Abstract
Food intake is regulated by signals from peripheral organs, but the way these are integrated remains uncertain. Cholecystokinin (CCK) from the intestine and leptin from adipocytes interact to inhibit food intake. Our aim was to examine the hypothesis that these interactions occur at the level of vagal afferent neurons via control of the immediate early gene EGR1. We now report that CCK stimulates redistribution to the nucleus of early growth response factor-1 (EGR1) in these neurons in vivo and in culture, and these effects are not dependent on EGR1 synthesis. Leptin stimulates EGR1 expression; leptin alone does not stimulate nuclear translocation, but it strongly potentiates the action of CCK. Ghrelin inhibits CCK-stimulated nuclear translocation of EGR1 and leptin-stimulated EGR1 expression. Expression of the gene encoding the satiety peptide cocaine- and amphetamine-regulated transcript (CARTp) is stimulated by CCK via an EGR1-dependent mechanism, and this is strongly potentiated by leptin. Leptin potentiated inhibition of food intake by endogenous CCK in the rat in conditions reflecting changes in EGR1 activation. The data indicate that by separately regulating EGR1 activation and synthesis, CCK and leptin interact cooperatively to define the capacity for satiety signaling by vagal afferent neurons; manipulation of these interactions may be therapeutically beneficial.
Cholecystokinin and leptin interact co-operatively to regulate the transcription factor EGR1 in vagal afferent neurons thereby providing new insight into the mechanisms controlling food intake.
The peripheral signals that control nutrient ingestion and delivery to the small intestine include leptin and other adipokines from fat cells, insulin from the islets of Langerhans, and a variety of hormones from the gastrointestinal tract (1). In the case of the latter, enteric hormones such as cholecystokinin (CCK) from the proximal small intestine and peptide YY3-36 and glucagon-like peptide-1 from ileum and colon slow nutrient delivery to the small intestine by inhibiting gastric emptying or food intake (2); conversely, the gastric hormone ghrelin has the opposite effects in stimulating food intake and gastric emptying, thereby accelerating nutrient delivery to the small intestine (3). In both cases, afferent neurons of the vagus nerve represent a potential primary target. Multiple interactions are now recognized between the gut hormones and other factors at the level of vagal afferent neurons. Specifically, the actions of CCK on food intake or vagal afferent nerve discharge are potentiated by leptin and gastric distension but inhibited by orexin A (4,5,6,7); they may also be inhibited by ghrelin, although this remains controversial (7,8,9).
Recent work indicates that the interactions between different signaling systems at the level of vagal afferent neurons include changes in expression of genes encoding both neuropeptide transmitters and G protein-coupled receptors. For example, CCK acts at CCK1 receptors to stimulate the expression of cocaine- and amphetamine-regulated transcript (CARTp) and of the Y2 receptor, both of which are linked to inhibition of food intake and suppresses the expression of melanin-concentrating hormone (MCH), MCH-1 receptors, and cannabinoid (CB)-1 receptors, which are linked to stimulation of food intake (10,11,12,13). In all these cases, the action of CCK is inhibited by ghrelin (12,14). The transcriptional effects of CCK on CART expression are mediated in part by phosphorylation of cAMP response element-binding protein (CREB), and the inhibitory effect of ghrelin involves exclusion of phospho-CREB from the nucleus (12).
In a variety of neurons and other cells, the immediate early gene EGR1 (early growth response factor-1) plays a role in transcriptional responses to cell stimulation. Also known as Zif/268, NGFI-A, TIS8, or Krox 24, EGR1 is rapidly induced by growth factors and cell depolarization (15,16), but in some cells, including neurons, there is also constitutive expression (17). In the present study, we examined the cellular basis of interactions between CCK, leptin, and ghrelin for control of gene expression in vagal afferent neurons involving EGR1. The data indicate that CCK induces nuclear localization of EGR1 independently of expression, whereas leptin stimulates expression but not nuclear localization; ghrelin inhibits both. Cooperative interactions between CCK and leptin in controlling EGR1 regulate expression of CART.
Materials and Methods
Animals
Studies were performed using adult male Wistar rats (225–300 g) housed at 22 C under a 12-h light, 12-h dark cycle with ad libitum access to food and water, unless stated otherwise. Studies were conducted in compliance with the appropriate United Kingdom Home Office personal and project licenses and with the institutional ethical review processes of the University of Liverpool and the Institutional Animal Use and Care Committee, University of California, Davis.
Peptides and drugs
CCK8s and ghrelin were obtained from Bachem (St. Helens, UK), and leptin was obtained from Sigma (Poole, Dorset, UK). Lorglumide was a gift from Rotta Pharmaceuticals (Milan, Italy). Phorbol-12-myristate-13-acetate ester (PMA), cycloheximide, and actinomycin D were obtained from Sigma. Ro32-0432 and AG490 were obtained from Calbiochem (La Jolla, CA). U0126 was obtained from Cell Signaling (Danvers, MA).
Plasmids
The expression plasmid pCMVzif, containing mouse EGR1 cDNA under the cytomegalovirus promoter was a kind gift from Dr. Gerald Thiel (18). A construct consisting of the promoter sequence of EGR1 coupled to luciferase (EGR1-luc) was a kind gift from Dr. David Cohen (19). Constructs consisting of the CART promoter linked to luciferase (CART-luc) were kind gifts from Dr. Michael Kuhar (20). To investigate the importance of the putative EGR1 response element (2380 bp upstream of transcription) for CCK-mediated CART transcription, the core of this site was mutated from gtgggg to acaaaa in a 3451-bp CART-luc construct using a QuikChange II XL site-directed mutagenesis kit (Stratagene Europe, Amsterdam, The Netherlands).
Fasting-feeding experiments
Rats were fasted for 24 h (water ad libitum) and subsequently refed for up to 2 h. Some fasted animals received CCK8s (10 nmol, ip) or saline and were killed 1 h later. In addition, some rats that were fasted for 24 h received the CCK1 receptor antagonist lorglumide (10 mg/kg, ip) 15 min before refeeding and were killed 1 h after refeeding. Rats were killed by CO2 inhalation, and nodose ganglia were rapidly immersed in 4% paraformaldehyde for 1 h at 22 C, followed by 25% sucrose in PBS overnight at 4 C.
Food intake studies
Three different food intake studies were performed. In each experiment, rats were fasted for 13 h (10 h light cycle, 3 h dark cycle). All experiments were performed during the dark cycle. In study 1, rats received a priming dose of leptin (80 μg/kg, ip) or saline followed 2 h later by CCK8s (10 nmol, ip) or saline, and preweighed Purina chow was given. In study 2, rats received leptin (80 μg/kg, ip) or CCK8s (10 nmol/kg), alone or in combination, or saline (400 μl, ip) immediately before preweighed Purina chow was given. In study 3, rats received leptin (80 μg/kg, ip) or lorglumide (8 mg/kg, ip), alone or in combination, or saline (400 μl, ip) and were presented with a 3-g pellet of Purina chow for 40 min; thereafter, preweighed Purina chow was given. In each experiment, food intake was determined by measuring the difference between the preweighed standard chow and the weight of chow and spill at 20-min intervals.
Nodose neuron cultures
Nodose ganglia were dissected under aseptic conditions and digested for 75 min at 37 C in 5 ml of Ca2+- and Mg2+-free Hanks’ balanced salt solution containing 0.8–1 mg/ml collagenase type Ia (Roche Diagnostics, Indianapolis, IN) as previously described (12). Cells were dispersed by gentle trituration through siliconized Pasteur pipettes, washed twice with HEPES-buffered DMEM containing 10% fetal calf serum (Hyclone/Perbio Science, Cramlington, UK) supplemented with 3% antibiotic/antimycotic solution, plated onto four-well chamber slides, and maintained in HEPES-buffered DMEM with 10% fetal calf serum at 37 C in 5.5% CO2, and the medium was changed every 48 h. For many experiments, cells were transferred to serum-free medium for 2 h before experimental treatments. These treatments included stimulation with CCK8s (up to 10 nm), PMA (100 nm), or leptin (up to 30 ng/ml); putative inhibitors including Ro32-0432 (10 nm), actinomycin D (2 μg/ml), cycloheximide (10 μg/ml), UO126 (10 μm), AG490 (50 μm), and ghrelin (10 nm) were added to the media 30 min before stimulation.
Immunohistochemistry
Cryostat sections of fixed nodose ganglia (5–10 μm) were mounted on Polysine-coated slides (Polysine; MenzelGlaser, Braunschweig, Germany) and processed for immunohistochemistry (12). Cultured neurons were fixed in 4% paraformaldehyde in PBS and processed for immunohistochemistry with affinity-purified rabbit polyclonal antibody to CARTp (Phoenix Laboratories, San Antonio, TX), rabbit polyclonal antibody to EGR1 (Santa Cruz Biotechnology, Santa Cruz, CA), or goat anti-phospho-signal transducer and activator of transcription-3 (pSTAT3) (Tyr 705) (Santa Cruz Biotechnology). Secondary antibodies were used as appropriate and included donkey antirabbit Ig conjugated to fluorescein isothiocyanate and donkey antigoat Ig labeled with Texas Red (Jackson ImmunoResearch, West Grove, PA). Specificity of immunostaining was determined by omitting the primary antibody and by preincubation with an excess of appropriate peptide where available. Samples were mounted in Vectashield with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Peterborough, UK) for nuclear localization and examined using an Axiopan Universal microscope (Zeiss, Oberkochen, Germany); images were processed using the Axio Vision 3.0 imaging system (Zeiss). Samples were also analyzed using an ImageXpress Micro imaging system (Molecular Devices, Wokingham, Berkshire, UK) with MetaXpress 2.0 software and the cell scoring module to quantify number and intensity of positive cells. The total number of cells was determined using DAPI, and immunoreactive cells were identified by setting a threshold fluorescence determined by the mean fluorescent intensity of control cells. In experimental treatments, cells were considered positive when their fluorescent intensity was more than 2 sd above the mean intensity of control conditions. Typically, 150 cells were analyzed for each condition from three different rats. Comparisons were between samples run at the same time and with the same instrument settings.
Luciferase promoter-reporter studies
Vagal afferent neurons were cultured in full medium for 48 h in four-well plates. The medium was removed, and cells were transfected with 0.25–1 μg DNA per well, using CombiMag (OZ Biosciences, Marseille, France) together with Transfast reagent (Promega, Southampton, Hampshire, UK), on a magnetic plate according to the manufacturer’s instructions. Cells were further incubated for 24–48 h before stimulation. Cells were lysed after overnight stimulation, and the luciferase activity was measured by dual luciferase assay (Promega) according to the manufacturer’s instructions in a Lumat LB9507 luminometer (Berthold, Redbourne, Herts, UK). Results are presented as fold increase over unstimulated control, so 1.0 signifies no change in luciferase activity.
RNA interference
EGR1 expression was knocked down in vagal afferent neurons using a specific small interfering RNA (siRNA) targeting sense, 5′-CCUUUUCUCCUAGGACAAUtt-3′ (Ambion, Austin, TX) as previously described (21). Cells were transfected with 50 nm siRNA using 3 μl SilenceMag (OZ Biosciences) for each well and incubated for 15 min on a magnetic plate. Cells were incubated for 48 h at 37 C before stimulation.
Statistics
Results are expressed as mean ± se, and comparisons between samples were made using ANOVA and Student’s t test as appropriate.
Results
CCK stimulates EGR1 nuclear localization in vagal afferent neurons in vivo
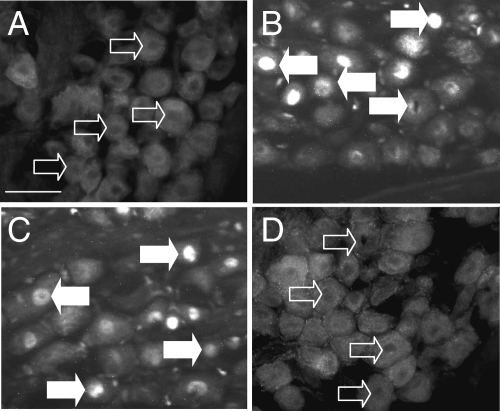
In initial studies, we examined the localization of EGR1 in vivo in vagal afferent neurons of rats fasted for 24 h. Neurons serving the gastrointestinal tract are localized to the mid and caudal regions of the nodose ganglion (22), and in these regions, there was typically a diffuse cytoplasmic staining of EGR1, and over 90% of neurons were negative for EGR1 in the nucleus (Fig. 1A). However, administration of CCK8s (10 nmol, ip) to fasted rats increased the proportion of neurons exhibiting nuclear EGR1 within 1 h (Fig. 1B). In addition, EGR1 nuclear localization was restored in rats refed for 1 h after food withdrawal for 24 h (Fig. 1C), and this was inhibited by administration of the CCK-1 receptor antagonist lorglumide (Fig. 1D), compatible with a role for endogenous CCK in mediating nutrient-dependent nuclear localization of EGR1.
Figure 1.
CCK-dependent nuclear localization of EGR1 in vagal afferent neurons. A, EGR1 is excluded from the nucleus of neurons after fasting for 24 h and is diffusely distributed in the cytoplasm; B, nuclear localization of EGR1 is increased in fasted rats 1 h after an ip injection of 10 nmol CCK8s; C and D, nuclear localization of EGR-1 was also increased in fasted rats (24 h) by refeeding for 1 h (C), but this was inhibited by administration of the CCK1 receptor antagonist lorglumide (10 mg/kg, ip) 15 min before refeeding (D). Representative images from experiments with four to six rats in each group are shown. Open arrows indicate cells with EGR1 excluded from the nucleus, filled arrows show cells exhibiting EGR1 nuclear localization. Scale bar, 50 μm (A–D).
Mechanisms regulating CCK-induced EGR1 nuclear localization
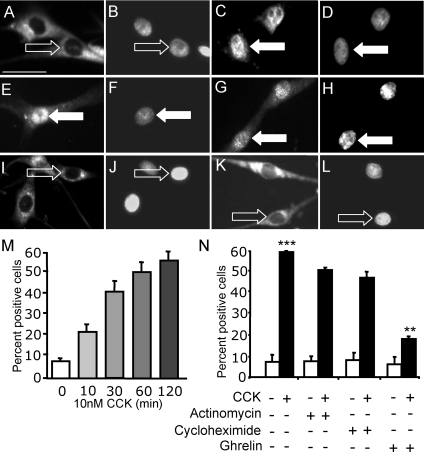
Previous work has shown that approximately 80% of cells were positive for neuron-specific enolase in cultures of nodose ganglia such as those used for the present studies (12). When vagal afferent neurons were cultured in serum-free medium, there was cytoplasmic staining of EGR1 but little or no nuclear localization. In the presence of CCK8s (10 nm), there was a time-dependent redistribution of EGR1 immunoreactivity to the nucleus (Fig. 2). A statistically significant increase in the number of neurons exhibiting nuclear EGR1 was detectable after 10 min stimulation, and 1–2 h after exposure to CCK8s, the response was maximal (Fig. 2). Neither the transcriptional inhibitor actinomycin D nor the translational inhibitor cycloheximide inhibited CCK8s-induced EGR1 nuclear localization (Fig. 2). However, the orexigenic hormone ghrelin markedly inhibited CCK8s-stimulated EGR1 redistribution to the nucleus (Fig. 2K).
Figure 2.
CCK induces EGR1 translocation to the nucleus in cultured vagal afferent neurons, and this does not depend on EGR1 expression. Vagal afferent neurons cultured for 72 h and stained with antibody to EGR1 (A, C, E, G, I, and K) and DAPI (B, D, F, H, J, and L) in respective fields. A and B, Nuclear EGR1 staining was undetectable after transfer to serum-free medium for 2 h; C and D, nuclear EGR1 was readily detectable after treatment with 10 nm CCK8s (1 h) in serum-free medium; E–H, the effect of CCK was not inhibited by pretreatment with the transcriptional inhibitor actinomycin D (2 μm) (E and F) or the translational inhibitor cycloheximide (10 μg/ml) (G and H); I–L, nuclear EGR1 was undetectable after treatment with ghrelin (10 nm) (I and J), but ghrelin (10 nm) inhibited the effect of 10 nm CCK8s on nuclear localization of EGR1 (K and L). Arrows identify a representative neuron in each pair of images: filled arrows show EGR1 localization to the nucleus, and open arrows show EGR1 excluded from the nucleus. M, Time course of CCK8s-induced nuclear translocation of EGR1; N, quantification of immunohistochemical data described above represented as the percentage of total cells exhibiting nuclear EGR1. ***, P < 0.001 compared with control; **, P < 0.01 compared with response to CCK8s. Representative images from six independent experiments are shown. Scale bar, 50 μm (A–L).
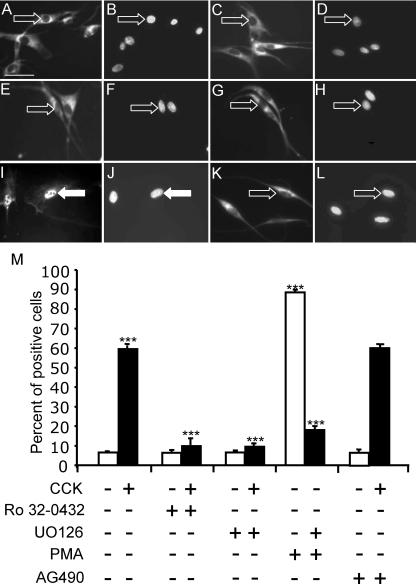
Some actions of CCK on vagal afferent neurons are mediated by protein kinase C (PKC) (12), and so we examined the effect of the PKC inhibitor Ro32-0432 (1 μm) on the distribution of EGR1. In unstimulated neurons, Ro32-0432 had no effect on the cytoplasmic localization of EGR1; in contrast, the effect of CCK8s on the redistribution to the nucleus was completely inhibited by Ro32-0432 (Fig. 3). Stimulation of PKC with PMA also stimulated nuclear localization, consistent with a role for PKC in controlling nuclear EGR1 in these neurons (Fig. 3). Inhibition of the MAPK signaling cascade after either CCK8s or PMA stimulation blocked the nuclear localization of EGR1, suggesting that Erk1/2 activation was downstream of PKC in mediating the action of CCK on EGR1 localization.
Figure 3.
CCK induces EGR1 localization to the nucleus through activation of PKC and Erk1/2 in vagal afferent neurons. Vagal afferent neurons cultured for 72 h and stained with antibody to EGR1 (A, C, E, G, I, and K) and DAPI (B, D, F, H, J, and L) in respective fields (see Fig. 2 for further details). A–D, The PKC inhibitor Ro32-0432 (1 μm) on its own had no effect on EGR1 localization (A and B) but inhibited the action of 10 nm CCK8s (1 h) on nuclear localization of EGR1 (C and D); E–H, the inhibitor of Erk1/2 activation U0126 (10 μm) had no effect on EGR1 translocation on its own (E and F) but inhibited the effect of 10 nm CCK8s (G and H); I–L, nuclear EGR1 localization was readily detectable after treatment with 100 nm PMA (1 h) (I and J), and this effect was inhibited by 10 μm U0126 (K and L). In A–L, arrows identify a representative neuron in each pair of images: filled arrows show EGR1 nuclear localization, and open arrows show EGR1 exclusion from the nucleus. M, Quantification of data in A–L represented as the percentage of total cells exhibiting nuclear EGR1 and response to CCK8s (10 nm) in the presence of AG490 (50 nm), an inhibitor of STAT3. ***, P < 0.001 compared with the relevant control. Representative images from six independent experiments are shown. Scale bar, 50 μm (A–L).
CCK and leptin act cooperatively to induce EGR1 nuclear translocation in cultured vagal afferent neurons
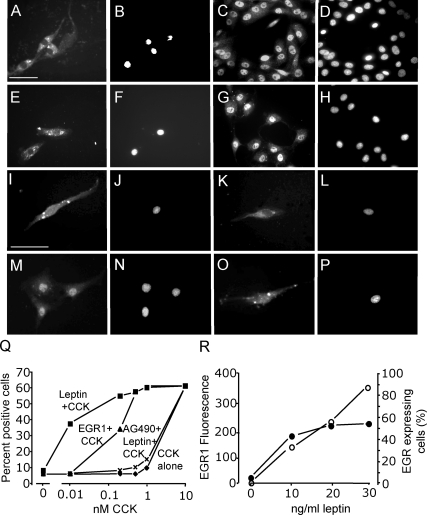
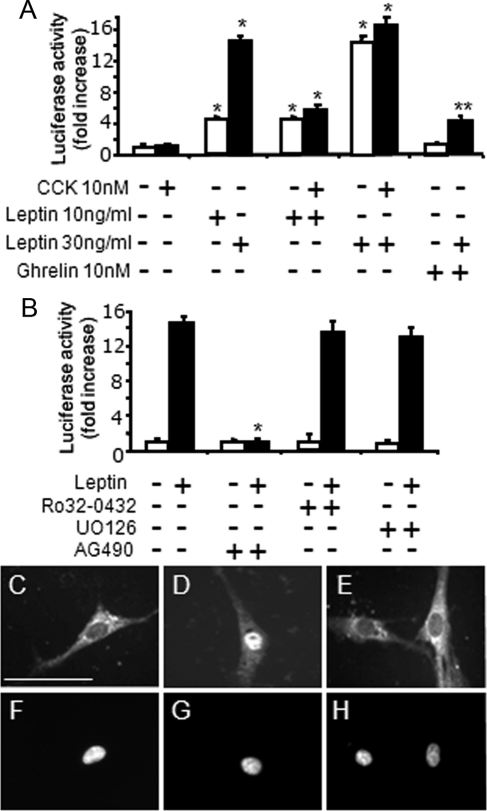
In neurons in serum-free medium, the concentration of CCK8s required for EGR1 redistribution to the nucleus was 10 nm; at concentrations of 1 nm and lower, there was little or no detectable effect of CCK8s on EGR1 distribution (Fig. 4). In contrast, leptin on its own (10–30 ng/ml) had no effect on EGR1 translocation (Fig. 4), but it markedly potentiated the action of CCK in stimulating nuclear localization of EGR1. Thus, at 10 pm CCK8s in the presence of 10 ng/ml leptin, the proportion of cells exhibiting nuclear EGR1 was still more than 50% maximal (Fig. 4). Leptin acts via activation of STAT3 in vagal afferent neurons (23), and the STAT3 inhibitor AG490 reversed the effect of leptin in potentiating CCK-evoked nuclear localization of EGR1 (Fig. 4). To determine whether the action of CCK was enhanced by increased EGR1 abundance, we overexpressed EGR1 by transfection of neurons with an EGR1 expression vector. In transfected neurons, EGR1 localization was predominantly cytoplasmic. Importantly, however, cells overexpressing EGR1 were more sensitive to the action of CCK in stimulating nuclear redistribution (Fig. 4). Because EGR1 expression is stimulated by a number of cytokines and growth factors, we asked whether leptin might act to increase EGR1 synthesis. There was a concentration-dependent increase in EGR1 abundance in a significant subset of vagal afferent neurons compatible with stimulation of synthesis (Fig. 4).
Figure 4.
The action of CCK on nuclear translocation of EGR1 is potentiated by overexpression of EGR1 and by leptin. Vagal afferent neurons cultured for 72 h and stained with antibody to EGR-1 (A, C, E, G, I, K, M, and O) and DAPI (B, D, F, H, J, L, N, and P) in respective fields. A and B, Transfection with an EGR1 overexpression vector was associated with EGR1 immunoreactivity in the cytoplasm but not nuclear translocation; C and D, addition of 10 nm CCK8s induced EGR1 localization to the nucleus in overexpressing neurons; E and F, neurons stimulated with leptin (30 ng/ml) exhibited EGR1 localization to the cytoplasm, but not nucleus; G and H, stimulation with both leptin (30 ng/ml) and CCK8s (10 nm) led to nuclear EGR1; I and J, with lower concentrations of leptin (10 ng/ml), there was again EGR1 in the cytoplasm; K and L, low doses of CCK8s (1 nm) had no effect of EGR1 translocation; M and N, importantly, 1000-fold lower concentrations of CCK8s (10 pm) in the presence of 10 ng/ml leptin induced the nuclear translocation of EGR1; O and P, there was no effect of 10 ng/ml leptin on EGR1 nuclear translocation in cells stimulated with very low concentrations of CCK8s (2 pm); Q, quantification of cells exhibiting nuclear EGR1 staining in response to varying concentrations of CCK8s alone; CCK8s and leptin (10 ng/ml); CCK8s, leptin (10 ng/ml), and AG490 (50 nm); or CCK8s and EGR1 overexpression; R, the intensity of EGR1 fluorescence in vagal afferent neurons is shown in response to leptin: left, mean fluorescent intensity (○) in cells where fluorescence was more than 2 sd above mean of unstimulated cells; right, numbers of cells (•) with fluorescent intensity more than 2 sd above the mean of unstimulated cells (n = 6).
Regulation of EGR1 expression
To explore the control of EGR1 expression in more detail, we therefore examined the action of CCK and leptin on an EGR1-luc promoter-reporter construct transfected into cultured vagal afferent neurons. In keeping with the data presented above, CCK had no effect on EGR1-luc expression (Fig. 5); similarly, PMA had no effect on EGR1-luc expression (1.04 ± 0.25 relative to control, 1.00). In contrast, leptin at 10 and 30 ng/ml significantly stimulated EGR1-luc expression, and this was inhibited by ghrelin (Fig. 5A). Leptin-induced EGR1 expression was not influenced by CCK or by inhibitors of PKC or Erk1/2 activation but was inhibited by the STAT3 inhibitor AG490 (Fig. 5B). Because leptin actions on vagal afferent neurons are thought to be mediated by translocation of pSTAT3 to the nucleus (23), and because ghrelin inhibited nuclear translocation of EGR1, we examined the effect of ghrelin on the distribution of pSTAT3 in cultured neurons in serum-free medium. In unstimulated cells, pSTAT3 was excluded from the nucleus, but in response to leptin, there was a redistribution leading to intense nuclear staining (Fig. 5, C and D); ghrelin blocked the action of leptin in stimulating pSTAT3 nuclear localization (Fig. 5E).
Figure 5.
Leptin stimulates EGR1 promoter activity in vagal afferent neurons via STAT3, and ghrelin inhibits STAT3 translocation to the nucleus. A, Cultured vagal afferent neurons transfected with EGR1-luc do not exhibit increased luciferase activity in response to treatment with 10 nm CCK8s (16 h); however, 10 and 30 ng/ml leptin dose-dependently increased luciferase activity (*, P < 0.05 compared with control), and CCK8s had no further effect. Preincubation (30 min) with 10 nm ghrelin inhibited leptin-induced EGR1-luc activity (**, P < 0.05 compared with leptin alone). B, The PKC inhibitor Ro32-0432 and the inhibitor of Erk1/2 activation U0126 had no effect on leptin-induced (30 ng/ml) expression of EGR1-luc, whereas the STAT3 inhibitor AG490 prevented the action of leptin (*, P < 0.001 compared with response to leptin alone). C–H, Vagal afferent neurons cultured for 72 h and stained with antibody to STAT3 (C–E) and DAPI (F–H) in respective fields. Nuclear STAT3 staining was undetectable after transfer to serum-free medium for 2 h (C and F). Nuclear STAT3 was readily detectable after treatment with 30 ng/ml leptin in serum-free medium (D and G), whereas ghrelin (10 nm) in the presence of 30 ng/ml leptin inhibited nuclear localization of STAT3 (F and H). Representative images from six independent experiments are shown. Scale bar, 50 μm.
EGR1 regulates CART synthesis
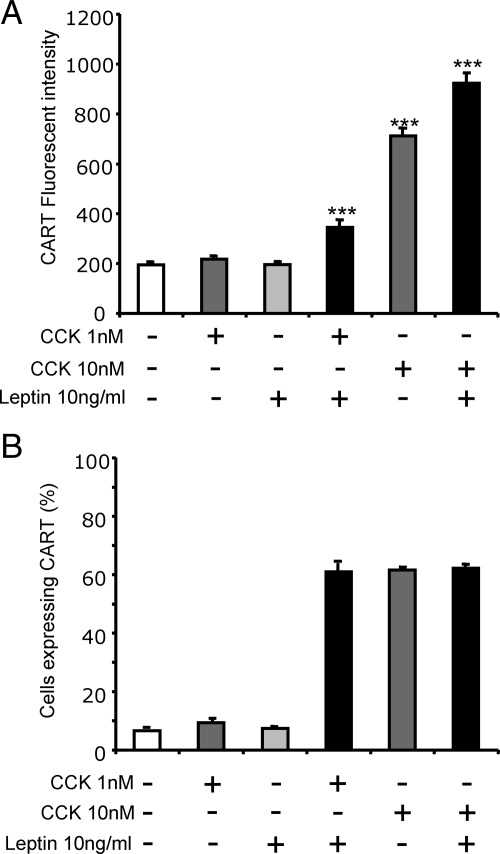
To determine the functional significance of the action of CCK and leptin on EGR1 in vagal afferent neurons, we examined a putative target, namely expression of the satiety peptide CARTp. As previously reported (12), in vagal afferent neurons in serum-free medium, CCK8s (1 nm) had little effect on either the number of cells expressing CARTp (which was <10% of the total) or the abundance of CARTp in these cells, but at 10 nm, approximately 60% of cells expressed CARTp, and there was increased abundance within these cells (Fig. 6A). In the presence of leptin (10 ng/ml), there was no change in CARTp abundance in vagal afferent neurons in serum-free medium. However, leptin significantly increased the CARTp response to 1 nm CCK8s, and the number of cells responding was similar to that in response to 10 nm CCK8s alone (Fig. 6).
Figure 6.
Leptin potentiates CCK-induced CART abundance in vagal afferent neurons. Neurons were cultured for 72 h and stained with antibody to CART. A, CART fluorescence in cells with a fluorescent intensity more than 2 sd above control were quantified. Neither 1 nm CCK8s nor 10 ng/ml leptin alone had an effect on the abundance of CART immunoreactivity compared with unstimulated cells; however, there was a significant increase in CART abundance when vagal afferent neurons were stimulated with both 1 nm CCK8s and 10 ng/ml leptin. The response to 10 nm CCK8s was further increased by leptin. B, The percentage of cells exhibiting fluorescent intensity more than 2 sd above control was increased to comparable proportions when stimulated with 10 nm CCK8s, 1 nm CCK8s, and 10 ng/ml leptin, or 10 nm CCK8s and 10 ng/ml leptin, whereas no significant increase was observed with either 1 nm CCK8s or 10 ng/ml leptin alone compared with unstimulated cells (n = 3).
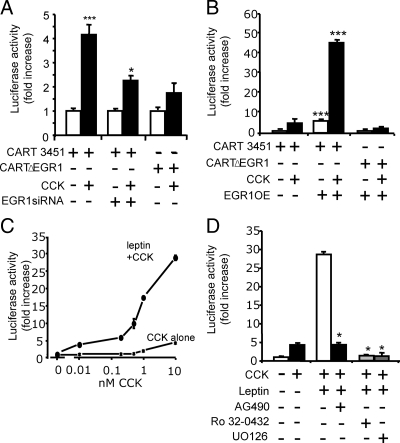
To further study cellular mechanisms, we examined the response of a CART-promoter luciferase-reporter construct after transfection into vagal afferent neurons. In response to CCK8s (10 nm, 16 h), there was a 4.2 ± 0.4-fold increase in expression of a 3.45-kb CART-luc vector. Mutation of a putative EGR1 response element (CARTΔEGR1) reduced the response to CCK8s, and inhibition of EGR1 synthesis by siRNA treatment also significantly reduced the action of CCK8s in stimulating CART-luc (Fig. 7A). We then examined the effect of overexpression of an EGR1 construct on CART-luc; there was an increase in basal expression of CART-luc of 6.8 ± 2.2-fold in cells cotransfected with an EGR1 vector, and in the presence of 10 nm CCK8s, the expression of CART-luc was stimulated 56.2 ± 1.3-fold (Fig. 7B). However, after mutation of a putative EGR1 site in the CART promoter (CARTΔEGR1) and cotransfection with the EGR1 expression vector, there was no change in basal expression, and the dramatic increase in response to CCK8s was abolished (Fig. 7C). We then asked whether leptin potentiated CART-luc responses to CCK8s. In the presence of 10 ng/ml leptin, there was a substantial increase in the response to 1000-fold lower concentrations of CCK8s (10 pm) and a 7-fold increase in the maximal response (Fig. 7C). The potentiating effects of CCK8s and leptin on CART expression were inhibited by AG490, Ro32-0432, and U0126 (Fig. 7D).
Figure 7.
EGR1 regulates CART promoter activity. A, In cultured vagal afferent neurons transfected with 3.45 kb CART-luc promoter-reporter construct, incubation with 10 nm CCK8s (16 h) increased luciferase activity compared with unstimulated cells. Mutation of a putative EGR1 binding site in the CART-luc (CARTΔEGR1) vector reduced the response to CCK, and so too did pretreatment with EGR1 siRNA (***, P < 0.001 compared with control; *, P < 0.05). B, Cotransfection of CART-luc with an EGR1 expression vector (EGR1OE) dramatically increased the response to CCK (***, P < 0.001) and also increased basal expression; however, when the EGR1 expression vector was cotransfected with CARTΔEGR1, there was no change in basal expression and no additional response to CCK. C, Concentration-response relationships for CART-luc with graded concentrations of CCK8s alone and in the presence of 10 ng/ml leptin showing potentiating between CCK8s and leptin, which together stimulated approximately 7-fold increases in maximal response and induced significant responses at 10 pm CCK8s. D, The synergistic effects of leptin on CCK-induced CART-luc were inhibited by the STAT3 inhibitor AG490 and by Ro32-0432 and U0126 (*, P < 0.05 compared with CCK8s and leptin).
Interactions between leptin and CCK for control of food intake
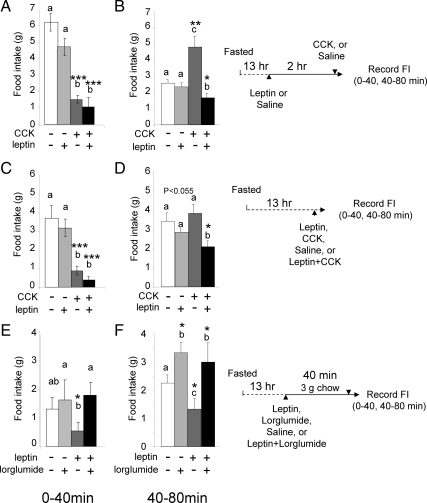
The data presented above imply that there might be peripheral interactions between leptin and CCK for control of food intake in the rat working over periods of a few hours. To test this possibility, we first designed experiments to test interactions of peripherally administered leptin and CCK over a period of 2 h (based on the time course of neurochemical changes reported above). When leptin was administered 2 h before CCK8s (both ip) and animals then immediately refed, there was significantly prolongation of the satiety effect of CCK8s (Fig. 8, A and B). When leptin and CCK8s were administered together, there was no change in the response to CCK over the first 40 min (which is when the satiating action of CCK8s is maximal), but there was a significant prolongation of the action of CCK8s, consistent with a longer-term change in sensitivity (Fig. 8, C and D). Finally, we asked whether there were comparable interactions between leptin and endogenous CCK by using the CCK-1 receptor antagonist lorglumide and a small preloading meal to release endogenous CCK. There was no difference in food intake over 0–40 min between animals that received lorglumide or saline (Fig. 8E); however, leptin reduced food intake significantly compared with rats receiving lorglumide alone or lorglumide with leptin. At 40–80 min (Fig. 8F), rats treated with lorglumide or lorglumide with leptin ate significantly more than saline-treated rats. Moreover, leptin alone inhibited food intake compared with saline-treated rats. The data suggest that leptin potentiates the satiety effect of endogenous CCK over periods up to 80 min after a small meal.
Figure 8.
Leptin augments the inhibitory action of CCK on food intake. Cumulative food intake (in grams) is shown. A and B, Rats fasted for 13 h received a priming dose of leptin (80 μg/kg, ip), or saline, followed 2 h later by CCK8s (10 nmol, ip) or saline and refeeding; food intake was then recorded for 80 min. A, CCK8s alone inhibited food intake compared with saline over 0–40 min, and previous administration of leptin had no effect on this. B, There was increased food intake compared with control over 40–80 min after CCK8s (which is a compensatory response for inhibition over 0–40 min); leptin alone had no effect but significantly enhanced inhibition of food intake by CCK, thereby prolonging its satiety effects. C and D, Rats fasted for 13 h received leptin (80 μg/kg, ip) or CCK8s (10 nmol/kg) alone or in combination immediately before refeeding. Leptin alone did not significantly change food intake compared with saline. C, CCK8s inhibited food intake over 0–40 min, and leptin had no effect on this. D, Leptin alone had no effect on food intake over 40–80 min but significantly increased the effect of CCK in reducing food intake, thereby prolonging its satiety effects. E and F, Rats fasted for 13 h received leptin (80 μg/kg, ip) or lorglumide (8 mg/kg, ip) alone or in combination and were allowed to eat 3 g of food in 40 min; thereafter, ad libitum food intake was allowed and recorded. E, After 40 min, rats receiving leptin alone ate significantly less than those receiving leptin and lorglumide or saline. F, At 40–80 min, lorglumide significantly increased food intake compared with saline, leptin inhibited food intake compared with saline, and lorglumide inhibited the effect of leptin. Results are shown as means ± se (n = 6); letters denote significant differences between groups at that time point, i.e. a vs. b; c vs. a or b; ab not significantly different from a or b; *, P < 0.05; **, P < 0.01; ***, P < 0.001 (repeated ANOVA).
Discussion
The present study identifies the immediate early gene EGR1 as a target for cooperative actions between CCK and leptin, and inhibitory actions of ghrelin, in vagal afferent neurons. In particular, the data indicate that leptin stimulates EGR1 expression but not its translocation to the nucleus. In contrast, the action of CCK is directed at stimulation of EGR1 nuclear translocation. The gastric orexigenic peptide ghrelin inhibits both CCK-stimulated EGR1 nuclear localization, and leptin-stimulated pSTAT3 nuclear localization, thereby inhibiting EGR1 synthesis. The functional significance of these interactions can be seen in CCK-stimulated expression of the satiety peptide CARTp, which is mediated in part by EGR1 and occurs at concentrations similar to those in plasma (24) in the presence of leptin but requires 1000-fold higher concentrations of CCK in the absence of leptin. The results provide a basis for interpreting the mechanisms that account for synergistic interactions of peripheral leptin in prolonging and potentiating the satiating actions of CCK.
The delivery of fat and protein to the small intestine stimulates release of CCK, which acts at CCK1 receptors on vagal afferent neurons to inhibit food intake and delay gastric emptying (2,25). These actions are generally considered to be important for short-term control of food intake. Within the central nervous system, there is integration of these signals with other peripheral signals implicated in longer-term control of food intake including leptin (26). For instance, there is evidence that leptin acts within both the hypothalamus and the brainstem to increase sensitivity to CCK (27,28,29). In addition, however, previous studies have shown potentiation between leptin and CCK at the level of vagal afferent neurons, notably with respect to vagal afferent discharge and to changes in intracellular calcium (5,30). The present data indicate that there are leptin-CCK interactions at the level of vagal afferent neurons that extend beyond acute control of neuronal discharge and that include changes in gene expression. The findings identify EGR1 as a cellular target for these interactions and suggest that leptin and CCK cooperate by separately controlling synthesis of EGR1 and translocation to the nucleus where a downstream target is the satiety neuropeptide transmitter CARTp. There is evidence that the latter is released by vagal afferent neurons in response to CCK and that it acts as an autocrine modulator to augment the satiety effects of CCK (21); increased CARTp abundance is therefore consistent with prolongation of the satiety effect of CCK by peripheral leptin pretreatment.
It is well established that EGR1 is rapidly induced in many cell lines in response to growth factor stimulation. In the central nervous system, there is constitutive expression in some neurons, but there is also rapid induction of EGR1 in seizure models and with neuronal stimulation (16,17,31). There are three zinc fingers in a DNA-binding domain in EGR1, and these together with basic flanking sequences are thought to act as nuclear localization signals (32,33). Although expression of EGR1 is frequently associated with its localization to the nucleus, this is not invariably the case. For example, in prostate cancer cells, nuclear localization is a feature of benign cells, whereas malignant cells exhibited cytoplasmic localization (34). The present identification of separate cell signaling systems associated with EGR1 induction and nuclear localization suggests that there are more sophisticated regulatory mechanisms in primary neurons than previously supposed and that modulation of EGR1 function can be a site of interaction of different signaling pathways.
In vagal afferent neurons cultured in serum-free medium for 2 h or longer, and in vivo after fasting, EGR1 localization is predominantly cytoplasmic. However, both in vivo and in vitro CCK relatively rapidly stimulated relocation to the nucleus. In cultured neurons, the action of CCK was not blocked by actinomycin D or cycloheximide and therefore did not require transcription or translation. The effects were, however, dependent on activation of PKC and Erk1/2. Importantly, the effect of CCK required concentrations that were approximately 1000 times higher than those achieved in the circulation after a meal (24). On its own, leptin did not stimulate nuclear accumulation of EGR1, but it strongly stimulated EGR1 expression, and it strongly potentiated the capacity of CCK to induce EGR1 redistribution so that when combined with leptin, 1000-fold lower concentrations of CCK stimulated EGR1 redistribution to the nucleus. Overexpression of EGR1 in unstimulated cells was also associated with a remarkable potentiation of the action of CCK on both nuclear localization and induction of CART, consistent with the idea that the interaction between CCK and leptin was attributable to increased EGR1 abundance in response to the latter.
Leptin is produced in gastric epithelial cells, as well as adipocytes, and functional leptin receptors (ObR) are expressed on vagal afferent neurons (23,35,36). It is thought that the peripheral terminals of these neurons in the stomach are likely to be exposed to relatively high concentrations of leptin produced from adjacent epithelial cells. Stimulation of vagal afferent neurons with leptin induced nuclear translocation of pSTAT3, but CCK had no effect on pSTAT3 translocation. In the hypothalamus, leptin has been shown to stimulate EGR1 expression, and in CHO cells, this is mediated in part by activation of Erk1/2 (37). Although the effects of CCK on EGR1 nuclear localization were blocked by inhibition of Erk1/2 activation, this was not accompanied by increased expression, indicating that in nodose neurons, this pathway is not on its own sufficient to induce EGR1 expression. We suggest, therefore, that in vivo, leptin is responsible for setting the level of EGR1 abundance in vagal afferent neurons and so determining the magnitude of responses to CCK after ingestion of a meal.
In the immediate postprandial period, there are rising plasma concentrations of CCK and falling concentrations of ghrelin providing the conditions for interactions between the two hormones. Ghrelin has been shown to inhibit CCK-evoked vagal afferent nerve discharge and to suppress the effect of CCK in stimulating expression of CART and inhibiting expression of CB1, MCH1, and MCH (8,14). In a previous study, we showed that in CCK-stimulated vagal afferent neurons, ghrelin was associated with exclusion of phospho-CREB from the nucleus (12). The present observation that ghrelin also inhibits CCK-stimulated nuclear localization of EGR1 and leptin-stimulated nuclear translocation of pSTAT3 indicates that control of transcription factor redistribution to the nucleus might be a common mechanism of action of ghrelin the basis of which is presently unknown.
The present data indicate a role for EGR1 in the induction of CART, in addition to the phosphorylation of CREB noted previously (12). Although there may well be direct effects of phospho-CREB and EGR1 on the CART promoter, there may also be indirect interactions because in both TF-1 cells and in human aortic endothelial cells, CREB has been shown to activate EGR1 (38,39). In vagal afferent neurons, these events are part of a phenotypic switch mediated by CCK by which neurons change from expression of MCH, MCH1 receptor, and CB1 in the absence of CCK to CART and Y2 receptors in the presence of CCK (10,11,12,13,14). The mechanisms responsible for the switch have remained unclear, and the present data now show that EGR1 is one candidate mediator. The data therefore suggest a new therapeutic target in seeking to control food intake that may be of relevance in obesity.
Acknowledgments
We are grateful to Rotta Pharmaceuticals for a gift of lorglumide and to Gerald Thiel, David Cohen, and Michael Kuhar for gifts of plasmids.
We thank the Medical Research Council, the Biotechnology and Biological Sciences Research Council, and the National Institutes of Health for financial support.
Footnotes
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 9, 2010
Abbreviations: CARTp, Cocaine- and amphetamine-regulated transcript; CB, cannabinoid; CCK, cholecystokinin; CREB, cAMP response element-binding protein; DAPI, 4′,6-diamidino-2-phenylindole; EGR1, early growth response factor-1; MCH, melanin-concentrating hormone; PKC, protein kinase C; PMA, phorbol-12-myristate-13-acetate ester; pSTAT3, phospho-signal transducer and activator of transcription-3; siRNA, small interfering RNA.
References
- Murphy KG, Bloom SR 2006 Gut hormones and the regulation of energy homeostasis. Nature 444:854–859 [DOI] [PubMed] [Google Scholar]
- Dockray GJ 2004 Gut endocrine secretions and their relevance to satiety. Curr Opin Pharmacol 4:557–560 [DOI] [PubMed] [Google Scholar]
- Cummings DE, Overduin J 2007 Gastrointestinal regulation of food intake. J Clin Invest 117:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GJ, McHugh PR, Moran TH 1993 Gastric loads and cholecystokinin synergistically stimulate rat gastric vagal afferents. Am J Physiol 265:R872–R876 [DOI] [PubMed] [Google Scholar]
- Peters JH, Karpiel AB, Ritter RC, Simasko SM 2004 Cooperative activation of cultured vagal afferent neurons by leptin and cholecystokinin. Endocrinology 145:3652–3657 [DOI] [PubMed] [Google Scholar]
- Barrachina MD, Martínez V, Wang L, Wei JY, Taché Y 1997 Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci USA 94:10455–10460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Lal S, Spiller D, Jiang W, Thompson D, Attwood S, Saeed S, Grundy D, Varro A, Dimaline R, Dockray GJ 2003 Localization of orexin-1 receptors to vagal afferent neurons in the rat and humans. Gastroenterology 124:129–139 [DOI] [PubMed] [Google Scholar]
- Date Y, Toshinai K, Koda S, Miyazato M, Shimbara T, Tsuruta T, Niijima A, Kangawa K, Nakazato M 2005 Peripheral interaction of ghrelin with cholecystokinin on feeding regulation. Endocrinology 146:3518–3525 [DOI] [PubMed] [Google Scholar]
- Arnold M, Mura A, Langhans W, Geary N 2006 Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci 26:11052–11060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ 2004 Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci 24:2708–2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ 2006 Feeding-dependent depression of melanin-concentrating hormone and melanin-concentrating hormone receptor-1 expression in vagal afferent neurones. Neuroscience 137:1405–1415 [DOI] [PubMed] [Google Scholar]
- de Lartigue G, Dimaline R, Varro A, Dockray GJ 2007 Cocaine- and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J Neurosci 27:2876–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, de Lartigue G, Raybould HE, Morris R, Dimaline R, Varro A, Thompson DG, Dockray GJ 2008 Cholecystokinin regulates expression of Y2 receptors in vagal afferent neurons serving the stomach. J Neurosci 28:11583–11592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ 2006 Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol Gastrointest Liver Physiol 290:G1289–G1297 [DOI] [PubMed] [Google Scholar]
- Sukhatme VP, Cao XM, Chang LC, Tsai-Morris CH, Stamenkovich D, Ferreira PC, Cohen DR, Edwards SA, Shows TB, Curran T 1988 A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell 53:37–43 [DOI] [PubMed] [Google Scholar]
- Cole AJ, Saffen DW, Baraban JM, Worley PF 1989 Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature 340:474–476 [DOI] [PubMed] [Google Scholar]
- Beckmann AM, Wilce PA 1997 Egr transcription factors in the nervous system. Neurochem Int 31:477–510 [DOI] [PubMed] [Google Scholar]
- Thiel G, Schoch S, Petersohn D 1994 Regulation of synapsin I gene expression by the zinc finger transcription factor zif268/egr-1. J Biol Chem 269:15294–15301 [PubMed] [Google Scholar]
- Cohen DM, Gullans SR, Chin WW 1996 Urea inducibility of egr-1 in murine inner medullary collecting duct cells is mediated by the serum response element and adjacent Ets motifs. J Biol Chem 271:12903–12908 [DOI] [PubMed] [Google Scholar]
- Dominguez G, Lakatos A, Kuhar MJ 2002 Characterization of the cocaine- and amphetamine-regulated transcript (CART) peptide gene promoter and its activation by a cyclic AMP-dependent signaling pathway in GH3 cells. J Neurochem 80:885–893 [DOI] [PubMed] [Google Scholar]
- De Lartigue G, Dimaline R, Varro A, Raybould H, De la Serre CB, Dockray GJ 2010 Cocaine and amphetamine regulated transcript mediates the actions of cholecystokinin on rat vagal afferent neurons. Gastroenterology 138:1479–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey KA, Williams RG, Dockray GJ 1984 Sensory substance P innervation of the stomach and pancreas. Demonstration of capsaicin-sensitive sensory neurons in the rat by combined immunohistochemistry and retrograde tracing. Gastroenterology 87:914–921 [PubMed] [Google Scholar]
- Buyse M, Ovesjö ML, Goïot H, Guilmeau S, Péranzi G, Moizo L, Walker F, Lewin MJ, Meister B, Bado A 2001 Expression and regulation of leptin receptor proteins in afferent and efferent neurons of the vagus nerve. Eur J Neurosci 14:64–72 [DOI] [PubMed] [Google Scholar]
- Forster ER, Dockray GJ 1992 The role of cholecystokinin in inhibition of gastric emptying by peptone in the rat. Exp Physiol 77:693–699 [DOI] [PubMed] [Google Scholar]
- Moran TH, Kinzig KP 2004 Gastrointestinal satiety signals. II. Cholecystokinin. Am J Physiol Gastrointest Liver Physiol 286:G183–G188 [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW 2006 Central nervous system control of food intake and body weight. Nature 443:289–295 [DOI] [PubMed] [Google Scholar]
- Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Baskin DG, Schwartz MW 2005 Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest 115:703–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Baskin DG, Schwartz MW 2009 Hindbrain leptin receptor stimulation enhances the anorexic response to cholecystokinin. Am J Physiol Regul Integr Comp Physiol 297:R1238–R1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, Grill HJ 2010 Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab 11:77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Taché Y, Sheibel AB, Go VL, Wei JY 1997 Two types of leptin-responsive gastric vagal afferent terminals: an in vitro single-unit study in rats. Am J Physiol 273:R833–R837 [DOI] [PubMed] [Google Scholar]
- Saffen DW, Cole AJ, Worley PF, Christy BA, Ryder K, Baraban JM 1988 Convulsant-induced increase in transcription factor messenger RNAs in rat brain. Proc Natl Acad Sci USA 85:7795–7799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gashler AL, Swaminathan S, Sukhatme VP 1993 A novel repression module, an extensive activation domain, and a bipartite nuclear localization signal defined in the immediate-early transcription factor Egr-1. Mol Cell Biol 13:4556–4571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheny C, Day ML, Milbrandt J 1994 The nuclear localization signal of NGFI-A is located within the zinc finger DNA binding domain. J Biol Chem 269:8176–8181 [PubMed] [Google Scholar]
- Mora GR, Olivier KR, Cheville JC, Mitchell Jr RF, Lingle WL, Tindall DJ 2004 The cytoskeleton differentially localizes the early growth response gene-1 protein in cancer and benign cells of the prostate. Mol Cancer Res 2:115–128 [PubMed] [Google Scholar]
- Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, Lewin MJ 1998 The stomach is a source of leptin. Nature 394:790–793 [DOI] [PubMed] [Google Scholar]
- Burdyga G, Spiller D, Morris R, Lal S, Thompson DG, Saeed S, Dimaline R, Varro A, Dockray GJ 2002 Expression of the leptin receptor in rat and human nodose ganglion neurones. Neuroscience 109:339–347 [DOI] [PubMed] [Google Scholar]
- Bjørbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, Neel BG, Myers Jr MG, Flier JS 2001 Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem 276:4747–4755 [DOI] [PubMed] [Google Scholar]
- Sakamoto KM, Fraser JK, Lee HJ, Lehman E, Gasson JC 1994 Granulocyte-macrophage colony-stimulating factor and interleukin-3 signaling pathways converge on the CREB-binding site in the human egr-1 promoter. Mol Cell Biol 14:5975–5985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao F, Tan M, Xu X, Cui MZ 2008 Histamine induces Egr-1 expression in human aortic endothelial cells via the H1 receptor-mediated protein kinase Cδ-dependent ERK activation pathway. J Biol Chem 283:26928–26936 [DOI] [PMC free article] [PubMed] [Google Scholar]