Abstract
Recently, a subset of neurons was identified in the arcuate nucleus of the hypothalamus that colocalize three neuropeptides, kisspeptin, neurokinin B, and dynorphin, each of which has been shown to play a critical role in the central control of reproduction. Growing evidence suggests that these neurons, abbreviated as the KNDy subpopulation, are strongly conserved across a range of species from rodents to humans and play a key role in the physiological regulation of GnRH neurons. KNDy cells are a major target for steroid hormones, form a reciprocally interconnected network, and have direct projections to GnRH cell bodies and terminals, features that position them well to convey steroid feedback control to GnRH neurons and potentially serve as a component of the GnRH pulse generator. In addition, recent work suggests that alterations in KNDy cell peptides may underlie neuroendocrine defects seen in clinical reproductive disorders such as polycystic ovarian syndrome. Taken together, this evidence suggests a key role for the KNDy subpopulation as a focal point in the control of reproductive function in health and disease.
A subset of neurons in the arcuate nucleus of the hypothalamus, which co-localize kisspeptin, neurokinin B and dynorphin (“KNDy” cells), plays a critical role in the central control of reproductive function in health and disease.
Although it has been nearly 40 yr since the discovery of GnRH and its central role in reproduction (1), identification of the afferent neuronal groups and pathways through which internal hormonal signals (e.g. gonadal steroids, stress hormones, and nutrient signals) and external cues in the environment (e.g. social cues and day length) regulate GnRH release is still a major unresolved issue in the field of neuroendocrinology. Much of the recent focus on afferent control of GnRH secretion has been upon kisspeptin neurons located in the preoptic area (POA) and hypothalamus, based on the ability of mutations of the kisspeptin receptor to cause hypogonadotropic hypogonadism in humans and animal models (2,3). Compelling evidence now suggests that kisspeptin plays a key role in conveying the feedback effects of gonadal steroid hormones on GnRH neurosecretory activity during puberty (4,5), the estrous cycle (6), and seasonal reproductive transitions (7). In the past year, another neuropeptide, neurokinin B (NKB), has come under the same intense spotlight. Although NKB had been studied for its role in steroid feedback control of GnRH release since the 1990s (8,9,10), its recent celebrity comes from the discovery that human mutations in the gene encoding this peptide (called TAC3), or its receptor (TACR3), like that of the kisspeptin system, leads to a defect in the control of GnRH release and subsequent hypogonadism (11,12). In 2007 (13), a key observation was made when both NKB and kisspeptin, along with a third peptide, dynorphin (DYN), were shown to be colocalized in a single subpopulation in the hypothalamic arcuate nucleus (ARC) of the sheep (Fig. 1). DYN is an endogenous opioid peptide (EOP) that appears to mediate the inhibitory feedback control of progesterone on GnRH secretion (14,15). Thus, a single subpopulation of neurons in the ARC contains three distinct neuropeptides, each of which has been strongly implicated in the feedback regulation of GnRH neurons; for ease of reference [and because of its paronomastic relationship to Hershey’s kiss, the origin of kisspeptin (16)], we abbreviated the name of this cell group as the KNDy (coexpressing kisspeptin, NKB, and DYN) subpopulation.
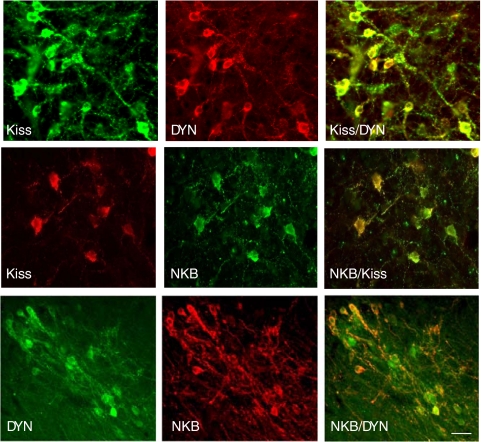
Figure 1.
Fluorescent images showing immunocytochemical colocalization of kisspeptin (Kiss) and DYN, kisspeptin and NKB, and DYN and NKB in KNDy cells of the ovine ARC. Bar, 20 μm. (Modified from Refs. 13 and 43.)
Although each of these three peptides is found in separate sets of neurons in other brain regions, recent evidence suggests that the colocalization of KNDy peptides seen in the ARC is unique among brain regions and is conserved across multiple mammalian species that include the rat (17), mouse (18), sheep (13), and goat (19) (for complete list of references, see Supplemental Table 1 published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). Complementary studies (albeit not yet multiple-label studies) suggest that kisspeptin, NKB, and DYN are also colocalized in a single subpopulation in the human infundibular (ARC) nucleus (20). Functional evidence has accumulated in parallel with these anatomical observations and strongly suggest that KNDy cells constitute a conserved, central node in the control of GnRH secretion, playing a key role in normal physiological control of reproduction as well as in abnormalities leading to reproductive endocrine disorders. In this minireview, we will provide an overview of this research, focusing on the anatomical features of KNDy cells and evidence for their roles in steroid feedback control of GnRH neurons in the generation of episodic GnRH secretion and in reproductive health and disease. It should be noted that this focus, and space constraints, preclude a detailed consideration of kisspeptin signaling; interested readers are directed to an excellent recent review on this subject (16).
Anatomical Features of KNDy Cells
A key neuroanatomical observation leading to the identification of the KNDy subpopulation was that each of the component neuropeptides (kisspeptin, NKB, and DYN) when examined in dual-label studies showed a very high degree of colocalization with gonadal hormone steroid receptors, specifically the α-isoform of the estrogen receptor (ERα) (17,21,22,23), the progesterone receptors (PR) (24), and the androgen receptor (25,26). The high degree of colocalization of KNDy cells with each of these receptors (e.g. in sheep, >95% for ERα and PR and >85% for androgen receptor) is particularly noteworthy when compared with other neuropeptide cell types in the ARC, such as β-endorphin (20% of which colocalize ERα) (27), neuropeptide Y (15% of which colocalize ERα) (28), and A12 dopaminergic cells (5–15% of which colocalize ERα) (29).
The question of whether KNDy cells directly contact GnRH neurons is key to understanding their functional role but is difficult to examine using standard tract-tracing techniques due to the scattered distribution of the GnRH population. Because colocalization of the three KNDy peptides in the ARC appears to be unique among brain areas examined to date, colocalization of multiple KNDy peptides in the same axon terminal can be used to determine the efferent targets of this subpopulation. Using this approach, we have demonstrated in the sheep that KNDy cells provide direct inputs to GnRH neurons in the POA as well as in the mediobasal hypothalamus (MBH) at the level of their cell bodies and dendrites (Fig. 2, A and B). This is consistent with data in the rat (30,31), mouse (32), rhesus monkey (33), and human (34) showing that kisspeptin, NKB, or DYN fibers directly contact GnRH cell bodies, although it is not known from these studies whether the inputs derive specifically from KNDy cells or from single-labeled NKB or kisspeptin cells located elsewhere. This evidence that KNDy neurons project to GnRH cell bodies in the POA is consistent with work in ewes using retrograde tract tracing that demonstrated ERα-containing neurons in the ARC projecting to the POA (35). Although one study concluded that ARC neurons do not project directly to POA GnRH neurons (36), the anterograde tracer in these two ewes appears to have been injected into the rostral ARC, where there are few KNDy cell bodies (13).
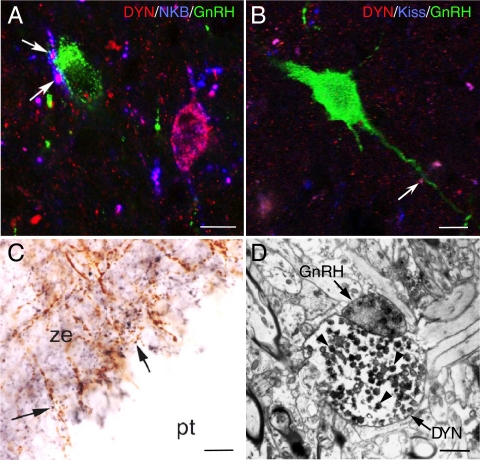
Figure 2.
Evidence that KNDy cells directly contact GnRH cell bodies (A and B) and terminals in the median eminence (C and D) in the sheep. A and B, Confocal images (1-μm optical sections) of triple-labeled sections through the ovine MBH showing DYN/NKB-positive (A) and DYN/Kiss-positive (B) axon terminals in close contact with a GnRH soma (A) and dendrite (B), respectively. Note, in A, the presence of a DYN/NKB-positive KNDy cell body nearby. Bars in A and B, 10 μm. C and D, Light microscopic (C) and electron microscopic (D) evidence of direct contacts between DYN and GnRH-positive terminals in the median eminence of a luteal-phase ewe. The section shown in C was processed for dual-label immunoperoxidase detection of DYN (blue-black) and GnRH (brown) and shows close associations (arrows) between DYN and GnRH fibers. D is an electron micrograph of dual-labeled DYN/GnRH section through the median eminence, generated using a preembedding dual-immunoperoxidase technique (110). A terminal containing DYN-positive dense-core vesicles (arrowheads) is seen in direct contact to a GnRH terminal that contains large neurosecretory granules. pt, Pars tuberalis; ze, zona externa of the median eminence. Bars in C and D, 10 μm (C) and 2 μm (D).
There is also evidence that KNDy cells project to the median eminence in rodents, monkeys, and sheep and establish close contacts with GnRH terminals in the external zone (30,33,37), although whether these terminals contain the appropriate receptors remains to be determined. In the sheep, fibers containing KNDy peptides form contacts onto GnRH terminals at both the light microscopic and electron microscopic level (Fig. 2, C and D), and preliminary results from retrograde tracing studies have confirmed that these inputs arise from KNDy cells of the ARC (38). Similarly, in the rat and monkey, there are close contacts between terminals containing kisspeptin or NKB and GnRH terminals in the median eminence at both the light microscopic (33,37) and electron microscopic (30) level, and anterograde tracer injections into the rat ARC label KNDy peptide-containing axons projecting to the median eminence (39).
Thus, a body of evidence (see Supplemental Table 2) strongly suggests that KNDy cells, and individual KNDy peptides, can influence the activity of GnRH cells by acting directly at the level of their cell bodies and/or their neurosecretory terminals in the median eminence. Recent studies on the stimulatory effects of kisspeptin are consistent with actions at both the median eminence (40) and GnRH cell bodies in the POA (41,42). However, evidence that some KNDy projections directly contact GnRH cells and terminals does not preclude the possibility that projections of KNDy cells to interneurons, such as single-labeled DYN or kisspeptin cells in the POA (13,43), play a major role in the control of GnRH release.
One of the most interesting features of KNDy cells is that most appear to receive input from other KNDy cells and thus form a reciprocally interconnected network within the ARC (17,24,39). KNDy-KNDy cell connections have been detected in the sheep and rat ARC (17,24), where axon terminals labeled for one or more KNDy peptides are in direct contact with KNDy-positive cell bodies. In the sheep, these contacts are seen in more than 90% of all KNDy neurons and have been confirmed at the electron microscopic level as representing bona fide synapses (24). Reciprocal contacts are absent among single-labeled DYN cells (24) and appear much less frequently among single-labeled kisspeptin cells (Lehman, M. N., unpublished observations) located in the POA in sheep; hence, this feature appears to be another distinguishing characteristic of the KNDy subpopulation. Based on this, it has been hypothesized that the KNDy network may play a role in the generation of episodic GnRH (see below) (18,44) as well as in the coordination/amplification of responses to internal and external signals that KNDy cells are attuned to (e.g. gonadal steroids). In either case, one would predict that appropriate postsynaptic receptors to KNDy peptides should also be expressed within KNDy cells. At present, this appears to be true for at least two of the three KNDy peptide receptors: the high-affinity receptor for NKB (NK3R) is colocalized in KNDy cells in rats (37), mice (18), and sheep (45), and the κ-EOP receptor (KOR), the opioid receptor subtype with highest specificity for DYN, is expressed within KNDy neurons in the mouse (18). Although the kisspeptin receptor (Kiss1r) is expressed predominantly in GnRH cells, it is also found in other hypothalamic regions but, at least in mice, is not expressed in the ARC nucleus, suggesting a lack of colocalization within KNDy cells (46).
Although we have defined KNDy cells on the basis of the presence of three peptides, this subpopulation may well contain other neurotransmitters as well as other receptors. Evidence in rats (30) and sheep (47) indicates that KNDy cells and their terminals colocalize the vesicular glutamate transporter-2 (vGLUT-2), suggesting that they are glutamatergic as well as peptidergic in phenotype. A majority of vGLUT-2-positive cells in the ARC of the sheep, like KNDy cells, are colocalized with ERα (48), and given the role of glutamate in conveying the feedback influence of estradiol (E2) during the preovulatory GnRH surge (49), it is tempting to speculate that coordinated release of kisspeptin and glutamate provide dual stimulatory signals to activate either the KNDy subpopulation and/or GnRH neurons during the follicular phase of the estrous cycle. Although there is evidence that N-methyl-d-aspartic acid receptors are expressed in GnRH cell bodies (50,51) as well as in GnRH terminals at the level of the median eminence (52), it is not known whether KNDy cells express these or other glutamate receptor subtypes.
Role in Steroid Feedback
In females, two modes of GnRH/LH secretion occur at different times during the ovarian cycle (53). Tonic GnRH/LH is secreted in an episodic pattern throughout most of the cycle and is controlled by the negative feedback actions of ovarian steroids, with E2 inhibiting pulse amplitude and progesterone inhibiting pulse frequency (54). The preovulatory GnRH/LH surge occurs at the end of the follicular phase and is induced by the positive feedback actions of high E2 concentrations from the preovulatory follicle(s). There is growing evidence implicating KNDy neurons as mediators for ovarian steroid feedback, but different peptide components within these neurons appear to mediate different types of steroid feedback.
Recent work has provided strong support that kisspeptin in ARC neurons mediates the negative feedback actions of E2 (16). In mice (16,23,55), sheep (56), and primates (57), in situ hybridization techniques have demonstrated that ovariectomy (OVX) increases and E2 decreases the number of Kiss1-expressing neurons in the ARC but not in more rostral areas (16,23,55,56), and a similar increase is observed in postmenopausal women (57). In mice, this effect of E2 is mediated by ERα (23), the receptor implicated in the negative feedback actions of E2 (58). Moreover, in this species, E2 inhibition of both tonic LH secretion and Kiss1 mRNA in the ARC occur via a nonclassical mechanism that does not involve estrogen response element signaling (59). Quantitative RT-PCR has also demonstrated an increase in Kiss1 mRNA in the ARC after OVX in primates (60) and possibly rats (RNA was extracted from tissue containing both the POA and ARC) (61). Using a similar approach, OVX increased and E2 treatment inhibited ARC Kiss1 mRNA in sheep (62). Moreover, a strong positive correlation between ARC Kiss1 mRNA and LH pulse amplitude was observed (62), suggesting that E2 inhibits GnRH/LH pulse amplitude by suppressing kisspeptin release from KNDy neurons during the breeding season.
Kisspeptin in KNDy neurons has also been implicated in the photoperiod-controlled changes in E2 negative feedback responsible for seasonal breeding in the ewe (53). Kisspeptin expression in the ARC, but not the POA, is suppressed under inhibitory photoperiod (63), and exogenous kisspeptin induces ovulation in anestrous ewes (64), suggesting that the low levels of this neuropeptide is limiting fertility at this time of year. Kisspeptin appears to play a similar role in seasonal breeding in male Syrian hamsters (65) but not in male Siberian hamsters (66). In contrast to its important role in E2 negative feedback, it seems unlikely that kisspeptin plays a role in the negative feedback actions of progesterone. Progesterone had no effect on Kiss1 mRNA in ewes (56,62), and there was no correlation between Kiss1 mRNA and LH pulse frequency (62).
Although the kisspeptin produced by KNDy neurons does not appear to mediate progesterone negative feedback, there is considerable evidence that DYN from KNDy cells plays this role. It is now generally accepted (53,54,67) that the inhibition of GnRH/LH pulse frequency by progesterone is mediated by an inhibitory EOP, and recent work in the ewe has implicated DYN as this EOP. Data supporting this hypothesis include 1) virtually all KNDy neurons contain PR (24); 2) local administration of an antagonist to the DYN receptor (KOR) to the MBH increased LH pulse frequency in luteal phase ewes, whereas antagonists to other EOP receptors did not (14); 3) OVX decreased prepro-DYN mRNA in the ARC (15); and 4) local administration of the PR antagonist RU486 to the ARC disrupted the negative feedback actions of progesterone (Goodman, R. L., unpublished data). This hypothesis is also consistent with work in humans that EOP mediate progesterone negative feedback (67) and that expression of prepro-DYN mRNA in ARC neurons increased in postmenopausal women (68). However, the data in rodents are contradictory. Pharmacological evidence supports an inhibitory role for DYN (18,69), but prepro-DYN mRNA in the ARC is inhibited by ovarian steroids in mice (18), and knockout of the KOR decreased LH secretion in OVX mice (18). It is also unlikely that ARC DYN plays a role in E2 negative feedback in mice because the effects of this steroid on DYN expression occur via the classical estrogen response element (59), which is not required for inhibition of LH secretion by E2 in this species. Thus, although DYN appears to mediate progesterone negative feedback in ewes and primates, its role in rodents is less clear, possibly because of the abbreviated luteal phase in cycling rats and mice.
Although there may be minor species differences in neural mediators of the negative feedback actions of ovarian steroids, there are major differences between rodents and larger mammals in the neural systems mediating the positive feedback actions of E2. In rodents, E2 acts in the anteroventral periventricular nucleus (AVPV) and adjacent areas (70) to induce the preovulatory GnRH/LH surge, and there is strong evidence that AVPV kisspeptin neurons play a critical role in this action of E2 (16). In contrast, in sheep (71) and primates (72,73), the positive feedback action of E2 occurs in the MBH, probably in either the ventromedial nucleus or the ARC. Moreover, recent work using the early immediate gene product Fos as an index of neural activation has implicated KNDy neurons in sheep in the induction of the preovulatory surge. The expression of Fos in KNDy neurons is dramatically increased both 1 h after injection of a surge-inducing dose of E2 (74) and during the preovulatory LH surge in ovary-intact ewes (47). Interestingly, Fos was also elevated in kisspeptin neurons in the POA in the latter, but not the former, ewes. This observation, together with the recent report that Kiss1 mRNA is increased in these neurons late in the follicular phase (74), suggests that this POA kisspeptin subpopulation may also contribute to the stimulation of GnRH secretion during the surge. Although these data implicate KNDy neurons in both the initial and final phases of E2 positive feedback, they do not indicate which neurotransmitter is involved. One obvious possibility is kisspeptin; exogenous kisspeptin induced an LH surge in follicular phase ewes (64), and Kiss1 mRNA levels increased in KNDy neurons late in the follicular phase (74). Glutamate is another possibility (53), and vGLUT-2 is colocalized in kisspeptin terminals that contact POA and MBH GnRH neurons (47). NKB is the third possibility because we have observed that activation of NK3R with an agonist (senktide) consistently stimulates LH secretion in the ewe (75). Even a brief treatment with senktide during the follicular phase produced a prolonged increase in LH concentrations to levels close to those seen during the preovulatory LH surge. The recent report that mutations in the gene for NK3R are associated with infertility in humans (11) is also consistent with a key stimulatory role for NKB. In contrast to sheep and humans, NKB appears to be predominantly inhibitory in rodents because senktide suppressed LH secretion in both rats (76) and mice (18).
Based on these data, the current working hypothesis for the role of KNDy neurons in the preovulatory LH surge in ewes is that E2 acts on these neurons to trigger a sequence of events that leads to release of NKB and kisspeptin 12–18 h later that then drives GnRH secretion during the surge. As noted above, the stimulatory as well as inhibitory effects of steroids mediated by KNDy neurons may be conveyed directly to GnRH neurons via KNDy efferents (Figs. 2 and 3) or indirectly via projections to other neurons, which then in turn project to GnRH cells or terminals. The data implicating KNDy neurons in both positive and negative feedback actions of E2 in ewes raise intriguing questions as to how the same set of neurons can have both inhibitory and stimulatory effects on GnRH secretion. The two most likely explanations are that subsets of KNDY neurons mediate different actions of E2 or that the same set of neurons respond differently to low and high concentrations of this steroid, perhaps through different intracellular signaling mechanisms (77). It is also important to note that other neural systems, possibly including noradrenergic neurons in the brainstem and somatostatin-containing neurons in the ventromedial nucleus, have also been implicated in the positive feedback actions of E2 in sheep (53); thus, it is likely E2 that acts via multiple pathways to induce the preovulatory LH surge in the ewe, as it does in the rat.
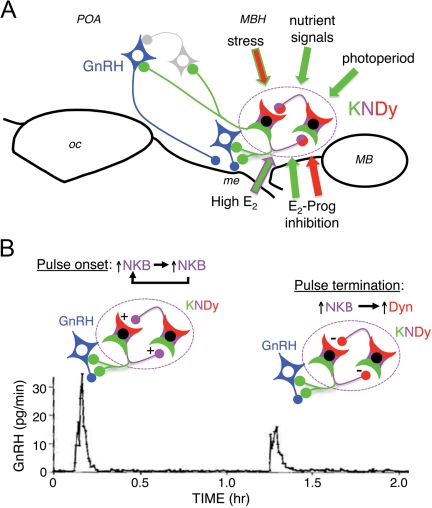
Figure 3.
A, Schematic diagram showing potential regulatory signals conveyed by KNDy cells to GnRH neurons and the KNDy peptides (kisspeptin, green; NKB, magenta; DYN, red) currently implicated in each action. Note that in some cases (e.g. positive feedback actions of E2 and stress), two colors indicate that more than one KNDy peptide may be involved. In addition to direct contacts onto GnRH neurons in the POA and MBH, the potential for indirect influence via interneurons (gray cell) is also shown. MB, Mammillary bodies; me, median eminence; oc, optic chiasm; Prog, progesterone. B. Hypothetical model by which synchronous activity among KNDy cells may regulate GnRH pulse frequency (see text for details). GnRH pulse data was redrawn from Moenter et al. (85). Prog, Progesterone.
In summary, KNDy neurons appear to play a key role in steroid negative feedback in a number of species, but their role in E2 positive feedback is more species specific. There is now strong and growing evidence that KNDy neurons mediate the positive feedback actions of E2 in ewes, although other neural systems are also involved. In contrast, it is equally clear that KNDy neurons are not part of the mechanisms responsible for the LH surge in rodents. Data in primates showing that E2 acts in the MBH and that kisspeptin and NKB are necessary for normal fertility raise the possibility that KNDy neurons participate in the positive feedback actions of E2, but there is no direct evidence for such a role in primates at this time.
Role in Generation of Episodic GnRH Secretion
Although the importance of the episodic pattern of GnRH secretion has been recognized for several decades (50), the mechanisms responsible for this pattern in vivo remain unclear. There is strong in vitro evidence that GnRH neurons are capable of episodic secretion without external input (78,79), but the ability of genetic disruptions of kisspeptin signaling to produce infertility argues that episodic secretion of GnRH in vivo is dependent on some input. The observation that KNDy neurons form an interconnected network presumably capable of producing a synchronous burst of firing has led to speculation that they may represent an important component of the hypothalamic pulse generator that drives episodic secretion of GnRH (18,19). The evolutionary conservation of KNDy neurons, and the data that at least two of their neuropeptides (kisspeptin and NKB) are essential for normal GnRH secretion in humans (2,3,11,12), point to a key role for this neural population in reproductive neuroendocrinology. Lesion data (54), and recordings of multi-unit electrical activity (MUA) coincident with LH pulses (80), point to the MBH as the site of the hypothalamic pulse generator in a number of species. Although speculative, the following observations allow the development of a testable working hypothesis for how KNDy neurons could help synchronize the activity of the GnRH neurons responsible for episodic secretion in sheep: 1) KNDy neurons contain NK3R in sheep (45) and mice (18); 2) NK3R agonists increase LH secretion in ewes (75) and MUA in goats (19); 3) ovine GnRH neurons contain Kiss1r (69) but not NK3R (45); 4) kisspeptin is required for episodic LH secretion (44,81) and kisspeptin pulses correlate with those of GnRH in the primate median eminence (82), but kisspeptin does not affect MUA in goats (83); 5) a nonspecific EOP receptor antagonist increases the amplitude and duration, but does not affect the rising phase, of each GnRH pulse in ewes and increases GnRH secretion between pulses (84); and 6) a KOR antagonist increased the frequency of MUA in goats (19).
Based on these observations, we propose that synchronous activity of KNDy neurons is controlled by stimulatory actions of NKB and inhibitory actions of DYN on these neurons and that their output to GnRH neurons is primarily via kisspeptin. The model shown in Fig. 3 predicts that each GnRH pulse is triggered by an initial increase in NKB from a few KNDy neurons, which stimulates further NKB release; the resulting positive feedback loop produces release of kisspeptin onto GnRH neurons and hence the extremely rapid increase in GnRH secretion that occurs at the onset of a pulse (85). NKB stimulation of KNDy neurons is predicted to also stimulate release of DYN, and the inhibitory actions of this EOP on KNDy neurons first begins to hold kisspeptin release in check and, after a few minutes, completely suppresses the activity of KNDy neurons, terminating the GnRH secretory episode and preventing any GnRH secretion between pulses. The action of DYN on KNDy neurons will also suppress DYN release, which eventually allows increased firing and the NKB release that triggers the next GnRH pulse. DYN could also act directly on GnRH neurons, but the model (Fig. 3) proposes an action on KNDy neurons based on data in rodents that KNDy neurons contain KOR (18), whereas GnRH neurons do not (86) (at this time, there are no data on the cellular location of KOR in ewes). Thus, we speculate that kisspeptin in KNDy cells is the output that drives GnRH pulses, that NKB is the trigger that initiates synchronous firing of KNDy neurons and the onset of each pulse, and that DYN is the peptide that shuts off the firing of KNDy neurons and terminates each pulse. It is interesting to note that a similar, albeit somewhat more complicated, model has recently been proposed for the role of KNDy neurons in episodic GnRH secretion in mice (18).
The hypothesis that episodic release of kisspeptin from KNDy neurons is important for pulsatile secretion of GnRH is apparently not consistent with reports that brief exposure of murine GnRH cell bodies to kisspeptin produced a sustained increase in firing rate lasting more than 20 min (41,42). One simple, albeit speculative, explanation for this apparent paradox is that in mice, kisspeptin actions at the cell body drive the preovulatory GnRH surge, whereas actions at the GnRH terminals (18,40) are important for episodic GnRH secretion. In ewes, KNDy neurons could act on GnRH terminals in the median eminence, because, as noted above, ovine KNDy neurons project to this area (38). Alternatively, GnRH cell bodies in the MBH receive abundant input from KNDy neurons and are selectively activated (based on Fos expression) when episodic LH secretion is stimulated (87).
Although this model must be rigorously tested, it does provide a simple explanation for the differential roles of kisspeptin and DYN in the negative feedback actions of E2 and progesterone, respectively, described above. Because kisspeptin release from KNDy neurons is driving GnRH release during a pulse, E2 inhibition of kisspeptin expression would be expected to inhibit GnRH pulse amplitude. In contrast, DYN terminates and prevents GnRH secretion between pulses. Thus, a stimulation of DYN release by progesterone might be expected to prolong the interval between pulses and thus reduce GnRH pulse frequency.
Role in Humans
Given the evolutionarily conserved role that KNDy cells appear to serve in the negative feedback control of GnRH secretion, and the observation that mutations in the genes for at least two components of KNDy cell signaling are associated with human infertility (2,3,11,12), it would not be surprising if other clinical neuroendocrine disorders were at least partly due to malfunctions of this cell group. One such disease that may be tied in part to the KNDy subpopulation is polycystic ovarian syndrome (PCOS). PCOS is among the most common of adult reproductive endocrine disorders (88) and is characterized by both reproductive and metabolic deficits (89), the former of which include defects in the ability of gonadal steroid hormones to exert appropriate feedback control on GnRH neurons (90). PCOS is likely a disease of prenatal origin (91), based on epidemiological evidence, suggesting that excess androgens during fetal life can lead to an increased risk of this disorder later in adulthood (92). Animal models of PCOS include the prenatally androgenized ewe (89), monkey (93), rat (94), and mouse (95), and prenatal testosterone (T) treatment of female sheep leads to a constellation of deficits that very closely resemble the symptoms of PCOS in women (89). Among these, again, are pronounced deficits in the gonadal hormone feedback control of GnRH neurons, including the inhibitory influence of progesterone on GnRH pulses and the stimulatory influence of E2, leading to the generation of the preovulatory GnRH/LH surge (96,97).
Based on the functional role of the KNDy subpopulation in mediating progesterone negative feedback in sheep (14), we recently tested the hypothesis that alterations in KNDy cell peptides may be associated with the prenatal T model of PCOS in this species (98). Prenatal T treatment between d 30 and 90 of fetal life (the sheep gestation period is 147 d) resulted in long-lasting changes in KNDy cell peptide expression in these animals examined as adults, reducing by half the number of NKB and DYN cells; in contrast, the number of kisspeptin cells remained at levels comparable to that seen in the KNDy subpopulation of normal control females (98). The results suggest that an imbalance between inhibitory (DYN) and stimulatory (kisspeptin) neuropeptides in this subpopulation may be responsible for the deficits in progesterone negative feedback seen in this model and that normalizing the balance of KNDy peptide expression and release in prenatal T sheep may help to ameliorate the neuroendocrine deficit. Kisspeptin antagonists (99) may therefore represent a potential clinical treatment for PCOS and other disorders where pulse frequency is elevated (90,100). Observations that microinjections of kisspeptin antagonists into the ARC are capable of inhibiting LH pulse frequency (44,81) are consistent with this possibility as well as the proposed role of KNDy cells as part of the GnRH pulse generator (see above).
Although we have focused here on alterations in KNDy cells that may underlie reproductive disease, there is also compelling evidence that changes in KNDy cells occur as a part of the process of normal aging in association with menopause. Specifically, it has long been known that in the brains of postmenopausal women, there is selective hypertrophy of neurons of the infundibular (arcuate) nucleus of the human hypothalamus (101). Rance and colleagues have shown in single-label studies that the large majority of these hypertrophied neurons each contain KISS1 (57), NKB (8), and DYN (68) as well as ERα (102) mRNA; thus, they likely represent KNDy cells of the human hypothalamus. In postmenopausal women, there is increased gene expression of NKB (8) and KISS1 (57) in these cells, along with decreased gene expression of DYN (68), consistent with an alteration in the balance between stimulatory (kisspeptin and NKB) and inhibitory (DYN) KNDy peptides that would lead to the GnRH and LH hypersecretion characteristic of postmenopausal women (20). Because similar changes in KNDy peptide gene expression are seen in young OVX monkeys (20), it may be that these changes in postmenopausal women are a response to the ovarian failure and depletion of ovarian steroids that occurs during menopause. Thus, KNDy cells likely play a key role in normal physiological regulation of steroid negative feedback in humans, just as in experimental animals.
Conclusion and Unresolved Questions
There is now strong evidence that KNDy neurons and their projections play a central role in steroid negative feedback of GnRH secretion in rodents, sheep, and primates and growing evidence that they also participate in the positive feedback actions of E2 to induce the preovulatory GnRH/LH surge in the ewe (Fig. 3). Although the actions of NKB remain controversial, it is clear that kisspeptin stimulates and DYN inhibits GnRH secretion. This raises the possibility that differential effects of ovarian steroids on these two peptides or their receptors could have a dramatic effect on the overall control of GnRH release. This in turn points to the need for studies to determine the effects of steroids on expression of receptors for KNDy peptides in GnRH neurons and whether individual KNDy peptides are sequestered in the same or separate secretory vesicles.
Other possible functions for these neurons remain largely speculative at this time. However, there is evidence that KNDY neurons may mediate the effects of nutritional status and stress on the reproductive neuroendocrine axis. KNDy neurons contain leptin receptors (103) and nutrient restriction inhibits Kiss1 mRNA expression in rodents (104,105). Similarly, ovine KNDy neurons contain glucocorticoid receptors (106), and recent work in the rat has implicated ARC kisspeptin neurons in the suppression of GnRH in response to a variety of stressors (107). Thus, KNDy cells may serve as a central node in the control of GnRH secretion, acting as conduits for a variety of intrinsic and extrinsic regulatory signals (Fig. 3A).
Finally, the important role that KNDy neurons appear to play in the control of the GnRH system is interesting in light of the history of reproductive neuroendocrinology. The ARC was the hypothalamic nucleus that Ernst Knobil (108,109) and others originally identified as the site of the GnRH pulse generator and has long been considered a key locus in steroidal control of GnRH secretion. A central role for KNDy cells in the control of GnRH secretion is therefore a return of the ARC to the neuroendocrine spotlight, albeit at a level of greater cellular detail in both phenotype and circuitry. Understanding how the synthesis and release of individual KNDy peptide is orchestrated within KNDy neurons and their projections, and ultimately translated into control of the reproductive neuroendocrine axis, will likely have key relevance for a wide range of issues affecting reproductive health and disease.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grants R01 HD39916 (to M.N.L. and R.L.G.), P01 HD44232 (to M.N.L.) and Canadian Institutes of Health Research Operating Grant 86744 (to M.N.L.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 25, 2010
Abbreviations: ARC, Arcuate nucleus; DYN, dynorphin; E2, estradiol; EOP, endogenous opioid peptide; ERα, α-isoform of the estrogen receptor; KOR, κ-EOP receptor; MBH, mediobasal hypothalamus; MUA, multi-unit electrical activity; NKB, neurokinin B; NK3R, high-affinity receptor for NKB; OVX, ovariectomy; PCOS, polycystic ovarian syndrome; POA, preoptic area; PR, progesterone receptor; T, testosterone; vGLUT-2, vesicular glutamate transporter-2.
References
- Schally AV, Arimura A, Kastin AJ, Matsuo H, Baba Y, Redding TW, Nair RM, Debeljuk L, White WF 1971 Gonadotropin-releasing hormone: one polypeptide regulates secretion of luteinizing and follicle-stimulating hormones. Science 173:1036–1038 [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E 2003 Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno Jr JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley Jr WF, Aparicio SA, Colledge WH 2003 The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- Plant TM 2006 The role of KiSS-1 in the regulation of puberty in higher primates. Eur J Endocrinol 155(Suppl 1):S11–S16 [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, García-Galiano D, Tena-Sempere M 2007 Neuroendocrine factors in the initiation of puberty: the emergent role of kisspeptin. Rev Endocr Metab Disord 8:11–20 [DOI] [PubMed] [Google Scholar]
- Maeda K, Adachi S, Inoue K, Ohkura S, Tsukamura H 2007 Metastin/kisspeptin and control of estrous cycle in rats. Rev Endocr Metab Disord 8:21–29 [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Smith JT, Caraty A, Goodman RL, Lehman MN 2009 Kisspeptin and seasonality in sheep. Peptides 30:154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance NE, Young 3rd WS 1991 Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology 128:2239–2247 [DOI] [PubMed] [Google Scholar]
- Sahu A, Kalra SP 1992 Effects of tachykinins on luteinizing hormone release in female rats: potent inhibitory action of neuropeptide K. Endocrinology 130:1571–1577 [DOI] [PubMed] [Google Scholar]
- Rance NE, Bruce TR 1994 Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology 60:337–345 [DOI] [PubMed] [Google Scholar]
- Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK 2009 TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guran T, Tolhurst G, Bereket A, Rocha N, Porter K, Turan S, Gribble FM, Kotan LD, Akcay T, Atay Z, Canan H, Serin A, O'Rahilly S, Reimann F, Semple RK, Topaloglu AK 2009 Hypogonadotropic hypogonadism due to a novel missense mutation in the first extracellular loop of the neurokinin B receptor. J Clin Endocrinol Metab 94:3633–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ 2007 Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 148:5752–5760 [DOI] [PubMed] [Google Scholar]
- Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN 2004 Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology 145:2959–2967 [DOI] [PubMed] [Google Scholar]
- Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN 2005 Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic acid levels in a subset of dynorphin neurons in the sheep. Endocrinology 146:1835–1842 [DOI] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA 2009 Kisspeptin signaling in the brain. Endocr Rev 30:713–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke MC, Letts PA, Krajewski SJ, Rance NE 2006 Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol 498:712–726 [DOI] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA 2009 Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H 2010 Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 30:3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance NE 2009 Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides 30:111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE 2000 Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology 141:4218–4225 [DOI] [PubMed] [Google Scholar]
- Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A 2006 Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor α. Neurosci Lett 401:225–230 [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA 2005 Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN 2002 Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology 143:4366–4374 [DOI] [PubMed] [Google Scholar]
- Ciofi P, Krause JE, Prins GS, Mazzuca M 1994 Presence of nuclear androgen receptor-like immunoreactivity in neurokinin B-containing neurons of the hypothalamic arcuate nucleus of the adult male rat. Neurosci Lett 182:193–196 [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA 2005 Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984 [DOI] [PubMed] [Google Scholar]
- Lehman MN, Ebling FJ, Moenter SM, Karsch FJ 1993 Distribution of estrogen receptor-immunoreactive cells in the sheep brain. Endocrinology 133:876–886 [DOI] [PubMed] [Google Scholar]
- Skinner DC, Herbison AE 1997 Effects of photoperiod on estrogen receptor, tyrosine hydroxylase, neuropeptide Y, and β-endorphin immunoreactivity in the ewe hypothalamus. Endocrinology 138:2585–2595 [DOI] [PubMed] [Google Scholar]
- Lehman MN, Karsch FJ 1993 Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and beta-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology 133:887–895 [DOI] [PubMed] [Google Scholar]
- Ciofi P, Leroy D, Tramu G 2006 Sexual dimorphism in the organization of the rat hypothalamic infundibular area. Neuroscience 141:1731–1745 [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K 2005 Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 146:4431–4436 [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE 2006 Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM 2008 Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology 149:4387–4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl SK, Amstalden M, Coolen L, Fitzgerald M, Lehman M 2009 Dynorphin immunoreactive fibers contact GnRH neurons in the human hypothalamus. Reprod Sci 16:781–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubillon M, Delaleu B, Tillet Y, Caraty A, Herbison AE 1999 Localization of estrogen-receptive neurons projecting to the GnRH neuron-containing rostral preoptic area of the ewe. Neuroendocrinology 70:228–236 [DOI] [PubMed] [Google Scholar]
- Pompolo S, Rawson JA, Clarke IJ 2001 Projections from the arcuate/ventromedial region of the hypothalamus to the preoptic area and bed nucleus of stria terminalis in the brain of the ewe; lack of direct input to gonadotropin-releasing hormone neurons. Brain Res 904:1–12 [DOI] [PubMed] [Google Scholar]
- Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE 2005 Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol 489:372–386 [DOI] [PubMed] [Google Scholar]
- Amstalden M, Billings HJ, Hileman SM, McManus CJ, Valent M, Coolen LM, Goodman RL, Lehman MN, Location of dynorphin neurons that project to the external zone of the median eminence in the sheep. Proc 35th Annual Meeting of the Society for Neuroscience, Washington, DC, 2005 (Abstract 759.14) [Google Scholar]
- Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE 2010 Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience 166:680–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH 2008 Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology 149:3926–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM 2008 Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 149:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Moenter SM 2010 Kisspeptin increases γ-aminobutyric acidergic and glutamatergic transmission directly to gonadotropin-releasing hormone neurons in an estradiol-dependent manner. Endocrinology 151:291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foradori CD, Amstalden M, Goodman RL, Lehman MN 2006 Colocalisation of dynorphin A and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol 18:534–541 [DOI] [PubMed] [Google Scholar]
- Li XF, Kinsey-Jones JS, Cheng Y, Knox AM, Lin Y, Petrou NA, Roseweir A, Lightman SL, Milligan SR, Millar RP, O'Byrne KT 2009 Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS One 4:e8334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN 2010 Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol 22:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, de Tassigny X, Doran J, Colledge WH 2010 Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology 151:312–321 [DOI] [PubMed] [Google Scholar]
- Merkley Coolen LM, Jackson L, Goodman RL, Lehman MN, Evidence for transcriptional activation of arcuate kisspeptin neurons, and glutamatergic input to kisspeptin during the preovulatory GnRH surge of the sheep. Program of the 91st Annual Meeting of The Endocrine Society, Washington, DC, 2009 (Abstract P3-220) [Google Scholar]
- Pompolo S, Pereira A, Scott CJ, Fujiyma F, Clarke IJ 2003 Evidence for estrogenic regulation of gonadotropin-releasing hormone neurons by glutamatergic neurons in the ewe brain: an immunohistochemical study using an antibody against vesicular glutamate transporter-2. J Comp Neurol 465:136–144 [DOI] [PubMed] [Google Scholar]
- Mahesh VB, Brann DW 2005 Regulatory role of excitatory amino acids in reproduction. Endocrine 28:271–280 [DOI] [PubMed] [Google Scholar]
- Gore AC, Wu TJ, Rosenberg JJ, Roberts JL 1996 Gonadotropin-releasing hormone and NMDA receptor gene expression and colocalization change during puberty in female rats. J Neurosci 16:5281–5289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BH, Gore AC 2002 N-Methyl-d-aspartate receptor subunit expression in GnRH neurons changes during reproductive senescence in the female rat. Endocrinology 143:3568–3574 [DOI] [PubMed] [Google Scholar]
- Yin W, Mendenhall JM, Bratton SB, Oung T, Janssen WG, Morrison JH, Gore AC 2007 Novel localization of NMDA receptors within neuroendocrine gonadotropin-releasing hormone terminals. Exp Biol Med (Maywood) 232:662–673 [PubMed] [Google Scholar]
- Goodman RL, Inskeep EI 2006 Neuroendocrine control of the ovarian cycle of the sheep. In: Neill JD, ed. Knobil and Neill’s physiology of reproduction. 3rd ed. Amsterdam: Elsevier; 2389–2447 [Google Scholar]
- Karsch FJ 1987 Central actions of ovarian steroids in the feedback regulation of pulsatile secretion of luteinizing hormone. Annu Rev Physiol 49:365–382 [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA 2006 Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 26:6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Clay CM, Caraty A, Clarke IJ 2007 KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 148:1150–1157 [DOI] [PubMed] [Google Scholar]
- Rometo AM, Krajewski SJ, Voytko ML, Rance NE 2007 Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 92:2744–2750 [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Walker VR, Korach KS 2003 Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ERα but not ERβ. Mol Endocrinol 17:1039–1053 [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA 2009 Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci 29:9390–9395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Jessen HM, Auger AP, Terasawa E 2009 Postmenopausal increase in KiSS-1, GPR54, and luteinizing hormone releasing hormone (LHRH-1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides 30:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernández-Fernández R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M 2004 Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 145:4565–4574 [DOI] [PubMed] [Google Scholar]
- Goodman RL, Rao A, Smith JT, Clarke IJ, Negative feedback control of Kiss-1 gene expression by estradiol and progesterone in the ewe. Proc First World Conference on Kisspeptin Signaling in the Brain. Cordoba, Spain, 2008 [Google Scholar]
- Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN 2008 Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology 149:5770–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraty A, Smith JT, Lomet D, Ben Saïd S, Morrissey A, Cognie J, Doughton B, Baril G, Briant C, Clarke IJ 2007 Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology 148:5258–5267 [DOI] [PubMed] [Google Scholar]
- Simonneaux V, Ansel L, Revel FG, Klosen P, Pévet P, Mikkelsen JD 2009 Kisspeptin and the seasonal control of reproduction in hamsters. Peptides 30:146–153 [DOI] [PubMed] [Google Scholar]
- Greives TJ, Kriegsfeld LJ, Demas GE 2008 Exogenous kisspeptin does not alter photoperiod-induced gonadal regression in Siberian hamsters (Phodopus sungorus). Gen Comp Endocrinol 156:552–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferin M, Van Vugt D, Wardlaw S 1984 The hypothalamic control of the menstrual cycle and the role of endogenous opioid peptides. Recent Prog Horm Res 40:441–485 [DOI] [PubMed] [Google Scholar]
- Rometo AM, Rance NE 2008 Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol 20:1376–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo RV 1990 κ-Opioid receptor involvement in the regulation of pulsatile luteinizing hormone release during early pregnancy in the rat. J Neuroendocrinol 2:685–691 [DOI] [PubMed] [Google Scholar]
- Herbison AE 2008 Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev 57:277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraty A, Fabre-Nys C, Delaleu B, Locatelli A, Bruneau G, Karsch FJ, Herbison A 1998 Evidence that the mediobasal hypothalamus is the primary site of action of estradiol in inducing the preovulatory gonadotropin releasing hormone surge in the ewe. Endocrinology 139:1752–1760 [DOI] [PubMed] [Google Scholar]
- Krey LC, Lu KH, Bulter WR, Hotchkiss J, Piva F, Knobil E 1975 Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. II. GH and cortisol secretion. Endocrinology 96:1088–1093 [DOI] [PubMed] [Google Scholar]
- Weick RF 1981 Induction of the luteinizing hormone surge by intrahypothalamic application of estrogen in the rhesus monkey. Biol Reprod 24:415–422 [DOI] [PubMed] [Google Scholar]
- Smith JT, Li Q, Pereira A, Clarke IJ 2009 Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology 150:5530–5538 [DOI] [PubMed] [Google Scholar]
- Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, McManus CM, Nestor CC, Jacobs BH, Goodman RL 2010 Neurokinin B acts via the Neurokinin 3 receptor in the retrochiasmatic area to stimulate luteinzing hormone secretion in sheep. Endocrinology doi:10.1210/en.2010-0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval-Guzmán T, Rance NE 2004 Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res 1026:307–312 [DOI] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL 2007 Nonclassical estrogen receptor α signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci USA 104:8173–8177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krsmanoviæ LZ, Stojilkoviæ SS, Merelli F, Dufour SM, Virmani MA, Catt KJ 1992 Calcium signaling and episodic secretion of gonadotropin-releasing hormone in hypothalamic neurons. Proc Natl Acad Sci USA 89:8462–8466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E, Keen KL, Mogi K, Claude P 1999 Pulsatile release of luteinizing hormone-releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology 140:1432–1441 [DOI] [PubMed] [Google Scholar]
- Nishihara M, Takeuchi Y, Tanaka T, Mori Y 1999 Electrophysiological correlates of pulsatile and surge gonadotrophin secretion. Rev Reprod 4:110–116 [DOI] [PubMed] [Google Scholar]
- Goodman RL HS, Nestor CC, Holaskova I, Connors JM, Millar RP, Collen LM, Lehman MN, Kisspeptin actions in the arcuate nucleus of ewes are necessary for episodic GnRH secretion. Proc 39th Annual Meeting of the Society for Neuroscience, Chicago, IL, 2009 (Abstract 665.612) [Google Scholar]
- Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E 2008 An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 149:4151–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda KI, Okamura H 2009 Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol 21:813–821 [DOI] [PubMed] [Google Scholar]
- Goodman RL, Parfitt DB, Evans NP, Dahl GE, Karsch FJ 1995 Endogenous opioid peptides control the amplitude and shape of gonadotropin-releasing hormone pulses in the ewe. Endocrinology 136:2412–2420 [DOI] [PubMed] [Google Scholar]
- Moenter SM, Brand RC, Karsch FJ 1992 Dynamics of gonadotropin-releasing hormone (GnRH) secretion during the GnRH surge: insights into the mechanism of GnRH surge induction. Endocrinology 130:2978–2984 [DOI] [PubMed] [Google Scholar]
- Sannella MI, Petersen SL 1997 Dual label in situ hybridization studies provide evidence that luteinizing hormone-releasing hormone neurons do not synthesize messenger ribonucleic acid for μ, κ, or δ opiate receptors. Endocrinology 138:1667–1672 [DOI] [PubMed] [Google Scholar]
- Boukhliq R, Goodman RL, Berriman SJ, Adrian B, Lehman MN 1999 A subset of gonadotropin-releasing hormone neurons in the ovine medial basal hypothalamus is activated during increased pulsatile luteinizing hormone secretion. Endocrinology 140:5929–5936 [DOI] [PubMed] [Google Scholar]
- Setji TL, Brown AJ 2007 Polycystic ovary syndrome: diagnosis and treatment. Am J Med 120:128–132 [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Abbott DH, Padmanabhan V 2007 Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord 8:127–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC 1998 Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 83:582–590 [DOI] [PubMed] [Google Scholar]
- Barker DJ 1990 The fetal and infant origins of adult disease. BMJ 301:1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman RJ, Dewailly D, Legro RS, Hickey TE 2007 Polycystic ovary syndrome. Lancet 370:685–697 [DOI] [PubMed] [Google Scholar]
- Abbott DH, Barnett DK, Bruns CM, Dumesic DA 2005 Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update 11:357–374 [DOI] [PubMed] [Google Scholar]
- Mannerås L, Cajander S, Holmäng A, Seleskovic Z, Lystig T, Lönn M, Stener-Victorin E 2007 A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology 148:3781–3791 [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM 2004 Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci USA 101:7129–7134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JE, Birch RA, Foster DL, Padmanabhan V 2002 Prenatal exposure of the ovine fetus to androgens sexually differentiates the steroid feedback mechanisms that control gonadotropin releasing hormone secretion and disrupts ovarian cycles. Arch Sex Behav 31:35–41 [DOI] [PubMed] [Google Scholar]
- Sharma TP, Herkimer C, West C, Ye W, Birch R, Robinson JE, Foster DL, Padmanabhan V 2002 Fetal programming: prenatal androgen disrupts positive feedback actions of estradiol but does not affect timing of puberty in female sheep. Biol Reprod 66:924–933 [DOI] [PubMed] [Google Scholar]
- Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN 2010 The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology 151:301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP 2009 Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci 29:3920–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank SK, McCartney CR, Marshall JC 2006 The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update 12:351–361 [DOI] [PubMed] [Google Scholar]
- Sheehan HL, Kovács K 1966 The subventricular nucleus of the human hypothalamus. Brain 89:589–614 [DOI] [PubMed] [Google Scholar]
- Rance NE, McMullen NT, Smialek JE, Price DL, Young 3rd WS 1990 Postmenopausal hypertrophy of neurons expressing the estrogen receptor gene in the human hypothalamus. J Clin Endocrinol Metab 71:79–85 [DOI] [PubMed] [Google Scholar]
- Smith JT, Acohido BV, Clifton DK, Steiner RA 2006 KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol 18:298–303 [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernández-Fernández R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M 2005 Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology 146:3917–3925 [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M 2006 KiSS-1 and reproduction: focus on its role in the metabolic regulation of fertility. Neuroendocrinology 83:275–281 [DOI] [PubMed] [Google Scholar]
- Oakley AE, Coolen LM, Lehman MN, Wagenmaker ER, Karsch FJ,Are dynorphin neurons in the arcuate nucleus responsive to cortisol and influenced by the combined presence of cortisol and estradiol? Program of the 91st Annual Meeting of The Endocrine Society, Washington, DC, 2009 (Abstract P2-273) [Google Scholar]
- Kinsey-Jones JS, Li XF, Knox AM, Wilkinson ES, Zhu XL, Chaudhary AA, Millian SR, Lightman SL, O'Byrne KT 2009 Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. J Neuroendocrinol 21:20–29 [DOI] [PubMed] [Google Scholar]
- Plant TM, Krey LC, Moossy J, McCormack JT, Hess DL, Knobil E 1978 The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female rhesus monkey (Macaca mulatta). Endocrinology 102:52–62 [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Wilson R, Kesner JS, Knobil E 1986 Hypothalamic localization of multiunit electrical activity associated with pulsatile LH release in the rhesus monkey. Neuroendocrinology 44:168–171 [DOI] [PubMed] [Google Scholar]
- Horvath TL, Naftolin F, Leranth C 1992 β-Endorphin innervation of dopamine neurons in the rat hypothalamus: a light and electron microscopic double immunostaining study. Endocrinology 131:1547–1555 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.