Abstract
Forkhead box O1 (FoxO1) is a transcription factor that mediates the inhibitory effect of insulin on target genes in hepatic metabolism. Hepatic FoxO1 activity is up-regulated to promote glucose production during fasting and is suppressed to limit postprandial glucose excursion after meals. Increased FoxO1 activity augments the expression of insulin receptor (IR) and IR substrate (IRS)2, which in turn inhibits FoxO1 activity in response to reduced insulin action. To address the underlying physiology of such a feedback loop for regulating FoxO1 activity, we delivered FoxO1-ADA by adenovirus-mediated gene transfer into livers of adult mice. FoxO1-ADA is a constitutively active allele that is refractory to insulin inhibition, allowing us to determine the metabolic effect of a dislodged FoxO1 feedback loop in mice. We show that hepatic FoxO1-ADA production resulted in significant induction of IR and IRS2 expression. Mice with increased FoxO1-ADA production exhibited near glycogen depletion. Unexpectedly, hepatic FoxO1-ADA production elicited a profound unfolded protein response, culminating in the induction of hepatic glucose-regulated protein 78 (GRP78) expression. These findings were recapitulated in primary human and mouse hepatocytes. FoxO1 targeted GRP78 gene for trans-activation via selective binding to an insulin responsive element in the GRP78 promoter. This effect was counteracted by insulin. Our studies underscore the importance of an IR and IRS2-dependent feedback loop to keep FoxO1 activity in check for maintaining hepatic glycogen homeostasis and promoting adaptive unfolded protein response in response to altered metabolism and insulin action. Excessive FoxO1 activity, resulting from a dislodged FoxO1 feedback loop in insulin resistant liver, is attributable to hepatic endoplasmic reticulum stress and metabolic abnormalities in diabetes.
The forkhead transcriptional factor FoxO1 plays an important role in coupling hepatic insulin resistance to endoplasmic reticulum stress in obesity and type 2 diabetes.
Forkhead box O1 (FoxO1) belongs to a superfamily of transcription factors that is characterized by a highly conserved winged-helix DNA binding motif, termed “forkhead” domain, including FoxO1, FoxO3, FoxO4, and FoxO6 in mammals (1,2), abnormal dauer formation (DAF) 16 in Caenorhabditis elegans (3), and dFoxO in Drosophila (4). These forkhead proteins are substrates of serine-threonine kinase/protein kinase B and serum/glucocorticoid regulated kinase, playing important roles in mediating insulin action on the expression of genes involved in cell growth, differentiation, metabolism, and longevity (1,2,3,4,5). Insulin exerts its inhibitory effect on target gene expression via a highly conserved insulin responsive element (IRE) with its core motif 5′-TG/ATTTT/G-3′ in the promoter (1,2,6). In response to reduced insulin action, FoxO proteins reside in the nucleus and bind as a trans-activator to IRE, enhancing promoter activity. In response to insulin stimulation, FoxO proteins are phosphorylated through the phosphatidylinositol kinase-dependent pathway, resulting in FoxO nuclear exclusion and inhibition of target gene expression (7,8,9,10,11,12,13). This phosphorylation-dependent subcellular redistribution serves as an acute mechanism for insulin to regulate FoxO transcriptional activity for rapid adaptation to metabolic shift from fasting to refeeding states (1,2,5,6,14). Except for FoxO6 (15), all members of the FoxO superfamily undergo insulin-dependent phosphorylation and nuclear exclusion. Failure in FoxO phosphorylation results in its permanent nuclear localization and constitutive gene expression (1,2,6,7,16,17,18). Indeed, it has been shown that unbridled FoxO1 activity, resulting from an impaired ability of insulin to phosphorylate FoxO1, promotes the overproduction of gluconeogenic enzymes PEPCK and G6PC (1,5,19,20,21,22,23), as well as apolipoprotein C-III and microsomal triglyceride transfer protein, two key functions in very low-density lipoprotein (VLDL)-triglyceride metabolism (24,25,26). This effect accounts in part for the concurrent pathogenesis of fasting hyperglycemia and hypertriglyceridemia in insulin-resistant subjects with visceral obesity and type 2 diabetes (6,27,28).
There is anecdotic evidence that FoxO1 activity is subject to feedback regulation, but the underlying physiology remains elusive. FoxO1 is shown to stimulate the expression of its upstream effector gene encoding insulin receptor (IR), which in turn activates insulin signaling and inhibits FoxO1 activity (29,30). It is postulated that such a feedback loop serves as a mechanism for enhancing cellular sensitivity to insulin during fasting and priming starved cells for nutrient availability. Implicit in this assumption is that FoxO1 activity is up-regulated in serum-starved cells (29,30) and in liver of fasted mice (17). However, this hypothesis seems at odds with the clinical data showing that prolonged fasting (60 h) elicits peripheral insulin resistance with a concomitant induction in plasma free fatty acid (FFA) levels in healthy subjects (31). A 16-h fast results in increased lipid accumulation in liver without affecting insulin sensitivity in mice (32). Excessive FoxO1 activity also results in hepatosteatosis in mice (5,17,21,26,33,34).
To address the physiological significance of the FoxO1 feedback loop, we delivered FoxO1-ADA by adenovirus-mediated gene transfer into liver of adult mice. FoxO1-ADA is a constitutively active allele that is not subject to insulin inhibition due to point mutations in the three conserved phosphorylation sites (T24A, S253D, and S316A) of FoxO1 polypeptide chain (10,19). As a result, this system would disengage the effect of insulin on FoxO1 activity, allowing us to determine the metabolic consequence of a dislodged FoxO1 feedback loop in adult mice. We show that hepatic FoxO1-ADA production resulted in a significant induction of IR and IR substrate (IRS)2. Unexpectedly, hepatic FoxO1-ADA production selectively enhanced the expression of glucose-regulated protein 78 (GRP78), a molecular chaperone that resides in the endoplasmic reticulum (ER) and functions as an ER stress sensor to maintain ER homeostasis (35,36,37). This effect correlated with near depletion of hepatic glycogen content in mice with elevated FoxO1-ADA production. We recapitulated these findings in cultured hepatocytes with elevated FoxO1-ADA production. Furthermore, we show that FoxO1 stimulated GRP78 promoter activity via specific binding to its consensus IRE motif within the GRP78 promoter. This effect was counteracted by insulin. Mutations or deletion of the IRE motif resulted in abolition of FoxO1-mediated induction of GRP78 expression. In addition, we show that palmitate, a predominant saturated form of FFA that is known to elicit ER stress, augmented hepatic FoxO1 activity and induced GRP78 production. Palmitate-mediated induction of FoxO1 and GRP78 production was reversed to normal in response to 4-phenyl butyric acid (PBA), a pharmacological chaperone that is effective for mitigating cellular ER stress (38). Moreover, enhanced binding of FoxO1 to GRP78 promoter was detectable in insulin resistant liver, correlating with augmented hepatic FoxO1 activity and increased GRP78 production in obese db/db mice.
These results characterize GRP78 as a molecular target of FoxO1, underscoring the importance of FoxO1 in hepatic ER homeostasis. ER is the principal organelle for the biosynthesis of proteins and steroids and for the production of VLDL particles. Perturbation of ER homeostasis, such as the accumulation of misfolded proteins, deprivation of glucose, or altered glycosylation, often triggers adaptive unfolded protein response (UPR), also known as ER stress (35,36,37,39,40,41,42). Unresolved ER stress results in cellular apoptosis (36,43,44,45,46,47). Although UPR is intertwined with impaired insulin action and there is emerging evidence that excessive ER stress is attributable to insulin resistance (35,48,49), the underlying mechanism remains elusive. Our results together with previous data indicate that FoxO1 integrates hepatic insulin action to GRP78 expression for regulating UPR in a pathway that is orchestrated through the IR/IRS2-dependent FoxO1 feedback loop. We suggest that the FoxO1 feedback loop is crucial for keeping FoxO1 activity in check, a safeguarding mechanism for maintaining ER homeostasis and averting the deleterious effect of unrestrained FoxO1 activity on glucose and lipid metabolism.
Materials and Methods
Animal studies
CD1 mice were obtained from Charles River Laboratory (Wilmington, MA). For blood chemistry, mice were fasted for 16 h, and tail vein blood samples were collected into capillary tubes precoated with potassium-EDTA (Sarstedt, Nümbrecht, Germany) for the preparation of plasma or determination of blood glucose levels using Glucometer Elite (Bayer, Mishawaka, IN) and plasma insulin using the ultrasensitive mouse insulin ELISA (ALPCO, Windham, NH). All procedures were approved by the Institutional Animal Care and Use Committee of University of Pittsburgh School of Medicine. Other methods, including statistics, were described in online Supplemental Materials and Methods (published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org).
Results
FoxO1 up-regulates hepatic IR and IRS2 expression in mice
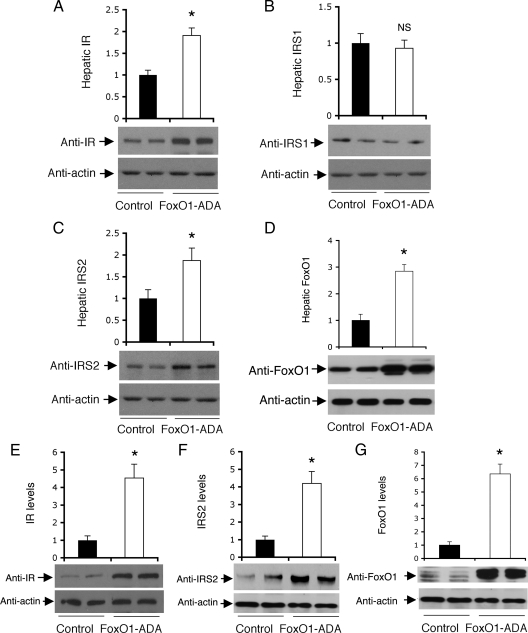
FoxO1 acts downstream of IR and IRS to mediate insulin action on hepatic gluconeogenesis. To investigate the effect of FoxO1 on IR and IRS expression, we delivered the constitutively active FoxO1-ADA allele into liver of mice using adenovirus-mediated gene transfer. Due to amino acid substitutions at the three conserved phosphorylation sites, FoxO1-ADA is refractory to insulin inhibition (19,26), allowing us to determine the net effect of FoxO1 on the expression of its upstream effectors, such as IR and IRS in liver. Male CD1 mice were stratified by body weight into two groups (n = 8 per group), which were treated with FoxO1-ADA and control vectors, as described (25). As shown in Fig. 1, hepatic FoxO1-ADA production resulted in a 2-fold induction in both IR and IRS2 protein levels. This effect correlated with a 2.5-fold elevation in hepatic FoxO1 activity in FoxO1-ADA group. In contrast, hepatic expression of IRS1 proteins remained unchanged in FoxO1-ADA vs. control vector-treated mice. These data suggest that FoxO1-ADA selectively up-regulated hepatic IR and IRS2 expression in mice.
Figure 1.
Effect of FoxO1-ADA on hepatic IR and IRS expression. Male CD1 mice of 10 wk old were stratified by body weight and randomly assigned to two groups (n = 8), which were iv injected with Adv-FoxO1-ADA or Adv-null vector at 1.5 × 1011 pfu/kg body weight. One week after vector administration, mice were fasted for 16 h and killed. Liver tissues were subjected to immunoblot analysis for the determination of hepatic levels of IR (A), IRS1 (B), IRS2 (C), and FoxO1 (D). HepG2 cells at 90% confluence were transduced with Adv-FoxO1-ADA or Adv-null vector at a fixed MOI (100 pfu/cell) in six-well plates. Each condition was run in triplicate. After a 24-h incubation, cells were subjected to immunoblot analysis using anti-IR (E), anti-IRS2 (F), and FoxO1 (G). IRS1 was undetectable due to its extremely low basal expression in HepG2 cells. *, P < 0.05 vs. control. NS, Not significant.
FoxO1 stimulates IR and IRS2 expression in cultured hepatocytes
To corroborate the above findings, we treated HepG2 cells in the presence and absence of FoxO1-ADA production, followed by the determination of IR and IRS expression. FoxO1-ADA production resulted in more than 4-fold induction in both IR (Fig. 1E) and IRS2 (Fig. 1F) protein levels, correlating with a 6-fold elevation of FoxO1 activity in FoxO1-ADA vector-treated HepG2 cells (Fig. 1G). Due to extremely low basal IRS1 expression, we could not detect IRS1 protein expression in HepG2 cells. Although FoxO1 activity is inhibited by insulin via an IR- and IRS-dependent mechanism, the present findings of FoxO1-mediated induction of IR and IRS2 are consistent with the idea that hepatic FoxO1 activity is tightly regulated via a feedback loop (30).
Correlation of FoxO1 activity with IR and IRS2 expression in fasted liver
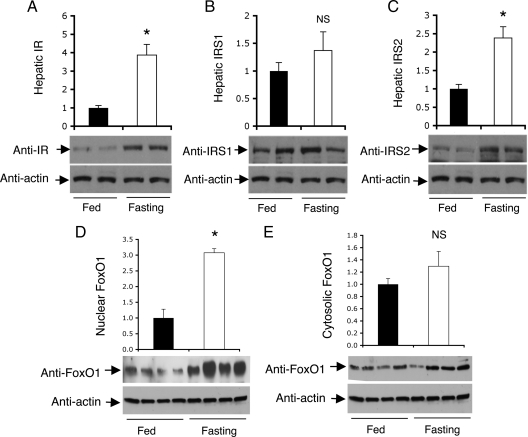
To understand the underlying physiology of the FoxO1 feedback loop, we determined the expression of hepatic IR, IRS1, and IRS2 in mice under different physiological conditions. Based on previous observations that FoxO1 protein expression along with its nuclear localization is increased, correlating with its enhanced activity in promoting gluconeogenesis in response to fasting (17), we hypothesized that increased FoxO1 activity would stimulate IR and IRS2 expression in fasted livers. Two groups of male CD1 mice (n = 6 per group) were maintained under fed conditions or fasted for 16 h, followed by determining IR and IRS protein levels in liver. As shown in Fig. 2, IR and IRS2 protein levels were significantly raised when the metabolic state was shifted from fed to an overnight fasting. This effect correlated with elevated hepatic FoxO1 activity, culminating in significantly increased FoxO1 nuclear localization in hepatocytes of fasted mice, in accordance with our previous observations (17). A small nonsignificant increase in hepatic IRS1 protein levels was detected in fasted mice.
Figure 2.
Hepatic IR and IRS expression in fed and fasting states. Male CD1 mice (10 wk old) were fasted for 16 h (n = 6) or fed ad libitum (n = 6). Liver tissue was collected from killed mice for the preparation of liver protein extracts. Aliquots of 20-μg liver proteins were subjected to immunoblot assay. Hepatic IR (A), IRS1 (B), and IRS2 (C) protein levels were determined. Additional aliquots of liver tissue (20 mg) were homogenized for the preparation of nuclear and cytoplasmic fractions, followed by the determination of FoxO1 in the nucleus (D) and cytoplasm (E) of hepatocytes in fed and fasted mice. *, P < 0.05 vs. control. NS, Not significant.
Effect of FoxO1 on hepatic glycogen metabolism
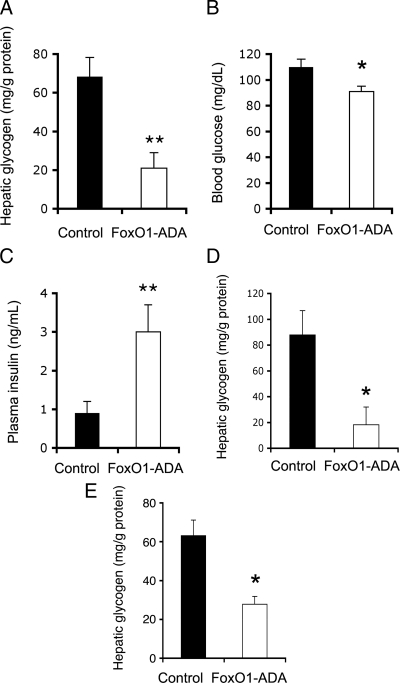
To address the underlying pathophysiology of FoxO1 feedback loop, we probed the metabolic consequence of unbridled FoxO1 activity in liver, resulting from a lack of FoxO1 feedback inhibition. We determined hepatic glycogen content in FoxO1-ADA vector-treated mice. Due to its insensitivity to insulin inhibition, FoxO1-ADA production provides a scenario of a circuit breakdown in FoxO1 feedback loop. As a result, mice with increased FoxO1-ADA production in liver displayed a 3.5-fold reduction in hepatic glycogen content after an overnight fasting (Fig. 3A). Hepatic glycogen was nearly depleted (<10 mg/g liver protein) in three out of eight FoxO1-ADA vector-treated mice, in comparison with hepatic glycogen content (>70 mg/g liver protein) in control vector-treated mice. This effect correlated with the ability of FoxO1 to stimulate G6PC production and promotes gluconeogenesis and glycogenolysis in liver (1,23,26). No significant differences in body weight were detected after 1 wk of hepatic FoxO1-ADA production, ruling out the possibility that the observed alterations in hepatic glycogen metabolism were secondary to body weight changes in FoxO1-ADA vector-treated mice.
Figure 3.
Effect of FoxO1-ADA on hepatic glycogen content. Mice were killed after a 16-h fasting after 1 wk of hepatic FoxO1-ADA production as described in Fig. 1. Aliquots of liver tissues (40 mg) were used for the determination of hepatic glycogen content in FoxO1-ADA (n = 8) and control (n = 8) groups (A). Fasted blood samples were used for the determination of fasting blood glucose levels (B) and plasma insulin levels (C). Human primary hepatocytes were transduced with Adv-null or Adv-FoxO1-ADA vector (MOI, 100 pfu/cell) in 12-well microplates. Each condition was run in six replicates. After a 16-h incubation, cells were harvested for the determination of hepatic glycogen content (D). Likewise, mouse primary hepatocytes were transduced with Adv-null or Adv-FoxO1-ADA vector (MOI, 100 pfu/cell) in six-well microplates. Each condition was run in 10 replicates. After a 16-h incubation, cells were harvested for the determination of hepatic glycogen content (E). *, P < 0.05 and **, P < 0.001 vs. control.
Furthermore, FoxO1-ADA vector-treated mice exhibited relatively lower blood glucose levels (Fig. 3B). This effect was inversely correlated with significantly increased fasting plasma insulin levels (Fig. 3C). Similar effects have been observed in adult mice with increased FoxO1 activity in liver in previous studies (17,26).
To underpin the above findings, we cultured human primary hepatocytes in 12-well collagen-coated microplates at the density of 1 × 106 cells/well in the presence of FoxO1-ADA or control vector at a fixed dose [multiplicity of infection (MOI), 200 plaque-forming unit (pfu)/cell]. After a 16-h incubation, cells were harvested for the determination of intracellular glycogen. Adenovirus-mediated FoxO1-ADA production resulted in a 5-fold reduction of glycogen content in human primary hepatocytes (Fig. 3D). Likewise, we treated mouse primary hepatocytes with 200 MOI of Adv-null vector or Adv-FoxO1-ADA, followed by the determination of hepatic glycogen. Consistent with the observation in human primary hepatocytes, mouse primary hepatocytes with FoxO1 gain-of-function exhibited significantly reduced glycogen content (Fig. 3E).
Impact of FoxO1 on hepatic ER stress
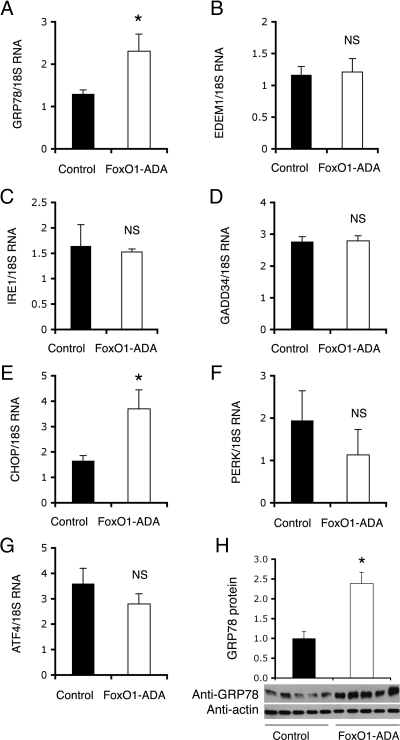
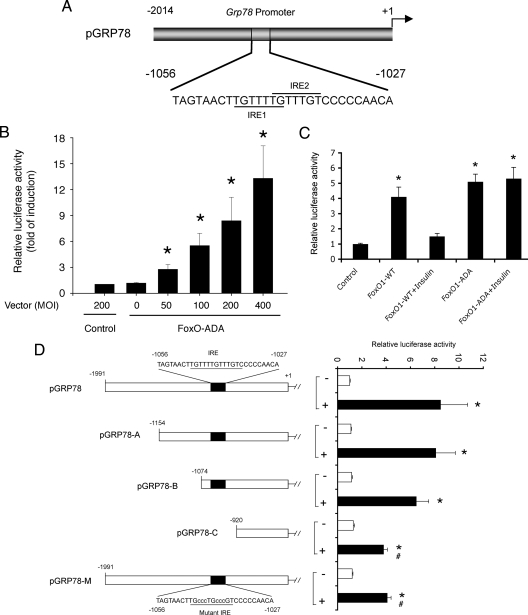
To further understand the physiology underlying FoxO1-mediated feedback regulation of hepatic insulin signaling, we investigate the impact of FoxO1-ADA on hepatic ER stress, a cellular response that is associated with altered hepatic metabolism in obesity and diabetes (37,39,41,42,50,51). As shown in Fig. 4, hepatic FoxO1-ADA production resulted in a selective induction of GRP78 and CCAAT-enhancer-binding protein homology protein (CHOP). In contrast, no significant alterations were seen in hepatic expression of other functions involved in ER stress, including the activating transcription factor (ATF)4, ER degradation enhancer, mannosidase α-like 1, growth arrest and DNA damage-inducing protein 34, inositol requiring 1 (IRE1), and protein kinase R-like ER kinase (PERK). Because GRP78 is an ER stress sensor, we chose to focus our studies on GRP78. We confirmed that GRP78 protein levels were also significantly up-regulated in response to FoxO1-ADA production in liver (Fig. 4H). These findings raised the hypothesis that FoxO1 plays a significant role in coupling hepatic insulin action to ER stress. Implicit in this hypothesis is the presence of two tandem IRE within the mouse GRP78 promoter (Fig. 5A). Likewise, four tandem IRE motifs were detected in the human GRP78 promoter (Supplemental Fig. 1), which is suggestive of an evolutionally conserved mechanism.
Figure 4.
Effect of FoxO1-ADA on hepatic ER stress responses. Aliquots of liver tissues (30 mg) were used for the preparation of total RNA, which was subjected to real-time quantitative RT-PCR assay for determining hepatic mRNA levels of GRP78 (A), ER degradation enhancer, mannosidase α-like (B), IRE1 (C), growth arrest and DNA damage-inducing protein 34 (D), CHOP (E), PERK (F), and ATF4 (G). Additional aliquots of liver tissue were homogenized for the preparation of total hepatic proteins, followed by immunoblot assay for the determination of hepatic GRP78 protein levels (H). *, P < 0.01 vs. control. NS, Not significant.
Figure 5.
Effect of FoxO1 on hepatic ER stress responses. Schematic depiction of the mouse GRP78 promoter. The two intertwined IRE sequences (IRE1 and IRE2) were highlighted (A). FoxO1 dose-dependent induction of GRP78 promoter activity (B). HepG2 cells were cotransfected with 2-μg pGRP78 encoding GRP78 promoter-directed luciferase reporter system and 2-μg pCMV-LacZ vector in the presence of Adv-FoxO1-ADA vector at different doses ranging from 50 to 400 pfu/cell or Adv-null at 200 pfu/cell in culture medium. After a 24-h incubation, cells were assayed for luciferase and β-galactosidase (β-gal) activities. The relative promoter activity, defined as the ratio of luciferase to β-gal activities, was determined (B). Effect of insulin on GRP78 promoter activity. HepG2 cells were cotransfected with 2-μg pGRP78 encoding GRP78 promoter-directed luciferase reporter system and 2-μg pCMV-LacZ vector in the presence of wild-type FoxO1 (FoxO1-WT) or its constitutive active allele FoxO1-ADA production. After a 24-h incubation in the presence or absence of insulin (100 nm), cells were assayed for determining the relative luciferase activity using β-gal activity as a control (C). Characterization of FoxO1 target site in GRP78 promoter. HepG2 cells were cotransfected with pCMV-LacZ plus either wild-type or mutant GRP78 promoter-directed luciferase reporter system in the presence of Adv-null or Adv-FoxO1-ADA vector at a fixed dose (100 pfu/cell). The relative luciferase activity for each construct was determined after a 24-h incubation (D). Data were obtained from five to eight experiments. *, P < 0.001 vs. basal states; #, P < 0.05 vs. control wild-type promoter construct.
To address this hypothesis, we cloned the mouse GRP78 promoter in a luciferase reporter assay system in pGL3. The resulting plasmid pGRP78 was transfected to HepG2 cells in the presence or absence of FoxO1-ADA production. As shown in Fig. 5B, FoxO1-ADA production stimulated GRP78 promoter activity in a dose-dependent manner.
FoxO1 mediates insulin action on hepatic GRP78 expression
To test the ability of FoxO1 to mediate insulin action on GRP78 expression, we transfected pGRP78 into HepG2 cells that were pretransduced with adenoviral vectors expressing wild-type FoxO1 or FoxO1-ADA mutant in the presence or absence of insulin (100 nm) in culture medium. After a 24-h incubation, cells were harvested for determination of luciferase activity. As shown in Fig. 5C, FoxO1 production resulted in a 4-fold induction of GRP78 promoter activity. This effect was counteracted by insulin, consistent with the ability of insulin to promote FoxO1 phosphorylation and translocation from the nucleus to cytoplasm (1,2,6). FoxO1-ADA production also contributed to a significant induction (5-fold) of GRP78 promoter activity. Unlike its wild-type counterpart, FoxO1-ADA mediated induction of GRP78 promoter activity was indifferent to insulin inhibition (Fig. 5C). This effect correlated with the inability of FoxO1-ADA to undergo insulin-dependent phosphorylation and nuclear exclusion (1,2,6).
FoxO1 targets GRP78 promoter for trans-activation
To test the hypothesis that FoxO1 targets GRP78 gene for trans-activation, we mapped FoxO1 target site within the GRP78 promoter. We generated a series of promoter variants with deletions of the upstream region of the GRP78 promoter. Using the luciferase reporter assay, we determined the activity of promoter variants in HepG2 cells in the presence and absence of FoxO1-ADA production. As shown in Fig. 5D, deletion of DNA up to −1074 nucleotide (nt) into the upstream region of GRP78 promoter did not affect promoter activity after FoxO1-ADA production in HepG2 cells. Further deletion up to −920 nt in the GRP78 promoter resulted in a significant reduction in promoter activity in response to FoxO1-ADA production. These results are in line with the presence of two IRE motifs conjoined within the region (−1074/−920 nt) of the GRP78 promoter. To strengthen these findings, we altered the IRE sequence in the GRP78 promoter by site-directed mutagenesis. After sequencing confirmation (Supplemental Fig. 2), the resulting mutant promoter was assessed for its ability to respond to FoxO1-ADA production. As expected, mutations in the IRE region significantly attenuated the promoter activity in response to FoxO1-ADA induction (Fig. 5D).
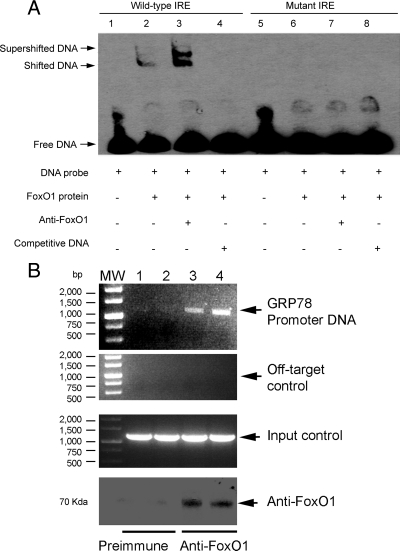
Molecular association of FoxO1 with GRP78 promoter DNA
To consolidate the above results, we performed EMSA to visualize the molecular association between FoxO1 and GRP78 IRE DNA. We prepared FoxO1-enriched nuclear protein extract from FoxO1-expressing HepG2 cells as described (25). Aliquots of FoxO1-containing protein extract were incubated with a 24-bp GRP78 IRE DNA sequence that was prelabeled with biotin, followed by chemiluminescent EMSA. As shown in Fig. 6A, the migration of the GRP78 IRE DNA was significantly retarded in the presence of FoxO1 in 6% native polyacrylamide gels. Addition of anti-FoxO1 antibody to the reaction mixture resulted in a supershifted DNA band. To confirm the specificity of FoxO1-IRE DNA interaction, we included nonlabeled IRE DNA at 100-fold higher concentrations as competitors in the reaction, demonstrating that IRE DNA shift was abolished. As control, we performed EMSA with a mutant IRE DNA containing six base substitutions. No shifted and supershifted DNA bands were detectable in the EMSA, indicating that mutations in the GRP78 IRE motif abrogated its ability to associate with FoxO1 protein.
Figure 6.
Molecular association of FoxO1 with GRP78 promoter. FoxO1 binding to DNA. Aliquots of FoxO1 protein extracts (5 μg) were incubated with biotin-labeled DNA probe, followed by chemiluminescent EMSA. FoxO1 protein lysates were prepared from HepG2 cells that were pretransduced with FoxO1 vector (MOI, 200 pfu/cell). DNA probe was derived from a 24-bp DNA covering the consensus IRE (−1056/−1023 nt) of the mouse GRP78 promoter (lanes 1–4). A mutant DNA probe with altered IRE motif was used as a control (lanes 5–8) in the EMSA (A). ChIP assay. HepG2 cells were transfected with pGRP78 in the presence of FoxO1 vector at an MOI of 100 pfu/cell in triplicate. After a 24-h incubation, cells were cross-linked with 1% formaldehyde, followed by ChIP assay using preimmune rabbit IgG (lane 1 and 2) or rabbit anti-FoxO1 antibody (lanes 3 and 4). Immunoprecipitates were subjected to PCR analysis using a pair of primers flanking the IRE sequence in the GRP78 promoter. As a negative control, the immunoprecipitates were subjected to PCR analysis using a pair of off-target primers flanking a distal region (−4671/−4652 nt) that is devoid of the consensus IRE motif in the upstream region of the GRP78 promoter. As a positive control, aliquots of input DNA samples (1 μl) were used in PCR analysis. In addition, aliquots of immunoprecipitates were subjected to anti-FoxO1 immunoblot analysis for confirming the presence of FoxO1 protein. Data were from three independent repeats (B). MW, molecular weight.
To further underpin these data, we employed chromatin immunoprecipitation (ChIP) assay to examine the molecular interaction between FoxO1 and the GRP78 promoter. We transfected pGRP78 into HepG2 cells that were pretransduced with FoxO1 vector, followed by ChIP assay using rabbit anti-FoxO1 antibody or preimmune rabbit sera. The immunoprecipitates were subjected to immunoblot assay for detecting immunoprecipitated FoxO1 and PCR analysis for visualizing coimmunoprecipitated DNA. As shown in Fig. 6B, specific DNA corresponding to the proximal region (−1074/+1 nt) of the GRP78 promoter was amplified by PCR using primers flanking the GRP78 IRE motif. In contrast, the immunoprecipitates derived from preimmune sera were negative in the same PCR assay. As an input control, aliquots of cell lysates (1 μl) before immunoprecipitation were analyzed. Specific DNA bands corresponding to the GRP78 promoter were detected (Fig. 6B). In addition, we performed PCR analysis using a pair of off-target primers flanking a distal region (−4671/−4652 nt) that is devoid of the consensus IRE motif in the GRP78 promoter. No specific DNA was amplified in the immunoprecipitates derived from preimmune IgG or anti-FoxO1 antibody (Fig. 6B).
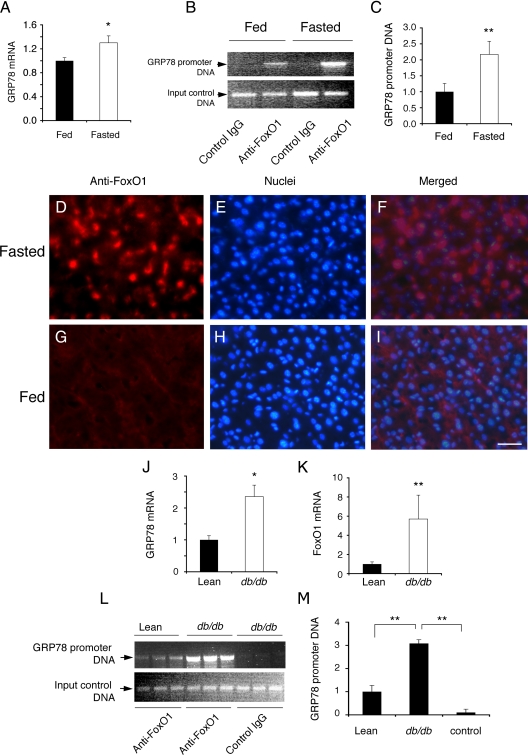
FoxO1 interacts with GRP78 promoter in liver
To recapitulate the above finding in vivo, we performed ChIP assay on liver tissues of fed and fasted mice. Because FoxO1 activity is induced in fasted liver, we reasoned that this effect would translate into an induction of GRP78 expression in fasted mice. As shown in Fig. 7, A–C, positive association of FoxO1 with GRP78 promoter DNA was detected in liver. This effect was induced in mice after a 16-h fast, correlating with a significant induction of hepatic GRP78 mRNA levels in fasted mice.
Figure 7.
FoxO1 association with GRP78 in liver. Male CD1 mice (10 wk old, n = 6 per group) were fasted for 16 h or fed ad libitum. Liver tissue was collected from killed mice for the preparation of total hepatic RNA, followed by real-time quantitative RT-PCR assay for the determination of GRP78 mRNA levels (A). Aliquots of liver tissue (20 mg) were subjected to ChIP assay using anti-FoxO1 or control anti-β-galactosidase antibodies. Immunoprecipitates were analyzed by PCR using specific primers flanking the IRE DNA motif in the GRP78 promoter in fed and fasted liver (B). The relative amount of GRP78 promoter DNA immunoprecipitated from fed and fasted liver was determined (C). Additionally, frozen liver sections (8 μm) were immunostained with anti-FoxO1 antibody for visualizing FoxO1 in the liver under fasting (D–F) and fed (G–I) conditions. Male obese db/db (16 wk old, n = 6 per group) and age-/sex-matched lean db/+ littermates were killed, and liver tissue (20 mg) was used for the preparation of total hepatic RNA, followed by real-time quantitative RT-PCR analysis for determining GRP78 (J) and FoxO1 (K) expression. Furthermore, aliquots of liver tissue (20 mg) were subjected to ChIP assay using anti-FoxO1 or control anti-β-galactosidase antibodies. Immunoprecipitates were analyzed by PCR using specific primers flanking the IRE DNA motif in the GRP78 promoter (L). The relative amount of GRP78 promoter DNA immunoprecipitated from the liver of lean and obese db/db mice was determined (M). *, P < 0.05; **, P < 0.001 vs. control. Scale bar in D–I, 20 μm.
To ascertain the finding of increased FoxO1 activity in fasted liver, we subjected liver tissue of fed and fasted mice to immunohistochemistry. FoxO1 was predominantly localized in the nucleus of hepatocytes in fasted mice (Fig. 7, D–F). In contrast, FoxO1 was expressed at basal level under fed conditions (Fig. 7, G–I).
To reinforce the idea that FoxO1 targets GRP78 gene for trans-activation, we investigated the interaction of FoxO1 with GRP78 promoter in insulin resistant liver of obese db/db mice. When compared with heterozygous lean db/+ mice, obese db/db mice exhibited significantly increased GRP78 expression (Fig. 7J), accompanied by a 5-fold induction of FoxO1 expression in liver (Fig. 7K). This effect correlated with a marked induction in molecular association between FoxO1 and GRP78 promoter DNA in insulin resistant liver of obese db/db mice (Fig. 7, L and M). These results are consistent with our previous observations that FoxO1 becomes deregulated in insulin resistant liver, as reflected in its increased nuclear redistribution in hepatocytes of db/db mice (24,25).
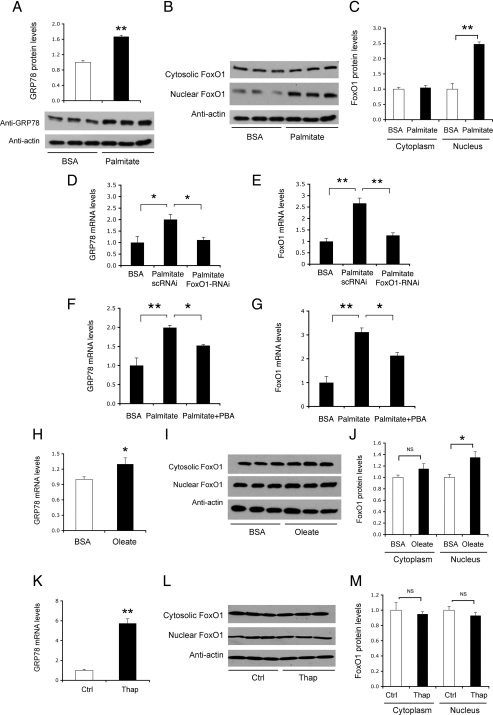
FoxO1 links saturated fat, but not thapsigargin, to ER stress
To further illustrate the underlying pathophysiology of FoxO1-mediated induction of GRP78 expression, we determined the expression levels of GRP78 and FoxO1 proteins in HepG2 cells that pretreated with palmitate (250 μm), the predominant saturated form of FFA that is deleterious to hepatic insulin signaling (52,53,54,55). Palmitate treatment resulted in a significant induction of GRP78 protein expression, which is indicative of ER stress in HepG2 cells (Fig. 8A). This effect correlated with a 2-fold induction of FoxO1 production in the nucleus of palmitate-treated HepG2 cells (Fig. 8, B and C). In accordance with these findings, Wei et al. (54) show that palmitate promotes CHOP production and elicits ER stress in cultured H4IIE cells.
Figure 8.
FoxO1 links palmitate, but not thapsigargin, to ER stress. HepG2 cells were treated with 250 μm of BSA-bound palmitate or BSA for 16 h (n = 3 for each condition), followed by immunoblot analysis using anti-GRP78 antibody (A). In addition, treated cells were subjected to nuclear and cytosolic fractionation. Aliquots (20 μg) of nuclear and cytosolic proteins were analyzed by anti-FoxO1 immunoblot assay (B), followed by the determination of cytosolic vs. nuclear FoxO1 protein levels (C). Separately, palmitate-treated cells were transduced with 100 MOI of Adv-FoxO1-RNAi encoding FoxO1-specific siRNA or control Adv-FoxO1-scRNAi vector encoding scrambled siRNA. After a 16-h incubation, cells were subjected to real-time quantitative (q)RT-PCR analysis for determining GRP78 (D) and FoxO1 (E) expression. Similarly, HepG2 cells were treated with palmitate (250 μm) in the presence and absence of PBA (1 mm) in culture medium. After a 2-h incubation, cells were subjected to real-time qRT-PCR analysis for the determination of GRP78 (F) and FoxO1 (G) expression. Likewise, HepG2 cells were treated with 500 μm of BSA-bound oleate or BSA for 16 h (n = 3 for each condition), followed by real-time qRT-PCR assay using specific primers of GRP78 or 18S rRNA (H). Treated cells were subjected to anti-FoxO1 immunoblot assay (I) for the determination of cytosolic vs. nuclear FoxO1 protein levels (J). Additionally, HepG2 cells were treated with 250 nm of thapsigargin for 16 h. Control cells were mock treated (n = 3 for each condition). Treated cells were subjected to real-time qRT-PCR assay for the determination of GRP78 mRNA levels (K) and to anti-FoxO1 immunoblot assay (L) for the determination of cytosolic vs. nuclear FoxO1 protein levels (M). *, P < 0.05 and **, P < 0.001 vs. control. NS, Not significant; Ctrl, control group; Thap, thapsigargin group.
To correlate FoxO1 activity with GRP78 induction, we employed small interfering RNA (siRNA)-mediated gene silencing approach to ablate FoxO1 expression in HepG2 cells, using Adv-FoxO1-siRNA vector as described (25). In response to siRNA-mediated FoxO1 knockdown, palmitate-mediated induction of GRP78 expression was significantly attenuated (Fig. 8, D and E), underscoring the importance of FoxO1 in palmitate-mediated induction of ER stress.
To further underpin the importance of FoxO1 in ER stress, we incubated palmitate-treated HepG2 cells in the absence and presence of 1-mm PBA, a pharmacological chaperone that is shown to reduce cellular ER stress and improve insulin sensitivity in rodent models of type 2 diabetes (38). PBA treatment ameliorated palmitate-elicited ER stress, as evidenced by the significant reduction of GRP78 expression (Fig. 8F). This effect was accompanied by a concomitant reduction of FoxO1 expression in palmitate-treated HepG2 cells (Fig. 8G). PBA treatment also attenuated palmitate-mediated induction of CHOP, X-box binding protein 1, ATF4, eukaryotic translation initiation factor 2 α, PERK, and ATF6 expression to different extents in HepG2 cells (Supplemental Fig. 3).
As control, we incubated HepG2 cells in the absence and presence of monounsaturated fat oleate (500 μm), followed by analysis of GRP78 and FoxO1 expression. In contrast to palmitate, oleate treatment resulted in a relatively milder induction of GRP78 expression (∼30%) (Fig. 8H), accompanied by a small increase of nuclear FoxO1 protein levels in oleate-treated HepG2 cells (Fig. 8, I and J).
Thapsigargin is a potent inducer of ER stress. It raises cytosolic calcium concentration by blocking the ability of cells to pump calcium into ER lumen, resulting in overt ER stress (56). To test whether FoxO1 is responsible for thapsigargin-induced ER stress, we cultured HepG2 cells in the absence and presence of thapsigargin (250 nm), followed by determination of FoxO1 and GRP78 expression levels. As expected, thapsigargin treatment resulted in about a 6-fold induction of GRP78 expression (Fig. 8K). In contrast, no significant differences in FoxO1 expression levels were detected in control and thapsigargin-treated HepG2 cells (Fig. 8, L and M). Together, these data suggest that FoxO1 played a significant role in coupling saturated fat, but not thapsigargin, to ER stress.
Discussion
FoxO1 has emerged as an important transcriptional factor that integrates hepatic insulin signaling to target genes in hepatic metabolism. Abundantly expressed in liver, FoxO1 controls insulin-dependent inhibition of PEPCK and G6PC, two key enzymes that are involved in gluconeogenesis (1,2). In response to fasting, FoxO1 activity is enhanced, culminating in its increased nuclear localization in liver. This effect stimulates PEPCK and G6PC expression and promotes hepatic glucose production to maintain fasting blood sugar levels within the physiological range (1,2). In response to refeeding, FoxO1 undergoes insulin-dependent phosphorylation and nuclear exclusion, resulting in inhibition of PEPCK and G6PC production in liver. This effect limits hepatic glucose production and prevents prolonged postprandial blood glucose excursion after meals. Such a reciprocal mechanism is essential for liver to adjust the rate of hepatic glucose in response to insulin and nutrient availability.
A substrate of serine-threonine kinase/protein kinase B, FoxO1 is shown to stimulate the expression of its upstream effectors IR and IRS2, setting a feedback loop that negatively regulates FoxO1 activity in liver (Supplemental Fig. 4). It has been proposed that such a feedback loop serves as an adaptive mechanism to enhance insulin sensitivity in fasting states to program starved cells for forthcoming nutrients (29). This notion seems counterintuitive, because fasting tends to desensitize peripheral tissues to insulin to minimize carbohydrate metabolism in favor of survival in the face of famine (31). In this study, we probed the biological consequence of a dislodged FoxO1 feedback loop for better understanding of the physiology that underlies FoxO1-mediated feedback regulation of IR and IRS2 in liver. Using adenovirus-mediated gene transfer approach, we achieved hepatic production of FoxO1-ADA, a constitutive active allele that is able to evade IR- and IRS2-facilitated feedback regulation. We show that hepatic FoxO1-ADA production 1) resulted in a significant induction of IR and IRS expression, 2) caused near depletion of hepatic glycogen content, and 3) induced GRP78 production in liver. These findings were recapitulated in cultured HepG2 cells, as well as human and mouse primary hepatocytes with elevated FoxO1 activity. Furthermore, we show that hepatic IR and IRS2 expression was significantly up-regulated, correlating with the induction of FoxO1 activity in liver of fasted mice. Our data corroborate the idea that hepatic FoxO1 activity is subject to feedback regulation in an IR- and IRS2-dependent manner. We illustrate that the FoxO1 feedback loop plays an important role in limiting hepatic FoxO1 activity to prevent potential glycogen depletion and ER stress in liver (Supplemental Fig. 4).
Another important finding derived from this study is the revelation of the mechanism by which FoxO1 mediates insulin-dependent regulation of GRP78, an ER stress sensor molecule. FoxO1 targets GRP78 gene for trans-activation, and this effect is counteracted by insulin. Although ER stress is closely associated with insulin resistance, the underlying mechanism remains obscure. We show that FoxO1 activity is increased, accompanied by a significant induction of GRP78 expression in insulin resistant liver of obese db/db mice. Our interpretation is that in response to insulin resistance, hepatic FoxO1 activity is enhanced, culminating in its increased nuclear localization. This effect acts to stimulate hepatic production of GRP78, which in turn functions to resolve ER stress in liver. Thus, FoxO1-mediated induction of GRP78 plays an important role in adaptive UPR activation in response to attenuated insulin action in liver. Consistent with this notion is the observation that hepatic FoxO1 activity is significantly elevated (17), correlating with a marked induction of GRP78 expression in liver of high fat-induced obese mice (50). Weight loss-mediated improvement in insulin sensitivity is associated with the reduction of hepatic GRP78 expression and ER stress in obese subjects (41). Adenovirus-mediated hepatic GRP78 overproduction is sufficient to mitigate ER stress and enhance hepatic insulin sensitivity in diabetic db/db mice (37).
However, this view is challenged by a recent study showing that GRP78 haploinsufficiency attenuates high fat-induced insulin resistance and obesity in C57BL/6J mice, implicating a direct role of GRP78 in ER stress (57). It is noteworthy that GRP78 haploinsufficiency also promotes chronic UPR, resulting in a compensatory induction of other chaperones, such as GRP94 and protein disulfide isomerase in GRP78+/− heterozygous mice. This compensatory mechanism along with augmented residual UPR caused by GRP78 loss-of-function may contribute to the amelioration of diet-induced obesity and insulin resistance in GRP78+/− heterozygous mice.
Although thapsigargin, palmitate, and oleate elicited variable degrees of ER stress, as reflected by increased GRP78 expression in HepG2 cells, only palmitate-mediated ER stress was coupled with a marked induction of FoxO1 production. These results are novel, suggesting that FoxO1-mediated induction of GRP78 production and UPR is specific to overload of polyunsaturated fat. These results are in line with the observation by Kamagate et al. (25), who report that in response to increased lipid load, FoxO1 activity is enhanced, which in turn promotes hepatic MTP production and VLDL-triglyceride secretion. This raises a fundamental question: Why the liver cannot rid itself of excessive lipids, and avoid hepatic ER stress and steatosis by accelerating VLDL secretion in the face of lipid excess in subjects with visceral obesity or type 2 diabetes? An important insight to this question is gained from the study by Ota et al. (39), who show that hepatic VLDL production is sensitive to ER stress in a parabolic manner. Moderate ER stress does induce VLDL-triglyceride secretion, protecting liver from ER stress-induced steatosis. However, excessive ER stress in response to prolonged exposure to lipids impairs the ability of liver to secrete triglycerides. This effect contributes to lipid accumulation in liver, exacerbating hepatic steatosis (39). Indeed, this lipid-induced hepatic ER stress is concomitant with steatosis in both genetic and dietary models of obese mice (39,50,58), as well as in high fructose-fed hamsters (33,59,60).
Although FoxO1 was shown to bind and stimulate GRP78 promoter activity, neither deletion nor mutations of the consensus FoxO1 binding site abolished GRP78 promoter activity. These results are suggestive of additional mechanisms that may account for increased GRP78 production and ER stress in response to increased FoxO1 activity in HepG2 cells. Further studies are warranted to dissect the underlying mechanism of FoxO1-mediated regulation of GRP78 expression for better understanding of the molecular basis that couples ER stress to insulin resistance in obesity and type 2 diabetes.
An ER-resident protein, GRP78 remains bound to IRE1, PERK, and ATF6 in unstressed cells. In response to the accumulation of misfolded proteins in ER lumen, GRP78 dissociates from IRE1, PERK, and ATF6, triggering UPR for attenuating the rate of protein synthesis and promoting the induction of genes encoding ER chaperones (35,49,61). Thus, GRP78 is hailed as an ER chaperone for sensing stress signal and mounting UPR to resolve ER stress. GRP78 also plays a critical role in targeting misfolded proteins for proteasomal degradation, which is reviewed as an ER quality-control mechanism (40,61,62). In addition to its sensitivity to disruption in protein folding, the ER lumen is vulnerable to alterations in oxidizing redox potential (63), luminal calcium homeostasis (64), and excessive lipid accumulation (39,65). Thus, there are multiple routes leading to the induction of ER stress. Unresolved ER stress is deleterious to cell growth and metabolism (41,66,67,68). Mice with genetic ablation of the regulatory subunit p85α of phosphatidylinositol kinase in the liver exhibit impaired hepatic insulin action, accompanied by profound ER stress in response to tunicamycin administration (48). Chemical chaperone-mediated inhibition of ER stress is shown to improve glucose metabolism and enhance insulin sensitivity in type 2 diabetic mice (38).
In conclusion, our data consolidate the idea that hepatic FoxO1 activity is subject to feedback regulation. Unchecked FoxO1 activity, resulting from molecular defects in the FoxO1 feedback loop, is deleterious to hepatic metabolism, culminating in unrestrained glycogen breakdown and excessive ER stress in liver. Previous studies show that FoxO1 plays a pivotal role in mediating insulin-dependent regulation of hepatic glucose and VLDL production. FoxO1 dysregulation, resulting from an impaired ability to curb FoxO1 activity, is attributable to hepatic glucose and triglyceride overproduction, accounting in part for the dual pathogenesis of fasting hyperglycemia and hypertriglyceridemia in insulin resistant subjects with obesity and/or type 2 diabetes (1,2,5,6,27,34). Our present data, together with previous findings, suggest that the FoxO1 feedback loop may serve as a safeguarding mechanism for keeping FoxO1 activity in check to avert hepatic glycogen depletion and ER stress.
Supplementary Material
Acknowledgments
We thank Dr. Domenico Accili (Columbia University, New York, NY) for providing FoxO1 vectors.
Footnotes
This work was supported in part by American Diabetes Association and National Institutes of Health (NIH) Grant DK066301. S.C.S. and R.G. were supported by the NIH Contract N01-DK-7-0004/HHSN26700700004C for the Liver Tissue Cell Distribution System at the University of Pittsburgh School of Medicine.
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 25, 2010
Abbreviations: ATF, Activating transcription factor; ChIP, chromatin immunoprecipitation; CHOP, CCAAT-enhancer-binding protein homology protein; ER, endoplasmic reticulum; FFA, free fatty acid; FoxO1, forkhead box O1; GRP78, glucose-regulated protein 78; IR, insulin receptor; IRS, IR substrate; IRE, insulin responsive element; IRE1, inositol requiring 1; MOI, multiplicity of infection; nt, nucleotide; PBA, 4-phenyl butyric acid; PERK, protein kinase R-like ER kinase; pfu, plaque-forming unit; siRNA, small interfering RNA; UPR, unfolded protein response; VLDL, very low-density lipoprotein.
References
- Accili D, Arden KC 2004 FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117:421–426 [DOI] [PubMed] [Google Scholar]
- Barthel A, Schmoll D, Unterman TG 2005 FoxO proteins in insulin action and metabolism. Trends Endocrin Met 16:183–189 [DOI] [PubMed] [Google Scholar]
- Lee SS, Kennedy S, Tolonen AC, Ruvkun G 2003 DAF-16 target genes that control C. elegans life-span and metabolism. Science 300:644–647 [DOI] [PubMed] [Google Scholar]
- Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M 2004 Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 429:562–566 [DOI] [PubMed] [Google Scholar]
- Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, White MF 2008 Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab 8:65–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamagate A, Dong HH 2008 FoxO1 integrates insulin signaling to VLDL production. Cell Cycle 7:3162–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs 3rd WH, Meisenhelder J, Hunter T, Cavenee WK, Arden KC 1999 Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA 96:7421–7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rena G, Prescott AR, Guo S, Cohen P, Unterman TG 2001 Roles of the forkhead in rhabdomyosarcoma (FKHR) phosphorylation sites in regulating 14-3-3 binding, transactivation and nuclear targetting. Biochem J 354:605–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae J, Park BC, Accili D 1999 Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a wortmannin-sensitive pathway. J Biol Chem 274:15982–15985 [DOI] [PubMed] [Google Scholar]
- Nakae J, Barr V, Accili D 2000 Differential regulation of gene expression by insulin and IGF-1 receptors correlates with phosphorylation of a single amino acid residue in the forkhead transcription factor FKHR. EMBO J 19:989–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham SK, Suwanichkul A, Scheimann AO, Yee D, Jackson JG, Barr FG, Powell DR 1999 FKHR binds the insulin response element in the insulin-like growth factor binding protein-1 promoter. Endocrinology 140:3140–3146 [DOI] [PubMed] [Google Scholar]
- Schmoll D, Walker KS, Alessi DR, Grempler R, Burchell A, Guo S, Walther R, Unterman TG 2000 Regulation of glucose-6-phosphatase gene expression by protein kinase B α and the forkhead transcription factor FKHR. J Biol Chem 36324–36333 [DOI] [PubMed] [Google Scholar]
- Zhang X, Gan L, Pan H, Guo S, He X, Olson ST, Mesecar A, Adam S, Unterman TG 2002 Phosphorylation of serine 256 suppresses transactivation by FKHR (FOXO1) by multiple mechanisms. Direct and indirect effects on nuclear/cytoplasmic shuttling and DNA binding. J Biol Chem 277:45276–45284 [DOI] [PubMed] [Google Scholar]
- Van Der Heide LP, Hoekman MF, Smidt MP 2004 The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J 380:297–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heide LP, Jacobs FM, Burbach JP, Hoekman MF, Smidt MP 2005 FoxO6 transcriptional activity is regulated by Thr26 and Ser184, independent of nucleo-cytoplasmic shuttling. Biochem J 391:623–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rena G, Guo S, Cichy SC, Unterman TG, Cohen P 1999 Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J Biol Chem 274:17179–17183 [DOI] [PubMed] [Google Scholar]
- Qu S, Altomonte J, Perdomo G, He J, Fan Y, Kamagate A, Meseck M, Dong HH 2006 Aberrant forkhead box O1 function is associated with impaired hepatic metabolism. Endocrinology 147:5641–5652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Gan L, Pan H, Kan D, Majeski M, Adam SA, Unterman TG 2004 Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO1: characterization of phosphorylation- and 14-3-3-dependent and -independent mechanisms. Biochem J 378:839–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae J, Kitamura T, Silver DL, Accili D 2001 The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest 108:1359–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altomonte J, Richter A, Harbaran S, Suriawinata J, Nakae J, Thung SN, Meseck M, Accili D, Dong H 2003 Inhibition of Foxo1 function is associated with improved fasting glycemia in diabetic mice. Am J Physiol 285:E718–E728 [DOI] [PubMed] [Google Scholar]
- Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matika R, Xiao X, Franks R, Heidenreich KA, Sajan MP, Farese RV, Stolz DB, Tso P, Koo SH, Montminy M, Unterman TG 2006 FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem 281:10105–10117 [DOI] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM 2003 Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature 423:550–555 [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D 2007 Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab 6:208–216 [DOI] [PubMed] [Google Scholar]
- Altomonte J, Cong L, Harbaran S, Richter A, Xu J, Meseck M, Dong HH 2004 Foxo1 mediates insulin action on ApoC-III and triglyceride metabolism. J Clin Invest 114:1493–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamagate A, Qu S, Perdomo G, Su D, Kim DH, Slusher S, Meseck M, Dong HH 2008 FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest 118:2347–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Han S, Kitamura T, Accili D 2006 Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest 116:2464–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks DJ, Dong HH 2009 FoxO1 and hepatic lipid metabolism. Curr Opin Lipidol 20:217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks JD, Sparks CE 2008 Overindulgence and metabolic syndrome: is FoxO1 a missing link? J Clin Invest 118:2012–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig O, Tjian R 2005 Transcriptional feedback control of insulin receptor by dFOXO/FOXO1. Gene Dev 19:2435–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig O, Tjian R 2006 Nutrient availability and growth: regulation of insulin signaling by dFOXO/FOXO1. Cell Cycle 5:503–505 [DOI] [PubMed] [Google Scholar]
- van der Crabben SN, Allick G, Ackermans MT, Endert E, Romijn JA, Sauerwein HP 2008 Prolonged fasting induces peripheral insulin resistance, which is not ameliorated by high-dose salicylate. J Clin Endocrinol Metab 93:638–641 [DOI] [PubMed] [Google Scholar]
- Heijboer AC, Donga E, Voshol PJ, Dang ZC, Havekes LM, Romijn JA, Corssmit EP 2005 Sixteen hours of fasting differentially affects hepatic and muscle insulin sensitivity in mice. J Lipid Res 46:582–588 [DOI] [PubMed] [Google Scholar]
- Qu S, Su D, Altomonte J, Kamagate A, He J, Perdomo G, Tse T, Jiang Y, Dong HH 2007 PPARα mediates the hypolipidemic action of fibrates by antagonizing FoxO1. Am J Physiol 292:E421–E434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Alemán JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, Cohen DE, King GL, Ginsberg HN, Kahn CR 2008 Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab 7:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS 2010 Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140:900–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bánhegyi G, Baumeister P, Benedetti A, Dong D, Fu Y, Lee AS, Li J, Mao C, Margittai E, Ni M, Paschen W, Piccirella S, Senesi S, Sitia R, Wang M, Yang W 2007 Endoplasmic reticulum stress. Ann NY Acad Sci 1113:58–71 [DOI] [PubMed] [Google Scholar]
- Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferré P, Foufelle F 2009 GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest 119:1201–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS 2006 Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313:1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota T, Gayet C, Ginsberg HN 2008 Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest 118:316–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W, Kohen-Avramoglu R, Mhapsekar S, Tsai J, Austin RC, Adeli K 2005 Glucosamine-induced endoplasmic reticulum stress promotes ApoB100 degradation: evidence for Grp78-mediated targeting to proteasomal degradation. Arterioscl Throm Vas 25:571–577 [DOI] [PubMed] [Google Scholar]
- Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, Klein S 2009 Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 58:693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werstuck GH, Lentz SR, Dayal S, Hossain GS, Sood SK, Shi YY, Zhou J, Maeda N, Krisans SK, Malinow MR, Austin RC 2001 Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J Clin Invest 107:1263–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R, Emi M, Tanabe K, Murakami S 2006 Role of the unfolded protein response in cell death. Apoptosis 11:5–13 [DOI] [PubMed] [Google Scholar]
- Rao RV, Hermel E, Castro-Obregon S, del Rio G, Ellerby LM, Ellerby HM, Bredesen DE 2001 Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. J Biol Chem 276:33869–33874 [DOI] [PubMed] [Google Scholar]
- Rao RV, Poksay KS, Castro-Obregon S, Schilling B, Row RH, del Rio G, Gibson BW, Ellerby HM, Bredesen DE 2004 Molecular components of a cell death pathway activated by endoplasmic reticulum stress. J Biol Chem 279:177–187 [DOI] [PubMed] [Google Scholar]
- Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ 2007 Endoplasmic reticulum stress contributes to β cell apoptosis in type 2 diabetes. Diabetologia 50:752–763 [DOI] [PubMed] [Google Scholar]
- Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, Kitakaze M 2004 Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation 110:705–712 [DOI] [PubMed] [Google Scholar]
- Winnay JN, Boucher J, Mori MA, Ueki K, Kahn CR 2010 A regulatory subunit of phosphoinositide 3-kinase increases the nuclear accumulation of X-box-binding protein-1 to modulate the unfolded protein response. Nat Med 16:438–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Walter P 2007 Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–529 [DOI] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS 2004 Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306:457–461 [DOI] [PubMed] [Google Scholar]
- Schenk S, Saberi M, Olefsky JM 2008 Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 118:2992–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Yang R, Tarr PT, Wu PH, Handschin C, Li S, Yang W, Pei L, Uldry M, Tontonoz P, Newgard CB, Spiegelman BM 2005 Hyperlipidemic effects of dietary saturated fats mediated through PGC-1β coactivation of SREBP. Cell 120:261–273 [DOI] [PubMed] [Google Scholar]
- Sinha S, Perdomo G, Brown NF, O'Doherty RM 2004 Fatty acid-induced insulin resistance in L6 myotubes is prevented by inhibition of activation and nuclear localization of nuclear factor κB. J Biol Chem 279:41294–41301 [DOI] [PubMed] [Google Scholar]
- Wei Y, Wang D, Topczewski F, Pagliassotti MJ 2006 Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol 291:E275–E281 [DOI] [PubMed] [Google Scholar]
- Wang D, Wei Y, Pagliassotti MJ 2006 Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology 147:943–951 [DOI] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drøbak BK, Hanley MR, Dawson AP 1990 Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci USA 87:2466–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, Jung DY, Jun JY, Li J, Luo S, Ko HJ, Kim JK, Lee AS 2010 Grp78 heterozygosity promotes adaptive unfolded protein response and attenuates diet-induced obesity and insulin resistance. Diabetes 59:6–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, Ozawa K, Ogawa S, Hori M, Yamasaki Y, Matsuhisa M 2005 Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem 280:847–851 [DOI] [PubMed] [Google Scholar]
- Morand JP, Macri J, Adeli K 2005 Proteomic profiling of hepatic endoplasmic reticulum-associated proteins in an animal model of insulin resistance and metabolic dyslipidemia. J Biol Chem 280:17626–17633 [DOI] [PubMed] [Google Scholar]
- Zhang L, Perdomo G, Kim DH, Qu S, Ringquist S, Trucco M, Dong HH 2008 Proteomic analysis of fructose-induced fatty liver in hamsters. Metabolism 57:1115–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D 2000 Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2:326–332 [DOI] [PubMed] [Google Scholar]
- Oyadomari S, Yun C, Fisher EA, Kreglinger N, Kreibich G, Oyadomari M, Harding HP, Goodman AG, Harant H, Garrison JL, Taunton J, Katze MG, Ron D 2006 Cotranslocational degradation protects the stressed endoplasmic reticulum from protein overload. Cell 126:727–739 [DOI] [PubMed] [Google Scholar]
- Merksamer PI, Trusina A, Papa FR 2008 Real-time redox measurements during endoplasmic reticulum stress reveal interlinked protein folding functions. Cell 135:933–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciani DS, Gwiazda KS, Yang TL, Kalynyak TB, Bychkivska Y, Frey MH, Jeffrey KD, Sampaio AV, Underhill TM, Johnson JD 2009 Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and β-cell death. Diabetes 58:422–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage AT, Walter LA, Shi Y, Khan MI, Kaneto H, Capretta A, Werstuck GH 2010 Hexosamine biosynthesis pathway flux promotes endoplasmic reticulum stress, lipid accumulation, and inflammatory gene expression in hepatic cells. Am J Physiol 298:E499–E511 [DOI] [PubMed] [Google Scholar]
- Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ 2008 Chop deletion reduces oxidative stress, improves β cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest 118:3378–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS 2007 Endoplasmic reticulum stress and inflammation in obesity and type 2 diabetes. Novartis Found Symp 286:86–94 [DOI] [PubMed] [Google Scholar]
- Scheuner D, Kaufman RJ 2008 The unfolded protein response: a pathway that links insulin demand with β-cell failure and diabetes. Endocr Rev 29:317–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.