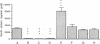Abstract
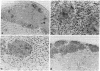
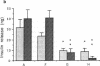
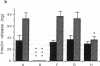
Allogeneic mouse islets or xenogeneic rat islets, or fetal porcine islets were implanted under the renal capsule of C57BL/6 mice either alone or carefully mixed with syngeneic islets. With this experimental model the syngeneic islets, although not rejected themselves, are exposed to cytokines and inflammatory mediators released during either allograft or xenograft rejection. No differences in insulin content could be observed between mixed islet grafts and pure syngeneic islet grafts 6 wk after transplantation. Neither was there any morphological evidence of a non-specific destruction of syngeneic islets. These findings suggest that the mechanisms of both allograft and xenograft rejections are highly specific. The hormone release from the mixed syngeneic-allogeneic grafts was similar to that from pure syngeneic islet grafts. In contrast, a pronounced impairment of both the first and second phases of insulin release was observed 2 wk after implantation in mixed syngeneic-xenogeneic islet grafts. When perfusing the mixed islet graft after completed rejection of the concordant xenogeneic rat islets (6 wk after implantation), the insulin release from the remaining syngeneic mouse islets was identical to that of control grafts. However, syngeneic mouse islets exposed to the rejection mechanism of the discordant xenogenic pig islet-like cell clusters did not attain a complete functional recovery.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auchincloss H., Jr Xenogeneic transplantation. A review. Transplantation. 1988 Jul;46(1):1–20. doi: 10.1097/00007890-198807000-00001. [DOI] [PubMed] [Google Scholar]
- Dallman M. J., Roake J., Hughes D., Toogood G., Morris P. J. Sequential analysis of IL-2 gene transcription in renal transplants. Transplantation. 1992 Mar;53(3):683–685. doi: 10.1097/00007890-199203000-00038. [DOI] [PubMed] [Google Scholar]
- Ganda O. P., Srikanta S., Brink S. J., Morris M. A., Gleason R. E., Soeldner J. S., Eisenbarth G. S. Differential sensitivity to beta-cell secretagogues in "early," type I diabetes mellitus. Diabetes. 1984 Jun;33(6):516–521. doi: 10.2337/diab.33.6.516. [DOI] [PubMed] [Google Scholar]
- Gress R. E., Nathenson S. G., Lucas P. J. Fine specificity of xenogeneic antigen recognition by human T cells. Transplantation. 1989 Jul;48(1):93–98. doi: 10.1097/00007890-198907000-00022. [DOI] [PubMed] [Google Scholar]
- Grill V., Herberg L. Glucose- and arginine-induced insulin and glucagon responses from the isolated perfused pancreas of the BB-Wistar diabetic rat. Evidence for selective impairment of glucose regulation. Acta Endocrinol (Copenh) 1983 Apr;102(4):561–566. doi: 10.1530/acta.0.1020561. [DOI] [PubMed] [Google Scholar]
- Hanenberg H., Kolb-Bachofen V., Kantwerk-Funke G., Kolb H. Macrophage infiltration precedes and is a prerequisite for lymphocytic insulitis in pancreatic islets of pre-diabetic BB rats. Diabetologia. 1989 Feb;32(2):126–134. doi: 10.1007/BF00505185. [DOI] [PubMed] [Google Scholar]
- Hao L. M., Wang Y., Gill R. G., La Rosa F. G., Talmage D. W., Lafferty K. J. Role of lymphokine in islet allograft rejection. Transplantation. 1990 Mar;49(3):609–614. doi: 10.1097/00007890-199003000-00025. [DOI] [PubMed] [Google Scholar]
- Heding L. G. Determination of total serum insulin (IRI) in insulin-treated diabetic patients. Diabetologia. 1972 Aug;8(4):260–266. doi: 10.1007/BF01225569. [DOI] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. Potassium ions and the secretion of insulin by islets of Langerhans incubated in vitro. Biochem J. 1968 Jun;108(1):17–24. doi: 10.1042/bj1080017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kano Y., Kanatsuna T., Nakamura N., Kitagawa Y., Mori H., Kajiyama S., Nakano K., Kondo M. Defect of the first-phase insulin secretion to glucose stimulation in the perfused pancreas of the nonobese diabetic (NOD) mouse. Diabetes. 1986 Apr;35(4):486–490. doi: 10.2337/diab.35.4.486. [DOI] [PubMed] [Google Scholar]
- Kaufman D. B., Platt J. L., Rabe F. L., Dunn D. L., Bach F. H., Sutherland D. E. Differential roles of Mac-1+ cells, and CD4+ and CD8+ T lymphocytes in primary nonfunction and classic rejection of islet allografts. J Exp Med. 1990 Jul 1;172(1):291–302. doi: 10.1084/jem.172.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsgren O., Sandler S., Landström A. S., Jansson L., Andersson A. Large-scale production of fetal porcine pancreatic isletlike cell clusters. An experimental tool for studies of islet cell differentiation and xenotransplantation. Transplantation. 1988 Mar;45(3):509–514. doi: 10.1097/00007890-198803000-00001. [DOI] [PubMed] [Google Scholar]
- Krams S. M., Falco D. A., Villanueva J. C., Rabkin J., Tomlanovich S. J., Vincenti F., Amend W. J., Melzer J., Garovoy M. R., Roberts J. P. Cytokine and T cell receptor gene expression at the site of allograft rejection. Transplantation. 1992 Jan;53(1):151–156. doi: 10.1097/00007890-199201000-00031. [DOI] [PubMed] [Google Scholar]
- Kröncke K. D., Kolb-Bachofen V., Berschick B., Burkart V., Kolb H. Activated macrophages kill pancreatic syngeneic islet cells via arginine-dependent nitric oxide generation. Biochem Biophys Res Commun. 1991 Mar 29;175(3):752–758. doi: 10.1016/0006-291x(91)91630-u. [DOI] [PubMed] [Google Scholar]
- Lechler R. I., Lombardi G., Batchelor J. R., Reinsmoen N., Bach F. H. The molecular basis of alloreactivity. Immunol Today. 1990 Mar;11(3):83–88. doi: 10.1016/0167-5699(90)90033-6. [DOI] [PubMed] [Google Scholar]
- Lee K. U., Amano K., Yoon J. W. Evidence for initial involvement of macrophage in development of insulitis in NOD mice. Diabetes. 1988 Jul;37(7):989–991. doi: 10.2337/diab.37.7.989. [DOI] [PubMed] [Google Scholar]
- Leiter E. H. Murine macrophages and pancreatic beta cells. Chemotactic properties of insulin and beta-cytostatic action of interleukin 1. J Exp Med. 1987 Oct 1;166(4):1174–1179. doi: 10.1084/jem.166.4.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrup-Poulsen T., Bendtzen K., Nerup J., Dinarello C. A., Svenson M., Nielsen J. H. Affinity-purified human interleukin I is cytotoxic to isolated islets of Langerhans. Diabetologia. 1986 Jan;29(1):63–67. doi: 10.1007/BF02427283. [DOI] [PubMed] [Google Scholar]
- Mandrup-Poulsen T., Helqvist S., Mølvig J., Wogensen L. D., Nerup J. Cytokines as immune effector molecules in autoimmune endocrine diseases with special reference to insulin-dependent diabetes mellitus. Autoimmunity. 1989;4(3):191–234. doi: 10.3109/08916938909003049. [DOI] [PubMed] [Google Scholar]
- Martinez O. M., Krams S. M., Sterneck M., Villanueva J. C., Falco D. A., Ferrell L. D., Lake J., Roberts J. P., Ascher N. L. Intragraft cytokine profile during human liver allograft rejection. Transplantation. 1992 Feb;53(2):449–456. doi: 10.1097/00007890-199202010-00035. [DOI] [PubMed] [Google Scholar]
- Maryanski J. L., Pala P., Corradin G., Jordan B. R., Cerottini J. C. H-2-restricted cytolytic T cells specific for HLA can recognize a synthetic HLA peptide. Nature. 1986 Dec 11;324(6097):578–579. doi: 10.1038/324578a0. [DOI] [PubMed] [Google Scholar]
- McEvoy R. C. Changes in the volumes of the A-, B-, and D-cell populations in the pancreatic islets during the postnatal development of the rat. Diabetes. 1981 Oct;30(10):813–817. doi: 10.2337/diab.30.10.813. [DOI] [PubMed] [Google Scholar]
- O'Connell P. J., Pacheco-Silva A., Nickerson P. W., Muggia R. A., Bastos M., Kelley V. R., Strom T. B. Unmodified pancreatic islet allograft rejection results in the preferential expression of certain T cell activation transcripts. J Immunol. 1993 Feb 1;150(3):1093–1104. [PubMed] [Google Scholar]
- Pierson R. N., 3rd, Winn H. J., Russell P. S., Auchincloss H., Jr Xenogeneic skin graft rejection is especially dependent on CD4+ T cells. J Exp Med. 1989 Sep 1;170(3):991–996. doi: 10.1084/jem.170.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roep B. O., Kallan A. A., De Vries R. R. Beta-cell antigen-specific lysis of macrophages by CD4 T-cell clones from newly diagnosed IDDM patient. A putative mechanism of T-cell-mediated autoimmune islet cell destruction. Diabetes. 1992 Nov;41(11):1380–1384. doi: 10.2337/diab.41.11.1380. [DOI] [PubMed] [Google Scholar]
- Sachs D. H., Bach F. H. Immunology of xenograft rejection. Hum Immunol. 1990 Jun;28(2):245–251. doi: 10.1016/0198-8859(90)90025-k. [DOI] [PubMed] [Google Scholar]
- Sandler S., Eizirik D. L., Svensson C., Strandell E., Welsh M., Welsh N. Biochemical and molecular actions of interleukin-1 on pancreatic beta-cells. Autoimmunity. 1991;10(3):241–253. doi: 10.3109/08916939109001895. [DOI] [PubMed] [Google Scholar]
- Schwizer R. W., Leiter E. H., Evans R. Macrophage-mediated cytotoxicity against cultured pancreatic islet cells. Transplantation. 1984 Jun;37(6):539–544. doi: 10.1097/00007890-198406000-00002. [DOI] [PubMed] [Google Scholar]
- Simeonovic C. J., Ceredig R., Wilson J. D. Effect of GK1.5 monoclonal antibody dosage on survival of pig proislet xenografts in CD4+ T cell-depleted mice. Transplantation. 1990 May;49(5):849–856. doi: 10.1097/00007890-199005000-00002. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Stock P. G., Ascher N. L., Chen S., Field J., Bach F. H., Sutherland D. E. Evidence for direct and indirect pathways in the generation of the alloimmune response against pancreatic islets. Transplantation. 1991 Oct;52(4):704–709. doi: 10.1097/00007890-199110000-00023. [DOI] [PubMed] [Google Scholar]
- Sutton R., Gray D. W., McShane P., Dallman M. J., Morris P. J. The specificity of rejection and the absence of susceptibility of pancreatic islet beta cells to nonspecific immune destruction in mixed strain islets grafted beneath the renal capsule in the rat. J Exp Med. 1989 Sep 1;170(3):751–762. doi: 10.1084/jem.170.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C. T., Unanue E. R. The costimulatory function of antigen-presenting cells. Immunol Today. 1990 Feb;11(2):49–55. doi: 10.1016/0167-5699(90)90018-5. [DOI] [PubMed] [Google Scholar]