Abstract
Oscillations in intracellular calcium levels have been described in GnRH-1 neurons in both prenatal and adult cells. However, differences have been reported in the mechanisms underlying these [Ca2+]i oscillations, dependent on the model used. The goal of this study was to address whether these changes depend on the maturation status of GnRH-1 neurons by assaying prenatal GnRH-1 cells maintained in explants, at two different developmental stages. This report documents an increase in the frequency of [Ca2+]i oscillations between 1 and 3 wk of in vitro maturation. During the early stage, [Ca2+]i oscillations are blocked by tetrodotoxin and are mainly triggered by excitatory neurotransmitters, γ-aminobutyric acid (GABA), and glutamate. In contrast, in the later stage, some cells exhibit residual tetrodotoxin-insensitive [Ca2+]i oscillations, which are sustained by action potential-independent GABA and glutamate release. The strength of these two excitatory inputs remained relatively constant during the maturation process, and the increase in frequency of [Ca2+]i oscillations observed at the later stage is due to a novel excitatory input carried by cholecystokinin. Together, these data indicate developmentally regulated release and interactions of neurotransmitters (known regulators of GnRH-1 cells in adults) and point to extrinsic factors regulating GnRH-1 cellular physiology.
Calcium oscillations in GnRH-1 neurons are triggered by neurotransmitters.
GnRH-1 released at the median eminence is the final central output that controls reproduction by triggering pituitary hormone secretion that stimulates the gonads. It is clear that GnRH-1 neurons must release GnRH-1 in a pulsatile manner to preserve the responsiveness of pituitary gonadotropes. However, the mechanisms underlying the rhythmic activity of the GnRH-1 network remain unclear.
GnRH-1 neurons are few (∼800 in mouse) and not confined to a classic anatomical nucleus but rather are found in a continuum within the forebrain. In rodents, the GnRH-1 cells are located from the olfactory bulbs to the caudal hypothalamus (1). To assess their cellular physiology and to address neuronal activity of the GnRH-1 neurons, different models have been developed, including immortalized cell lines GT1s (2), nasal explants (3,4,5,6), and acute brain slices from GnRH neuron-tagged mice (7,8,9). In all models, oscillations in intracellular calcium ([Ca2+]i) baseline were observed (9,10,11,12). GT1 cells were derived from a tumor at the rostral boundary of the optic chiasm (2), whereas GnRH-1 cells in explants never entered the central nervous system (CNS) and are obtained prenatally (11,12) (reviewed in Ref. 13). Although both of these models certainly retain developmental characteristics, it has been shown for each that the cells are mature enough to exhibit 1) pulsatile release (6,14,15,16,17) and 2) synchronized [Ca2+]i events, concomitant with release (17,18) or occurring with the same periodicity as GnRH-1 release (11,12). Both these characteristics suggest a relationship between [Ca2+]i oscillations and secretion. In fact, the [Ca2+]i oscillations described in GnRH-1 neurons in acute postnatal brain slices (9) were consistent with those reported in prenatal GnRH-1 neurons in nasal explants (12,19). Thus, [Ca2+]i oscillations appear to be an early property of the GnRH-1 cell, independent of CNS input, and maintained postnatally. However, differences in the mechanisms triggering [Ca2+]i oscillations appeared between primary GnRH-1 cells obtained from prenatal (19) and postnatal mice (9). Thus, the goal of this paper was to identify properties of GnRH-1 neurons that may mature as a function of development.
This report indicates an increase in the frequency of [Ca2+]i oscillations detected during in vitro development and details the mechanisms responsible for this increase. We show that the response to tetrodotoxin (TTX) changes in GnRH-1 cells between 1 and 3 wk, abolishing the majority of [Ca2+]i oscillations at 7 days in vitro (div), whereas producing only a partial blockade at 21 div. Preventing the activation of both γ-aminobutyric acid (GABA)A and α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate (AMPA)/kainate receptors was also sufficient to block the majority of oscillations at 7 div whereas only partially blocked oscillations at 21 div. These data suggested that either GABA and/or glutamate inputs to GnRH-1 cells changed over development or another mechanism triggering oscillations was present at 21 div. GABAergic inputs remained relatively constant during development, whereas glutamatergic inputs increased but only slightly. Thus, their relative contribution to [Ca2+]i oscillations in GnRH-1 cells was attenuated over development, because the overall oscillation rate increased. An additional input that changed throughout development was cholecystokinin (CCK)ergic. Removal of CCK receptor activation abrogated all amino acid-independent oscillations at 21 div. These data indicate developmental changes in neuronal inputs controlling [Ca2+] oscillations, without any change in the properties of the GnRH-1 neuronal calcium oscillator itself.
Materials and Methods
Animals
All mice were killed in accordance with National Institutes of Health, National Institute of Neurological Stroke and Disorders guidelines. Nasal pits of embryonic d 11.5 staged NIH Swiss mice were isolated under aseptic conditions and adhered onto coverslips by a plasma (Cocalico Biologicals, Reamstown, PA)/bovine thrombin (20 U/ml; Sigma, St. Louis, MO) clot as previously described (5). Nasal explants were maintained at 37 C in a defined serum-free medium (SFM) (5) in a humidified atmosphere with 5% CO2. On culture d 3, fresh media containing fluorodeoxyuridine (8 × 10−5 m; Sigma) were given for 3 days to inhibit proliferation of dividing olfactory neurons and nonneuronal explant tissue. On culture d 6, and every 2 days afterward, the medium was changed for fresh SFM. Explants were used between 6 and 24 div (Fig. 1A).
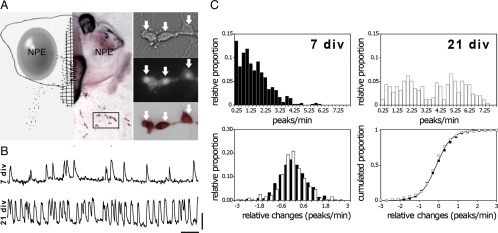
Figure 1.
GnRH-1 neurons exhibit different [Ca2+]i oscillation patterns over development. A, left panel, Schematic representation (left side) and low magnification of a nasal explant (right side) obtained from E11.5 mouse and maintained for 21 d and then immunostained for GnRH-1 (brown). NPE, Nasal pit epithelium; NMC, nasal midline cartilage. GnRH-1 neurons (dots in schematic representation and brown in image) migrate from NPE and follow olfactory axons to the NMC and off the explant into the periphery. The boxed area delimits a typical field of cells selected for calcium recording. Cells are shown at higher magnification in right panel. Calcium imaging recordings were performed on GnRH-1 cells maintained for 7 or 21 div. Cells, identified by their bipolar morphology (right upper panel), were loaded with a calcium-sensitive dye (right middle panel), and after recording, the phenotype was confirmed by immunocytochemistry (right lower panel). Arrows indicate same cells in all fields. B, Representative 10-min calcium imaging recordings from cell at 7 div (upper trace) and 21 div (lower trace), in SFM, show spontaneous baseline oscillations in intracellular calcium level. X bar, 1 min; y bar, 200 U of OD. C, upper panel, Normalized distribution of [Ca2+]i oscillations of the GnRH-1 population at 7 div (left) and 21 div (right) indicates a wider distribution in the raw oscillatory frequency at 21 than at 7 div. Lower panel, Distribution and cumulated distribution of spontaneous changes in [Ca2+]i oscillations between 7 div (black symbols) and 21 div (open symbols) shows that the pattern of [Ca2+]i oscillations does not change spontaneously over the recording periods by more than 1.8 peaks/min at both ages.
Calcium imaging
Calcium imaging recordings were performed between 6 and 10 div and 20 and 24 div (Fig. 1B). The Calcium Green-1 AM (Molecular Probes, Eugene, OR) was diluted to 2.7 mm concentration in 80% dimethylsulfoxide and 20% pluronic F-127 solution (Molecular Probes). This solution was diluted 1:200 with SFM to a final Calcium Green-1 concentration of 13.5 μm. Explants, maintained at 37 C in a 5% CO2 humidified incubator, were incubated with this loading solution for 20 min, then washed twice with fresh SFM (10 min each). Explants were mounted into a perfusion chamber and were continuously superfused at a rate of approximately 280 μl/min, using a peristaltic pump (Spectra Hardware, Inc., Westmoreland City, PA). To maintain consistency throughout the experimental paradigm, the same medium, in which the explants were maintained, was used during the recording periods as control medium and solvent for drugs [SFM; F12: basal medium Eagle (BME) 1:1] (5). Calcium Green-1 was visualized using an inverted Nikon microscope, through a ×20 fluorescence objective and a digital charge-coupled device camera (Retiga; Qimaging, Burnaby, Canada) connected to a computer. Experiments were piloted by imaging software (IPLab Spectrum; Scanalytics, Inc., Rockville, MD), controlling the shutter (Uniblitz; Vincent Associates, Rochester, NY) and acquiring pictures every second. Excitation wavelengths were provided through a medium-width excitation bandpass filter at 465–495 nm, and emission was monitored through a 40-nm bandpass centered on 535 nm. Fluctuations in [Ca2+]i were analyzed a posteriori with IPLab software. Each cell, individually identified, was circled. Calcium Green-1 fluorescence intensity was plotted and analyzed with MATLAB (Mathworks, Natick, MA). Calcium recording experiments were subdivided into three to four periods for analysis [control period (5 min), treatment period (6 min or two times 3 min), and washout period (5 min)]. All recordings were terminated by a 40 mm KCl stimulation to ensure the viability of the recorded cells.
Statistical analysis
Calcium oscillations reflecting electrical activity (20) were measured as an indication of GnRH-1 neuronal activity. A calcium fluctuation was first identified when a value was greater than the five previous and five subsequent points. Then, to be considered as a calcium oscillation (peak), that calcium fluctuation had to be greater than the mean of the five previous and five next points plus a minimal value (which was determined as four times greater than the sd calculated in 11-point-windows floating over a 5-min recording in the presence of TTX at 7 div). The frequency of calcium oscillations was calculated as the number of detected calcium peaks/min. Because the distribution of the frequencies of calcium oscillations is skewed, Wilcoxon-Mann-Whitney U test (nonparametric) was used to compare cell populations. ANOVA was used to compare the degree of inhibition of a drug between ages. Cell proportions were compared using a Fisher’s exact test. Unless otherwise indicated, a paired t test was done comparing peaks/min of the individual cells (n ≥ 28 recorded cells) in an experiment (N; at least two different animals, mostly from independent litters), during control vs. treatment periods. Significance threshold was set at P < 0.05. Data are presented as mean ± sem.
Drugs
(−)−Bicuculline (BIC) chloride (a GABAA receptor antagonist), saclofen (SAC) (a GABAB receptor antagonist), thapsigargin [sarcoplasmic/endoplasmic reticulum (Ca2+) ATPase inhibitor], TTX (a voltage-gated sodium channel blocker), flufenamic acid (FFA) [transient receptor potential (TRP) inhibitor], nifedipine (NIF) (a L-type calcium channel blocker), nimodipine (NIM) (a L-type calcium channel blocker), and proglumide (PRG) (CCK receptor antagonist) were obtained from Sigma. 2-Aminoethoxydiphenylborane (2-APB) [inositol triphosphate receptor (IP3R) inhibitor], pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (P2-type purinergic receptor antagonist), D-2-amino-5-phosphonovalerate (AP5) [N-methyl-d-aspartate (NMDA) glutamatergic receptor antagonist], and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (non-NMDA glutamatergic receptor antagonist) were obtained from Tocris (Avonmouth, UK). All stock solutions (SFM, ethanol, or dimethylsulfoxide) were stored at −20 C, and solutions were prepared before each experiment by diluting stock solutions (1:500 to 1:2000; dilutions for which solvents do not affect basal oscillations) (19) into SFM [used for culturing explants (5)]. Ca2+-free SFM was made by substituting F12 medium with Ca2+-free F12 medium and adding of 500 μm EGTA. Because [Ca2+] in SFM is normally about 1 mm, CaCl2 was not substituted by another salt.
Immunocytochemistry
The recorded cells were verified as GnRH-1 positive via immunocytochemistry as previously described (Fig. 1A) (5). Briefly, after imaging, the explants were fixed (4% formaldehyde, 1 h), rinsed in PBS, and placed in cryoprotectant (21) until staining. Cryoprotected explants were washed in PBS, blocked in 10% normal goat serum and 0.3% Triton X-100 for 1 h, washed several times in PBS, and then incubated overnight (4 C) in GnRH-1 antibody (1:3000, SW-1) (22). The next day, explants were washed in PBS, incubated in biotinylated secondary antibody (1 h, 1:500 in PBS and 0.3% Triton X-100; Vector Laboratories, Inc., Burlingame, CA), washed in PBS, and processed for avidin-biotin-horseradish peroxidase and 3′3-diaminobenzidine cytochemistry. Once stained, recorded fields were compared with the stained explants and only GnRH-1-positive cells analyzed [the majority of cells loaded with calcium green are in fact GnRH-1 positive (12)]. Following similar staining procedures, explants (at 7 and 21 div, both recorded and nonrecorded) were also evaluated for CCK (previously used in Ref. 23; 1:8000, CCK-8; ImmunoStar, Inc., Hudson, WI) and glutamate [vesicular glutamate transporter (VGLUT)2; 1:18,000; Synaptic Systems, Göttingen, Germany]. Double label immunocytochemistry (CCK/GnRH-1 and VGLUT2/GnRH-1) was performed using 3′3-diaminobenzidine and SG substrate (Vector Laboratories) as described previously (23).
Results
Spontaneous [Ca2+]i oscillations
Spontaneous oscillations of the baseline intracellular calcium ([Ca2+]i) were observed in GnRH-1 neurons at both 7 and 21 div (Fig. 1B). A higher frequency of [Ca2+]i oscillations was observed at 21 div [1.54 ± 0.03 peaks/min at 7 div (n = 1231, n = 42), 3.7 ± 0.06 peaks/min at 21 div (n = 1477, n = 55); P < 0.05, ANOVA], consistent with Moore et al. (12). These data suggest that 1) changes in the calcium oscillator, 2) appearance/augmentation of excitatory inputs, and/or 3) loss of inhibitory inputs occurs in this in vitro preparation during a 2-wk developmental time window. To test these hypotheses, cells from both ages were challenged with pharmacological agents.
Because the average frequency of [Ca2+]i oscillations increased between ages, the sensitivity to pharmacological agents needed to be assessed by a value that represented the change in oscillatory frequency compared with the control period. The overall impact on the frequency (loss or gain) was kept in peaks/min rather than expressed in percentage to minimize the influence of the initial baseline frequencies. Indeed, for a given specific input driving five oscillations at both ages, the percentage would artificially underestimate the input to the cells with higher frequencies [i.e. 5/10 at 7 div (50%) and 5/30 at 21 div (16%)]. This bias was avoided by evaluating changes in peaks/min to compare responses at different ages.
To use this approach, it was first necessary to characterize the spontaneous variations in the frequency of [Ca2+]i oscillations during a control period (SFM) at both ages. Examination of the GnRH-1 population at 7 and 21 div revealed a greater heterogeneity in the frequency of [Ca2+]i oscillations at 21 div compared with 7 div (Wilcoxon-Mann-Whitney U test, P < 0.05) (Fig. 1C, top panels). Two phenomena could account for this heterogeneity: a greater variability between the cells and/or a greater variability within each single cell over time. Examination of the changes in the frequency of [Ca2+]i oscillations that cells spontaneously exhibited over a recording period revealed no difference between 7 and 21 div (Wilcoxon-Mann-Whitney U test, P > 0.05) (Fig. 1C, bottom panels), i.e. independent of the initial baseline frequency of a cell, it varied within the same range over the recording period. These data support the first hypothesis that variability between the cells increases with age, accounting for changes in the GnRH oscillator and/or inputs. Because spontaneous activity remained constant during the 16-min recording periods at both ages, comparisons between similar treatments across ages were made in changes in peaks/min to compare the effect of a drug affecting both ages.
Voltage-gated sodium channels and [Ca2+]i oscillations
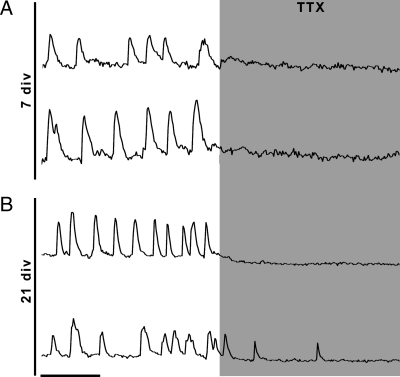
A recent study has shown that the majority of spontaneous [Ca2+]i oscillations in GnRH-1 neurons are blocked by TTX in explants at 7 div during a similar recording period (19). Jasoni et al. (9) have described TTX-sensitive, as well as TTX-insensitive, oscillations in GnRH-1 neurons in adult acute brain slices, dependent on the activation of IP3R. Thus, the sensitivity to TTX was evaluated over development (Fig. 2). As previously described, TTX (1 μm) inhibited, in a reversible manner, [Ca2+]i oscillations at 7 div (P < 0.05) (Table 1). Similarly, [Ca2+]i oscillations were also inhibited at 21 div (P < 0.05) (Table 1). Although TTX blocked more oscillations at 21 div than at 7 div (7 div, −1.38 ± 0.07 peaks/min; 21 div, −3.38 ± 1.19 peaks/min; P < 0.05, ANOVA), the number of TTX-resistant oscillations was significantly lower at 7 than 21 div (TTX: 7 div, 0.12 ± 0.03 peaks/min vs. 21 div, 0.68 ± 0.11 peaks/min; P < 0.05, ANOVA), suggesting a TTX-insensitive mechanism triggering [Ca2+]i oscillations in GnRH-1 cells at 21 div.
Figure 2.
Dependency on voltage-gated sodium channels changes during development. A, Blockage of voltage-gated sodium channels with TTX is complete at 7 div. B, Representative single explant at 21 div, TTX completely inhibits oscillations in some cells (upper trace), but only a partial blockade is seen in others (lower trace). X bar, 1 min.
Table 1.
Characterization of [Ca2+]i oscillations of GnRH-1 neurons over development
| 7 div
|
21 div
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Experiment | Control peaks/min | Treatment peaks/min | n | N | Control peaks/min | Treatment peaks/min | n | N |
| SFM/TTX | 1.50 ± 0.07 | 0.12 ± 0.03a | 159 | 4 | 4.05 ± 0.18 | 0.68 ± 0.11a | 131 | 3 |
| SFM/2-APB | 1.17 ± 0.12 | 1.29 ± 0.11 | 60 | 3 | 4.89 ± 0.20 | 4.45 ± 0.21a | 86 | 2 |
| SFM/FFA | 1.87 ± 0.22 | 1.74 ± 0.20 | 30 | 2 | 3.83 ± 0.43 | 2.33 ± 0.41a | 28 | 2 |
| SFM/NIF | 1.50 ± 0.11 | 1.06 ± 0.08a | 106 | 2 | 3.64 ± 0.23 | 1.53 ± 0.14a | 67 | 2 |
| SFM/BIC | 1.40 ± 0.09 | 0.50 ± 0.06a | 133 | 3 | 3.27 ± 0.25 | 2.10 ± 0.25a | 66 | 2 |
| SFM/SAC | 1.49 ± 0.10 | 1.62 ± 0.11 | 143 | 2 | 3.44 ± 0.26 | 3.82 ± 0.23a | 72 | 2 |
| SAC/+BIC | 1.62 ± 0.11 | 0.75 ± 0.07a | 143 | 2 | 3.82 ± 0.23 | 1.44 ± 0.17a | 72 | 2 |
| BIC/+SAC | 0.58 ± 0.11 | 0.56 ± 0.09 | 63 | 3 | 1.34 ± 0.18 | 1.45 ± 0.18 | 77 | 3 |
| SFM/CNQX | 2.46 ± 0.19 | 1.97 ± 0.19a | 59 | 3 | 4.35 ± 0.24 | 3.48 ± 0.23a | 68 | 5 |
| SFM/BIC+CNQX | 1.21 ± 0.15 | 0.24 ± 0.06a | 30 | 2 | 4.08 ± 0.28 | 1.96 ± 0.24a | 73 | 5 |
| SFM/PRG | 1.32 ± 0.11 | 1.32 ± 0.10 | 62 | 3 | 4.67 ± 0.32 | 2.56 ± 0.33a | 37 | 3 |
Significant effect of treatment compared with control within the same age group (paired t test, P < 0.05).
Calcium sources required for calcium oscillations
To evaluate the role of intracellular calcium stores, the sensitivity to 2-APB (a compound known to block IP3R and TRP cation (TRPC) channels and stimulate TRP vanilloid channels) was tested. 2-APB failed to block [Ca2+]i oscillations at either age. However, it attenuated the frequency of [Ca2+]i oscillations at 21 div (P < 0.05) (Table 1). These data suggest the involvement of IP3R in [Ca2+]i oscillations in older GnRH-1 cells. Because 2-APB is also a TRP blocker (24), the paradigm was repeated with FFA (100 μm), a compound known to block TRPC channels (24). Like 2-APB, FFA failed to inhibit [Ca2+]i oscillations at 7 div (P > 0.05) (Table 1) but partially blocked oscillations at 21 div (P < 0.05) (Table 1). The inhibition observed with FFA at 21 div was greater than the inhibition induced by 2-APB (FFA, −1.50 ± 0.19 peaks/min vs. 2-APB, −0.33 ± 0.09 peaks/min; P < 0.05, ANOVA). These data suggest that inhibition by 2-APB occurred via blockade of TRPC rather than IP3R and that TRPC are involved in GnRH-1 neuronal activity at later stages. Consistent with previous data obtained using thapsigargin, a sarcoplasmic/endoplasmic reticulum (Ca2+) ATPase inhibitor at 7 div (19), at 21 div, the intracellular calcium stores are not a major source of calcium for generating oscillations in GnRH-1 cells (data not shown).
Because [Ca2+]i oscillations were reduced by TTX at 21 div and depolarization can subsequently activate L-type voltage-gated calcium channels (VGCC) and promote calcium influx, the effect of VGCC blockers, NIF (1 μm), and NIM (10 μm) were investigated. Application of NIF partially blocked [Ca2+]i oscillations at both ages (P < 0.05 at both ages) (Table 1). However, NIF inhibition was greater at 21 div (7 div, −0.44 ± 0.08 peaks/min vs. 21 div, −2.11 ± 0.21 peaks/min; P < 0.05, ANOVA). Similar results were observed with NIM (data not shown), indicating a greater role of VGCC in the genesis of [Ca2+]i oscillations in older GnRH-1 cells, as well as the requirement of extracellular calcium.
Characterization of TTX-resistant [Ca2+]i oscillations at 21 div
A subpopulation of GABAergic neurons has been identified in the nasal placodes in vivo and is maintained in nasal explants in vitro (25). GABAergic input is a major excitatory input to GnRH-1 neurons at 7 div, via activation of GABAA receptors (12,19,20,26,27), and in acute brain slices, TTX-insensitive miniatures, mainly GABAergic, tonically drive GnRH-1 neurons (28). Therefore, to evaluate whether the persistence of [Ca2+]i oscillations in TTX at 21 div (in 30% of the cells tested) was due to tonic GABAergic inputs which could override voltage-gated sodium channels, BIC (20 μm) was combined with TTX (Fig. 3A). Although some TTX-insensitive oscillations were inhibited by BIC (P < 0.05) (Table 2), two types of responses were observed. TTX plus BIC silenced one population of TTX-resistant GnRH-1 cells (∼30% of the cells tested), suggesting that in these cells, the mechanism for action potential-independent release of GABA is sufficient to keep the calcium oscillator active at 21 div. However, another population of GnRH-1 cells (∼70% of the cells tested) continued to show [Ca2+]i oscillations in the presence of TTX and BIC. This specific cell population was examined further.
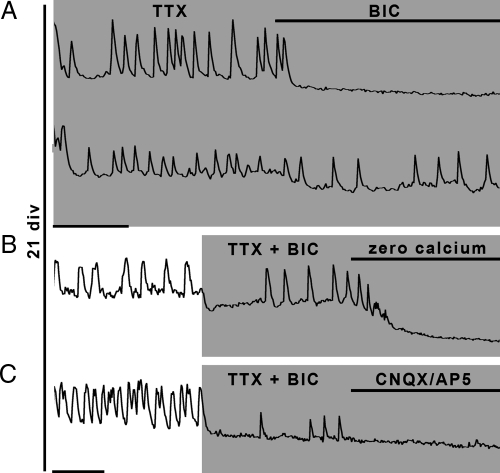
Figure 3.
Activity-independent release of excitatory amino acids in older cultures. A, At 21 div, the TTX-insensitive [Ca2+]i oscillations can be completely (upper trace), or partially (lower trace), blocked by a GABAA antagonist (BIC), indicating an additional oscillator that is independent of voltage-gated sodium channels and GABAergic input. B, Removing extracellular calcium does stop the BIC-resistant and TTX-resistant oscillations. C, The BIC- and TTX-insensitive oscillations are blocked by glutamatergic receptor blockers (CNQX/AP5). X bar, 1 min.
Table 2.
Characterization of TTX-resistant [Ca2+]i oscillations at 21 div
| Experiment | Control peaks/min | Treatment peaks/min | n | N |
|---|---|---|---|---|
| TTX/+BIC | 2.77 ± 0.30 | 1.96 ± 0.25a | 44 | 4 |
| TTX+BIC/+Ca free | 1.07 ± 0.21 | 0.41 ± 0.07a | 62 | 2 |
| TTX+BIC/+PPADS | 0.54 ± 0.16 | 0.45 ± 0.18 | 49 | 2 |
| TTX+BIC/+AP5+CNQX | 0.63 ± 0.18 | 0.17 ± 0.07a | 46 | 2 |
| TTX+BIC+AP5/+CNQX | 1.29 ± 0.46 | 0.25 ± 0.0.13a | 36 | 2 |
| BIC+CNQX/+PGR | 1.96 ± 0.24 | 0.63 ± 0.19a | 73 | 5 |
Significant effect of treatment compared with control within the same age group (paired t test, P < 0.05).
Characterization of TTX- and BIC-resistant [Ca2+]i oscillations at 21 div
To determine whether the TTX- and BIC-resistant [Ca2+]i oscillations were dependent on extracellular calcium, calcium-free SFM was superfused in the presence of TTX and BIC (Fig. 3B). The remaining oscillations were inhibited (P < 0.05) (Table 2). Therefore, in these GnRH-1 cells, calcium influx through the plasma membrane, insensitive to L-type VGCC blockers (data not shown), maintained the calcium oscillator.
Functional ionotropic purinergic (P2X) receptors have been described in prenatal primate GnRH-1 cells (29), and P2X receptors are involved in pacemaking in pituitary gonadotrophs (30). Blockade of P2X receptors by pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS) (10 μm) failed to block oscillations (P > 0.05) (Table 2), rejecting P2X as a membrane pathway in this population of GnRH-1 cells. Alternatively, calcium influx could occur through ionotropic glutamatergic receptors, because 1) cells immunoreactive for VGLUT were detected in the developing olfactory system (31), 2) GnRH-1 cells respond to glutamate at later in vitro stages (26), and 3) calcium entry via glutamate receptors is described in adult GnRH-1 neurons (32). Glutamatergic inputs were tested on TTX- and BIC-resistant [Ca2+]i oscillations (Fig. 3C). Oscillations were blocked by a cocktail of NMDA (AP5; 10 μm) and non-NMDA (CNQX; 10 μm) glutamatergic blockers (P < 0.05) (Table 2). Consecutive application of AP5 then AP5+CNQX showed that non-NMDA glutamatergic channels were responsible for the oscillations (P < 0.05) (Table 2) rather than NMDA channels (P > 0.05) (Table 2). CNQX alone was sufficient to induce the same blockade (data not shown).
Excitatory amino acids and calcium oscillations
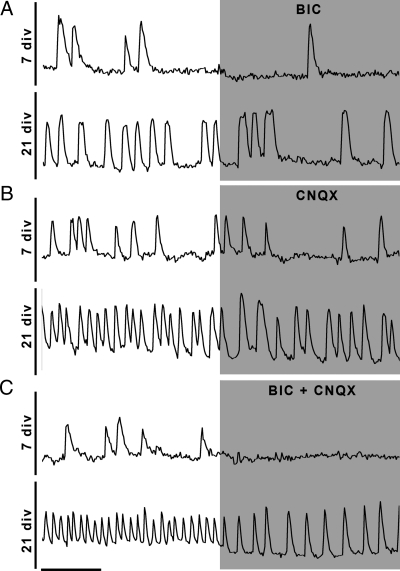
Because both GABAergic and glutamatergic inputs contributed to the activity of the GnRH-1 oscillator at 21 div, an increase in these excitatory inputs could account for the observed increase in GnRH-1 neuronal activity. To determine whether the increase in [Ca2+]i oscillations between 7 and 21 div was due to an increase in GABAergic input, the effect of BIC was reevaluated across ages (Fig. 4A). In agreement with previous data (12), BIC significantly reduced the frequency of [Ca2+]i oscillations at both ages (P < 0.05) (Table 1). The change induced by BIC was similar at both ages (−0.91 ± 0.08 peaks/min in BIC at 7 div, −1.17 ± 0.22 peaks/min at 21 div; P > 0.05, ANOVA). These data indicate an increase in GnRH-1 neuronal activity occurs independent of A-type GABAergic input.
Figure 4.
Inputs differentially modulate GnRH-1 neurons during development. A, Blockade of A-type GABAergic receptors with BIC inhibits [Ca2+]i oscillations at both ages with a similar magnitude. B, The blockade of AMPA/kainate glutamatergic receptors with CNQX inhibits [Ca2+]i oscillations with a greater magnitude at 21 div. C, The simultaneous blockade of amino acids receptors inhibits the majority of [Ca2+]i oscillations at 7 div (upper trace), but an input remains at 21 div (lower trace). X bar, 1 min.
GABAB receptors regulate GABAergic inputs by inhibiting pre- and/or postsynaptic cells (33) and are involved in the modulation of GnRH-1 neuronal activity in explants (12). A decrease in GABAB activation over development could explain an increase in GnRH-1 neuronal activity. To determine whether this feedback loop changed over development, experiments were performed with the GABAB antagonist, SAC (10 μm). SAC increased [Ca2+]i oscillations only at 21 div (P < 0.05) (Table 1). Application of BIC after SAC still reduced [Ca2+]i oscillations in GnRH-1 neurons at both ages (P < 0.05) (Table 1). No changes were detected when SAC was added after BIC (P > 0.05 for both groups) (Table 1). Together, and in accordance with a delayed development of GABAB receptors, in contrast to GABAA receptors, in CNS neurons (34), these experiments indicate that postsynaptic GABAB receptors become functional at later stages. Overall, the GABAergic input remains constant and does not explain the increase in the frequency of [Ca2+]i oscillations detected in GnRH-1 neurons at 21 div.
Because glutamatergic input triggered the TTX- and BIC-insensitive [Ca2+]i oscillations at 21 div, its contribution in [Ca2+]i oscillations was also reevaluated at both ages (Fig. 4B). Application of CNQX partially inhibited the [Ca2+]i oscillations at 7 and 21 div (P < 0.05 for both ages) (Table 1). Changes in oscillatory frequency appeared higher at 21 div (7 div, −0.49 ± 0.13 peaks/min vs. 21 div, −0.86 ± 0.15 peaks/min; P < 0.05, ANOVA) but not sufficient to account for the robust increase in GnRH-1 neuronal activity.
Data indicated no major changes in either GABAergic nor glutamatergic inputs during development. Therefore, the remaining component triggering [Ca2+]i oscillations was evaluated by coapplication of BIC and CNQX (Fig. 4C). Notably, although the removal of these two inputs blocked [Ca2+]i oscillations at 7 div (P < 0.05) (Table 1), indicating that GABA and glutamate are the major two excitatory inputs required to maintain the GnRH-1 calcium oscillator active, more oscillations remained at 21 div (7 div, 0.24 ± 0.06 peaks/min vs. 21 div, 1.96 ± 0.24 peaks/min; P < 0.05, ANOVA), revealing an additional excitatory input.
Age-dependent modulation of GnRH-1 neurons by CCK
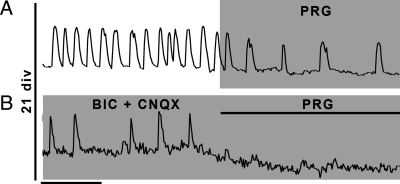
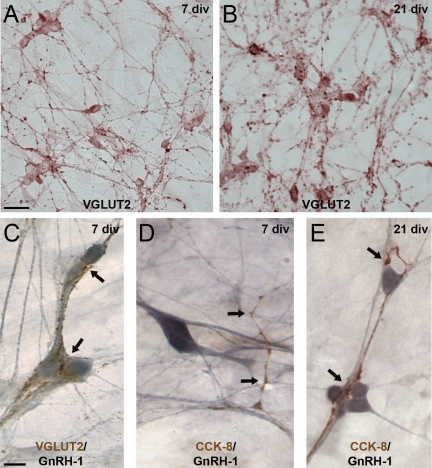
Olfactory axons immunoreactive for CCK emerge from the olfactory pit in vivo (23). Both CCK and functional receptors have been described in nasal explants (23,35) having an effect on GnRH-1 neuronal activity. Therefore, a change in CCK modulation as a function of time could account for an increase in GnRH-1 neuronal activity. Application of proglumide (100 nm), a CCK receptor antagonist, identified CCK as an age-dependent excitatory input to GnRH-1 neurons. PRG had no effect on the frequency of [Ca2+]i at 7 div (P > 0.05) (Table 1), whereas it decreased the frequency of [Ca2+]i oscillations at 21 div (Fig. 5A) (P < 0.05) (Table 1). To determine whether the CCKergic input was the only one that appeared at 21 div, PRG was subsequently applied to BIC+CNQX-inhibited GnRH-1 cells (Fig. 5B). The majority of the remaining oscillations were inhibited (P < 0.05) (Table 1). To investigate the increase in CCKergic input further, immunocytochemistry was performed for CCK and a VGLUT (VGLUT2) in 7 and 21 div explants (n = 6 and 7, respectively, for both staining) (Fig. 6). As expected from pharmacological data, no obvious changes were detected in glutamatergic staining pattern (Fig. 6, A–C). In contrast, more CCKergic fibers were consistently detected in older explants. Analysis revealed that CCKergic fibers in 7 div explants were rarely apposed to GnRH-1 cells (∼10%; n = 47/518; N = 5) (Fig. 6D), whereas in the older explants, CCKergic fibers were detected entwined between GnRH-1 cells (∼30%; n = 122/426; N = 8; Fisher’s exact test, P < 0.05) (Fig. 6E), supporting the calcium imaging data.
Figure 5.
CCK input excites GnRH-1 neurons at 21 div. A, Blockade of CCKergic receptors with PRG inhibits [Ca2+]i oscillations at 21 div. B, CCKergic input is responsible for the BIC- and CNQX-insensitive oscillations at 21 div. X bar, 1 min.
Figure 6.
In contrast to VGLUT2, immunostaining for CCK reveals more cellular interactions with GnRH-1 neurons in older nasal explants. Explants stained at 7 div (A) and 21 div (B) for VGLUT2 (brown) do not exhibit major differences either in fiber densities nor bundling. C, At both ages, numerous VGLUT2 fibers (brown) appose GnRH-1 cells (blue; only 7 div shown). In contrast, although CCKergic fibers are detected at both ages, their contacts with GnRH-1 cell bodies are less frequent at 7 div (D) than at 21 div (E). Scale bars, 10 μm (upper panels) or 5 μm (lower panels).
Discussion
The rhythmic activity of GnRH-1-secreting neurons is fundamental for reproductive function. However, the mechanisms underlying this pattern are unknown, e.g. intrinsic and/or driven activity. The present study evaluated developmental changes that GnRH-1 neurons undergo independent of the CNS. Our data show an increase in the activity of GnRH-1 neurons during development and an age-dependent sensitivity to TTX. Initially, TTX blocked most [Ca2+]i oscillations, although 2 wk later, some GnRH-1 cells remained sensitive to TTX, whereas others were only partially inhibited. The TTX-insensitive oscillations were driven primarily by both GABAergic and glutamatergic inputs, via the activation of GABAA and AMPA/kainate receptors, respectively. At 1 wk, these inputs are mainly responsible for the activity of the GnRH-1 neuronal oscillator but are disrupted by TTX. No dramatic changes in these inputs were detected after two additional weeks of development. Both inputs remained excitatory to the GnRH-1 cells, and their contribution to the overall activity of the GnRH-1 cells did not change. However, with development, new CCKergic inputs take part in the genesis of the oscillations detected in GnRH-1 cells. These experiments indicate that modulation of GnRH-1 neurons changes over development and demonstrate that neuronal inputs are required to trigger the majority of [Ca2+]i oscillations.
Over the past years, evidence has accumulated, indicating that GnRH-1 neurons possess an intrinsic oscillator. These data included pulsatile GnRH-1 release in preoptic area/hypothalamic explants, whose primary inputs, known to be widely spread (36), were predominantly cut off, the GT1 GnRH-1 immortalized cell line (14) and GnRH-1 cells maintained in nasal explants models (6,15,16,17), the later two devoid of CNS inputs. In agreement with a GnRH-1 intrinsic oscillator, [Ca2+]i oscillations have been described in GnRH-1 neurons, initially in GT1 cells (10), then in GnRH-1 neurons maintained in nasal explants (11,37). However, because GT1 cells were derived from a tumor and actively divide (2), and nasal explants GnRH-1 neurons are prenatal (4,5), and the fact that [Ca2+]i transients are a common feature of developing neurons (38), the physiological relevance of these oscillations remained uncertain. Recent work using a genetically encoded calcium dye targeted to GnRH-1 neurons revealed [Ca2+]i oscillations in GnRH-1 neurons in adult mouse acute brain slices (9), suggesting that oscillations are an intrinsic property of GnRH-1 neurons. To discriminate developmental and intrinsic features, it seemed important to analyze developing GnRH-1 neurons to look for similarities and divergences between oscillatory mechanisms.
In this study, spontaneous oscillations were observed at two different developmental stages, 7 and 21 div. Consistent with a previous study, the frequency of [Ca2+]i oscillations increased during this time window (12), indicating changes in the control of the GnRH-1 neuronal oscillator during development. A recent study showed that the majority of [Ca2+]i oscillations were inhibited when voltage-gated sodium channels were blocked in prenatal GnRH-1 neurons maintained for 7 div in explants (19). In contrast, a partial inhibition was described in adult GnRH-1 neurons (9), suggesting the existence of an oscillator independent of action potentials in adult cells. Thus, the sensitivity to TTX of GnRH-1 neurons was reevaluated in explants at 1 and 3 wk. In contrast with the observation made at the early developmental stage, after 3 wk, two responses were detected within the GnRH-1 neuronal population. In one group of GnRH-1 cells, the [Ca2+]i oscillations were inhibited by the application of TTX as seen at 7 div. In the other group of GnRH-1 cells, the majority of [Ca2+]i oscillations remained, indicative of either 1) greater sensitivity of the cellular oscillator to the same inputs, i.e. facilitation, and/or 2) greater inputs to the same cellular oscillator.
Extrasynaptic GABAA receptors can be constitutively activated by a surrounding GABA spillover (39), and evidence exists in favor of this mechanism in GnRH-1 neurons (40). The effect of a GABAA antagonist on the TTX-independent [Ca2+]i oscillations revealed only a partial inhibition. Thus, at the later developmental stage, some [Ca2+]i oscillations in GnRH-1 neurons can be initiated in presence of TTX by GABAA-dependent depolarization. However, the remaining oscillations were triggered by a TTX- and BIC-insensitive, but extracellular calcium-sensitive mechanism.
GnRH-1 cells in explants derived from monkey have been shown to express purinergic P2X2 channels and respond to ATP (29). The calcium influx evoked by ATP on P2X2-transfected GT1 cells has been described as being voltage sensitive and voltage insensitive (41). Therefore, the potential activation of P2X2 on the TTX- and BIC-insensitive [Ca2+]i oscillations in GnRH-1 cells at 3 wk was investigated. Blockade of P2X receptors failed to block the TTX- and BIC-insensitive oscillations. Activation of glutamatergic receptors was then evaluated and revealed a constitutive glutamatergic input via non-NMDA ionotropic receptors. This observation is consistent with the activation of glutamatergic receptors in GnRH-1 cells during in vivo development (42), the emergence of cells immunoreactive for the VGLUT from the olfactory placode (31), and the responsiveness to exogenous glutamatergic agonists in prenatal GnRH-1 cells (26) and in adult GnRH-1 cells (32). An increase in excitatory amino acid inputs could have explained the increase in GnRH-1 neuronal activity observed at 21 div. Our data indicate that glutamatergic and GABAergic inputs remain constant, implying the participation of a new mechanism, at 3 wk, that triggers oscillations in a group of GnRH-1 cells.
During development, CCK-positive axons are present in the migratory path that GnRH-1 cells follow in nasal regions (23). Both prenatally and in the adult, GnRH-1 cells express CCK receptors, and the signaling pathway is functional at 7 div (23). Blockade of the CCK receptor for a short exposure period (3 min) did not induce any effects on [Ca2+]i oscillations in GnRH-1 cells at 7 div but identified CCKergic input as the additional mechanism triggering oscillations at 21 div. In previous work (35), CCK receptor antagonists activated GnRH-1 neurons at 7 div within a 30-min period. The mechanism of this action remains unknown and may involve indirect modulation. Excitatory effects of CCK are well documented within the brain. Gαq/11 coupling, production of inositol triphosphate, and increase in [Ca2+]i level are described in heterologous systems expressing CCK1R (43,44) or CCK2R (45,46). In amygdaloid neurons, the CCK-induced increase in cell excitability requires the activation of TRP channels (47), and in vagal terminals in the solitary nucleus, CCK-induced [Ca2+]i oscillations are partially inhibited by TTX, L-type VGCC blockers, A- and C-protein kinase blockers (48). Consistent with these pathways, our data indicate a greater sensitivity of GnRH-1 neurons to both TRPC and L-type VGCC blockers at 21 div, concomitant with the change in CCKergic inputs.
The three cell populations described in the present study are known to arise from the olfactory placode [GABAergic (25), glutamatergic (31), and CCKergic (23)] during the same developmental window as the GnRH-1 cell population. Our immunocytochemical data support cell-to-cell interactions, and the observation of [Ca2+]i frequency specific to individual cells or in individual cultures supports the idea of inputs developing in parallel to GnRH-1 neuronal network. Furthermore, previous data obtained in monkey explants indicate that the frequency of [Ca2+]i oscillations was independent of the speed of perfusion (11), supporting the specificity of the intercellular communication established in the model rather than passive diffusion of irrelevant substances from the tissue mass to the medium. In agreement with this, previous reports show that exogenous application of CCK had no effect on the frequency of [Ca2+]i oscillations at 7 div (35). Finally, GABA and glutamate are two cell populations well described as primary inputs to GnRH-1 neurons (49,50). Although CCK fibers contact GnRH neurons in vivo (35), the physiological relevance of CCKergic input remains uncertain in vivo, because subfertility is not reported in CCK1R deficient mice (51). However, central infusion of CCK stimulates the GnRH-1 pulse generator in goats (52) and release in monkeys (53), which support the role of CCK as a natural input to GnRH-1 cells.
Footnotes
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke.
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 16, 2010
Abbreviations: AMPA, α-Amino-3-hydroxyl-5-methyl-4-isoxazolepropionate; AP5, D-2-amino-5-phosphonovalerate; 2-APB, 2-aminoethoxydiphenylborane; BIC, bicuculline; CCK, cholecystokinin; CNS, central nervous system; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; div, days in vitro; FFA, flufenamic acid; GABA, γ-aminobutyric acid; IP3R, inositol triphosphate receptor; NIF, nifedipine; NIM, nimodipine; NMDA, N-methyl-d-aspartate; PRG, proglumide; P2X, functional ionotropic purinergic; SAC, saclofen; SFM, serum-free medium; TRP, transient receptor potential; TRPC, TRP cation; TTX, tetrodotoxin; VGCC, voltage-gated calcium channels; VGLUT, vesicular glutamate transporter.
References
- Hoffman GE, Lee WS, Wray S 1992 Gonadotropin releasing hormone (GnRH). In: Nemeroff CB, ed. Neuroendocrinology. Boca Raton, FL: CRC Press; 185–218 [Google Scholar]
- Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI 1990 Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 5:1–10 [DOI] [PubMed] [Google Scholar]
- Daikoku S, Koide I, Chikamori-Aoyama M, Shimomura Y 1993 Migration of LHRH neurons derived from the olfactory placode in rats. Arch Histol Cytol 56:353–370 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Quanbeck CD, Schulz CA, Burich AJ, Luchansky LL, Claude P 1993 A primary cell culture system of luteinizing hormone releasing hormone neurons derived from embryonic olfactory placode in the rhesus monkey. Endocrinology 133:2379–2390 [DOI] [PubMed] [Google Scholar]
- Fueshko S, Wray S 1994 LHRH cells migrate on peripherin fibers in embryonic olfactory explant cultures: an in vitro model for neurophilic neuronal migration. Dev Biol 166:331–348 [DOI] [PubMed] [Google Scholar]
- Duittoz AH, Batailler M 2000 Pulsatile GnRH secretion from primary cultures of sheep olfactory placode explants. J Reprod Fertil 120:391–396 [PubMed] [Google Scholar]
- Spergel DJ, Krüth U, Hanley DF, Sprengel R, Seeburg PH 1999 GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci 19:2037–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM 2000 Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology 141:412–419 [DOI] [PubMed] [Google Scholar]
- Jasoni CL, Todman MG, Strumia MM, Herbison AE 2007 Cell type-specific expression of a genetically encoded calcium indicator reveals intrinsic calcium oscillations in adult gonadotropin-releasing hormone neurons. J Neurosci 27:860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales TG, Sanderson MJ, Charles AC 1994 GABA has excitatory actions on GnRH-secreting immortalized hypothalamic (GT1-7) neurons. Neuroendocrinology 59:297–308 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Schanhofer WK, Keen KL, Luchansky L 1999 Intracellular Ca(2+) oscillations in luteinizing hormone-releasing hormone neurons derived from the embryonic olfactory placode of the rhesus monkey. J Neurosci 19:5898–5909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore Jr JP, Shang E, Wray S 2002 In situ GABAergic modulation of synchronous gonadotropin releasing hormone-1 neuronal activity. J Neurosci 22:8932–8941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S 2002 Development of gonadotropin-releasing hormone-1 neurons. Front Neuroendocrinol 23:292–316 [DOI] [PubMed] [Google Scholar]
- Krsmanović LZ, Stojilković SS, Merelli F, Dufour SM, Virmani MA, Catt KJ 1992 Calcium signaling and episodic secretion of gonadotropin-releasing hormone in hypothalamic neurons. Proc Natl Acad Sci USA 89:8462–8466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E, Keen KL, Mogi K, Claude P 1999 Pulsatile release of luteinizing hormone-releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology 140:1432–1441 [DOI] [PubMed] [Google Scholar]
- Funabashi T, Daikoku S, Shinohara K, Kimura F 2000 Pulsatile gonadotropin-releasing hormone (GnRH) secretion is an inherent function of GnRH neurons, as revealed by the culture of medial olfactory placode obtained from embryonic rats. Neuroendocrinology 71:138–144 [DOI] [PubMed] [Google Scholar]
- Constantin S, Caraty A, Wray S, Duittoz AH 2009 Development of gonadotropin-releasing hormone-1 secretion in mouse nasal explants. Endocrinology 150:3221–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez L, Villalobos C, Boockfor FR, Frawley LS 2000 The relationship between pulsatile secretion and calcium dynamics in single, living gonadotropin-releasing hormone neurons. Endocrinology 141:2012–2017 [DOI] [PubMed] [Google Scholar]
- Constantin S, Caligioni CS, Stojilkovic S, Wray S 2009 Kisspeptin-10 facilitates a plasma membrane-driven calcium oscillator in GnRH-1 neurons. Endocrinology 150:1400–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin S, Wray S 2008 Gonadotropin-releasing hormone-1 neuronal activity is independent of cyclic nucleotide-gated channels. Endocrinology 149:279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson Jr RE, Wiegand SJ, Clough RW, Hoffman GE 1986 Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7:155–159 [DOI] [PubMed] [Google Scholar]
- Wray S, Gähwiler BH, Gainer H 1988 Slice cultures of LHRH neurons in the presence and absence of brainstem and pituitary. Peptides 9:1151–1175 [DOI] [PubMed] [Google Scholar]
- Giacobini P, Kopin AS, Beart PM, Mercer LD, Fasolo A, Wray S 2004 Cholecystokinin modulates migration of gonadotropin-releasing hormone-1 neurons. J Neurosci 24:4737–4748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE 2007 SnapShot: mammalian TRP channels. Cell 129:220 [DOI] [PubMed] [Google Scholar]
- Wray S, Fueshko SM, Kusano K, Gainer H 1996 GABAergic neurons in the embryonic olfactory pit/vomeronasal organ: maintenance of functional GABAergic synapses in olfactory explants. Dev Biol 180:631–645 [DOI] [PubMed] [Google Scholar]
- Kusano K, Fueshko S, Gainer H, Wray S 1995 Electrical and synaptic properties of embryonic luteinizing hormone-releasing hormone neurons in explant cultures. Proc Natl Acad Sci USA 92:3918–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin S, Wray S 2008 Gonadotropin-releasing hormone-1 neuronal activity is independent of hyperpolarization-activated cyclic nucleotide-modulated channels but is sensitive to protein kinase a-dependent phosphorylation. Endocrinology 149:3500–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim JA, Skynner MJ, Pape JR, Herbison AE 2000 Late postnatal reorganization of GABA(A) receptor signalling in native GnRH neurons. Eur J Neurosci 12:3497–3504 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Keen KL, Grendell RL, Golos TG 2005 Possible role of 5′-adenosine triphosphate in synchronization of Ca2+ oscillations in primate luteinizing hormone-releasing hormone neurons. Mol Endocrinol 19:2736–2747 [DOI] [PubMed] [Google Scholar]
- Zemkova H, Balik A, Jiang Y, Kretschmannova K, Stojilkovic SS 2006 Roles of purinergic P2X receptors as pacemaking channels and modulators of calcium-mobilizing pathway in pituitary gonadotrophs. Mol Endocrinol 20:1423–1436 [DOI] [PubMed] [Google Scholar]
- Honma S, Kawano M, Hayashi S, Kawano H, Hisano S 2004 Expression and immunohistochemical localization of vesicular glutamate transporter 2 in the migratory pathway from the rat olfactory placode. Eur J Neurosci 20:923–936 [DOI] [PubMed] [Google Scholar]
- Constantin S, Jasoni CL, Wadas B, Herbison AE 2010 γ-Aminobutyric acid and glutamate differentially regulate intracellular calcium concentrations in mouse gonadotropin-releasing hormone neurons. Endocrinology 151:262–270 [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R 2007 GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev 87:1215–1284 [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL 1997 GABAA, NMDA and AMPA receptors: a developmentally regulated ‘menage a trois’. Trends Neurosci 20:523–529 [DOI] [PubMed] [Google Scholar]
- Giacobini P, Wray S 2007 Cholecystokinin directly inhibits neuronal activity of primary gonadotropin-releasing hormone cells through cholecystokinin-1 receptor. Endocrinology 148:63–71 [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne HJ, Todman MG, Korach KS, Greiner E, Pérez CA, Schütz G, Herbison AE 2006 Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore Jr JP, Wray S 2000 Luteinizing hormone-releasing hormone (LHRH) biosynthesis and secretion in embryonic LHRH. Endocrinology 141:4486–4495 [DOI] [PubMed] [Google Scholar]
- Spitzer NC, Root CM, Borodinsky LN 2004 Orchestrating neuronal differentiation: patterns of Ca2+ spikes specify transmitter choice. Trends Neurosci 27:415–421 [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z 2005 Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 6:215–229 [DOI] [PubMed] [Google Scholar]
- Sim JA, Skynner MJ, Herbison AE 2001 Heterogeneity in the basic membrane properties of postnatal gonadotropin-releasing hormone neurons in the mouse. J Neurosci 21:1067–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimizu TA, Van Goor F, Tomić M, Wong AO, Tanoue A, Tsujimoto G, Stojilkovic SS 2000 Characterization of calcium signaling by purinergic receptor-channels expressed in excitable cells. Mol Pharmacol 58:936–945 [DOI] [PubMed] [Google Scholar]
- Simonian SX, Herbison AE 2001 Differing, spatially restricted roles of ionotropic glutamate receptors in regulating the migration of GnRH neurons during embryogenesis. J Neurosci 21:934–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weerth A, Pisegna JR, Huppi K, Wank SA 1993 Molecular cloning, functional expression and chromosomal localization of the human cholecystokinin type A receptor. Biochem Biophys Res Commun 194:811–818 [DOI] [PubMed] [Google Scholar]
- Ulrich CD, Ferber I, Holicky E, Hadac E, Buell G, Miller LJ 1993 Molecular cloning and functional expression of the human gallbladder cholecystokinin A receptor. Biochem Biophys Res Commun 193:204–211 [DOI] [PubMed] [Google Scholar]
- Ito M, Matsui T, Taniguchi T, Tsukamoto T, Murayama T, Arima N, Nakata H, Chiba T, Chihara K 1993 Functional characterization of a human brain cholecystokinin-B receptor. A trophic effect of cholecystokinin and gastrin. J Biol Chem 268:18300–18305 [PubMed] [Google Scholar]
- Lee YM, Beinborn M, McBride EW, Lu M, Kolakowski Jr LF, Kopin AS 1993 The human brain cholecystokinin-B/gastrin receptor. Cloning and characterization. J Biol Chem 268:8164–8169 [PubMed] [Google Scholar]
- Meis S, Munsch T, Sosulina L, Pape HC 2007 Postsynaptic mechanisms underlying responsiveness of amygdaloid neurons to cholecystokinin are mediated by a transient receptor potential-like current. Mol Cell Neurosci 35:356–367 [DOI] [PubMed] [Google Scholar]
- Rogers RC, Hermann GE 2008 Mechanisms of action of CCK to activate central vagal afferent terminals. Peptides 29:1716–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, MacLusky NJ, Sakamoto H, Shanabrough M, Naftolin F 1985 Glutamic acid decarboxylase-containing axons synapse on LHRH neurons in the rat medial preoptic area. Neuroendocrinology 40:536–539 [DOI] [PubMed] [Google Scholar]
- Goldsmith PC, Thind KK, Perera AD, Plant TM 1994 Glutamate-immunoreactive neurons and their gonadotropin-releasing hormone-neuronal interactions in the monkey hypothalamus. Endocrinology 134:858–868 [DOI] [PubMed] [Google Scholar]
- Raybould HE 2007 Mechanisms of CCK signaling from gut to brain. Curr Opin Pharmacol 7:570–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimaru T, Matsuyama S, Ohkura S, Mori Y, Okamura H 2003 Central cholecystokinin-octapeptide accelerates the activity of the hypothalamic gonadotropin-releasing hormone pulse generator in goats. J Neuroendocrinol 15:80–86 [DOI] [PubMed] [Google Scholar]
- Perera AD, Verbalis JG, Mikuma N, Majumdar SS, Plant TM 1993 Cholecystokinin stimulates gonadotropin-releasing hormone release in the monkey (Macaca mulatta). Endocrinology 132:1723–1728 [DOI] [PubMed] [Google Scholar]