Abstract
Although it has been observed that various cofactors modulate activity of the androgen receptor (AR), the specific relationship between AR cofactors and prostate development and functions has not been well studied. To determine whether AR cofactor p44/WDR77 is important in prostate growth and development, we examined prostate architecture in p44/WDR77-null mice and wild-type (WT) littermates. Prostate glands from p44/WDR77-deficient animals were not only smaller than those from WT mice but also had fewer branches and terminal duct tips and were deficient in production of secretory proteins. The p44/WDR77-null prostate tissue was less differentiated and hyperproliferative relative to WT littermates. In addition, the altered expression of androgen-regulated genes was observed in the p44/WDR77-null prostate. Thus, these results suggest that the AR cofactor p44/WDR77 plays important roles in prostate growth and differentiation by modulating AR-target gene expression.
The androgen receptor cofactor p44/WDR77 regulates expression of androgen receptor-target genes and is essential for the full differentiation of mouse prostate epithelial cells.
Prostate development occurs with outgrowth of the urogenital sinus epithelium into the surrounding mesenchyme to form epithelial buds that will become the main prostatic ducts. Studies have shown that mesenchymal androgens are necessary and sufficient for prostate ductal development (1). On the other hand, epithelial androgens are important for the final stages of morphogenesis and for the initiation and maintenance of secretory function in the prostate (2).
The adult prostate gland consists of glandular epithelium that is in close contact with an underlying stromal compartment. The epithelium is composed of two histological distinct layers. The secretory luminal layer is made up of tall columnar cells that are responsible for the production of prostate-specific antigen (PSA) [PSA is not expressed in the mouse prostate], prostate alkaline phosphatase, and kallikrein-2, which are secreted as part of the seminal fluid (3,4). Within the epithelial compartment, the androgen receptor (AR) directly inhibits epithelial cell proliferation, induces differentiation, and regulates prostate metabolic and secretory functions (5,6). Androgen-dependent genes that play key regulatory roles in prostate development or maintenance are poorly defined.
AR mediates androgen functions in the prostate (7,8,9,10,11). AR is a member of the nuclear receptor superfamily and, upon ligand binding, AR interacts with cognate androgen response elements (AREs) in genomic DNA and recruits various cofactors (12,13,14,15,16,17). Less is known about the function of these cofactors in the regulation of AR functions in vivo in the prostate.
We have purified and cloned a novel AR-interacting protein (p44/WDR77) (18). The p44/WDR77 cDNA encodes a protein (containing 342 amino acid residues) that is highly expressed in the luminal epithelium but not in basal epithelium and stromal cells (19). P44/WDR77 enhanced AR-dependent transcription and was recruited onto the promoters of the prostate-specific antigen (PSA) and p21 genes in the presence of the androgen (18,19). We found that the p44/WDR77 translocation from the nucleus to the cytoplasm is strongly associated with prostate tumorigenesis (19). When we forced nuclear localization of p44/WDR77 in prostate cancer cells, cell growth was inhibited because of G1 cell cycle arrest, which resulted from up-regulation of p21 gene expression and down-regulation of CDK2 gene expression (19,20). Loss of one copy of the p44/WDR77 gene resulted in prostatic hyperplasia (19). These studies suggest that p44/WDR77 plays important roles in the control of epithelial cell proliferation in the prostate.
In this report, we show that p44/WDR77 is required for prostate functions, because p44/WDR77-null mutants display defects in ductal morphogenesis and secretory protein production. Furthermore, p44/WDR77 regulates prostate epithelial proliferation, because its loss results in epithelial hyperproliferation. Finally, loss of p44/WDR77 expression altered expression of some androgen-dependent genes. Taken together, our results provide evidence that the AR cofactor p44/WDR77 functions to promote functional differentiation of epithelium and secretory protein production in the developing prostate by regulating expression of androgen-driven genes.
Materials and Methods
Animals
The p44/WDR77 genomic fragment of the intron 5 was replaced by a phosphoglycerate kinase neo-cassette (PGK-neo), which was surrounded by two loxP sequences. Additional loxP sequence was inserted in the intron 1 of the p44/WDR77 genomic DNA. This arrangement would ablate the NH2-terminal portion (amino acid residues 1–194) of p44/WDR77 in the presence of the Cre recombinase. The embryonic stem (ES) electroporation and blastocyst injection were performed by the Genetically Engineered Mouse Facility (GEMF) at M.D. Anderson Cancer Center. In total, 348 G418-resistant clones were screened by Southern blot analysis using the 3′-external probe. Five clones displayed evidence of homologous recombination of the disrupted p44/WDR77 gene. ES clones were microinjected into the blastocysts of female C57BL6/J mice. Germ-line chimeras were bred to C57BL6/J mice to generate heterozygous mutant (MT) F1 mice. The homogenous mice (p44/WDR77loxP/loxP) were obtained by the cross between the heterozygous F1 mice. A subsequent cross between p44/WDR77loxP/loxP wild type (WT) and PRR2Bi-Cre mice generated p44/WDR77loxP/loxP;Cre MT mice. For genotyping, the genomic DNA isolated from ES cells or mouse tails was subjected to Southern blot analysis with a 3′-external probe or used for PCR analysis (primer sequences are available on request). The prostate, testis, and penis were dissected from male mice and weighed using an analytic balance. Mice were identified by PCR on tail snips, as previously described (19).
Prostate separation and histology
The male mice were killed, and the prostate was freed from the other structures. To determine glandular detail, separation of glandular structures from stromal ones was performed according to the method of Sugimura et al. (21). The numbers of terminal ductal tips and branches (five WT and five MT mice) were determined in a blinded manner under a dissecting microscope. Anterior prostate (AP), dorsolateral prostate (DLP), and ventral prostate (VP) lobes of the prostate were fixed by immersion in 4% paraformaldehyde in PBS for 60 min. The tissues were then embedded in paraffin, and sections (4 μm) were cut and mounted on Super-frost Plus adhesion slides (Fisher Scientific, Pittsburgh, PA) for hematoxylin and eosin (H&E) and immunohistochemical staining.
In some cases, the prostate glands were removed en bloc (including the seminal vesicles, urethra, and bladder) and fixed with 4% paraformaldehyde in PBS at 4 C overnight and embedded in paraffin. Sections (4 μm) were cut and mounted on Super-frost Plus adhesion slides for staining with H&E.
The height of the luminal epithelium was measured under microscope. Cells from each lobe (30,31,32,33,34,35,36,37,38,39,40) (AP, DLP, or VP) of WT and MT prostates were measured. Three sections (500 μm apart) from each prostate lobe were analyzed. The means of heights of the luminal epithelium from three prostates were presented.
Immunohistochemistry
Antikeratin 18 (anti-K18)(1:50) and antikeratin 5 (anti-K5)(1:1000) were purchased from CHEMICON International (Temecula, CA). The antigen-purified anti-p44/WDR77 (1:100) and anti-AR (1:200) antibodies were described previously (18,22). The frozen tissue sections (8 μm) were treated with the AffinitPure Fab Fragment Goat Antimouse IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) overnight at 4 C before immunostaining with the anti-K18 antibody. Antibodies were applied to the prostate tissue sections and incubated overnight. A streptavidin-biotin peroxidase detection system was used according to the manufacturer’s instructions (Dako A/S, Grostrup, Denmark). For immunofluorescent staining, the Alexa 594-labeled antirabbit IgG antibody (1:1000) (DAKO Corp., Carpinteria, CA) was used. The fluorescent signals were observed under a cofocal microscope with a red (to detect the p44/WDR77 protein) or blue (to detect the nucleus) filter.
Bromodeoxyuridine (BrdU) labeling
Mice were injected with BrdU (0.1 mg/g of body weight·d) (Sigma-Aldrich, St. Louis, MO) ip for 3 d. The BrdU-labeled epithelial cells were detected employing a monoclonal anti-BrdU antibody (BD Pharmingen, Franklin Lakes, NJ). The labeled cells were calculated from three fields of each slide. Three sections from each prostate were analyzed to obtain the mean of BrdU-positive epithelial cells. The means of the proliferating cells from five prostates were presented.
Apoptosis assay
The in situ cell death detection kit (Promega Corp., Madison, WI) was used to detect apoptotic cells according to published procedures (23). Terminal deoxynucleotide transferase-mediated dUTP nick end labeling (TUNEL)-positive cells and total epithelial cells from each lobe (AP, DLP, or VP) of WT and MT prostates were accounted. The percentage of apoptotic cells were calculated. Three sections (500 μm apart) from each prostate lobe were analyzed. The means of percentage apoptotic cells from five prostates are presented.
Collection of secretion
Luminal material was collected from mouse prostate glands. The prostate was minced in 100 μl of PBS containing protease inhibitors (1 mm phenylmethane sulfonylfluoride, 10 μm leupeptin, 1.4 μm pepstatin A). The tissue was centrifuged at 16,000 × g for 2 min at 4 C, the supernatant was drawn off, and the protein concentrations were determined by using the Bradford method (Bio-Rad Laboratories, Hercules, CA) with BSA (Bio-Rad) as the standard. The supernatant was mixed with 100 μl of 2× sodium dodecyl sulfate (SDS) gel sample buffer. The samples were then heated to 100 C for 5 min and submitted to 10 to 20% SDS polyacryamide gradient gel. The gel was stained with Coomassie blue R 250.
DNA microarray
Prostate glands were obtained from age-matched MT (p44/WDR77loxP/loxP;Cre) and WT (p44/WDR77loxP/loxP) mice and submitted to Condon Biosciences (Houston, TX) for transcriptome profiling using Affymetrix Mouse Genome 430 2.0 chips. Array signals were harvested by the GeneArray Scanner (Affymetrix, Santa Clara, CA) and were processed by the Microarray Suite software (Affymetrix).
Castration and androgen administration
WT (n = 20) and MT (n = 20) male mice were castrated at 8 wk of age under anesthesia in accordance with the protocol approved by the International Animal Care and Use Committee. The mice were injected sc with testosterone enanthate (3.6 ng/g body weight) 7 d after the operation. The prostate glands were dissected at 0 (n = 10), 24 (n = 10), 48 (n = 10), and 72 (n = 10) h after testosterone replacement. Total RNA was isolated from prostate glands using the Trizol method and subjected to real-time PCR analysis.
Real-time PCR
The total RNAs were reverse transcribed, and the resulting cDNA products were PCR amplified with the RT2 real-time SyBR Green PCR master mix by the SmartCycler II (Cepheid, Sunnyvale, CA). The raw data procession and quantification were performed with the SmartCycler Software (version 2.0C). RT-PCR primer sets were purchased from SuperArray Bioscience.
Northern blot assay
Northern blot analysis was performed as previously described (23). The mRNAs were isolated from whole prostate glands of WT (n = 5) or MT (n = 5) male mice at the age of 2 months and transferred to a Hybond N+ membrane (Amersham Biosciences, Piscataway, NJ). The membrane was hybridized with cDNA probes of p44/WDR77 and β-actin genes.
Results
Generation of the mouse with the conditional deletion of the p44/WDR77 gene in the prostate
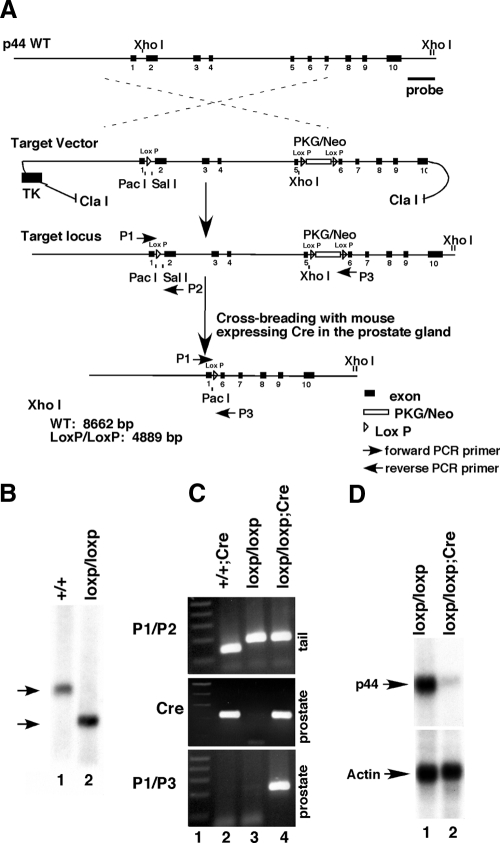
Because the p44/WDR77 gene is essential for mouse embryonic development and loss of two alleles of the p44/WDR77 gene is embryonically lethal (19), we decided to generate the mouse with complete deletion of the p44/WDR77 gene in the prostate by the loxP/Cre system. LoxP sequences were inserted into the endogenous p44/WDR77 locus flanking exon 2 to exon 5 (Fig. 1A). Exon 2 to exon 5 encodes the N-terminal 194 amino acid residues of the p44/WDR77 protein, and this sequence is essential for p44/WDR77 to regulate the AR-driven gene expression (data not shown). In addition, exon 1 and exon 6 are not in the same reading frame after the Cre-mediated recombination and RNA splicing. Thus, this strategy will avoid expression of any p44/WDR77 truncations from the p44/WDR77 locus after the Cre-mediated recombination.
Figure 1.
Generation of the Cre-mediated prostate-specific deletion of the p44/WDR77 gene. A, Diagram of the strategy to generate a prostate-specific p44/WDR77-deficient mouse. Wild allele of the mouse p44/WDR77 gene, the target vector with PGK-neo cassettes and loxP sites, and the predicted MT allele resulting from homologous and Cre-mediated recombination are presented. B, Southern blot analysis of offspring obtained by a heterozygous cross. The WT allele (8.7 kb) and MT allele (4.9 kb) are indicated by arrows. C, Gene typing by PCR. Genomic DNA was isolated from mouse tails and submitted for PCR analysis to detect the presence of the MT loci (the loxP site) (using primers 1 and 2) (top panel). Genomic DNA was isolated from mouse prostates and submitted for PCR analysis to detect the presence of the Cre gene (middle panel, lanes 2 and 4) or the Cre-mediated deletion of the p44/WDR77 gene (using primers 1 and 3) (bottom panel, lane 4). D, Northern blot analysis of p44/WDR77 and β-actin mRNAs in the prostate gland. The mRNA was isolated from the whole prostate glands of p44/WDR77loxP/loxP (n = 5; lane 1) or p44/WDR77loxP/loxP;Cre (n = 5; lane 2) mice, fractionated by electrophoresis, and transferred to a Hybond N+ membrane. The membrane was hybridized with a p44/WDR77 probe (top panel) or β-actin probe (bottom panel). P1, P2, P3, Primers 1, 2, and 3; TK, thymidine kinase.
Embryonic stem cells with a disrupted p44/WDR77 locus (p44/WDR77loxP/+) were injected into C57/B6 blastocysts. Chimeric mice were backcrossed to C57/B6 mice, and the germ-line transmission of the p44/WDR77loxP/loxP allele was confirmed by Southern blot (Fig. 1B, lane 2). PCR analysis of tail genomic DNA confirmed the existence of the LoxP site in p44/WDR77loxP/loxP mice (Fig. 1C, top panel, lane 3). In contrast to the embryonic lethal phenotype observed in p44/WDR77−/− mice (19), p44/WDR77loxP/loxP animals were viable, and no phenotypic changes were observed over a period up to 1 yr, suggesting that introducing the LoxP sites into the p44/WDR77 locus does not perturb the normal function of p44/WDR77. Real-time PCR analysis indicated that p44/WDR77 expression in the prostate of the p44/WDR77loxP/loxP mouse was the same as in the WT mouse prostate (data not shown).
To achieve the p44/WDR77 prostate-specific deletion, we crossed p44/WDR77loxP/loxP mice to the ARR2Pbi-Cre transgenic mouse line, in which the Cre recombinase is under the control of a modified rat prostate-specific probasin promoter (24). The original report indicated that Cre recombinase was highly expressed in all prostate lobes. To confirm prostate-specific p44/WDR77 deletion, the prostate glands from 2-month-old p44/WDR77loxP/loxP WT and p44/WDR77loxP/loxP;Cre MT mice were carefully dissected, and the status of p44/WDR77 deletion was examined by sensitive PCR and immunohistochemical analyses. PCR analysis confirmed the existence of the target locus in MT mice (Fig. 1C, top panel, lane 4) and of the Cre gene in the prostate of the MT mouse (Fig. 1C, middle panel, lane 4). The Cre-mediated p44/WDR77 deletion, as indicated by excision of exons 2–5 of the p44/WDR77 gene, was observed in the MT prostate gland (Fig. 1C, bottom panel, lane 4).
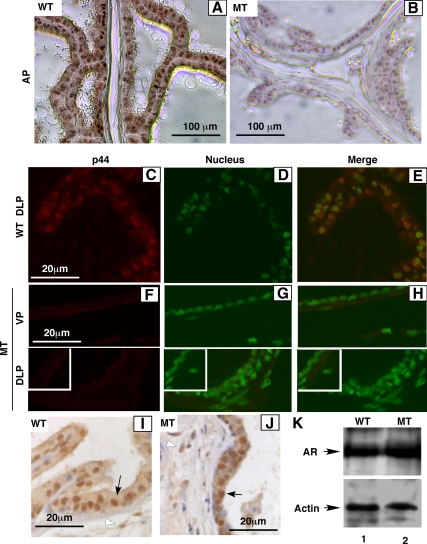
Northern blot analysis shows the Cre-mediated loss of p44/WDR77 mRNA expression in the whole prostate of the MT mouse (Fig. 1D, top panel, lane 2 vs. lane 1). Immunohistochemical staining with the anti-p44/WDR77 antibody demonstrated that the p44/WDR77 protein was expressed in the nucleus of epithelial cell of the prostate of the WT (n = 3) mouse (Fig. 2, A and C–E). The nuclear p44/WDR77 immunostaining signals were dramatically reduced in epithelial cells of the prostate of the MT (n = 3) mouse (Fig. 2, B and F–H). Further analysis indicated that expression of the p44/WDR77 protein was mostly lost in the MT mouse prostate at the age of 10 d. In contrast, AR expression in prostate epithelial (indicated by black arrow) and stromal (indicated by white arrow) cells was not affected by the p44/WDR77 gene deletion (Fig. 2, I–K). Thus, we achieved the Cre-mediated deletion of the p44/WDR77 gene in the mouse prostate.
Figure 2.
Conditional deletion of p44/WDR77 in the prostate luminal epithelium. Immunohistochemical staining of AP, DLP, and VP of the WT (A, C–E, and I; n = 3) and MT (B, F–H, and J; n = 3) mice with anti-p44/WDR77 (A–H) or anti-AR (I and J) antibody and the alkaline phosphophase-labeled (A, B, I, and J) or Alexa 594-labeled (C–H) antirabbit IgG antibody. Panels D and G and panels E and H show the nucleus staining and the merge of the nuclear staining with the p44/WDR77 immunostaining, respectively. Insets in F–H show the immunostaining without the first antibody as a negative control. The signals were detected with a streptavidin-biotin peroxidase system (A, B, I, and J) or observed under a fluorescent microscope (C–H). K, Shows Western blot analysis of AR in protein extracts (20 μg/per sample) prepared from WT (lane 1) or MT (lane 2) whole prostate gland.
Loss of the p44/WDR77 gene altered the prostate development and led to hyperproliferation of prostate epithelial cells
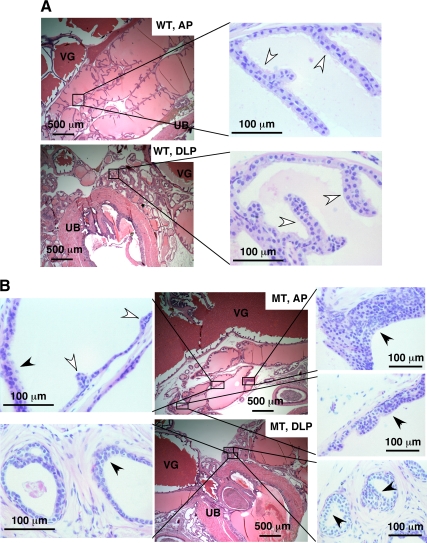
To determine whether the loss of the p44/WDR77 gene affected the mouse prostate, we kept a cohort of WT and MT animals for observation of the development and growth of the prostate gland. To avoid potential variations due to their genetic background, only mice from the F2 generation were used. At the age of 2 months, males of WT (n = 12) and MT (n = 12) littermates were killed. Prostate glands, including the seminal vesicles, urethra, and bladder, were carefully dissected en bloc from WT and MT mice, fixed, and embedded in paraffin (at the same orientation for WT and MT prostates). Samples were then sectioned from dorsal planes, and sections were placed on slides for histological analysis. Figure 3 shows views of AP and DLP prostates of the WT and MT mice. The MT AP (Fig. 3B) contained small glands and fewer epithelial enfoldings (indicated by white arrows) compared with the WT AP (Fig. 3A). The MT dorsolateral prostate (Fig. 3B) had a few glands and clusters of small ducts composed of a simple low columnar epithelium. These ducts resembled immature prostate. The MT ventral prostate showed similar phenotype (data not shown).
Figure 3.
Altered development and hyperplasia of the MT mouse prostate. H&E staining of the AP and DLP of WT (panel A; n = 12) and MT (panel B; n = 12) mice at the age of 2 months. Some epithelial enfoldings and hyperplastic lesions are indicated by white and black arrows, respectively. VG, Vesicular gland; UB, urinary bladder.
In addition, multifocal hyperproliferative epithelial cells were observed in every prostate lobe in all 12 MT male mice examined (Fig. 3B, indicated by black arrows). Multiple layers of epithelial cells were characteristic of these hyperplasic foci. In contrast, none of the 12 male WT mice had hyperproliferative epithelial cells (Fig. 3A). However, no prostate tumors developed in MT mice at ages up to 6 months (the oldest mice analyzed to date).
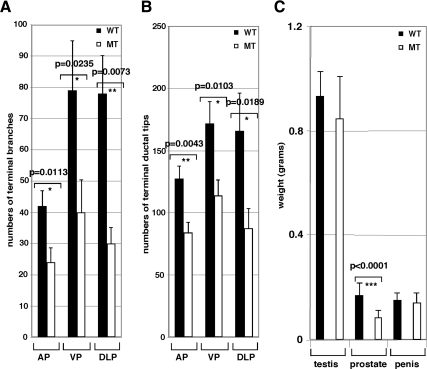
To further analyze ductal morphogenesis of the prostate, we used a microdissection technique (21). To quantify morphological differences, we counted the number of discernible branches and ductal tips of three prostate lobes (AP, DLP, and VP). The WT prostate ducts were well branched at the ends, whereas the prostate development of the MT mouse was significantly impaired with regard to the number of branches and terminal duct tips in three (AP, DLP, and VP) prostate lobes (Fig. 4, A and B). These results indicate that p44/WDR77 plays a critical role in the development of prostate branches and ductal tips. The deletion of both alleles of the p44/WDR77 gene led to a small prostate gland (about half the size of the WT prostate gland) but did not affect weights of testis and penis (Fig. 4C). We did not observed any histological defects in testis and penis in the MT mice. These results are consistent with the fact that the Cre is specifically expressed in prostate epithelial cells and there no off target effects on other androgen-target organs.
Figure 4.
The p44/WDR77 deletion decreased branches and terminal ductal tips of the prostate gland. A and B Branches and terminal ductal tips of the epithelial cords in AP, DLP, and VP (n = 5) were counted, and data are shown. C, Homozygous p44/WDR77 deletion leads to the small size of prostate. Organs were dissected from WT (n = 5) and MT (n = 10) mice and weighed. Data (mean ± sem) are expressed. The unpaired t test was performed, and the P values are shown.
Loss of p44/WDR77 expression altered the differentiation of prostate epithelial cells
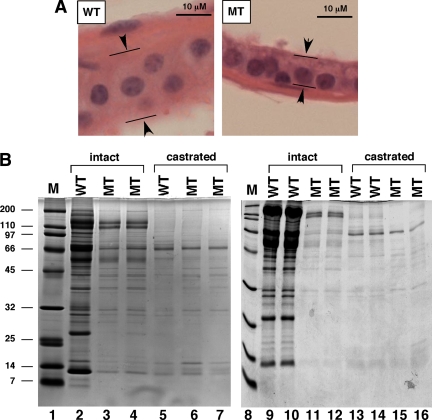
The secretory luminal layer of the prostatic glandular epithelium is made up of tall columnar cells that are responsible for the production of secretory proteins (3). The luminal layer in the WT prostate contained the normal tall columnar secretory cells (Fig. 5A, left panel). In contrast, the luminal layer in the MT prostate was composed of the cuboidal basal-like cell (Fig. 5A, right panel). The luminal epithelial cells in the MT prostates were decreased in height compared with those of the WT controls (Table 1). The MT epithelial cells lacked the prominent areas of lightly staining, apical cytoplasm seen in prostatic epithelial cells of the controls.
Figure 5.
Loss of p44/WDR77 expression decreased protein secretions in the mouse prostate. A, H&E-stained sections show a basal-like cuboidal epithelium in the MT prostate vs. the tall columnar secretory epithelium in the WT prostate. B, Analysis of secretory proteins from intact (lanes 2–4 and lanes 9–12) or castrated (5,6,7,13,14,15,16) WT (lanes 2, 5, 9, 10, 13, and 14) and MT (lanes 3, 4, 6, 7, 11, 12, 15, and 16) mouse prostate glands. Protein secretions were resolved on a 10–20% SDS-polyacrylamide gradient gel. An equal volume (5 μl) was loaded onto each lane. The migration of molecular weight markers (Bio-Rad) is indicated in lanes 1 and 8.
Table 1.
Heights of luminal epithelia of AP, DLP, and VP from WT (n = 5) and MT (n = 5) mice
| Range | Average | Standard deviation | |
|---|---|---|---|
| AP | |||
| WT | 5.5–9.9 | 6.89 | 0.99 |
| MT | 1.1–2.2 | 2.15 | 0.33 |
| DLP | |||
| WT | 2.2–7.7 | 5.32 | 1.10 |
| MT | 2.2–4.4 | 2.99 | 0.77 |
| VP | |||
| WT | 7.7–15.4 | 8.84 | 1.21 |
| MT | 1.1–4.4 | 2.21 | 0.88 |
The secretions from WT (n = 5) and MT (n = 5) prostates were prepared, and protein concentrations of the WT prostate secretions were 2.90 ± 0.14 mg/ml, whereas protein concentrations of the MT prostate secretions were 0.95 ± 0.05 mg/ml. SDS-PAGE analysis revealed that the density of protein bands in the secretions of MT mouse prostate was significantly less than that of the WT prostate (Fig. 5B, lanes 3, 4, 11, and 12 vs. lanes 2, 9, and 10). However, no significant differences in the secretions in the WT (0.54 ± 0.06 mg/ml) and MT (0.50 ± 0.09 mg/ml) prostates were observed when mice were castrated for 2 wk (Fig. 5B, lanes 6, 7, 15, and 16 vs. lanes 5, 13, and 14). These results suggest that the epithelium in the MT prostate is not fully functional.
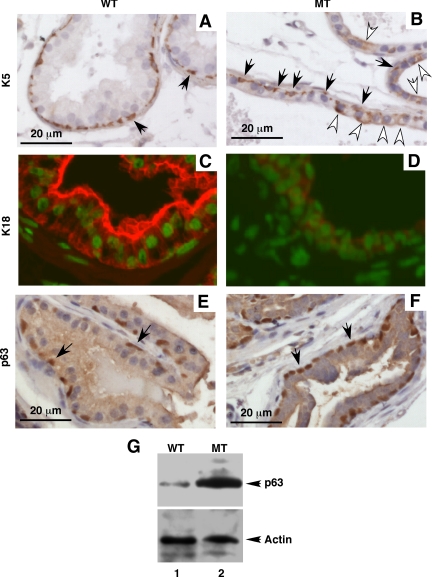
Antibodies to cytoskeleton proteins of the keratin subclasses have been used to study prostate cell differentiation (25). These studies demonstrated that changes in the levels of differentiation are accompanied by a distinct transition in the expression profile of individual keratins. Epithelial cells in the adult prostate could be characterized as basal cells (K52+/K18−), luminal cells (K5−/K182+), and intermediate cells (K5+/K18+). The intermediate cells are in an intermediate state of differentiation (26); they stem from basal cells and eventually differentiate into luminal cells. Sections of the WT and MT DLP prostate lobes were immunohistochemically stained with anti-K5 and anti-K18 antibodies. K5 was expressed in the basal cells (Fig. 6, A and B, indicated by black arrows) but absent in the luminal cells (Fig. 6A) of the WT prostate. However, K5 was strongly expressed in some luminal cells of the MT prostate (Fig. 6B, indicated by white arrows). K18 was positive in all luminal cells and negative in all basal cells of the WT prostate (Fig. 6C). In contrast, K18 was stained weakly in all luminal cells of the MT prostate (Fig. 6D). In contrast, the expression pattern of p63 (Fig. 6, E and F) did not altered but p63 expression levels (Fig. 6G) were significantly increased in the MT prostate epithelium. Similar results were observed with the AP and VP prostate lobes. These results suggest that loss of p44/WDR77 expression leads to improper epithelial differentiation, with miss-expression of K5 and a decreased expression of K18.
Figure 6.
The p44/WDR77 deletion altered expression of cytokeratines in the MT prostate. Anti-K5 (A and B), anti-K18 (C and D), and anti-p63 (E and F) stainings of prostate of WT (n = 3) and MT (n = 3) mice at the age of 2 months. The alkaline phosphophase-labeled (A, B, E, and F) or the Alexa 594-labeled (C and D) antirabbit IgG antibody was used. DAPI staining of the nucleus was merged with K18 immunostaining in C and D. The black and white arrows indicate the basal and epithelial cells, respectively. G, Shows Western blot analysis of p63 in protein extracts (20 μg/per sample) prepared from WT (lane 1) or MT (lane 2) whole prostate gland.
Loss of the p44/WDR77 gene led to hyperproliferation of prostate epithelial cells
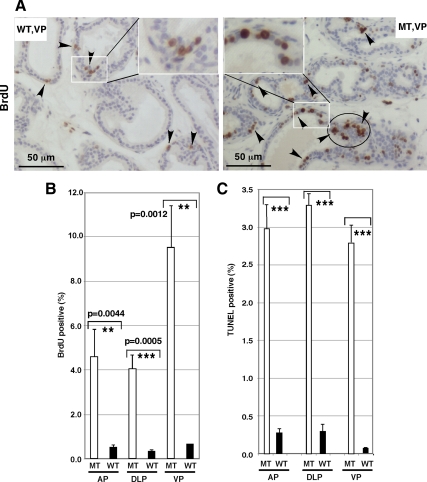
To check proliferation of prostate epithelial cells, we evaluated BrdU incorporation. BrdU incorporation was significantly higher in VP prostatic epithelium of MT mice (n = 5) than that of WT littermates (n = 5) (Fig. 7A, indicated by arrows). The observed significant increase in proliferation was evident in all prostatic lobes (Fig. 7B). We noticed that the BrdU-labeled cells do not distribute uniformly and rather are clustered in the hypeproliferative region (Fig. 7B, circled region). The BrdU-positive cells are mainly epithelial cells (Fig. 7A, insets).
Figure 7.
Loss of p44/WDR77 increased proliferation and apoptosis of prostate epithelial cells. A, In vivo proliferation was assessed using the BrdU incorporation assay. Sections of WT (left) and MT (right) prostate were immunostained with anti-BrdU antibody. The BrdU-positive cells are brown (indicated by black arrows). Insets show regions under high power. B, Percentage of BrdU-positive cells in VP, DLP, and VP of WT and MT mice. C, Apoptosis was assessed using the TUNEL assay with sections of WT and MT prostates. Percentage of apoptotic cells in WT and MT luminal epithelial cells are presented. Data (mean ± sem) from five WT and five MT prostates are expressed. The unpaired t test was performed; *** and ** indicate significant (P < 0.001 and P < 0.05) differences between WT and MT mice.
To evaluate cell death, we used TUNEL staining in WT (n = 5) and MT (n = 5) mouse prostates. We saw little apoptosis in the WT prostate epithelial cells. However, we saw considerable cell death in the MT prostatic epithelial cells (Fig. 7C).
To assess whether loss of the p44/WDR77 gene affects androgen-induced regenerative growth of the prostate, we castrated the male mice at 8 wk of age, 21 d later administered testosterone for 15 d, killed the mice, and dissected the prostate glands for histological analysis. Compared with the WT prostate gland, the MT prostate gland showed the similar phenotype (lacking epithelial enfoldings and containing small immature ducts) (data not shown.). Therefore, these results suggest that loss of the p44/WDR77 gene also affected the androgen-driven regeneration of the prostate gland.
The gene expression profile in the p44/WDR77-null mouse prostate
Prostate glands were obtained from age-matched MT (p44/WDR77loxP/loxP; Cre) and WT (p44/WDR77loxP/loxP) mice and submitted to Condon Biosciences (Houston, TX) for transcriptome profiling using Affymetrix Mouse Genome 430 2.0 chips. Statistical analysis of 39,000 transcripts (the list of genes is available at www.affymetrix.com) generated a list of 57 significantly (>4-fold) altered genes (Table 2). Expression of 39 genes was decreased in the p44/WDR77-null prostate, and expression of 18 genes was increased. The expression levels of selected genes were confirmed by real-time PCR analysis.
Table 2.
List of genes the expression of which was affected by loss of the p44 gene in the prostate
| Gene name | Fold change
|
||
|---|---|---|---|
| DNA chip | RT-PCR | Gene accession no. | |
| Androgen-driven secretion proteins | |||
| Seminal vesicle antigen (Sva) | −256.0 | NM_009299.1 | |
| Seminal vesicle secretion 3 (Svs.3) | −157.5 | −377.3 | NM_021363.1 |
| Seminal vesicle secretion 6 (Svs.6) | −21.1 | NM_013679.1 | |
| Seminal vesicle protein, secretion 2 (Svs.2) | −4.9 | BB807675 | |
| Mucosal defense proteins | |||
| Mucin glycoprptein MUC3 (Muc3) | −97.0 | −61.8 | AF027131.1 |
| Terfoil factor 3, intestinal (Tff3) | −17.1 | −41.1 | NM_011575.1 |
| crp-ductin (Dmbt1) | −10.5 | −20.8 | NM_007769.1 |
| Defensin β9 (Defb9) | −8.0 | BM936051 | |
| Chloride channel calcium activated 3 (Clca3) | −6.0 | NM_017474.1 | |
| Defensin β2 (Defb2) | −5.2 | −7.2 | NM_010030.1 |
| Defensin β1 (Defb1) | −4.2 | BC024380.1 | |
| Sperm-associated antigen 1 (Spag1) | −4.2 | NM_012031.1 | |
| CUB and zona pellucida-like domains 1 (Cuzd1) | −4.2 | NM_008411.2 | |
| Defensin β6 (Defb6) | +4.5 | NM_054074.1 | |
| Signaling proteins | |||
| Protein tyrosine kinase (Ptk6) | −13.9 | −102.5 | NM_009184.1 |
| Axonal-associated cell adhesion molecule (Cntn4) | −8.0 | −18.0 | NM_007518.1 |
| Serum/glucocorticoid regulated kinase 2 (Sgk2) | −6.4 | NM_013731.1 | |
| Interferon-inducible GTPase (Igtp) | −5.2 | −13.5 | NM_018738.1 |
| Colony stimulating factor 3 (Csf3) | +8.0 | NM_009971.1 | |
| Immune system | |||
| Orosomucoid 3 (Orm3) | −111.4 | NM_013623.1 | |
| Serum amyloid A1 (Saa1) | −68.5 | −14.9 | NM_009117.1 |
| POU domain associating factor 1 (Pou2af1) | −32.0 | NM_011136 | |
| CD244 natural killer cell receptor 2B4 (Cd244) | −29.8 | NM_018729.1 | |
| Killer cell lectin-like receptor member 5 (Klra5) | −12.9 | NM_008463.1 | |
| Immunoglobulin κ chain variable 28 (V28) | −12.1 | AV057155 | |
| C-type lectin, superfamily member 5 (Clec5a) | −8.0 | NM_021364.1 | |
| Galectin-6 (Lgals6) | −4.5 | AF026794.1 | |
| C-type lectin, superfamily member 8 (Clec4d) | −4.2 | NM_010819.1 | |
| T cell receptor α chain (Tcra) | −4.0 | U07662.1 | |
| Lipid metabolism | |||
| 2,3-Bisphosphoglycerate mutase (Bpgm) | −5.2 | NM_007563.1 | |
| α-2-Glycoprptein 1, zinc (Azgp1) | −4.5 | NM_013478.1 | |
| Proteinase inhibitors | |||
| Serine proteinase inhibitor, member 5 (Serpina5) | −4.0 | NM_008785.1 | |
| Serine peptidase inhibitor, member 3C (Serpina3c) | +24.2 | NM_008458.1 | |
| Serine protease inhibitor 1–2 (Serpina1b) | +4.2 | NM_009244.1 | |
| Unclassified proteins | |||
| CEA-related cell adhesion molecule 10 (Ceacam10) | −55.7 | −176.0 | NM_007675.1 |
| Deoxyribonuclease II β (Dnase2b) | −55.7 | NM_019957.1 | |
| Cytotoxic T-lymphocyte-associated protein 4 (Ctla4) | −11.3 | NM_009843.1 | |
| Brain-derived neurotrophic factor (Bdnf) | −9.1 | NM_007540.1 | |
| Chaperonin subunit 6b (Cct6b) | −6.4 | NM_009839.1 | |
| Hyaluronan and proteoglycan link protein 2 (Hapln2) | −6.4 | NM_022031.1 | |
| Tescalcin (Tcsc) | −5.6 | −5.35 | NM_021344.1 |
| Similar to solute carrier family 26 (Sic26a3) | −4.5 | −8.5 | BC022639.1 |
| β-Microseminoprotein (Msmb) | −4.0 | NM_020597.1 | |
| Keratocan (Kera) | +34.2 | NM_008438.1 | |
| Myocardin A (Bsac2A) | +14.9 | AF437877.1 | |
| Cellular retinoic acid binding protein I (Crabp1) | +12.1 | NM_013496.1 | |
| Aortic preferntially expressed gene 1 (Apeg1) | +8.0 | NM_007463.1 | |
| Integrin-binding sialoprotein (Ibsp) | +7.4 | NM_008318.1 | |
| Crystallin, γ C (Crygc) | +6.0 | NM_007775.1 | |
| Actin-binding protein frabin-β | +5.6 | AF402612.1 | |
| Adipocyte complement-related protein (Acrp30) | +4.9 | NM_009605.1 | |
| Thyroid hormone-responsive SPOT14 homolog (Thrsp) | +4.2 | NM_009381.1 | |
| Receptor activity-modifying protein 3 (Ramp3) | +4.2 | NM_019511.1 | |
| Secreted phosphoprotein (Spp1) | +4.0 | NM_009263.1 | |
| Melanocyte-specific gene 1 (Msg1) | +4.0 | U65091.1 | |
| Collagen pro-α-1 type I chain | +4.0 | U08020.1 | |
| Adipsin (Cfd) | +4.0 | NM_013459.1 | |
Products of genes (Sva, Svs2, Svs3, and Svs6) are secretions of prostate, and expression of Svs2 and Svs3 genes are androgen dependent (27). The second group of genes the expression of which was down-regulated in the p44/WDR77-null prostate includes components of a variety of mucus gels in the luminal aspect of surface epithelial cells. These are thought to provide a protective, lubricating barrier against particles and infectious agents at mucosal surfaces (28,29,30,31,32). The observed significant down-regulation of expression of these genes is consistent with the altered luminal differentiation of the p44/WDR77-null prostate. Expression of Tff3, Spag 1, Muc3, Defb1, and Defb2 genes is androgen regulated in LNCaP or in mouse prostate gland (27,33). Most significantly, the third group of genes (PTK6, CNTN4, SGK2, IGTP, CSF3), the expression of which was altered in the p44/WDR77-null prostate, is involved in the control of cell growth and differentiation. Ptk6, Csf3, and Igtp genes are regulated by androgens (27,33). The other 10 genes, the expression of which was decreased in the p44/WDR77-null prostate, are involved in immune reaction. Lgals6, Saa1, and V28 genes are androgen regulated (27,33). Among 57 genes the expression of which was affected by the p44/WDR77 gene deletion, 32 genes are androgen regulated in LNCaP cells, in mouse prostate, or in both, consistent with the function of p44/WDR77 as an AR cofactor.
Loss of the p44/WDR77 gene abolished the androgen-driven gene expression in the prostate
We studied androgen-response genes in the mouse prostate by investigating genes that were up-regulated or down-regulated by androgen replacement in the castrated mice. The previous studies indicated that in intact adult mice, mean serum testosterone levels were 8.8 nm, which were dramatically reduced upon castration (34). Administration of testosterone to castrated mice by sc injection of testosterone enanthate maintained serum testosterone levels above 8.8 nm for more than 6 d. The serum concentration of the androgen in castrated mice reached normal adult levels by 12 h after testosterone injection.
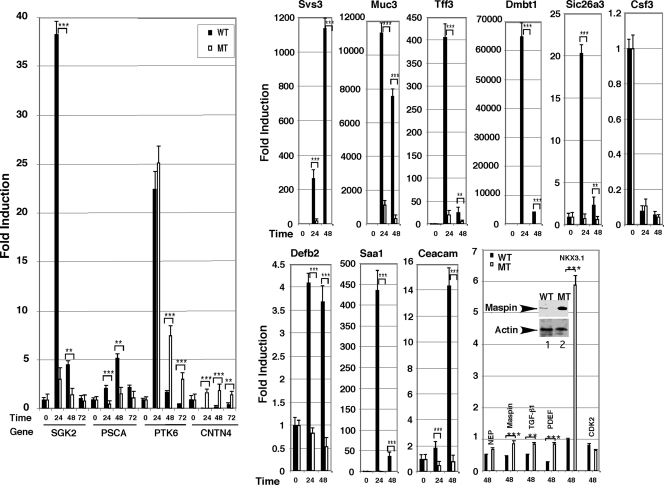
On the basis of this information, we castrated WT (p44/WDR77loxP/loxP) and MT (p44/WDR77loxP/loxP;Cre) mice and administrated testosterone enanthate 7 d later by sc injection. The 0-, 24-, 48-, and 72-h time points after androgen replacement were chosen for isolation of total RNAs from mouse prostate glands. Changes in genes expression induced by the androgen were then analyzed by real-time PCR. Expressions of SGK2, PSCA, and PTK6 genes were up-regulated by the androgen in the WT prostate (Fig. 8). Deletion of the p44/WDR77 gene dramatically decreased the androgen-induced expression of SGK2 and PSCA, but not PTK6 genes. Similarly, expressions of Svs3, Muc3, Tff3, Dmbt1, Defb2, Saa1, Ceacam10, and Sic26a3 were up-regulated in the WT prostate by the androgen (Fig. 8), whereas the androgen-driven expression of these genes was largely abolished in the MT prostate. In contrast, expression of Cntn4, Maspin, TGF-β1, and PDEF genes was suppressed by the androgen, and loss of p44/WDR77 expression relieved this inhibition. Western blot analysis demonstrated that Maspin protein levels were significantly higher in the MT prostate than those in the WT prostate (Fig. 8, inset). Csf3 and NEP genes were also down-regulated by the androgen, and loss of the p44/WDR77 gene did not significantly affect their expression. In the WT mouse, the androgen-induced expression of the NKX3.1 gene was observed at 72 h after androgen replacement. Deletion of the p44/WDR77 gene led to activation of the NKX3.1 gene at 24 h after androgen replacement, indicating that p44/WDR77 plays a negative role in the regulation of NKX3.1 gene expression soon after androgen administration. Although NKX3.1 gene expression in the p44/WDR77-null prostate was significantly lower that in the WT prostate (data not shown), loss of the p44/WDR77 gene resulted in the early expression of the NKX3.1 gene in response to the androgen administration. Thus, p44/WDR77 is involved in the regulation of some AR-target genes during mouse prostate regeneration and development.
Figure 8.
Loss of p44/WDR77 expression affects androgen-dependent gene expression. Real-time PCR analysis of gene expression in prostates of 7-d-castrated mice (WT, n = 3; MT, n = 3) treated with testosterone propionate for the indicated number of hours. The equation fold change = relative quantification of genes in the presence/absence of androgen. Data (mean ± sem) are expressed. Inset shows Western blot analysis of Maspin in protein extracts (20 μg/per sample) prepared from WT (lane 1) or MT (lane 2) whole prostate gland. The unpaired t test was performed; *** and ** indicate significant (P < 0.0001 and P < 0.001) differences between WT and MT mice.
Discussion
Our analysis provides evidence that AR cofactor p44/WDR77 plays an important role in normal prostate differentiation and development through modulation of AR-target gene expression. We have shown that p44/WDR77 is essential for normal morphogenesis and function of the prostate, whereas its inactivation in prostate epithelia leads to prostatic epithelial hyperproliferation, loss of cell differentiation, and altered expression of some AR-target genes. Taken together with the observation that p44/WDR77 translocation from the nucleus to the cytoplasm in prostate epithelial cells is associated with prostate tumorigenesis (19), we propose that p44/WDR77 in the nucleus is required for proper expression of AR-target genes to maintain the differentiation of prostate epithelial cells and prostate functions.
Prostate organogenesis is associated with androgen signaling from the earliest stages of prostate formation through mature functions. During embryogenesis, mesenchymal AR is required for prostate formation (35), whereas during adulthood, epithelial AR is required for secretory protein production (2). The prostate epithelial and stromal compartments act together as one functional unit. A complex signaling relationship between these stromal and epithelial tissue compartments plays an important role in regulating prostate development, growth, and function (5,6). Consistent with the fact that prostate epithelial cells express p44/WDR77, the MT luminal epithelial cells are cuboid-like cells that were not fully differentiated and significantly decreased production of secreted proteins in the prostate. Thus, p44/WDR77 plays a role in the cellular differentiation of the epithelium in the adult prostate.
The finding of this study is in accord with results obtained from the tissue recombination experiments using normal mesenchyme (M) plus epithelium (E) from androgen-insensitive (Tfm) mice (2). In wt-M+Tfm-E tissue recombinants, although the AR-deficient Tfm epithelium underwent ductal morphogenesis and epithelial proliferation, mature prostatic tissue was rarely detected. These tissue recombinants encompassed the same range of morphologies seen in normal mesenchyme and epithelium with atypical epithelial histodifferentiation and lacking the secretory proteins. Some small ducts had a low columnar epithelium, and other ducts had very large cystic ducts lined with cuboidal epithelium. The p44/WDR77-null prostate resembles that lacking epithelial AR in terms of less epithelial differentiation and hyperproliferation (36). In contrast, the absence of androgens (under castration) in both epithelial and stromal cells nearly completely abolished cell proliferation in the prostate (37). These observations further confirm the distinct roles of mesenchymal AR and epithelial AR (2,35).
The p44/WDR77 was identified by its physical association with AR in prostate cancer cells and regulated expression of AR-driven genes such as PSA, NKX3.1, PDEF, p21, and TGF-β1 in various assays (18,19). The chromatin immunoprecipitation analysis showed that p44/WDR77 was recruited onto the endogenous AR-target genes (PSA and p21) in an androgen-dependent manner. Thus, these observations define the positive cofactor function of p44/WDR77 for AR. Current research identified a subset of AR-regulated genes, the expression of which is regulated by p44. More resent studies indicate that transcriptional regulation occurs in RNA polymerase II hubs (38). In this view, some AR-regulated hubs, and not others, contain p44 that modulates the expression of a subset of genes.
The p44/WDR77-null mouse gave us an unique opportunity to further investigate the regulation of androgen-driven genes by the AR cofactor (p44/WDR77) in vivo. There are two alternative approaches to studying androgen-response genes in the mouse prostate: first, investigating genes that are up-regulated or down-regulated in the prostate by castration; and second, investigating genes that are up-regulated or down-regulated by androgen replacement in the castrated mice. We chose the second approach. One major advantage of this choice is that the induction of androgen-response genes is not affected by other factor(s) or hormone(s) secreted from the testis. In contrast, castration may cause gene expression changes that are regulated by nonandrogen testicular factors (39,40). Furthermore, the induction of androgen-response genes in the prostate by hormone replacement is more rapid (in days) than that by castration (in weeks), because the decay of androgen after castration takes time. Thus, this analysis would reveal genes that are truly regulated by the androgen other than genes the changes in expression of which resulted from alterations in cellular or tissue composition. This approach revealed 15 androgen-regulated genes the expressions of which were dependent on p44/WDR77 in the mouse prostate. Thus, our in vitro and in vivo studies firmly characterized p44/WDR77 functions as an AR cofactor to regulate expression of some androgen-dependent genes. Three lobes of mouse prostate are different in the term of androgen sensitivity and prostatic secretions. To investigate the lobe-specific functions of p44/WDR77, three individual lobes instead of the whole prostate should be used in future studies.
The androgen-signaling pathway plays a critical role in the growth and differentiation of the prostate gland and is a predominant target for prostate cancer therapies (41). It has been suggested that dysregulation of this signaling pathway contributes to prostate hyperplasia in mice (42). The fully differentiated luminal epithelial cells are polarized, and the prostatic epithelium loses this polarity during the progression of cancer (43). We showed previously that the p44/WDR77 translocation into the cytoplasm was strongly associated with the proliferation of prostatic epithelial cells and that the forced nuclear localization of p44/WDR77 seriously retarded the proliferation of prostate cancer cells (19,20). Thus, p44/WDR77 functions in the nucleus as a negative factor for cell growth. Consistent with these observations, the p44/WDR77-null prostate had epithelial cell hyperproliferation in every mouse observed. Our data implicate p44/WDR77 as a crucial mediator of the androgen-driven cytodifferentiation and production of secretory proteins in the prostate, thus inhibiting proliferation. In addition, the nuclear p44/WDR77 establishes or maintains prostatic epithelial cells in a differentiated/functional state through regulation of expression of a subset of (androgen-regulated) genes. Conversely, the p44/WDR77 cytoplasm localization (in prostate cancer) or decrease in p44/WDR77 levels in the nucleus by the loss of the p44/WDR77 gene would relieve this growth arrest and result in epithelial cell proliferation. The proliferating epithelial cells would then afford the opportunity for further genetic mutations to occur, thereby facilitating the progression to prostate cancer. These observations documented a novel role of p44/WDR77 in the control of prostatic epithelial cell differentiation and proliferation.
Acknowledgments
We thank Michael Worley for the critical editorial review and Dr. Jan Parker-Thornburg in the Genetically Engineered Mouse Facility at M. D. Anderson Cancer Center for generation of the p44/WDR77 gene-knockout mouse.
Footnotes
This work was supported, in part, by Grant 1R01 DK065156 01 (to Z.W.) from the National Institute of Diabetes and Digestive and Kidney Diseases and by the Institutional Research Grant from The University of Texas M.D. Anderson Cancer Center from the National Cancer Institute, National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 2, 2010
Abbreviations: AP, Anterior prostate; AR, androgen receptor; Brdu, bromodeoxyuridine; DLP, dorsolateral prostate; ES, embryonic stem; K5, keratin 5; K18, keratin 18; MT, mutant; PSA, prostate-specific antigen; SDS, sodium dodecyl sulfate; TUNEL, terminal deoxynucleotide transferase-mediated dUTP nick end labeling; VP, ventral prostate; WT, wild type.
References
- Cunha GR, Chung LW 1981 Stromal-epithelial interactions–I. Induction of prostatic phenotype in urothelium of testicular feminized (Tfm/y) mice. J Steroid Biochem 14:1317–1324 [DOI] [PubMed] [Google Scholar]
- Donjacour AA, Cunha GR 1993 Assessment of prostatic protein secretion in tissue recombinants made of urogenital sinus mesenchyme and urothelium from normal or androgen-insensitive mice. Endocrinology 132:2342–2350 [DOI] [PubMed] [Google Scholar]
- Rittenhouse HG, Finlay JA, Mikolajczyk SD, Partin AW 1998 Human Kallikrein 2 (hK2) and prostate-specific antigen (PSA): two closely related, but distinct, kallikreins in the prostate. Crit Rev Clin Lab Sci 35:275–368 [DOI] [PubMed] [Google Scholar]
- Isaacs JT, Coffey DS 1989 Etiology and disease process of benign prostatic hyperplasia. Prostate Suppl 2:33–50 [DOI] [PubMed] [Google Scholar]
- Liu AY, True LD, LaTray L, Nelson PS, Ellis WJ, Vessella RL, Lange PH, Hood L, van den Engh G 1997 Cell-cell interaction in prostate gene regulation and cytodifferentiation. Proc Natl Acad Sci USA 94:10705–10710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward SW, Del Buono R, Deshpande N, Hall PA 1992 A functional model of adult human prostate epithelium. The role of androgens and stroma in architectural organisation and the maintenance of differentiated secretory function. J Cell Sci 102:361–372 [DOI] [PubMed] [Google Scholar]
- Craft N, Sawyers CL 1998 Mechanistic concepts in androgen-dependence of prostate cancer. Cancer Metastasis Rev 17:421–427 [DOI] [PubMed] [Google Scholar]
- Craft N, Chhor C, Tran C, Belldegrun A, DeKernion J, Witte ON, Said J, Reiter RE, Sawyers CL 1999 Evidence for clonal outgrowth of androgen-independent prostate cancer cells from androgen-dependent tumors through a two-step process. Cancer Res 59:5030–5036 [PubMed] [Google Scholar]
- Jenster G 1999 The role of the androgen receptor in the development and progression of prostate cancer. Semin Oncol 26:407–421 [PubMed] [Google Scholar]
- Denmeade SR, Lin XS, Isaacs JT 1996 Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate 28:251–265 [DOI] [PubMed] [Google Scholar]
- McNeal JE 1988 Normal histology of the prostate. Am J Surg Pathol 12:619–633 [DOI] [PubMed] [Google Scholar]
- Brinkmann AO, Blok LJ, de Ruiter PE, Doesburg P, Steketee K, Berrevoets CA, Trapman J 1999 Mechanisms of androgen receptor activation and function. J Steroid Biochem Mol Biol 69:307–313 [DOI] [PubMed] [Google Scholar]
- Jänne OA, Moilanen AM, Poukka H, Rouleau N, Karvonen U, Kotaja N, Häkli M, Palvimo JJ 2000 Androgen-receptor-interacting nuclear proteins. Biochem Soc Trans 28:401–405 [PubMed] [Google Scholar]
- Gelmann EP 2002 Molecular biology of the androgen receptor. J Clin Oncol 20:3001–3015 [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C 2002 Androgen receptor (AR) coregulators: an overview. Endocr Rev 23:175–200 [DOI] [PubMed] [Google Scholar]
- Jia L, Berman BP, Jariwala U, Yan X, Cogan JP, Walters A, Chen T, Buchanan G, Frenkel B, Coetzee GA 2008 Genomic androgen receptor-occupied regions with different functions, defined by histone acetylation, coregulators and transcriptional capacity. PLoS One 3:e3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Li W, Liu XS, Carroll JS, Jänne OA, Keeton EK, Chinnaiyan AM, Pienta KJ, Brown M 2007 A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell 27:380–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosohata K, Li P, Hosohata Y, Qin J, Roeder RG, Wang Z 2003 Purification and identification of a novel complex which is involved in androgen receptor-dependent transcription. Mol Cell Biol 23:7019–7029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Wu H, Lee P, Wang Z 2006 Roles of the androgen receptor cofactor p44 in the growth of prostate epithelial cells. J Mol Endocrinol 37:283–300 [DOI] [PubMed] [Google Scholar]
- Peng Y, Chen F, Melamed J, Chiriboga L, Wei J, Kong X, McLeod M, Li Y, Li CX, Feng A, Garabedian MJ, Wang Z, Roeder RG, Lee P 2008 Distinct nuclear and cytoplasmic functions of androgen receptor cofactor p44 and association with androgen-independent prostate cancer. Proc Natl Acad Sci USA 105:5236–5241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura Y, Cunha GR, Donjacour AA 1986 Morphogenesis of ductal networks in the mouse prostate. Biol Reprod 34:961–971 [DOI] [PubMed] [Google Scholar]
- Yu X, Li P, Roeder RG, Wang Z 2001 Inhibition of androgen receptor-mediated transcription by amino-terminal enhancer of split. Mol Cell Biol 21:4614–4625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Lee P, Wang H, Gerald W, Adler M, Zhang L, Wang YF, Wang Z 2005 The androgen receptor directly targets the cellular Fas/FasL-associated death domain protein-like inhibitory protein gene to promote the androgen-independent growth of prostate cancer cells. Mol Endocrinol 19:1792–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, McKeehan K, Yu C, Ittmann M, McKeehan WL 2004 Chronic activity of ectopic type 1 fibroblast growth factor receptor tyrosine kinase in prostate epithelium results in hyperplasia accompanied by intraepithelial neoplasia. Prostate 58:1–12 [DOI] [PubMed] [Google Scholar]
- Schalken JA, van Leenders G 2003 Cellular and molecular biology of the prostate: stem cell biology. Urology 62 (Suppl 1):11–20 [DOI] [PubMed] [Google Scholar]
- Xue Y, Smedts F, Debruyne FM, de la Rosette JJ, Schalken JA 1998 Identification of intermediate cell types by keratin expression in the developing human prostate. Prostate 34:292–301 [DOI] [PubMed] [Google Scholar]
- Wang XD, Wang BE, Soriano R, Zha J, Zhang Z, Modrusan Z, Cunha GR, Gao WQ 2007 Expression profiling of the mouse prostate after castration and hormone replacement: implication of H-cadherin in prostate tumorigenesis. Differentiation 75:219–234 [DOI] [PubMed] [Google Scholar]
- Thornton DJ, Sheehan JK 2004 From mucins to mucus: toward a more coherent understanding of this essential barrier. Proc Am Thorac Soc 1:54–61 [DOI] [PubMed] [Google Scholar]
- Shirazi T, Longman RJ, Corfield AP, Probert CS 2000 Mucins and inflammatory bowel disease. Postgrad Med J 76:473–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazgier M, Hoover DM, Yang D, Lu W, Lubkowskia J 2006 Human β-defensins. Cell Mol Life Sci 49:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholfield DP, Simms MS, Bishop MC 2003 MUC1 mucin in urological malignancy. BJU Int 91:560–566 [DOI] [PubMed] [Google Scholar]
- Mathelin C, Tomasetto C, Rio MC 2005 [Trefoil factor 1 (pS2/TFF1), a peptide with numerous functions]. Bull Cancer 92:773–781 [PubMed] [Google Scholar]
- Hendriksen PJ, Dits NF, Kokame K, Veldhoven A, van Weerden WM, Bangma CH, Trapman J, Jenster G 2006 Evolution of the androgen receptor pathway during progression of prostate cancer. Cancer Res 66:5012–5020 [DOI] [PubMed] [Google Scholar]
- Mirosevich J, Bentel JM, Zeps N, Redmond SL, D'Antuono MF, Dawkins HJ 1999 Androgen receptor expression of proliferating basal and luminal cells in adult murine ventral prostate. J Endocrinol 162:341–350 [DOI] [PubMed] [Google Scholar]
- Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ, Sugimura Y 1987 The endocrinology and developmental biology of the prostate. Endocr Rev 8:338–362 [DOI] [PubMed] [Google Scholar]
- Wu CT, Altuwaijri S, Ricke WA, Huang SP, Yeh S, Zhang C, Niu Y, Tsai MY, Chang C 2007 Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci USA 104:12679–12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura Y, Cunha GR, Donjacour AA, Bigsby RM, Brody JR 1986 Whole-mount autoradiography study of DNA synthetic activity during postnatal development and androgen-induced regeneration in the mouse prostate. Biol Reprod 34:985–995 [DOI] [PubMed] [Google Scholar]
- Göndör A, Ohlsson R 2009 Chromosome crosstalk in three dimensions. Nature 461:212–217 [DOI] [PubMed] [Google Scholar]
- Dalton DP, Lee C, Huprikar S, Chmiel JS, Grayhack JT 1990 Non-androgenic role of testis in enhancing ventral prostate growth in rats. Prostate 16:225–233 [DOI] [PubMed] [Google Scholar]
- Darras FS, Lee C, Huprikar S, Rademaker AW, Grayhack JT 1992 Evidence for a non-androgenic role of testis and epididymis in androgen-supported growth of the rat ventral prostate. J Urol 148:432–440 [DOI] [PubMed] [Google Scholar]
- Santos AF, Huang H, Tindall DJ 2004 The androgen receptor: a potential target for therapy of prostate cancer. Steroids 69:79–85 [DOI] [PubMed] [Google Scholar]
- Magee JA, Abdulkadir SA, Milbrandt J 2003 Haploinsufficiency at the Nkx3.1 locus. A paradigm for stochastic, dosage-sensitive gene regulation during tumor initiation. Cancer Cell 3:273–283 [DOI] [PubMed] [Google Scholar]
- Gleason DM, Bottaccini MR, Reilly RJ 1977 Comparison of cystometrograms and urethral profiles with gas and water media. Urology 9:155–160 [DOI] [PubMed] [Google Scholar]