Abstract
To determine whether signaling through TNF and/or nuclear factor-κB contributes to bacterial lipopolysaccharide (LPS)-induced activation of type 2 iodothyronine deiodinase (D2) in tanycytes lining the floor and infralateral walls of the third ventricle, the effect of a TNF antagonist on D2 gene expression and LPS-induced Iκ-Bα expression in tanycytes were studied. Animals treated with soluble, rat, polyethylene glycol-conjugated TNF receptor type 1 (4 mg/kg body weight) before a single ip injection of LPS showed a significant reduction in circulating IL-6 levels but no effect on LPS-induced D2 mRNA in the majority of tanycytes with the exception of a subpopulation of α tanycytes in the wall of the third ventricle. LPS induced a rapid increase in Iκ-Bα mRNA in the pars tuberalis and a delayed response in α tanycytes but absent in all other tanycyte subsets. The LPS-induced increase in Iκ-Bα in the pars tuberalis was associated with increased TSHβ gene expression in this tissue, but cAMP response element-binding protein (CREB) phosphorylation was observed only in a subset of α tanycytes. These data suggest that TNF and nuclear factor-κB signaling are not the primary, initiating mechanisms mediating the LPS-induced D2 response in tanycytes, but may contribute in part to sustaining the LPS-induced D2 response in a subset of α tanycytes. We hypothesize that in addition to TSH, other factors derived from the pars tuberalis may contribute to LPS-induced D2 activation in tanycytes.
D2 expressed in tanycytes is highly regulated in response to bacterial LPS and may contribute to central hypothyroidism associated with infection.
Tanycytes are specialized, bipolar, ependymal cells lining the inferolateral borders (α tanycytes) and the floor (β tanycytes) of the third ventricle in the mediobasal hypothalamus (MBH) and becoming increasingly recognized to have important roles in neuroendocrine regulation (1,2). Recent studies in rats, mice, and rabbits have raised the possibility that endotoxin-induced up-regulation of type 2 iodothyronine deiodinase (D2) in tanycytes contributes to central hypothyroidism associated with infection (3,4), commonly referred to as the nonthyroidal illness syndrome (5). Because D2 is the major enzyme in the brain responsible for converting T4 to its more potent, biologically active metabolite, T3 (6), it is hypothesized that the endotoxin-induced increase in tanycyte D2 activity may cause tissue-specific thyrotoxicosis in the MBH by increasing the conversion of T4 to T3, ultimately leading to direct suppression of hypophysiotropic TRH neurons in the hypothalamic paraventricular nucleus (7,8).
D2 is expressed in tanycytes in all animal species studied thus far, including man (7,8), suggesting an important homeostatic function. Being at the interface of the cerebrospinal fluid (CSF) by nature of its location in the third ventricle, and the vascular system through long cytoplasmic projections that contact portal vessels and envelop blood vessels in the hypothalamus (1), tanycytes are in strategic position to extract T4 from the bloodstream or the CSF, convert T4 to T3, and then release T3 into the hypothalamus or back into the CSF to affect hypothalamic function (2,8,9,10). Evidence that changes in tanycyte D2 regulate hypothalamic tissue levels of T3 or affect hypophysiotropic TRH neurons have been shown in birds (11) and rats (12), respectively.
The precise mechanism(s) by which endotoxin increases tanycyte D2 activity remain unknown. The approximately 4-fold increase in tanycyte D2 mRNA and activity induced by bacterial lipopolysaccharide (LPS) is independent of the associated fall in circulating thyroid hormone levels (3), as well as the LPS-induced rise in corticosterone (13). Because in vitro studies have demonstrated that the nuclear factor-κB (NF-κB) second messenger system has a marked stimulatory effect on the activity of dio2 promoter through a single NF-κB binding site (14), LPS increases the TNF type 1 receptor (TNF-R1 or p55) in the MBH (15), and TNF-R1 activates NF-κB (16), we hypothesized that TNF may have an essential role in the LPS-induced activation of the dio2 gene in tanycytes. Accordingly, we studied the effect of LPS on tanycyte D2 activation in animals pretreated with soluble, rat, polyethylene glycol-conjugated TNF receptor type 1 (sTNF-R1), a TNF antagonist that binds TNF preventing it from interacting with cell surface receptors, and on Iκ-Bα gene expression, the latter as an index of NF-κB activation (15). We demonstrate that with the exception of a small, subset of tanycytes (α tanycytes) in the third ventricular wall, LPS-induced activation of D2 in most tanycytes is not mediated by TNF or NF-κB signaling. We further demonstrate that LPS markedly activates NF-κB signaling in the pars tuberalis. Because TSH secretion from the pars tuberalis is responsible for photoperiodic activation of tanycyte D2 in birds (17), we determined whether TSH contributes to indirect effects of cytokine signaling through the pars tuberalis on D2 activation in tanycytes.
Materials and Methods
Animals
Experiments were carried out on adult, intact male Sprague Dawley male rats (Taconic Farms, Germantown, NY), weighing 200–250 g. Animals were acclimatized to standard environmental conditions for at least 5 d (light between 0600 and 1800 h, temperature 22 ± 1 C) and given rat chow and tap water ad libitum. All experimental protocols were reviewed and approved by the Animal Welfare Committee at Tufts Medical Center and Tufts University School of Medicine.
Experiment 1: effect of LPS administration on D2 mRNA content in the MBH in animals pretreated with sTNF-R1
Animals were divided into three groups (n = 7–8 animals per group). The first two groups received a single ip injection of either LPS [250 μg/100 g body weight (BW), Sigma 0127:B8 dissolved in sterile saline; Sigma Chemical Co., St. Louis, MO] or an equivalent volume of sterile saline alone and then killed 9 h later, the time at which the increase in D2 mRNA peaks in tanycytes after LPS administration (13). The third group was first treated with sTNF-R1 (gift of Amgen, Inc., Thousand Oaks, CA) by ip injection (4 mg/kg BW), followed 120 min later by the LPS challenge as above. In preliminary experiments (n = 10), no effect of sTNF-R1 was observed on basal D2 mRNA levels in the MBH by quantitative in situ hybridization histochemistry compared with vehicle-treated controls [sTNF-R1 vs. control (integrated density units): 212 ± 2 vs. 230 ± 5 (P > 0.77)].
Experiment 2: effect of LPS on Iκ-Bα gene expression in the MBH
Animals were administered with a single ip injection of LPS (250 μg/100 g BW) or vehicle and killed at 3, 9, or 12 h later (n = 3 per group). Control animals (n = 5) were treated with an equal volume of vehicle ip (200 μl) and killed 3 and 12 h after injection.
Experiment 3: effect of LPS on TSHβ gene expression in the pars tuberalis and phospho cAMP response element-binding protein (CREB) immunoreactivity in the MBH
For studies on TSHβ gene expression in the pars tuberalis, animals were divided into two groups (n = 5 each). The control group received an ip injection of sterile saline, whereas the experimental group received an equal volume of bacterial LPS (250 μg/100 g BW, ip, in sterile saline). Both groups were killed 9 h later. For studies on phosphoCREB activation, animals were divided into five groups (n = 5 each). The control group received an ip injection of sterile saline, whereas the experimental groups were treated with LPS as above but killed at 2, 4, 6, and 9 h after LPS administration. The more frequent time intervals were chosen to assure immunocytochemical identification of phosphoCREB due to its transient nature after CREB activation. Animals were killed by perfusion fixation between 1500 and 1700 h.
Tissue processing
All animals were overdosed with pentobarbital (50 mg/kg; Ovation Pharmaceuticals, Inc., Deerfield, IL), blood obtained from the inferior vena cava, and then the animals perfused transcardially with 20 ml 0.01 m PBS (pH 7.4), containing 15,000 U/liter heparin sulfate, followed by 150 ml 4% paraformaldehyde in PBS. The brains were removed and postfixed by immersion in the same fixative for 2 h at room temperature. Tissue blocks containing the hypothalamus were cryoprotected in 25% sucrose/PBS at 4 C overnight, then snap frozen on dry ice. Serial 18-μm-thick coronal sections through the rostro-caudal extent of the median eminence were cut on a cryostat (Leica CM3050 S; Leica Microsystems, Nussloch GmbH, Germany) and either adhered to Superfrost/Plus glass slides (Fisher Scientific Co., Pittsburgh, PA) to obtain four sets of slides, each set containing every fourth section through the median eminence, or collected free floating in PBS. The slide-adhered tissue sections were desiccated overnight at 42 C and stored at −80 C until prepared for in situ hybridization histochemistry. Free-floating tissue sections were kept at 4 C until processed for double-labeling immunofluorescence.
IL-6 determination
Blood was collected in vacuum collection tubes (BD Vacutainer reference no. 367988; BD, Franklin Lakes, NJ) and then centrifuged at 3000 rpm for 15 min. Serum aliquots were stored at −80 C in polypropylene tubes until assayed and then thawed at room temperature and diluted 1:1 with RD5-16 calibrator diluent (rat ELISA system kit; Quantikine, CAT R6000B; R&D Systems, Inc., Minneapolis, MN). A standard curve run at the same time was created by diluting known amounts of rat IL-6 standard into RD5-16 calibrator diluent. The ELISA was performed following the manufacturer’s instructions, and optical densities were determined using a microplate reader set to 450 nm. Corrections of wavelength readings were accomplished by subtracting readings at 540 nm from the readings at 450 nm.
In situ hybridization histochemistry
In situ hybridization histochemistry was performed on every fourth section of the median eminence using an 800-bp single stranded [35S]-uridine 5′-triphosphate (UTP)-labeled cRNA probe complementary to the entire coding region of the rat D2 mRNA, a 1.114-kb single stranded [35S]-UTP labeled RNA probe complementary to the full-length coding sequence of the mouse Iκ-Bα mRNA (kindly provided by Serge Rivest) (15), or a 505-bp single stranded [35S]-UTP-labeled cRNA probe complementary to nucleotides 6 to 510 of the rat TSHβ mRNA (NM 013116, forward primer 5′-CCGAAGGGTATAAAATGAACAGAG-3′; reverse primer 5′-ACCAGATTGCATTGCCATTACAGT-3′). Hybridization was performed as previously reported (4) under plastic coverslips in a buffer containing 50% formamide, a 2-fold concentration of standard sodium citrate, 10% dextran sulfate, 0.5% sodium dodecyl sulfate, 250 μg/ml denatured salmon sperm DNA, and 5 × 105 cpm radiolabeled probe for 16 h at 56 C. Slides were dipped into Kodak NBT autoradiography emulsion diluted 1:1 in distilled water (Eastman Kodak, Rochester, NY), and the autoradiograms developed after 7 d of exposure for D2, 5 d for Iκ-Bα, and 1 d for TSHβ at 4 C. Specificity of the hybridization reactions were confirmed using sense probes, which resulted in the total absence of specific hybridization signal.
Double-labeling immunofluorescence
After washing in PBS, free-floating sections were pretreated with 0.5% H2O2 in PBS for 15 min to remove endogenous peroxidase activity, followed by 0.5% Triton X-100 in PBS for 20 min to improve the antibody penetration. The sections were then preincubated in 10% normal horse serum for 30 min, followed by incubation in a mixture of mouse monoclonal vimentin antiserum (catalog no. MAB3400, 1:5000; Chemicon, Bedford, MA) and antirabbit phosphoCREB-128-141 (1:10,000; gift of Marc R. Montminy, Salk Institute, La Jolla, CA) in 1% normal horse serum in PBS containing 0.08% sodium azide and 0.2% Kodak PhotoFlo (Eastman Kodak Co.) for 3 d at 4 C with continuous agitation on a rotary shaker. After washing in PBS, sections were incubated in biotinylated, goat, antirabbit IgG (1:400; Vector Laboratories, Burlingame, CA) for 2 h, immersed in avidin-biotin-peroxidase complex (Vector Elite kit, 1:1000; Vector Laboratories) for 2 h at room temperature, and the phosphoCREB signal was amplified using the tyramide signal amplification (TSA) kit according to the manufacturer’s instructions (NEN Life Science Products, Boston, MA). Sections were then incubated in a mixture of dichlorotriazinylamino-fluorescein (DTAF)-streptavidin (1:300; Jackson ImmunoResearch, West Grove, PA) and Cy3-conjugated donkey antimouse IgG (1:200; Jackson ImmunoResearch) overnight, mounted on Superfrost/Plus slides, and coverslipped with Vectashield mounting medium (Vector Laboratories). Specificity of the phosphoCREB and vimentin antiserum has been reported previously (18,19).
Image analysis
Autoradiograms were visualized with a Zeiss Axioplan 2 imaging microscope (Carl Zeiss Microimaging, Inc., Thornwood, NY) under dark-field illumination using a COHU 4912 video camera (COHU, Inc., San Diego, CA), and the images were analyzed with a Macintosh G4 computer using Scion Image software (National Institutes of Health, Bethesda, MD). For analysis of D2 mRNA, background density points were removed by thresholding the image, and integrated density values (density × area) of the MBH were measured in five rostro-caudal sequential sections through the median eminence for each animal extending from approximately −2.56 mm to −3.14 mm from the bregma based on the stereotaxic coordinates of Paxinos and Watson (20). For analysis of TSHβ mRNA, the means of integrated density values obtained from six clusters of TSHβ-expressing cells in anterior, mid, and caudal sections of the pars tuberalis were determined for each animal, and then the means of the integrated density values for each animal group were determined. Nonlinearity of radioactivity in the emulsion was evaluated by comparing density values with a calibration curve created from autoradiograms of known dilutions of the radiolabeled probes, immobilized on glass slides in 1.5% gelatin, fixed with 4% paraformaldehyde, and exposed and developed simultaneously with the in situ hybridization autoradiograms.
For double-labeling immunofluorescence, tissue sections were observed under Zeiss Axioplan 2 epifluorescence microscope using a dual filter set for DTAF and Cy3 (DTAF, excitation 490–505 nm, bandpass 510 nm, and emission 515–545 nm; Cy3, excitation 540–590 nm, bandpass 595 nm, and emission 600–660 nm). Images were captured using a Spot digital camera (Diagnostic Instruments, Sterling Heights, MI), double exposed while switching filter sets for each fluorochrome, and superimposed in Adobe Photoshop CS using a Macintosh G4 computer to create a composite image of the same field (Adobe, San Jose, CA).
Statistical analysis
Results are presented as mean ± sem. IL-6 measurements and in situ hybridization analyses for D2 mRNA were compared with one-way ANOVA followed by Newman-Keuls post hoc test using Prism 4 software (GraphPad Software, Inc., San Diego, CA). Hybridization analysis for TSHβ mRNA was performed using Student’s unpaired t test. In all analyses, a P value of less than 0.05 was considered statistically significant.
Results
Effect of LPS administration on immune activation
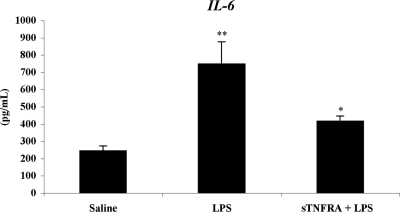
LPS administration induced a significant increase in serum IL-6 levels, rising approximately 3-fold compared with the values of vehicle-treated control animals [vehicle vs. 9 h LPS (pg/ml): 249 ± 24 vs. 752 ± 124, P < 0.01]. In contrast, when animals were pretreated with a single dose of sTNF-R1 before the administration of LPS, the rise in circulating IL-6 was significantly less than in control animals treated with LPS (9 h LPS vs. sTNF-R1: 752 ± 124 vs. 420 ± 26 pg/ml, P < 0.05) (Fig. 1). No significant difference was found between vehicle- and sTNF-R1/LPS-treated animals (P > 0.05).
Figure 1.
Effect of sTNF-R1 administration on IL-6 levels in LPS treated animals. Data represent the mean ± sem of six to eight animals. *, P < 0.05; **, P < 0.01.
D2 mRNA response to LPS in the MBH
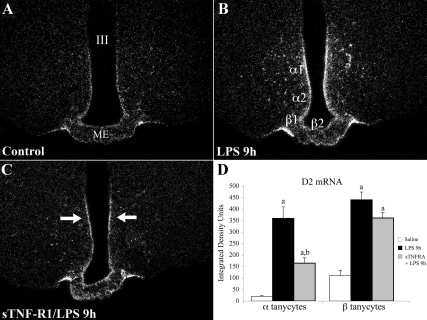
Silver grains representing D2 mRNA were distributed throughout the rostral-caudal extent of the median eminence in all tanycyte subpopulations, including α (α1, 2) tanycytes lining the inferolateral walls of the third ventricle and β (β1, 2) tanycytes lining the tuberoinfundibular sulci and floor of the third ventricle (Fig. 2A). D2 mRNA could also be seen in tanycyte cytoplasmic processes extending through the substance of the median eminence. LPS administration led to a marked increase in the density of silver grains accumulating along the walls and floor of the third ventricle, corresponding to the location of both α and β tanycytes (Fig. 2, A and B). Pretreatment with sTNF-R1 before the administration of LPS appeared to reduce the LPS-induced D2 mRNA response in α tanycytes, particularly in the region where α1 tanycytes reside, but there was no apparent reduction in β tanycytes (Fig. 2C). Densitometric analysis of the silver grain accumulation over α and β tanycytes confirmed a significant effect of LPS on D2 mRNA in both α and β tanycytes (Fig. 2D). Pretreatment with sTNF-R1 had no effect on the LPS-induced rise in D2 mRNA in β tanycytes. In addition, no significant differences were observed in D2 expression in animals pretreated with saline or sTNF-R1 before LPS administration [β tanycytes, control vs. LPS vs. sTNF-R1/LPS (integrated density units): 111 ± 22 vs. 440 ± 34 vs. 362 ± 23 (control vs. LPS, P < 0.001; control vs. sTNF-R1, P < 0.001; and LPS vs. sTNF-R1/LPS, P > 0.05)]. In contrast, although LPS significantly increased D2 mRNA in α tanycytes, the D2 increase was significantly less in animals pretreated with sTNF-R1 in comparison with saline pretreated controls [α tanycytes, control vs. LPS vs. sTNF-R1/LPS (integrated density units): 20 ± 4 vs. 359 ± 50 vs. 165 ± 24 (control vs. LPS, P < 0.001; control vs. sTNF-R1/LPS, P < 0.01; and LPS vs. sTNF-R1/LPS, P < 0.01)].
Figure 2.
Dark-field illumination photomicrographs of D2 mRNA in the MBH of animals receiving (A) saline (control), (B) 250 μg/100 g BW LPS, and (C) soluble TNF-R1 (sTNF-R1; 4 mg/kg BW) 2 h before of LPS administration. Note the increased expression of D2 mRNA in all tanycyte subpopulations (α and β) in the MBH after LPS administration (B) and a selective decrease on the lateral walls of the third ventricle in animals when sTNF-R1 was administered (arrows in C). D, Graph represents the densitometric analyses of animals in the saline, LPS, and TNF-R1-treated groups. a, Significantly different of saline, P < 0.001; b, significantly different of LPS, P < 0.001. III, Third ventricle; ME, median eminence; α1, α2, β1, and β2, subpopulations of tanycytes.
Effect of LPS on Iκ-Bα mRNA expression
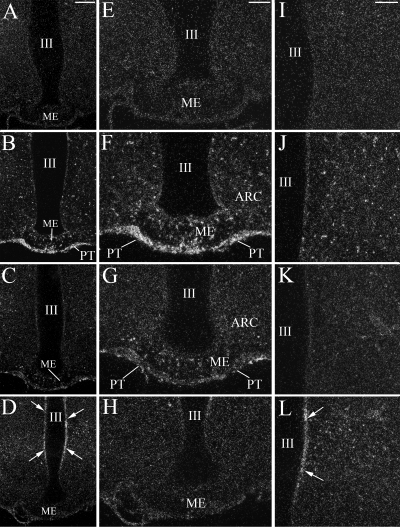
In saline-treated control animals, no apparent hybridization for Iκ-Bα mRNA was seen in the MBH or adjacent pars tuberalis (Fig. 3, A and E) and α or β tanycytes (Fig. 3, A, E, and I). In contrast, 3 h after the administration of LPS, a strong hybridization signal was present in the pars tuberalis with lesser signal intensity in isolated cells of the hypothalamic arcuate nucleus (Fig. 3, B and F). No hybridization signal was observed in either α or β tanycytes (Fig. 3, B and J). By 9 h after LPS treatment, Iκ-Bα mRNA hybridization signal in the pars tuberalis had diminished, and by 12 h, no hybridization signal was detectable in this region (Fig. 3, C, D, G, and H). No hybridization signal was observed in α or β tanycytes (Fig. 3, C and K) 9 h after LPS. Twelve hours after LPS treatment, however, Iκ-Bα mRNA appeared in the walls of the third ventricle, corresponding to the location of α tanycytes, primarily α1 tanycytes, but absent from β tanycytes (Fig. 3, D and L).
Figure 3.
Dark-field illumination photomicrographs of Iκ-Bα mRNA expression in the MBH of animals receiving (A, E, and I) saline or LPS for 3 h (B, F, and J), 9 h (C, G, and K), and 12 h (D, H, and L). Low-magnification photomicrographs are shown in first column, and high-magnification photomicrographs of the median eminence and wall of the third ventricle are shown in the adjacent columns. Note marked accumulation of silver grains over the pars tuberalis (PT) (B and F) 3 h after the administration of LPS. Iκ-Bα mRNA is only seen in a subset of tanycytes 12 h after LPS administration (arrows in D and L). III, Third ventricle; ME, median eminence; ARC, arcuate nucleus. Scale bar, 200 μm in A–D; 100 μm in E–H; and 50 μm in I–L.
Effect of LPS on TSHβ gene expression in the pars tuberalis
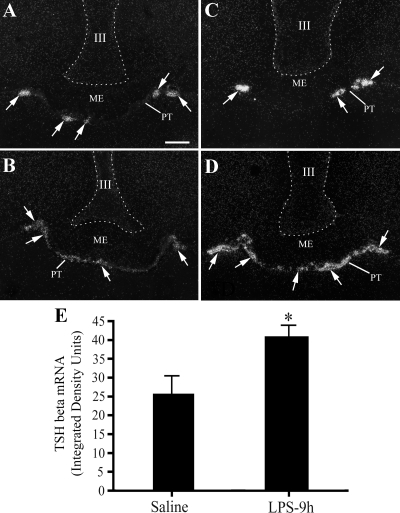
In view of the marked increase in Ικ-Βα mRNA in the pars tuberalis after LPS and recent evidence that TSHβ derived from the pars tuberalis is involved in photoperiod-induced D2 activation in the MBH in birds (17) and mice (21), we determined whether LPS also increases TSHβ mRNA in the pars tuberalis. Silver grains representing TSHβ mRNA were readily apparent in control tissue sections accumulating in clusters of cells in anterior and midportions of the pars tuberalis directly underlying the median eminence (Fig. 4A) and then becoming more confluent at its most caudal portions (Fig. 4B). After the administration of LPS, increased expression of TSHβ mRNA was apparent throughout the rostral-caudal extent of the pars tuberalis (Fig. 4, C and D). Densitometric analysis of the silver grain accumulation over clusters of TSHβ-expressing cells revealed a significant, near doubling of density values compared with saline-treated controls [control vs. LPS (integrated density units): 25.5 ± 5.0 vs. 40.7 ± 3.3; P < 0.05] (Fig. 4E).
Figure 4.
Dark-field illumination photomicrographs of TSHβ mRNA (arrows) in the pars tuberalis (PT) of animals receiving (A and B) saline (control) and (C and D) 250 μg/kg BW LPS. A and C correspond to a midrostral region of the PT, and B and D to a more caudal region. Note increased expression of TSHβ mRNA in the PT after LPS administration. E, Graph represents the densitometric analyses of control and 9 h LPS-treated animal groups. Ventricular borders are demarcated with dotted lines. *, P < 0.05. III, Third ventricle; ME, median eminence. Scale bar, 200 μm.
Effect of LPS on CREB phosphorylation in tanycytes
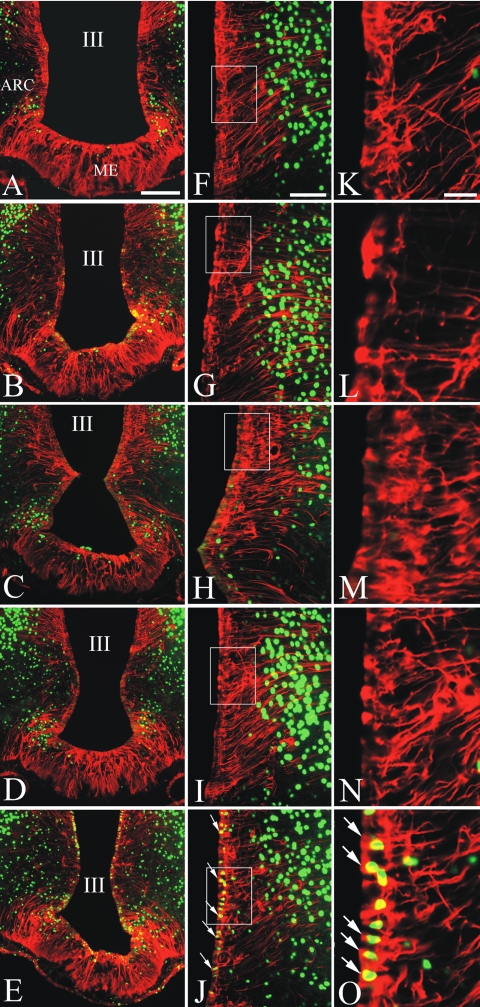
Because TSHβ is believed to signal to D2 by the activation of the cAMP-CREB second messenger system (17), we sought evidence for the activation of tanycytes by TSHβ by determining whether the phosphorylated form of CREB appears in the nucleus of tanycytes after the administration of LPS using double-labeling immunofluorescence. Tanycytes were clearly identified by vimentin immunofluorescence, filling both the cell body in the floor and infralateral walls of the third ventricle and in their cytoplasmic processes, extending into the substance of the arcuate and ventromedial nuclei and median eminence (Fig. 5). In saline-treated control animals, no phosphoCREB immunoreactivity was observed in the nucleus of any tanycyte subpopulation but was present in neurons in the adjacent arcuate nucleus (Fig. 5, A, F, and K). In addition, no phosphoCREB immunoreactivity was observed in the nucleus of either α or β tanycytes between 2 and 6 h after the administration of LPS (Fig. 5, B–D, G–I, and L–N). Nine hours after LPS administration, however, phosphoCREB immunolabeling was present in the nucleus of α1 and some α2 tanycytes, but conspicuously absent from β tanycytes (Fig. 5, E, J, and O).
Figure 5.
Immunofluorescence photomicrographs of the MBH showing the effect of LPS administration on phosphoCREB expression (green) in tanycytes (red) lining the floor and infralateral walls of the third ventricle (III). Control (A, F, and K), 2 h (B, G, and L), 4 h (C, H, and M), 6 h (D, I, and N), and 9 h (E, J, and O) after LPS administration. F–O, High magnification of the infralateral wall of the third ventricle corresponding to the left panels. PhosphoCREB expression primarily in α1 tanycytes (arrows) is seen only at 9 h after LPS administration. ME, Median eminence; ARC, arcuate nucleus. Scale bar, 200 μm in A–E; 100 μm in F–J; and 50 μm in K–O.
Discussion
These studies confirm our previous observations that endotoxin has a rapid and fairly uniform effect to increase D2 mRNA in tanycytes lining the floor and infralateral walls of the third ventricle (13) but demonstrate that TNF and signaling through NF-κB contributes to D2 activation in only a small subset of these cells. Support for the hypothesis that signaling through TNF and NF-κB might be responsible for the LPS-induced activation of D2 in tanycytes is suggested by the presence of NF-κB response element in the human and rat D2 gene (dio2) (4,14). In addition, in vitro studies demonstrate that coexpression of p65, a required component of the activated NF-κB heterodimer, together with the human dio2 5′-flanking region, leads to an abrupt and significant increase in the transcriptional activity of the hdio2 promoter (14). TNF also increases D2 activity in the anterior pituitary through a NF-κB dependent mechanism (22).
To assess the importance of TNF signaling for D2 activation in tanycytes by LPS in vivo, animals were pretreated with sTNF-R1 before the administration of LPS. This drug acts as a competitive inhibitor for the binding of TNF to cell surface TNF receptors, leading to inhibition of the biologic action of TNF (23). The efficacy of sTNF-R1 to diminish TNF signaling in response to LPS was established by a significant reduction in circulating levels of IL-6 when the administration of LPS was preceded by sTNF-R1 and to levels insignificantly different from control animals that did not receive LPS. Nevertheless, the anticipated D2 activation response in tanycytes in response to LPS was largely unaltered in sTNF-R1-pretreated animals with the exception of a small, discrete subpopulation of α tanycytes in the midportion of the third ventricle wall that showed a partial reduction in the D2 response.
Tanycytes are known to comprise a heterogeneous group of cells and can be divided into at least four different subtypes based on morphological and physiological features (24,25). According to the nomenclature of Akmayev and Popov (25), the subtypes include β1, β2, α1, and α2 tanycytes. β1 And β2 tanycytes line the floor and lateral extensions of the third ventricle and act as barrier cells, creating separate compartments between the CSF, median eminence, and adjacent hypothalamic arcuate nucleus, while simultaneously establishing an anatomical link between the CSF and portal capillary systems (1,26). The β tanycytes have important roles in reproductive function and thyroid hormone homeostasis by regulating the release of GnRH and TRH from axon terminals in the external zone of the median eminence (10,19,27) and generating T3 that may contribute to reproductive function in birds (11) and local feedback effects of thyroid hormone on hypophysiotropic TRH neurons and anterior pituitary thyrotrophs (12,28,29). In contrast, α1 and α2 tanycytes line the walls of the third ventricle and have no barrier properties (24). They are particularly noteworthy for the trajectory of their basal process directly into the hypothalamic ventromedial nucleus (α1) and arcuate nucleus (α2), regions of the brain that are major integrating centers for the regulation of appetite and energy homeostasis, as well as a number of other functions (30,31,32). Given these morphologic features and the ability of tanycytes to transport (33,34) and convert T4 to T3 as a result of their expression of thyroid hormone transporters (29), D2 (35), and D2 regulatory proteins (36), these cells may have a critical role in establishing normal hypothalamic levels of thyroid hormone. Indeed, in the Japanese quail, light induction of D2 in tanycytes is associated with a 10-fold increase in T3 content in the MBH (11). In addition, recent evidence suggests that the release of T3 from α tanycytes during fasting up-regulates uncoupling protein 2 in arcuate nucleus neurons, leading to the regulation of neuropeptide Y (37). Thyroid hormone also exerts direct effects on ventromedial nucleus neurons to regulate feeding (38).
The observation that the LPS-induced increase in D2 mRNA is reduced by sTNF-R1 in a small group of α tanycytes indicates that TNF participates in the mechanism by which LPS activates D2 gene expression but only in this tanycyte subset. The data do not distinguish between direct or indirect effects of TNF on this cell population, but the late onset of Iκ-Bα expression in the ventricular walls of the third ventricle 12 h after the administration of LPS relative to the maximal expression of tanycyte D2 mRNA at 9 h after LPS (13) suggests an indirect effect. The expression of Iκ-Bα is considered a sensitive marker for cytokine signaling through the NF-κB pathway, because it is rapidly resynthesized after release from NF-κB in the cytoplasm to resume its role as an endogenous inhibitory factor for NF-κB (39,40). Presumably, the late onset of NF-κB activation may contribute to sustaining the D2 response in this tanycyte subset rather than serve as the initiating factor.
Nevertheless, these data do suggest heterogeneity in the physiologic response of the tanycyte subsets. A similar selective regulation of D2 in α but not β tanycytes by corticosterone was previously reported by our group (13). The α tanycytes are known to have direct projections into the neuropil of the arcuate and ventromedial nuclei (1), regions of the brain that contain a high density of thyroid hormone receptors (41,42). Because α tanaycytes are the most sensitive to the effects of fasting-induced D2 up-regulation compared with other regions of the MBH (43), whereas β tanycytes, but not α tanycytes, are closely intermingled with TRH-producing axon terminals (19), it is feasible that α tanycytes may be primarily involved in the regulation of food intake and/or energy homeostasis, whereas β tanycytes are largely involved in feedback regulation of the hypothalamic-pituitary-thyroid axis.
In contrast to the subset of α tanycytes, no Iκ-Bα expression was identified in β tanycytes or the remainder of α tanycytes. These data indicate that signaling through TNF is not the major effector for tanycyte D2 activation after LPS and that other mechanisms are likely operable.
The dramatic increase in Iκ-Bα in the pars tuberalis that directly underlies tanycyte end-feet processes in the external zone of the median eminence, however, raises an intriguing possibility that these cells are a primary target for LPS and that products released from these cells are responsible for D2 activation in tanycytes. Credence for this hypothesis is given by recent studies in the Japanese quail showing the association between photoperiod-induced rise in pars tuberalis TSH β-subunit mRNA and the subsequent increase in tanycyte D2 gene expression as a mechanism that triggers long-day photoinduced seasonal breeding. In addition, intracerebroventricular administration of TSH rapidly induces expression of D2 in birds exposed to short day photoperiods, whereas TSH-β antiserum abolishes D2 activation in birds exposed to long-day photoperiods (17,21).
Despite these observations and the evidence herein that LPS rapidly increases TSHβ mRNA in the pars tuberalis, it is unlikely that TSH contributes in a major way to the LPS-induced D2 activation in tanycytes. This is based on the observation that TSH activation of the D2 promoter involves the cAMP signaling pathway and the binding of phosphoCREB to cAMP response elements in the D2 promoter (17,44). However, although phosphoCREB was observed in the nucleus of arcuate nucleus neurons after the administration of LPS, no phosphoCREB was observed in tanycytes 2, 4, or 6 h after injection. Only at 9 h after LPS administration was phosphoCREB observed in the nucleus of tanycytes but selectively in a subset of α tanycytes. Because these cells are located in the same distribution as those responsive to TNF inhibition, the possibility of an indirect effect of TNF on LPS-induced D2 activation via the release of TSH from the pars distalis can be raised. Alternatively, it is conceivable that other products released from the pars tuberalis might be involved in the activation of tanycyte D2 in response to LPS.
We conclude that activation of the D2 gene in tanycytes by LPS is not dependent upon signaling through TNF with the exception of a small subset of α tanycytes located in the third ventricular wall. The importance of NF-κB indirect effects on tanycytes mediated by the release of factors from the pars tuberalis other than TSH will require further investigation.
Acknowledgments
We thank to Laura Reilly (University of Aberdeen) for the plasmid containing the TSHβ DNA fragment.
Footnotes
Present address for E.S.: Dirección de Investigaciones en Neurociencias, Instituto Nacional de Psiquiatría, Ramón de la Fuente Muñíz, Calzada México-Xochimilco 101, 14370 México, Distrito Federal, México.
This work was supported by the National Institutes of Health Grant DK37021. G.W. is a recipient of a postdoctoral fellowship from the Hilda and Preston Davis Foundation.
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 25, 2010
Abbreviations: CREB, cAMP response element-binding protein; CSF, cerebrospinal fluid; D2, type 2 iodothyronine deiodinase; DTAF, dichlorotriazinylamino-fluorescein; LPS, lipopolysaccharide; MBH, mediobasal hypothalamus; NF-κB, nuclear factor-κB; TNF-R1, TNF type 1 receptor; sTNF-R1, soluble, rat, polyethylene glycol-conjugated TNF receptor type 1; UTP, uridine 5′-triphosphate.
References
- Rodríguez EM, Blázquez JL, Pastor FE, Peláez B, Peña P, Peruzzo B, Amat P 2005 Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol 247:89–164 [DOI] [PubMed] [Google Scholar]
- Lechan RM, Fekete C 2004 Feedback regulation of thyrotropin-releasing hormone (TRH): mechanisms for the non-thyroidal illness syndrome. J Endocrinol Invest 27:105–119 [PubMed] [Google Scholar]
- Fekete C, Sarkar S, Christoffolete MA, Emerson CH, Bianco AC, Lechan RM 2005 Bacterial lipopolysaccharide (LPS)-induced type 2 iodothyronine deiodinase (D2) activation in the mediobasal hypothalamus (MBH) is independent of the LPS-induced fall in serum thyroid hormone levels. Brain Res 1056:97–99 [DOI] [PubMed] [Google Scholar]
- Fekete C, Gereben B, Doleschall M, Harney JW, Dora JM, Bianco AC, Sarkar S, Liposits Z, Rand W, Emerson C, Kacskovics I, Larsen PR, Lechan RM 2004 Lipopolysaccharide induces type 2 iodothyronine deiodinase in the mediobasal hypothalamus: implications for the nonthyroidal illness syndrome. Endocrinology 145:1649–1655 [DOI] [PubMed] [Google Scholar]
- De Groot LJ 2006 Non-thyroidal illness syndrome is a manifestation of hypothalamic-pituitary dysfunction, and in view of current evidence, should be treated with appropriate replacement therapies. Crit Care Clin 22:57–86 [DOI] [PubMed] [Google Scholar]
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR 2002 Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89 [DOI] [PubMed] [Google Scholar]
- Lechan RM 2008 The dilemma of the nonthyroidal illness syndrome. Acta Biomed 79:165–171 [PMC free article] [PubMed] [Google Scholar]
- Fliers E, Alkemade A, Wiersinga WM, Swaab DF 2006 Hypothalamic thyroid hormone feedback in health and disease. Prog Brain Res 153:189–207 [DOI] [PubMed] [Google Scholar]
- Diano S, Naftolin F, Goglia F, Csernus V, Horvath TL 1998 Monosynaptic pathway between the arcuate nucleus expressing glial type II iodothyronine 5′-deiodinase mRNA and the median eminence-projective TRH cells of the rat paraventricular nucleus. J Neuroendocrinol 10:731–742 [DOI] [PubMed] [Google Scholar]
- Lechan RM, Fekete C 2007 Infundibular tanycytes as modulators of neuroendocrine function: hypothetical role in the regulation of the thyroid and gonadal axis. Acta Biomed 78(Suppl 1):84–98 [PubMed] [Google Scholar]
- Yoshimura T, Yasuo S, Watanabe M, Iigo M, Yamamura T, Hirunagi K, Ebihara S 2003 Light-induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature 426:178–181 [DOI] [PubMed] [Google Scholar]
- Coppola A, Hughes J, Esposito E, Schiavo L, Meli R, Diano S 2005 Suppression of hypothalamic deiodinase type II activity blunts TRH mRNA decline during fasting. FEBS Lett 579:4654–4658 [DOI] [PubMed] [Google Scholar]
- Sánchez E, Singru PS, Fekete C, Lechan RM 2008 Induction of type 2 iodothyronine deiodinase in the mediobasal hypothalamus by bacterial lipopolysaccharide: role of corticosterone. Endocrinology 149:2484–2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeöld A, Doleschall M, Haffner MC, Capelo LP, Menyhért J, Liposits Z, da Silva WS, Bianco AC, Kacskovics I, Fekete C, Gereben B 2006 Characterization of the nuclear factor-κB responsiveness of the human dio2 gene. Endocrinology 147:4419–4429 [DOI] [PubMed] [Google Scholar]
- Nadeau S, Rivest S 1999 Effects of circulating tumor necrosis factor on the neuronal activity and expression of the genes encoding the tumor necrosis factor receptors (p55 and p75) in the rat brain: a view from the blood-brain barrier. Neuroscience 93:1449–1464 [DOI] [PubMed] [Google Scholar]
- Zha J, Shu H 2002 Molecular mechanism of signaling by tumor necrosis factor. Sci China C Life Sci 45:113–119 [DOI] [PubMed] [Google Scholar]
- Nakao N, Ono H, Yamamura T, Anraku T, Takagi T, Higashi K, Yasuo S, Katou Y, Kageyama S, Uno Y, Kasukawa T, Iigo M, Sharp PJ, Iwasawa A, Suzuki Y, Sugano S, Niimi T, Mizutani M, Namikawa T, Ebihara S, Ueda HR, Yoshimura T 2008 Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature 452:317–322 [DOI] [PubMed] [Google Scholar]
- Sarkar S, Lechan RM 2003 Central administration of neuropeptide Y reduces α-melanocyte-stimulating hormone-induced cyclic adenosine 5′-monophosphate response element binding protein (CREB) phosphorylation in pro-thyrotropin-releasing hormone neurons and increases CREB phosphorylation in corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Endocrinology 144:281–291 [DOI] [PubMed] [Google Scholar]
- Sánchez E, Vargas MA, Singru PS, Pascual I, Romero F, Fekete C, Charli JL, Lechan RM 2009 Tanycyte pyroglutamyl peptidase II contributes to regulation of the hypothalamic-pituitary-thyroid axis through glial-axonal associations in the median eminence. Endocrinology 150:2283–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C 1986 The rat brain in stereotaxic coordinates. 2nd ed. Bowen Hills, Australia: Australian Academic Press [Google Scholar]
- Ono H, Hoshino Y, Yasuo S, Watanabe M, Nakane Y, Murai A, Ebihara S, Korf HW, Yoshimura T 2008 Involvement of thyrotropin in photoperiodic signal transduction in mice. Proc Natl Acad Sci USA 105:18238–18242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur A, Bauer K, Jarry H, Köhrle J 2000 Effects of proinflammatory cytokines on anterior pituitary 5′-deiodinase type I and type II. J Endocrinol 167:505–515 [DOI] [PubMed] [Google Scholar]
- Kerwin BA, Chang BS, Gegg CV, Gonnelli M, Li T, Strambini GB 2002 Interactions between PEG and type I soluble tumor necrosis factor receptor: modulation by pH and by PEGylation at the N terminus. Protein Sci 11:1825–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruzzo B, Pastor FE, Blázquez JL, Schöbitz K, Peláez B, Amat P, Rodríguez EM 2000 A second look at the barriers of the medial basal hypothalamus. Exp Brain Res 132:10–26 [DOI] [PubMed] [Google Scholar]
- Akmayev IG, Popov AP 1977 Morphological aspects of the hypothalamic-hypophyseal system. VII. The tanycytes: their relation to the hypophyseal adrenocorticotrophic function. An ultrastructural study. Cell Tissue Res 180:263–282 [DOI] [PubMed] [Google Scholar]
- Flament-Durand J, Brion JP 1985 Tanycytes: morphology and functions: a review. Int Rev Cytol 96:121–155 [DOI] [PubMed] [Google Scholar]
- Yasuo S, Watanabe M, Iigo M, Yamamura T, Nakao N, Takagi T, Ebihara S, Yoshimura T 2006 Molecular mechanism of photoperiodic time measurement in the brain of Japanese quail. Chronobiol Int 23:307–315 [DOI] [PubMed] [Google Scholar]
- Lechan RM, Fekete C 2005 Role of thyroid hormone deiodination in the hypothalamus. Thyroid 15:883–897 [DOI] [PubMed] [Google Scholar]
- Alkemade A, Friesema EC, Unmehopa UA, Fabriek BO, Kuiper GG, Leonard JL, Wiersinga WM, Swaab DF, Visser TJ, Fliers E 2005 Neuroanatomical pathways for thyroid hormone feedback in the human hypothalamus. J Clin Endocrinol Metab 90:4322–4334 [DOI] [PubMed] [Google Scholar]
- Simpson KA, Martin NM, Bloom SR 2009 Hypothalamic regulation of food intake and clinical therapeutic applications. Arq Bras Endocrinol Metabol 53:120–128 [DOI] [PubMed] [Google Scholar]
- Jo YH, Chua Jr S 2009 Transcription factors in the development of medial hypothalamic structures. Am J Physiol Endocrinol Metab 297:E563–E567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechan R, Fekete C 2010 Neuroendocrine and metabolic adaptations in the central nervous system to weight loss that facilitate weight re-gain. In: Freemark M, ed. Pediatric obesity. New York: Humana Press/Springer [Google Scholar]
- Heuer H, Maier MK, Iden S, Mittag J, Friesema EC, Visser TJ, Bauer K 2005 The monocarboxylate transporter 8 linked to human psychomotor retardation is highly expressed in thyroid hormone-sensitive neuron populations. Endocrinology 146:1701–1706 [DOI] [PubMed] [Google Scholar]
- Nakao N, Takagi T, Iigo M, Tsukamoto T, Yasuo S, Masuda T, Yanagisawa T, Ebihara S, Yoshimura T 2006 Possible involvement of organic anion transporting polypeptide 1c1 in the photoperiodic response of gonads in birds. Endocrinology 147:1067–1073 [DOI] [PubMed] [Google Scholar]
- Tu HM, Kim SW, Salvatore D, Bartha T, Legradi G, Larsen PR, Lechan RM 1997 Regional distribution of type 2 thyroxine deiodinase messenger ribonucleic acid in rat hypothalamus and pituitary and its regulation by thyroid hormone. Endocrinology 138:3359–3368 [DOI] [PubMed] [Google Scholar]
- Fekete C, Freitas BC, Zeöld A, Wittmann G, Kádár A, Liposits Z, Christoffolete MA, Singru P, Lechan RM, Bianco AC, Gereben B 2007 Expression patterns of WSB-1 and USP-33 underlie cell-specific posttranslational control of type 2 deiodinase in the rat brain. Endocrinology 148:4865–4874 [DOI] [PubMed] [Google Scholar]
- Coppola A, Liu ZW, Andrews ZB, Paradis E, Roy MC, Friedman JM, Ricquier D, Richard D, Horvath TL, Gao XB, Diano S 2007 A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell Metab 5:21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong WM, Martin NM, Smith KL, Gardiner JV, Connoley IP, Stephens DA, Dhillo WS, Ghatei MA, Small CJ, Bloom SR 2004 Triiodothyronine stimulates food intake via the hypothalamic ventromedial nucleus independent of changes in energy expenditure. Endocrinology 145:5252–5258 [DOI] [PubMed] [Google Scholar]
- Zabel U, Baeuerle PA 1990 Purified human I κB can rapidly dissociate the complex of the NF-κB transcription factor with its cognate DNA. Cell 61:255–265 [DOI] [PubMed] [Google Scholar]
- Zabel U, Henkel T, Silva MS, Baeuerle PA 1993 Nuclear uptake control of NF-κB by MAD-3, an I κB protein present in the nucleus. EMBO J 12:201–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puymirat J, Miehe M, Marchand R, Sarlieve L, Dussault JH 1991 Immunocytochemical localization of thyroid hormone receptors in the adult rat brain. Thyroid 1:173–184 [DOI] [PubMed] [Google Scholar]
- Lechan RM, Qi Y, Berrodin TJ, Davis KD, Schwartz HL, Strait KA, Oppenheimer JH, Lazar MA 1993 Immunocytochemical delineation of thyroid hormone receptor β2-like immunoreactivity in the rat central nervous system. Endocrinology 132:2461–2469 [DOI] [PubMed] [Google Scholar]
- Diano S, Naftolin F, Goglia F, Horvath TL 1998 Fasting-induced increase in type II iodothyronine deiodinase activity and messenger ribonucleic acid levels is not reversed by thyroxine in the rat hypothalamus. Endocrinology 139:2879–2884 [DOI] [PubMed] [Google Scholar]
- Unfried C, Ansari N, Yasuo S, Korf HW, von Gall C 2009 Impact of melatonin and molecular clockwork components on the expression of thyrotropin β-chain (Tshb) and the Tsh receptor in the mouse pars tuberalis. Endocrinology 150:4653–4662 [DOI] [PubMed] [Google Scholar]