Abstract
The question of whether to take hormone therapy (HT) will impact every woman as she enters reproductive senescence. In women, studies suggest that ovarian hormone loss associated with menopause has deleterious cognitive effects. Results from clinical studies evaluating whether estrogen-containing HT mitigates these effects, and benefits cognition, are discrepant. Type of menopause, surgical vs. transitional, impacts cognitive outcome in women. However, whether type of menopause impacts cognitive effects of HT has not been methodically tested in women or an animal model. We used the 4-vinylcyclohexene diepoxide rodent model of ovarian follicle depletion, which mimics transitional menopause, and the traditional rat model of menopause, ovariectomy, to cognitively test the most commonly prescribed estrogen therapy in the United States, conjugated equine estrogens (Premarin). Here we show conjugated equine estrogens benefited cognition in surgically menopausal rats, but, in contrast, impaired cognition in transitionally menopausal rats. Androstenedione, released from the residual transitional menopausal ovary, was positively associated with impaired performance, replicating our previous findings in 4-vinylcyclohexene diepoxide animals. The current findings are especially salient given that no clinical study testing cognition has methodically separated these two populations of menopausal women for analysis. That we now show surgical vs. transitional modes of menopause result in disparate cognitive effects of HT has implications for future research and treatments optimizing HT for menopausal women.
Using a rodent model, we find that the cognitive effects of conjugated equine estrogen hormone therapy depend on menopause type, and that elevated androstenedione levels are associated with impaired performance.
Conjugated equine estrogens (CEE; Premarin; Wyeth Pharmaceuticals Inc., Philadelphia, PA) is the most widely used estrogen component of hormone therapy (HT) in the United States, given since 1942 (1). Some studies have shown that CEE-containing therapy improved memory (2,3,4). Yet findings evaluating global cognitive function from the large placebo-controlled Women’s Health Initiative Memory Study (WHIMS) showed an increase in probable dementia risk in women aged 65 yr and older assigned to receive CEE+medroxyprogesterone acetate vs. control therapy (5), whereas CEE alone showed a nonsignificant increase in incidence of probable dementia (6,7). These and other clinical studies (8) indicated that CEE-containing therapy can result in both beneficial and detrimental actions on cognition in menopausal women. Recently we showed that CEE provided cognitive benefits when given via cyclic injections to middle-aged ovariectomized (OVX) rats (9). Specifically, CEE improved spatial working memory delayed match to sample (DMS) plus maze performance, attenuated overnight forgetting on the spatial reference memory Morris water maze (MWM), and prevented scopolamine-induced amnesia (9). Tonic CEE has also been shown to enhance spatial working memory retention on the DMS and water radial-arm (WRAM) mazes (23). Furthermore, a one-time CEE injection enhanced nonspatial working memory object recognition in OVX animals (10). Thus, preclinical findings support the cognitive-enhancing effects of CEE, at least in the rodent model that has undergone complete ovary removal via surgical menopause.
To determine variables impacting whether HT is detrimental or beneficial to brain and cognition, preclinical studies manipulated factors that could be influencing HT outcome, including OVX duration before treatment (11,12), age (13,14), dose (9,14), and type of estrogen used (9). Another factor influencing HT outcome may be etiology of menopause-induced ovarian hormone loss, i.e. surgical vs. transitional. There is evidence in women (15) and rats (16) that cognitive consequences of surgical menopause, when circulating ovarian hormone levels cease abruptly, differ from that of natural menopause, when ovarian hormone levels decrease in a transitional manner. In the rodent model, hormones released from the menopausal follicle-deplete ovary, notably androstenedione, impact cognitive function (16). These hormones could also modulate cognitive effectiveness of CEE-containing HT. In the WHIMS, although all women taking unopposed CEE had undergone removal of the uterus (hysterectomy), only a subset of these women had undergone removal of the ovaries (oophorectomy), resulting in the unopposed CEE group composed of women with different histories of menopause (6,7,17). It is also noted that only about 13% of menopausal women undergo surgical menopause; the majority of the remainder undergo transitional menopause from ovarian follicular depletion with retention of residual menopausal ovaries (http://www.menopause.org). There has been no human or animal model study evaluating whether etiology of menopause influences effectiveness of CEE-containing HT. This question is directly relevant to women’s health because every woman will undergo menopause as aging ensues, and CEE is the most commonly used estrogen component of HT, accounting for more than 20 million annual prescriptions (18).
Transitional hormone loss can be induced via the industrial chemical 4-vinylcyclohexene diepoxide (VCD), which produces ovarian follicular depletion in rodents by selectively destroying primordial and primary ovarian follicles via acceleration of the natural atresia process (19,20). Administration of VCD to rodents results in a follicle-deplete hormone profile similar to that seen in naturally menopausal women in that estrogen levels become deplete and the hormone milieu becomes androgen dominant because it is unopposed (20,21,22). Specifically, in VCD-treated rodents, interstitial ovarian tissue yields decreased, but still present, progesterone blood levels; because estrogen blood levels become deplete and androstenedione does not change (16), the endocrine milieu becomes androgen rich. Conversely, ovarian hormone loss is abrupt when menopause is surgical. Using the VCD model, we recently found that etiology of hormone loss influences cognition (16). Animals that had undergone transitional menopause exhibited superior cognitive scores, across multiple domains of function, compared with those that had undergone surgical menopause. Of note, this effect was apparent only when the follicle-deplete ovary was removed (16). Furthermore, higher blood levels of androstenedione, which are released from the follicle-deplete menopausal ovary, correlated with poorer memory performance in VCD animals (16). Together, these findings suggest that hormones released from the residual menopausal ovary negatively impact brain function. These residual hormones that are released from the ovary after transitional menopause could also impact the efficacy of subsequent HT, indicating that an animal that has undergone transitional menopause may have a different response to HT than one that has undergone surgical menopause, thereby yielding no circulating ovarian-derived hormones.
The current study is the first to evaluate the impact of CEE HT in two models of menopause: surgical (induced via OVX) and transitional (induced via VCD). We used a cognitive test battery tapping spatial working memory, which is a form of spatial short-term memory wherein spatial information needs to be updated, and spatial reference memory, which is a form of long-term memory and is tested as spatial information that remains constant. We hypothesized that OVX animals would show enhanced spatial working and reference memory performance after CEE treatment, based on our prior findings (9,23). We also hypothesized that animals that had undergone VCD-induced follicular depletion would not show this enhancement with CEE treatment based on our prior findings that the elevated androgen levels seen in VCD-treated transitionally menopausal rats correlated with impaired memory (16). Tests included the WRAM to assess spatial reference memory as well as working memory load increases across trials, the MWM to evaluate spatial reference memory, and the DMS place-learning task including temporal delays to evaluate high-demand memory retention. Furthermore, androstenedione was measured and correlated with maze scores. We hypothesized that higher androstenedione levels would be associated with impaired maze performance (16).
Materials and Methods
Subjects
Forty-three 7-month-old Fischer-344 female rats raised at the aging colony of the National Institute on Aging at Harlan Laboratories (Indianapolis, IN) were used. After arrival, rats were pair housed, had food and water ad libitum, and a 12-h light, 12-h dark cycle. Animals were 11 months old at maze initiation. All procedures were Institutional Animal Care and Use Committee approved and adhered to National Institutes of Health standards.
Experimental design and hormone manipulations
To make behavioral testing feasible, animals were run in two experimental waves, 1 month apart, with each treatment group represented in each wave. Rats were randomly divided into four groups (n in parentheses): OVX (9), OVX-CEE (9), VCD (12), and VCD-CEE (13). One week after arrival, animals received either VCD (160 mg/kg diluted in dimethylsulfoxide and saline at a volume of 2.5 μl/g body weight, ip; Sigma-Aldrich, St. Louis, MO) or vehicle injections for 15 d. VCD follicular depletion procedures were adapted from other reports (16,24). Seventy-two days after the first VCD injection (36 ± 1 d before behavioral testing), rats were anesthetized with isoflurane (Baxter HealthCare, Deerfield, IL) and received either OVX (OVX and OVX-CEE groups) or sham surgery (VCD and VCD-CEE groups). Bilateral-dorsolateral incisions were made in the skin and peritoneum, and ovaries and tips of uterine horns were removed. Muscle and skin were sutured. Sham surgery consisted of muscle/skin incision and suture only. Next, 18 ± 1 d after surgeries, hormone/vehicle treatment began. One sc injection was given for 2 consecutive days followed by 2 d off, a pattern repeated throughout the study (exactly as done in Ref. 9). Using this injection regimen, we have previously shown CEE to alter cognition in middle-aged OVX rats (9), and we and others have shown 17β-estradiol to impact hippocampal plasticity and memory (25,26,27). Animals received either cyclic treatment with vehicle injection (sesame oil, OVX, and VCD groups) or injectable CEE (30 μg), in its unconstituted powder form, as prescribed to women (Wyeth Pharmaceuticals Inc., Philadelphia, PA, obtained from a commercial pharmacy via veterinary prescription) dissolved in sesame oil. To confirm follicular depletion in VCD animals, complete OVX as well as CEE treatment, vaginal smears were taken after four CEE injections for 10 d before behavioral testing. Smears were classified as proestrus, estrous, metestrus, or diestrus (28). Behavioral testing started 18 d after initiation of CEE/vehicle injections.
Statistical analyses are noted in detail for each dependent variable described below in the specific maze sections. In general, each analysis included a two-between (hormone and menopause type) design using ANOVA. The hormone variable had two levels, vehicle or CEE, and menopause type had two levels, transitional or surgical. Whereas the main effects of hormone and menopause type are reported, we were particularly interested in the hormone × menopause type interaction to answer the primary question of the study regarding whether the effects of CEE differ with menopause etiology. Regression examined relationships between androstenedione and memory. Androstenedione was the predictor variable in the model, and the following were outcome variables: MWM distance collapsed across all trials/days, DMS postdelay errors, WRAM working memory correct, working memory incorrect, and reference memory errors (for all trials in block 4 and for postdelay trials).
WRAM
The WRAM was used to evaluate working memory and reference memory (29,30,31). The eight-arm WRAM had platforms hidden at the end of four arms. A subject had 3 min to find a platform. Once found, the animal remained on it for 15 sec and was returned to its heated cage for a 30-sec intertrial interval (ITI). The just-chosen platform was removed from the maze. These events were repeated until all four platforms were located. Consequently, there were four trials per day, with platformed arms reduced by one on each subsequent trial. Hence, working memory was increasingly taxed as trials progressed.
Each subject received one session per day for 12 d, with platform locations constant at the beginning of each session. Errors were quantified using orthogonal measures of working and reference memory errors (29,30,31,32,33,34,35). Working memory correct errors were first and repeat entries into arms from which a platform had been removed during that day. Reference memory errors were first entries into arms that never contained a platform. Working memory incorrect errors were repeat entries into reference memory arms. Testing days were blocked into four blocks (block 1, d 1–3; block 2, d 4–6; block 3, d 7–9; block 4, d 10–12), and each type of error was analyzed separately, using a two-between (hormone and menopause type), two-within (day and trial) repeated-measures ANOVA. Similar blocking methods have been used for WRAM analyses evaluating ovarian hormones (29,30,31,32,33,34,35).
On d 13, a 4-h delay was given between trials 2 and 3. The delay was analyzed separately for each error type, with performance on postdelay trials analyzed using two-tailed planned comparisons (OVX vs. OVX-CEE and VCD vs. VCD-CEE), alpha <0.05.
MWM
The MWM was a round water-filled tub (188 cm diameter) with a hidden platform (10 cm wide), remaining in a fixed location, testing spatial reference memory (36,37). The rat was dropped off at either north, south, east, or west and had 60 sec to locate the platform. After a 15-sec platform time, the rat was placed into its heated cage until the next trial. Rats were given four trials per day for 5 d. The ITI was 5–8 min. To evaluate whether rats localized the platform to the spatial location, after all test trials, a 60-sec probe trial was given whereby the platform was removed. A video camera/tracking system tracked and analyzed each rat’s path (Ethovision; Noldus Instruments, Wageningen, The Netherlands).
MWM analyses evaluated distance (centimeters) to the platform using a two-between (hormone and menopause type), two-within (days and trials), repeated-measures ANOVA. For probe trial data, percent distance in the previously platformed (target) quadrant was compared with the diagonally opposite quadrant (13) using repeated-measures (quadrant) ANOVA. Rats that learned the platform location should spend the greatest percent distance in the target vs. opposite quadrant.
DMS plus maze
The water-escape DMS task had a hidden platform at the end of one arm (each arm 38.1 × 12.7 cm). The platform location was the same within a day but changed across days. Rats received six trials per day, 90 sec trial time, 15 sec platform time, 30 sec ITI for 7 d. Trial 1 was the information trial, trial 2 was the working memory test trial, and trials 3–6 were recent memory test trials (38). An arm entry was counted when the tip of a rat’s snout reached a mark on the outside of the arm (not visible from the inside of the maze; 11 cm into the arm). Entry into any nonplatformed arm was counted as an error. The total number of errors were analyzed for each test trial. The seven initial learning days, including trials 2–6, were analyzed using a two-between (hormone and menopause type), two-within (day and trial), repeated-measures ANOVA to allow interpretation of potential hormone and menopause type main effects and interactions in the context of potentially complex interactions with initial testing days.
To evaluate high-demand memory retention, a 6-, 4-, and 8-h delay was instilled after the information trial on consecutive days. There was one postdelay test trial for each of the delays. The day after the 8-h delay, rats received 0.2 mg/kg ip scopolamine (Sigma-Aldrich), 20 min before the information trial. There was one postscopolamine test trial. Scopolamine, a muscarinic antagonist shown to have amnesic effect (39,40,41,42,43), was given to determine whether CEE impacted the ability to remember with this pharmacological challenge. Performance after the delay and scopolamine challenge was evaluated by analyzing the postdelay or postscopolamine trial, respectively, via two-tailed planned comparisons (OVX vs. OVX-CEE and VCD vs. VCD-CEE), alpha <0.05.
Ovarian histology and uterine weights
The uterus was cut above the cervical junction, trimmed of visible fat, and weighed (44). For animals with ovaries at the end of the study (VCD groups), ovaries were removed, trimmed of connective tissue and fat, and fixed in 10% formalin for 48 h. Tissue was processed for paraffin embedding, sectioned at 5 μm, mounted, and stained with hematoxylin and eosin. Corpus lutea were counted (Aristoplan compound microscope; Leitz, Charlotte, VT). Corpus lutea were analyzed only for VCD groups because they were the only groups to have ovaries at the end of the study.
Hormone assays
Blood was obtained via cardiocentesis at the time of decapitation. Serum was collected from a randomly chosen subset of animals from each group, and androstenedione levels were determined with an iodinated RIA using reagents obtained from Siemens (Los Angeles, CA). Assays were performed by the Core Endocrine Laboratory at Pennsylvania State University. Between-run imprecision averaged 10.2% at a mean concentration of 1.45 ng/ml, whereas the low-end sensitivity for this androstenedione method is 0.1 ng/ml.
Results
WRAM
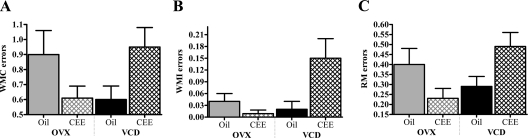
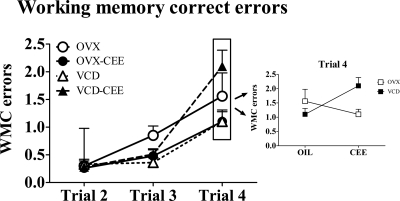
The WRAM maze evaluates spatial working and reference memory. There were no main effects of hormone or menopause type, nor were there hormone × menopause type interactions for working memory correct, working memory incorrect, or reference memory for blocks 1, 2, or 3. For block 4, the lattermost portion of testing, there was a hormone × menopause type interaction for working memory correct (F1,39 = 5.76; P < 0.05), working memory incorrect (F1,39 = 3.99; P = 0.05), and reference memory (F1,39 = 6.86; P = 0.01) with OVX animals given CEE making fewer working memory correct, working memory incorrect, and reference memory errors compared with those given oil, and VCD animals given CEE making more working memory correct, working memory incorrect, and reference memory errors compared with those given oil (Fig. 1). Thus, CEE treatment impaired animals that had undergone transitional ovarian hormone loss but benefited animals that had undergone abrupt surgical hormone loss. Group differences on the lattermost portion of WRAM testing due to various hormone manipulations have been observed previously in our laboratory, with most pronounced effects on the highest working memory load trial (29,45,46,47,48). Here we find a similar pattern of effects; there was a trial × hormone × menopause type interaction for block 4 for working memory correct (F2,78 = 3.85; P < 0.05). As working memory load increased, OVX animals given CEE made fewer working memory correct errors compared with OVX animals given oil, and VCD animals given CEE made more working memory correct errors compared with VCD animals given oil (Fig. 2). Because these data indicated that CEE aided the ability to better remember information at the highest memory load in OVX animals, and we have seen this effect with 17β-estradiol (29), we performed an additional analysis at the highest working memory load, trial 4. Again, we found a hormone × menopause type interaction (F1,39 = 5.46; P < 0.05), showing that CEE enhanced memory at this high memory load in OVX animals but impaired memory at this high memory load for VCD animals (Fig. 2). CEE treatment did not impact OVX or VCD animals for the delay.
Figure 1.
Mean ± se working memory correct (WMC) errors (A), working memory incorrect (WMI) errors (B), and reference memory (RM) errors (C) for each group (OVX, n = 9; OVX-CEE, n = 9; VCD, n = 12; VCD-CEE, n = 13) for the lattermost testing block (block 4). The significant hormone × menopause type interaction for each error type indicates that OVX animals given CEE made fewer WMC, WMI, and RM errors than those given oil, while VCD animals given CEE made more WMC, WMI, and RM errors compared with those given oil (P < 0.05). Therefore, treatment with CEE impaired animals undergoing transitional hormone loss but benefited animals undergoing abrupt hormone loss.
Figure 2.
Mean ± se working memory correct (WMC) errors for each group (OVX, OVX-CEE, VCD, and VCD-CEE) during the lattermost testing block (block 4). The inset shows mean ± se WMC errors for each group for trial 4. There was a significant trial × hormone × menopause type interaction for WMC errors, indicating that the pattern of change as trials increased differed with CEE treatment and menopause type. Further analyses revealed a hormone × menopause type interaction for trial 4, the trial with the highest working memory load. The data indicate that OVX animals given CEE were better able to handle the highest memory load than those given oil, and VCD animals given CEE were less able to handle the highest memory load than those given oil (P < 0.05). CEE therefore enhanced the ability to handle a more demanding memory load in animals with surgical, abrupt hormone loss but impaired the ability to handle a more demanding memory load in animals that had experienced transitional hormone loss.
DMS plus maze
The DMS plus maze is a measure of spatial working and recent memory. There were no main effects of hormone or menopause type nor hormone × menopause type interactions for initial learning (d 1–7).
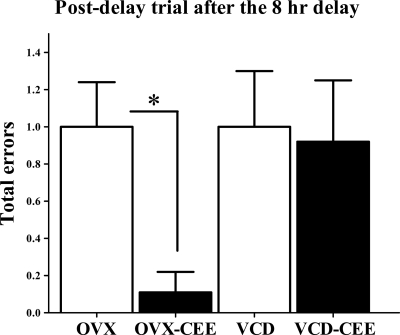
Because we initially hypothesized that CEE would enhance memory retention in OVX animals, an effect previously observed in our laboratory (23), and others have observed memory-enhancing effects of estrogens on memory retention in OVX animals (49,50), we conducted planned comparisons between OVX and OVX-CEE groups. For the 8-h delay, CEE enhanced performance in OVX animals, with the OVX-CEE group making fewer errors relative to the OVX group (t16 = 3.42; P < 0.005). In contrast, CEE did not impact postdelay performance in VCD animals (Fig. 3). There were no CEE effects for the 4- or 6-h delays (Supplemental Fig. 2) nor for the scopolamine challenge trial.
Figure 3.
Mean ± se total errors on the DMS plus maze 8-h postdelay trial. OVX-CEE animals made fewer errors relative to OVX animals after an 8-h delay (P < 0.005). CEE did not impact postdelay performance if animals had undergone transitional menopause via VCD treatment.
MWM
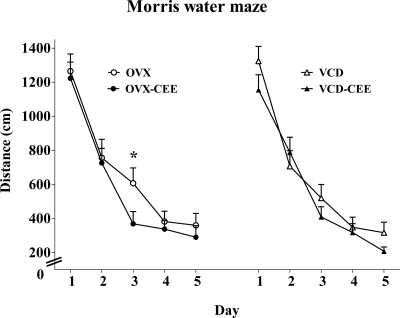
The MWM is a measure of spatial reference memory. There were no distance main effects for hormone or menopause type, nor was there a hormone × menopause type interaction. Upon inspection of the learning curves (Fig. 4), we noted that groups were disparate on d 3. Because we and others have shown MWM performance benefits of estrogens in middle-aged OVX animals (13,51), and we have shown faster learning with 17β-estradiol in middle-aged OVX rats (13), we further probed performance on d 3. We conducted a t test, set post hoc, comparing OVX and OVX-CEE groups, and another t test comparing VCD and VCD-CEE groups. On d 3, CEE enhanced performance in OVX animals (t16 = 2.09; P = 0.05), but not in VCD animals (P = 0.32).
Figure 4.
Mean ± se distance on the MWM for d 1–5. Group comparisons set post hoc revealed that on d 3 OVX-CEE rats showed decreased distance scores relative to OVX rats (P = 0.05). There was no CEE effect on this day for VCD-treated animals (P = 0.32). Thus, CEE enhanced performance in OVX rats but not VCD rats on d 3 of MWM testing, indicating faster learning by the OVX-CEE group.
For the probe trial, a higher percent distance was spent in the previously platformed vs. the opposite quadrant (quadrant main effect: F1,37 = 182.05; P < 0.0001). All groups localized the platform location by the end of testing (null quadrant × hormone × menopause type interaction: P > 0.11).
Vaginal smears, uterine weights, ovarian histology, and serum hormone levels
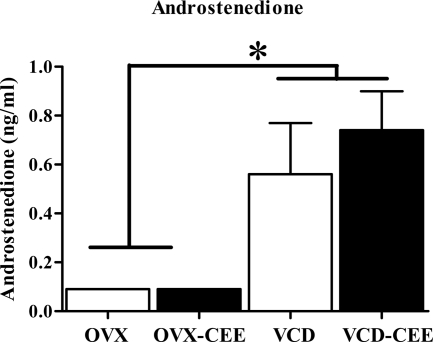
OVX animals not given CEE showed diestrous vaginal smears, with few, primarily leukocytic cells. VCD animals showed the expected pattern of vaginal smear profiles (16) because they did not cycle through the four cycle phases and exhibited smears of intermittent estrous and diestrous within a given subject. CEE animals, regardless of menopause type (VCD or OVX), showed estrous smears with primarily cornified cells, consistent with previous findings (9,16). Mean ± se uterine weights were: OVX (0.21 ± 0.01), OVX-CEE (0.35 ± 0.02), VCD (0.67 ± 0.06), and VCD-CEE (0.69 ± 0.08). Mean ± se corpus lutea were: VCD (3.78 ± 1.33) and VCD-CEE (1.44 ± .53). OVX animals had lower uterine weight relative to OVX-CEE animals (t15 = 5.78; P < 0.0001). As expected (16), uterine weights did not differ between VCD and VCD-CEE groups (t23 = 0.17; P = 0.86) nor did corpus lutea (t9 = 1.32; P = 0.23). VCD animals had higher androstenedione levels than OVX animals (VCD animals combined vs. OVX animals combined: t,15 = 3.84, P < 0.005) (Fig. 5).
Figure 5.
Mean ± se serum androstenedione levels for each group. Androstenedione levels were higher in VCD animals relative to OVX animals (P < 0.01).
Androstenedione levels and relationships with memory
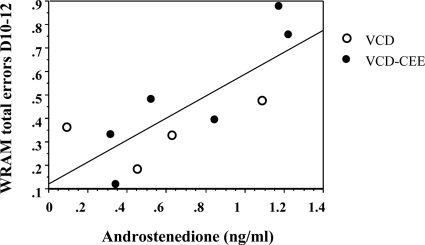
Figure 6 represents the scatterplot of the significant regression analysis. The two VCD-treated groups of animals were combined because levels of androstenedione between them did not differ. Higher androstenedione levels were associated with more WRAM working memory correct (B = 0.81, se = 0.29, P = 0.02, R = 0.70), working memory incorrect (B = 0.25, se = 0.09, P = 0.03, R = 0.68) and reference memory (B = 0.43, se = 0.13, P = 0.01, R = 0.75) errors on the last testing block (d 10–12). To simplify presentation, total combined errors (B = 0.47, se = 0.13, P = 0.008, R = 0.78) are presented (Fig. 6). Regressions examining relationships between androstenedione and the other memory measures analyzed were not significant.
Figure 6.
Regression analysis indicated that in VCD animals (VCD and VCD-CEE), higher androstenedione was associated with more WRAM working memory correct, working memory incorrect, and reference memory errors on the last testing block (P < 0.05). For ease of presentation, here we present only the combined graph with total errors (P < 0.01).
Discussion
In the current study, we demonstrated that the cognitive effects of CEE depend on whether menopause was surgical or transitional. Whereas we have previously shown that CEE benefits memory in older rats with a history of surgical menopause (9,23), the OVX rat models about 13% of women; the majority of women undergo transitional menopause and retain their residual follicle-deplete ovaries (21). Thus, we compared surgically menopausal rats to transitionally menopausal rats that retained their follicle-deplete ovaries for cognitive responsiveness to CEE. Here we show that CEE HT benefits cognition in surgically menopausal rats but, in contrast, is detrimental to cognition in transitionally menopausal rats.
Specifically, in OVX rats, CEE decreased reference memory errors, two orthogonal types of working memory errors, as well as working memory correct errors on the trial with the highest working memory load; VCD-induced menopausal rats showed the opposite effect on these measures, with errors increasing after CEE treatment. Moreover, after only one exposure to the platform, CEE-treated OVX rats showed better memory retention across a demanding 8-h delay relative to oil-treated OVX rats, whereas CEE exerted no retention benefit in VCD transitionally menopausal rats. The beneficial effects of CEE in surgically menopausal middle-aged rats, observed herein, concur with previous findings using surgically menopausal middle-aged rats whereby CEE enhanced memory retention on the DMS place task (9,23) and for nonspatial object memory (10). Thus, the three animal studies performed thus far testing CEE HT in middle-aged OVX rats are consistent in showing that CEE benefits cognition. This CEE benefit does not appear to translate to animals with a history of transitional menopause, effects which may be related to circulating androstenedione levels. Indeed, higher levels of androstenedione, which are released from the follicle deplete menopausal ovary, correlated with impaired working and reference memory. This replicates our prior finding of higher androstenedione levels correlating with impaired working memory in VCD transitionally menopausal rats (16).
CEE also provided some benefit on the MWM. This effect was found when an analysis, set post hoc, was performed and was specific to just d 3 of testing. CEE enhanced performance on this testing day in surgically menopausal animals but not in transitionally menopausal animals, indicating faster learning of the platform location after CEE with surgical menopause only. This effect was transient, with all groups’ scores merging on d 4 and no other group differences exhibited. A similar pattern has been noted previously in our laboratory, with 17β-estradiol enhancing MWM learning of the platform location in middle-aged OVX rats, with all groups’ scores merging by d 4 (13). We and others have also shown that in middle-aged OVX rats, 17β-estradiol (13,51) and CEE (9) enhances performance on the MWM, although not all studies show positive effects of estrogens on the MWM task (52,53). CEE, however, failed to protect against scopolamine challenge in the current study, an effect previously observed in our laboratory in OVX rats (9). These differences in CEE effects may be due to the numerous manipulations and number of days tested before scopolamine challenge in our previous study (28 d) (9) relative to the study herein (10 d).
The VCD model of ovarian follicular depletion closely models transitional menopause, the type of reproductive senescence experienced by the majority of women. VCD treatment results in a follicle-deplete rodent with elevated circulating gonadotropins, decreased but present progesterone, and depleted 17β-estradiol (16,20,22,24); the profile of these hormones is similar to what is seen in transitionally menopausal women who have kept their follicle-deplete ovaries (21). In transitionally menopausal women, studies indicate that androstenedione levels decrease but are still present, resulting in an increased androgen to estrogen ratio because estrogens are depleted (54). In the VCD rodent model, our prior studies (16,22) show no change in androstenedione levels relative to sham ovary-intact animals, resulting in an endocrine milieu with a relatively higher androgen to estrogen ratio because estrogens become deplete. The hormone environment is therefore androgen rich, similar to that seen in transitionally menopausal women (20). Furthermore, the VCD model enables methodical experimental evaluation while controlling for, or obviating, factors possibly influencing outcome in clinical studies such as age, socioeconomic status, and education. Thus, the VCD model of follicular depletion affords methodical evaluation of cognitive consequences of transitional ovarian hormone loss as a consequence of follicular depletion in an animal model.
Herein we confirmed the hormone status of all treatment groups by multiple measures. Complete OVX was confirmed via vaginal smears. VCD-induced follicular depletion was confirmed by vaginal smears and corpus lutea quantification, as shown previously (16). Also, as expected (16), VCD and VCD-CEE groups showed similar uterine weights relative to each other, whereas OVX-CEE animals showed elevated uterine weights relative to OVX animals. CEE-induced peripheral endocrine responsiveness was additionally evidenced by positive vaginal smears in all CEE-treated groups. Furthermore, androstenedione levels in VCD-treated animals (VCD and VCD-CEE) were elevated relative to OVX animals (OVX and OVX-CEE), consistent with previous findings (16).
The observation that CEE differentially affects cognitive outcome, depending on history of menopause, could help reconcile findings in the human HT and cognition literature. The WHIMS was designed to evaluate the effects of HT separately in women with a uterus, assigned to CEE+MPA, and women without a uterus, assigned to CEE alone, without specifically analyzing women depending on oophorectomy status (17). Separating women within the CEE-alone trial according to menopause type was not in the WHIMS protocol because it was not part of the original question driving the study (17). Findings from the WHIMS reporting a trend toward an increase in probable dementia and mild cognitive impairment in the CEE-alone group did not distinguish between women who had undergone transitional vs. surgical menopause in analyses (7). However, another report from the WHIMS evaluating global cognitive function did present bilateral oophorectomy as part of their demographics describing the women in the estrogen-alone arm (6). Importantly, for this evaluation, although 40% of women reported bilateral oophorectomy with hysterectomy, the remaining 60% comprised women who had hysterectomy and no oophorectomy as well as women whose oophorectomy status was not available, limiting interpretation of potential influence of oophorectomy (6).
Similarly, other smaller randomized, double-blind, placebo-controlled studies using unopposed CEE that did not account for menopause history also showed no benefit of CEE (55,56). Some randomly assigned controlled trials not distinguishing menopause type have shown benefits of CEE (57). Interestingly, the only human studies that included solely women who had surgical menopause (oophorectomy) have shown cognitive benefits of HT (58,59), consistent with our CEE findings in OVX rats. Reports of positive effects of HT in studies including only oophorectomized women, and the current findings of differential cognitive effects of CEE, depending on menopause history in the rodent model, collectively suggest that menopause history is a plausible factor that contributes to cognitive efficacy of HT.
There are plausible explanations for the finding that CEE is detrimental to cognition after transitional menopause. CEE may interact negatively with residual androgen hormones. We recently demonstrated that VCD animals showed impaired performance on tasks including working and recent memory, and they had higher androstenedione levels that correlated with impaired cognitive performance (16). In contrast, animals that were treated with VCD, followed by removal of residual androgen hormones via OVX, showed enhanced cognitive performance (16). Residual androgen hormones may therefore hinder cognitive performance, with CEE interacting negatively with residual androgens to exacerbate these deleterious effects. Supporting this tenet is the current replication (16) of the correlation that higher androstenedione is associated with worse maze performance. In postmenopausal women who still have their ovaries, there is a greater androgen to estrogen ratio (60). Whether the negative cognitive consequences of transitional menopause can be ameliorated by blocking residual androgens or their actions, alone or in combination with estrogen preparations, provides exciting new avenues for research and intervention.
Importantly, another factor that may influence the detrimental effects of CEE in transitional menopause may be time of CEE initiation. Animal studies using middle-aged OVX rats show that 17β-estradiol HT has a limited window during which it can be effective (11,12). It is therefore possible that HT initiated after menopause has ensued is detrimental to cognition. Indeed, a recent report found that in naturally menopausal women, HT initiated after menopause was detrimental to cognitive performance, whereas HT initiated before menopause was beneficial (61). Furthermore, another study found that HT was associated with a reduced risk of Alzheimer’s disease but only if HT exceeded 10 yr, being initiated earlier in menopause (62). Whether timing of CEE initiation impacts cognitive outcome during transitional menopause is a clinically relevant and poignant question (63); it is possible that the current negative findings of CEE HT in transitionally menopausal rats may be obviated with earlier initiation. Future studies will address this.
The finding that the effects of CEE are both beneficial and detrimental, depending on type of menopause, has implications for HT research in the laboratory and the clinic. Before this account, preclinical evaluations of the cognitive effects of any HT on menopause-related etiology of hormone loss were nonexistent. The most commonly used menopause model to examine cognition in animals is OVX. However, the current findings suggest that cognitive effects of HT using OVX animals may be most related to women undergoing abrupt surgical hormone loss because menopause history and state can lead to differential effects. Etiology of menopause should therefore be a consideration in future HT studies and interpretations. Furthermore, these data suggest that HT-induced cognitive effects via clinical intervention in menopausal women might be influenced by history of menopause type.
In conclusion, the current study indicates that the cognitive effects of CEE depend on etiology of menopausal hormone loss and that residual ovarian androgen hormones after transitional menopause may be associated with impaired performance. CEE enhanced maze performance in surgically menopausal rats but exerted the opposite effects in transitionally menopausal rats, impairing performance. This effect was shown on several orthogonal measures tapping multiple cognitive domains. These findings suggest that history of menopause impacts the cognitive effects of CEE HT, further indicating that history of menopause is an important parameter that should be considered in future HT experimental study designs and interpretation. We anticipate these findings to promote new avenues for preclinical and clinical HT research, and we hypothesize that etiology of ovarian hormone loss could influence the cognitive effects of HT in women.
Supplementary Material
Acknowledgments
We thank Candy Tsang, Melissa Scheldrup, Ian Crain, Joshua Talboom, Elizabeth Engler, Cynthia Zay, and Bronson Bowman for excellent experimental assistance. We also are grateful to Dr. Laurence Demers and the Core Endocrine Laboratory at Pennsylvania State University for performing the hormone assays.
Footnotes
This work was supported by Grant AG028084 (to H.A.B.-N.) and Diversity Supplement Grant AG028084 from the National Institute on Aging (to J.I.A.).
Disclosure Summary: J.I.A., B.B.B., S.N., S.M., and H.A.B.-N. have nothing to declare. L.P.M. has equity interests in Senestech, Inc.
First Published Online June 16, 2010
Abbreviations: CEE, Conjugated equine estrogens; DMS, delayed match to sample; HT, hormone therapy; ITI, intertrial interval; MWM, Morris water maze; OVX, ovariectomized; VCD, 4-vinylcyclohexene diepoxide; WHIMS, Women’s Health Initiative Memory Study; WRAM, water radial-arm maze.
References
- Stefanick ML 2005 Estrogens and progestins: background and history, trends in use, and guidelines and regimens approved by the U.S. Food and Drug Administration. Am J Med 118(Suppl 12B):64–73 [DOI] [PubMed] [Google Scholar]
- Campbell S, Whitehead M 1977 Oestrogen therapy and the menopausal syndrome. Clin Obstet Gynaecol 4:31–47 [PubMed] [Google Scholar]
- Ohkura T, Isse K, Akazawa K, Hamamoto M, Yaoi Y, Hagino N 1995 Long-term estrogen replacement therapy in female patients with dementia of the Alzheimer type: 7 case reports. Dementia 6:99–107 [DOI] [PubMed] [Google Scholar]
- Kantor HI, Michael CM, Shore H 1973 Estrogen for older women. Am J Obstet Gynecol 116:115–118 [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones 3rd BN, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J 2003 Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 289:2651–2662 [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J 2004 Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 291:2959–2968 [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH 2004 Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 291:2947–2958 [DOI] [PubMed] [Google Scholar]
- Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, Granek IA, Hogan P, Ockene JK, Shumaker SA 2006 Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab 91:1802–1810 [DOI] [PubMed] [Google Scholar]
- Acosta JI, Mayer L, Talboom JS, Zay C, Scheldrup M, Castillo J, Demers LM, Enders CK, Bimonte-Nelson HA 2009 Premarin improves memory, prevents scopolamine-induced amnesia and increases number of basal forebrain choline acetyltransferase positive cells in middle-aged surgically menopausal rats. Horm Behav 55:454–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA 2008 Conjugated equine estrogen enhances rats’ cognitive, anxiety, and social behavior. Neuroreport 19:789–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL 2006 Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology 147:607–614 [DOI] [PubMed] [Google Scholar]
- Gibbs RB 2000 Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging 21:107–116 [DOI] [PubMed] [Google Scholar]
- Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA 2008 Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem 90:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J 2003 Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol Aging 24:839–852 [DOI] [PubMed] [Google Scholar]
- Nappi RE, Sinforiani E, Mauri M, Bono G, Polatti F, Nappi G 1999 Memory functioning at menopause: impact of age in ovariectomized women. Gynecol Obstet Invest 47:29–36 [DOI] [PubMed] [Google Scholar]
- Acosta JI, Mayer L, Talboom JS, Tsang CW, Smith CJ, Enders CK, Bimonte-Nelson HA 2009 Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system. Endocrinology 150:4248–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker SA, Reboussin BA, Espeland MA, Rapp SR, McBee WL, Dailey M, Bowen D, Terrell T, Jones BN 1998 The Women’s Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials 19:604–621 [DOI] [PubMed] [Google Scholar]
- Hersh AL, Stefanick ML, Stafford RS 2004 National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA 291:47–53 [DOI] [PubMed] [Google Scholar]
- Springer LN, McAsey ME, Flaws JA, Tilly JL, Sipes IG, Hoyer PB 1996 Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol Appl Pharmacol 139:394–401 [DOI] [PubMed] [Google Scholar]
- Mayer LP, Devine PJ, Dyer CA, Hoyer PB 2004 The follicle-deplete mouse ovary produces androgen. Biol Reprod 71:130–138 [DOI] [PubMed] [Google Scholar]
- Timaras P, Quay W, Vernadakis A, eds. 1995 Hormones and aging. Boca Raton, New York, London, Tokyo: CRC Press [Google Scholar]
- Mayer LP, Dyer CA, Eastgard RL, Hoyer PB, Banka CL 2005 Atherosclerotic lesion development in a novel ovary-intact mouse model of perimenopause. Arterioscler Thromb Vasc Biol 25:1910–1916 [DOI] [PubMed] [Google Scholar]
- Engler-Chiurazzi E, Tsang C, Nonnenmacher S, Liang WS, Corneveaux JJ, Prokai L, Huentelman MJ, Bimonte-Nelson HA 2009 Tonic Premarin dose-dependently enhances memory, affects neurotrophin protein levels and alters gene expression in middle-aged rats. Neurobiol Aging 10.1016/j.neurobiolaging.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer LP, Pearsall NA, Christian PJ, Devine PJ, Payne CM, McCuskey MK, Marion SL, Sipes IG, Hoyer PB 2002 Long-term effects of ovarian follicular depletion in rats by 4-vinylcyclohexene diepoxide. Reprod Toxicol 16:775–781 [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS 1993 Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol 336:293–306 [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Bimonte-Nelson H, Neisewander JL, Conrad CD 2008 Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: evidence that the duration of hormone deprivation after ovariectomy compromises 17β-estradiol effectiveness in altering CA1 spines. Horm Behav 54:386–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol DL, Kolo LL 2002 Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci 116:411–420 [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL 2007 The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol 80:84–97 [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH 1999 Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology 24:161–173 [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH 2000 Sex differences in vicarious trial-and-error behavior during radial arm maze learning. Physiol Behav 68:495–499 [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Hyde LA, Hoplight BJ, Denenberg VH 2000 In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Physiol Behav 70:311–317 [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Nelson ME, Granholm AC 2003 Age-related deficits as working memory load increases: relationships with growth factors. Neurobiol Aging 24:37–48 [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Hunter CL, Nelson ME, Granholm AC 2003 Frontal cortex BDNF levels correlate with working memory in an animal model of Down syndrome. Behav Brain Res 139:47–57 [DOI] [PubMed] [Google Scholar]
- Hyde LA, Hoplight BJ, Denenberg VH 1998 Water version of the radial-arm maze: learning in three inbred strains of mice. Brain Res 785:236–244 [DOI] [PubMed] [Google Scholar]
- Hyde LA, Sherman GF, Hoplight BJ, Denenberg VH 2000 Working memory deficits in BXSB mice with neocortical ectopias. Physiol Behav 70:1–5 [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J 1982 Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683 [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC 2006 Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci 24:229–242 [DOI] [PubMed] [Google Scholar]
- Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL 1995 Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol Aging 16:149–160 [DOI] [PubMed] [Google Scholar]
- Moss DE, Rogers JB, Deutsch JA, Salome RR 1981 Time dependent changes in anterograde scopolamine-induced amnesia in rats. Pharmacol Biochem Behav 14:321–323 [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA 1997 Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol Learn Mem 68:172–188 [DOI] [PubMed] [Google Scholar]
- Dohanich GP, Fader AJ, Javorsky DJ 1994 Estrogen and estrogen-progesterone treatments counteract the effect of scopolamine on reinforced T-maze alternation in female rats. Behav Neurosci 108:988–992 [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Beninger RJ 2002 Water maze versus radial maze: differential performance of rats in a spatial delayed match-to-position task and response to scopolamine. Behav Brain Res 128:139–152 [DOI] [PubMed] [Google Scholar]
- Okaichi H, Jarrard LE 1982 Scopolamine impairs performance of a place and cue task in rats. Behav Neural Biol 35:319–325 [DOI] [PubMed] [Google Scholar]
- Ashby J, Odum J, Foster JR 1997 Activity of raloxifene in immature and ovariectomized rat uterotrophic assays. Regul Toxicol Pharmacol 25:226–231 [DOI] [PubMed] [Google Scholar]
- Huentelman MJ, Stephan DA, Talboom J, Corneveaux JJ, Reiman DM, Gerber JD, Barnes CA, Alexander GE, Reiman EM, Bimonte-Nelson HA 2009 Peripheral delivery of a ROCK inhibitor improves learning and working memory. Behav Neurosci 123:218–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Williams BJ, Granholm AC 2004 Ovarian hormones and cognition in the aged female rat: II. progesterone supplementation reverses the cognitive enhancing effects of ovariectomy. Behav Neurosci 118:707–714 [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Hunter CL, Price KL, Moore AB, Granholm AC 2003 Ovarian hormones and cognition in the aged female rat: I. Long-term, but not short-term, ovariectomy enhances spatial performance. Behav Neurosci 117:1395–1406 [DOI] [PubMed] [Google Scholar]
- Braden BB, Talboom JS, Crain ID, Simard AR, Lukas RJ, Prokai L, Scheldrup MR, Bowman BL, Bimonte-Nelson HA 2010 Medroxyprogesterone acetate impairs memory and alters the GABAergic system in aged surgically menopausal rats. Neurobiol Learn Mem 93:444–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL 2001 Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci 115:384–393 [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL 2004 Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm Behav 45:128–135 [DOI] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM 2002 Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the morris water maze. Horm Behav 42:284–293 [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Juraska JM 2000 Acute administration of estrogen and progesterone impairs the acquisition of the spatial morris water maze in ovariectomized rats. Horm Behav 38:234–242 [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Millard WJ, Simpkins JW 1994 Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res 644:305–312 [DOI] [PubMed] [Google Scholar]
- Davison SL, Bell R, Donath S, Montalto JG, Davis SR 2005 Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab 90:3847–3853 [DOI] [PubMed] [Google Scholar]
- Binder EF, Schechtman KB, Birge SJ, Williams DB, Kohrt WM 2001 Effects of hormone replacement therapy on cognitive performance in elderly women. Maturitas 38:137–146 [DOI] [PubMed] [Google Scholar]
- Ditkoff EC, Crary WG, Cristo M, Lobo RA 1991 Estrogen improves psychological function in asymptomatic postmenopausal women. Obstet Gynecol 78:991–995 [PubMed] [Google Scholar]
- Shaywitz SE, Naftolin F, Zelterman D, Marchione KE, Holahan JM, Palter SF, Shaywitz BA 2003 Better oral reading and short-term memory in midlife, postmenopausal women taking estrogen. Menopause 10:420–426 [DOI] [PubMed] [Google Scholar]
- Sherwin BB 1988 Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology 13:345–357 [DOI] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB 1992 Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology 17:485–495 [DOI] [PubMed] [Google Scholar]
- Simpson ER 2002 Aromatization of androgens in women: current concepts and findings. Fertil Steril 77(Suppl 4):S6–S10 [DOI] [PubMed] [Google Scholar]
- Greendale GA, Huang MH, Wight RG, Seeman T, Luetters C, Avis NE, Johnston J, Karlamangla AS 2009 Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology 72:1850–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JC 2002 Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA 288:2123–2129 [DOI] [PubMed] [Google Scholar]
- Maki PM, Sundermann E 2009 Hormone therapy and cognitive function. Hum Reprod Update 15:667–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.