extrinsically modulated by the HGP axis of the recipient mouse. Each intact recipient that had received eight hamster testis xenografts (8I) carried an average total of 239 mg of its own testis tissue plus 198 mg of xenografted hamster testis, leading to a total of 437 mg of available testis tissue. In contrast, each castrated recipient that had only received two hamster testis xenografts (2C) carried an average total weight of 67 mg of testis tissue. Nevertheless, the average bodyweight was 35.1 g in group 8I and 32.4 in group 2C. The average seminal vesicle weight in group 8I was 304 mg, and in group 2C, it was 184 mg. Although the total amount of testicular tissue differed drastically between these two experimental groups, body weight as well as seminal vesicle weights are within the range of intact healthy adult nude mice. The differences between these two extreme groups are statistically not significant. Thus, although the total amount of testis tissue is more than six times higher between group 8I and group 2C, the androgen-dependent anatomical parameters remain undisturbed, indicating that the total androgen release from the testicular tissues is comparable in both groups. This implicates that the recipient mouse’s HGP axis performs a tight control on steroidogenic activity in those tissues thus maintaining the bio-available androgen levels always within the physiologically normal range.
Thus, this xenografting model has allowed us to demonstrate that the growth of grafted hamster testis tissue is controlled intrinsically, whereas the steroidogenic activity of the grafts is tightly controlled by the hypothamalic-pituitary-gonadal axis of each recipient mouse.
We conclude that our hamster testis xenografting model provides a new and exciting tool to study testicular growth and the endocrine regulation of the hypothalamic-pituitary-gonadal axis.
Acknowledgments
We thank Dr. M. Rodriguez and his colleagues (Magee-Womens Research Institute) for excellent support with the rodent colony management.
Footnotes
This work was supported by a Lance Armstrong New Investigator grant (to J.E.), a Ph.D. scholarship from the Ernst Schering Foundation (to K.G.), start-up funds from the University of Muenster (to S.S.), and the National Institutes of Health U54 HD 008610 Center Grant (to S.S.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 16, 2010
Abbreviation: P, Pachytene spermatocytes.
References
- Orth JM, Higginbotham CA, Salisbury RL 1984 Hemicastration causes and testosterone prevents enhanced uptake of [3H] thymidine by Sertoli cells in testes of immature rats. Biol Reprod 30:263–270 [DOI] [PubMed] [Google Scholar]
- Simorangkir DR, de Kretser DM, Wreford NG 1995 Increased number of Sertoli and germ cells in adult rat testes induced by synergistic action of transient neonatal hyperthyroidism and neonatal hemicastration. J Reprod Fertil 104:207–213 [DOI] [PubMed] [Google Scholar]
- Putra DK, Blackshaw AW 1982 Morphometric studies on compensatory testicular hypertrophy in the rat after hemicastration. Aust J Biol Sci 35:287–293 [PubMed] [Google Scholar]
- Frankel AI, Wright WW 1982 The hemicastrated rat: definition of a model for the study of the regulation of testicular steroidogenesis. J Endocrinol 92:213–223 [DOI] [PubMed] [Google Scholar]
- Agee J, Parsa C, Huntrakoon M 1988 Morphologic response of rat Leydig cells to hemicastration. Arch Androl 20:1–9 [DOI] [PubMed] [Google Scholar]
- Frankel AI, Chapman JC, Cook B 1989 The testicular response to hemicastration in the male rat cannot be maintained in vitro. J Endocrinol 121:43–48 [DOI] [PubMed] [Google Scholar]
- Frankel AI, Chapman JC, Cook B 1989 Testes are asymmetric in the testicular hemicastration response of the male rat. J Endocrinol 122:485–488 [DOI] [PubMed] [Google Scholar]
- Meachem SJ, McLachlan RI, de Kretser DM, Robertson DM, Wreford NG 1996 Neonatal exposure of rats to recombinant follicle stimulating hormone increases adult Sertoli and spermatogenic cell numbers. Biol Reprod 54:36–44 [DOI] [PubMed] [Google Scholar]
- Ehmcke J, Gassei K, Schlatt S 2008 Ectopic testicular xenografts from newborn hamsters (Phodopus sungorus) show better spermatogenic activity in aged compared with young recipients. J Exp Zool A Ecol Genet Physiol 309:278–287 [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Li MW, Penedo MC, Meyers S, Dobrinski I 2004 Accelerated maturation of primate testis by xenografting into mice. Biol Reprod 70:1500–1503 [DOI] [PubMed] [Google Scholar]
- Jahnukainen K, Ehmcke J, Schlatt S 2006 Testicular xenografts: a novel approach to study cytotoxic damage in juvenile primate testis. Cancer Res 66:3813–3818 [DOI] [PubMed] [Google Scholar]
- Rathi R, Zeng W, Megee S, Conley A, Meyers S, Dobrinski I 2008 Maturation of testicular tissue from infant monkeys after xenografting into mice. Endocrinology 149:5288–5296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnukainen K, Ehmcke J, Hergenrother SD, Schlatt S 2007 Effect of cold storage and cryopreservation of immature non-human primate testicular tissue on spermatogonial stem cell potential in xenografts. Hum Reprod 22:1060–1067 [DOI] [PubMed] [Google Scholar]
- Ehmcke J, Schlatt S 2008 Animal models for fertility preservation in the male. Reproduction 136:717–723 [DOI] [PubMed] [Google Scholar]
- Schlatt S, Kim SS, Gosden R 2002 Spermatogenesis and steroidogenesis in mouse, hamster and monkey testicular tissue after cryopreservation and heterotopic grafting to castrated hosts. Reproduction 124:339–346 [DOI] [PubMed] [Google Scholar]
- Dobrinski I, Rathi R 2008 Ectopic grafting of mammalian testis tissue into mouse hosts. Methods Mol Biol 450:139–148 [DOI] [PubMed] [Google Scholar]
- Wistuba J, Mundry M, Luetjens CM, Schlatt S 2004 Cografting of hamster (Phodopus sungorus) and marmoset (Callithrix jacchus) testicular tissues into nude mice does not overcome blockade of early spermatogenic differentiation in primate grafts. Biol Reprod 71:2087–2091 [DOI] [PubMed] [Google Scholar]
- Schlatt S, Westernströer B, Gassei K, Ehmcke J 2010 Donor-host involvement in immature rat testis xenografting into nude mouse hosts. Biol Reprod 82:888–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassei K, Schlatt S, Ehmcke J 2006 De novo morphogenesis of seminiferous tubules from dissociated immature rat testicular cells in xenografts. J Androl 27:611–618 [DOI] [PubMed] [Google Scholar]
- Gassei K, Ehmcke J, Wood MA, Walker WH, Schlatt S 2010 Immature rat seminiferous tubules reconstructed in vitro express markers of Sertoli cell maturation after xenografting into nude mouse hosts. Mol Hum Reprod 16:97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L, Suggs LC, Norton YM, Welsh Jr TH, Wilker CE 1996 Effect of hypophysectomy, sex of host, and/or number of transplanted testes on Sertoli cell number and testicular size of syngeneic testicular grafts in Fischer rats. Biol Reprod 54:960–969 [DOI] [PubMed] [Google Scholar]
- Johnson L, Suggs LC, Norton YM, Zeh WC 1996 Effect of developmental age or time after transplantation on Sertoli cell number and testicular size in inbred Fischer rats. Biol Reprod 54:948–959 [DOI] [PubMed] [Google Scholar]