Abstract
To determine the effectiveness of stereotactic radiosurgery (SRS) treatment to central nervous system (CNS) hemangioblastomas in von Hippel–Lindau disease (VHL), we analyzed long-term results in VHL patients treated with SRS. Patients were enrolled in a prospective VHL natural history study, undergoing SRS treatment of CNS hemangioblastomas. Treatment regimens, serial clinical evaluations, and longitudinal imaging data were analyzed. Twenty VHL patients (10 males and 10 females) underwent SRS treatment of 44 CNS hemangioblastomas (39 cerebellar and 5 brainstem). Mean (±SD) age at treatment was 37.5 ± 12.0 years (range: 13–67). Mean follow-up was 8.5 ± 3.2 years (range: 3.0–17.6 years). All patients were alive at last follow-up. Mean treated tumor volume was 0.5 ± 0.7 cm3 (range: 0.01–3.6 cm3). Mean prescription dose was 18.9 Gy (range: 12–24 Gy) at the tumor margin. Local control rate at 2, 5, 10, and 15 years after SRS treatment was 91%, 83%, 61%, and 51%, respectively. Univariate analysis did not identify variables associated (P > .05) with worse tumor control at last follow-up. Thirty-three percent of SRS-treated small (<1.0 cm diameter), asymptomatic tumors progressed over a long-term follow-up. There were no long-term adverse radiation effects. Although SRS treatment of hemangioblastomas in VHL has a low risk for adverse radiation effects, it is associated with diminishing control over a long-term follow-up. These results indicate that SRS should not be used to prophylactically treat asymptomatic tumors and should be reserved for the treatment of tumors that are not surgically resectable.
Keywords: central nervous system hemangioblastoma, radiosurgery, treatment, von Hippel–Lindau disease
Central nervous system (CNS) hemangioblastomas represent the most common manifestation of von Hippel–Lindau disease (VHL) and occur in approximately 80% of patients. VHL-associated hemangioblastomas are multifocal and progressive. These tumors represent a major source of morbidity and mortality in VHL. Hemangioblastomas most frequently arise in the posterior fossa1,2 and 90% of VHL patients with a CNS hemangioblastoma will have multiple hemangioblastomas on imaging.3 VHL natural history studies have shown that hemangioblastomas exhibit a saltatory growth pattern characterized by the periods of growth interrupted by long quiescent periods and that new hemangioblastomas arise over time.2,4
Recently, the selection of stereotactic radiosurgery (SRS) over surgical resection to treat VHL-associated hemangioblastomas has become more common.5–7 However, the long-term safety and efficacy of SRS has not been determined. Previous studies demonstrate local control rates in VHL ranging from 83% to 100%, but these control rates represent limited follow-up.5–7 Because hemangioblastomas exhibit a saltatory growth pattern, accurate determination of the effectiveness of SRS in their management requires a long-term follow-up. To better define the effectiveness of SRS treatment of hemangioblastomas in VHL, we analyzed the findings of patients who underwent the SRS treatment of CNS hemangioblastomas in a long-term natural history study.
Clinical Materials and Methods
Patients
Patients enrolled in a prospective natural history study of CNS lesions in VHL (National Institutes of Health [NIH] protocol 00-N-0140) who underwent a single-session SRS of intracranial CNS hemangioblastomas performed using a gamma knife (GK) or linear accelerator (LINAC) unit were included (Table 1). This study was approved by the National Institutes for Neurological Disorders and Stroke Institutional Review Board. Informed consent was obtained from all patients. Patients had VHL diagnosed by clinical and genetic criteria.1,8
Table 1.
Patient and treatment characteristics
| Clinical characteristics | Value |
|---|---|
| Patient characteristics | |
| Number of patients | 20 |
| Mean age years (range) | 37.5 (13–67) |
| Gender | |
| Male (%) | 10 (50) |
| Female (%) | 10 (50) |
| Median pretreatment KPS (range) | 100 (70–100) |
| Treatment characteristics | |
| Tumors | |
| Treated | 44 |
| Untreated at time of radiosurgery | 55 |
| Treatment modality (number of patients) | |
| GK | 8 |
| LINAC | 12 |
| Mean margin dose (Gy) (range) | 18.9 (12–24) |
| Mean maximal dose (Gy) (range) | |
| GK | 34.6 (23–49) |
| LINAC | 25.1 (15–34) |
| Mean isodose normalization (%) (range) | |
| GK | 54 (35–85) |
| LINAC | 82 (70–96) |
| Mean tumors treated per session (range) | 2.2 (1–10) |
| Treated tumor location | |
| Cerebellum | 39 |
| Brainstem | 5 |
| Mean treated tumor volume (cm3) (range) | 0.5 (0.01–3.6) |
Abbreviations: KPS, Karnofsky performance status score; LINAC, Linear accelerator; GK, gamma knife.
Clinical and Imaging Evaluation
Clinical Evaluation
Patients underwent serial clinical and imaging evaluations at approximately 6–12-month intervals. Findings from physical, neurological, and imaging examinations were recorded at each visit. Karnofsky performance status (KPS) scale scores (0–100) were used to standardize clinical performance evaluations.9
Radiosurgical Treatment
SRS was performed using a 201-source 60Cobalt Leksell GK or 6-MV LINAC unit. GK treatment plans were generated from contrast-enhanced magnetic resonance imaging (MRI) obtained after stereotactic frame placement. The number of isocenters, maximum radiation dose, prescribed dose to the tumor margins, and isodose normalization were recorded. LINAC treatment plans were generated based on the registration of pretreatment contrast-enhanced MRI with contrast-enhanced CT scans obtained after stereotactic frame placement. The number of isocenters and radiation arcs, maximum radiation dose, prescribed dose to margins, and isodose line used for delivery were recorded.
Imaging Evaluation
Serial gadolinium-enhanced and unenhanced MRI (including T1-, T2-, fluid-attenuated inversion recovery sequences) was performed at each visit. Tumor location and volume (largest anteroposterior dimension × largest mediolateral dimension × largest dorsoventral dimension/2) based on gadolinium-enhanced T1-wieghted MRI were determined and recorded.10 Tumors were classified (based on maximum diameter) as small (<1.0 cm), medium (1.0–2.0 cm), or large (>2.0 cm).
Response to Treatment
Response to treatment was determined by measurement of tumor volume (solid component) on MRI and clinical assessment for symptom development associated with cyst growth. Progression was defined either by tumoral growth (>50% increase from pretreatment volume) or by symptomatic peritumoral cyst expansion necessitating resection. Regression was defined as greater than 50% reduction from pretreatment volume. Tumors were defined as stable if their volume remained within 50% of pretreatment volume. Treatment success included tumors that remained stable or regressed at last follow-up. Treatment failure was defined by tumors that progressed at last follow-up.
Statistical Analysis
Fisher's exact test was used to assess the relationship between categorical tumor level variables and tumor progression status during the follow-up period. Univariate logistic regression was performed when continuous predictors were considered. Survival analyses were used to analyze the time to event data and determine local control rates. Start time was defined by the time SRS was administered. Event time was recorded when tumor progression became evident. The Wilcoxon rank-sum test was used to compare independent variables between subsets of tumors. Analyses were performed on data obtained at 3 years after SRS and last follow-up. Statistical analyses were performed using the software R (R version 2.7.2, The R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was determined by a 2-sided P value of <.05.
Results
Patient Characteristics
Two hundred and fifty patients were included in the VHL natural history protocol (120 males and 130 females) (Table 1). Twenty patients (10 males and 10 females; 8%) received SRS treatment to 44 CNS hemangioblastomas. Mean (±SD) age at SRS treatment was 37.5 ± 12.0 years (range: 13–67 years). Mean follow-up was 8.5 ± 3.2 years (median: 8 years; range: 3–17.6 years). Prior to SRS treatment, 15 patients (75%) underwent a surgery for CNS hemangioblastomas (mean: 1.6 surgeries; range: 0–6 surgeries). Eleven (55%) patients required no further treatment for these completely resected tumors. Four patients (20%) developed local recurrence following operation at outside institutions and received SRS to these recurrent tumors. No patients were lost to follow-up.
Eleven patients (55%) underwent SRS for the treatment of a symptomatic hemangioblastoma, whereas 9 patients (45%) were asymptomatic at the time of SRS but had enlargement of at least 1 tumor by MRI. Symptomatic patients had a mean of 1.7 tumors (range: 1–3 tumors) treated and asymptomatic patients had a mean of 2.8 tumors (range: 1–10 tumors) treated. Symptoms associated with cerebellar hemangioblastomas included headache (50% of patients), ataxia (38%), and imbalance (25%). Symptoms associated with brainstem hemangioblastomas included paresthesias (33% of patients), dysphagia (33%), and dysgraphia (33%).
SRS Characteristics
SRS was performed using a GK (8 patients and 28 tumors) or LINAC (12 patients and 16 tumors). SRS was performed at the NIH (n = 8) or outside institutions (n = 12). Mean number of hemangioblastomas treated per session was 2.2 (range: 1–10 tumors). A mean prescribed dose of 18.9 Gy (range: 12–24 Gy) was delivered. GK delivered a mean maximum radiation dose of 34.6 Gy (range: 23–49 Gy) and mean prescribed dose of 18.1 Gy (range: 12–20 Gy) at tumor margins utilizing a mean isodose line of 54% (range: 35%–85%). A mean of 2.2 isocenters (range: 1–18 isocenters) was used per GK-treated tumor. LINAC delivered a mean maximum radiation dose of 25.1 Gy (range: 15–34 Gy) and mean prescribed dose of 20.0 Gy (range: 12–24 Gy) at tumor margins utilizing a mean isodose line of 82% (range: 70%–96%). A single isocenter and a mean of 4.9 non-coplanar arcs (range: 3–6 arcs) were used per LINAC-treated tumor.
Tumor Characteristics
Thirty-nine (88.6%) cerebellar and 5 (11.4%) brainstem (OBEX) hemangioblastomas were treated. Thirty-nine (88.6%) hemangioblastomas were naïve to treatment and 5 (11.4%) were postsurgical recurrences. Mean volume of all treated hemangioblastomas was 0.5 ± 0.7 cm3 (median: 0.1 cm3; range: 0.01–3.6 cm3), and mean maximum diameter was 0.9 ± 0.5 cm (median: 0.7 cm; range: 0.3–2.1 cm). Mean volume of cerebellar hemangioblastomas was 0.4 ± 0.6 cm3 (median: 0.1 cm3; range: 0.01–2.2 cm3). Mean volume of brainstem hemangioblastomas was 0.8 ± 1.5 cm3 (median: 0.1 cm3; range: 0.03–3.6 cm3). There were 30 (68%) small, 11 (25%) medium, and 3 (7%) large SRS-treated tumors.
Tumor Control
Overall Control
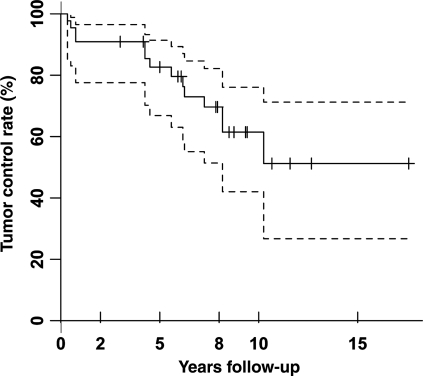
At last follow-up, 16 tumors (36%) were stable, 14 (32%) regressed, and 14 (32%) progressed (Fig. 1). Local control rates at 2, 5, 8, 10, and 15 years were 91%, 83%, 70%, 61%, and 51%, respectively (Fig. 2). Mean pretreatment volume of tumors that remained either stable or regressed (mean: 0.4 ± 0.5 cm3; median: 0.08 cm3; range: 0.01–2.0 cm3) was not significantly different from those that progressed (mean: 0.7 ± 1.1 cm3; median: 0.1 cm3; range: 0.02–3.6 cm3; P = .7). Analysis of tumors 3 years after treatment revealed larger tumor size was associated with progression but this association was not present at last follow-up (P = .049). SRS delivery system, dosage delivered, and independent tumor variables (presence of a cyst, recurrent tumor, follow-up time, initial size, and tumor location) were not associated with failure of tumor control at last follow-up (Table 2).
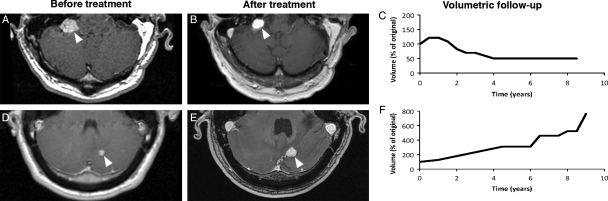
Fig. 1.
Axial, contrast-enhanced, T1-weighted, magnetic resonance imaging of regressing (A,B, arrowheads) and progressing (D,E, arrowheads) irradiated tumors in 2 patients with volumetric follow-up revealing significant (greater that 50%) volume loss (C) and gain (F).
Fig. 2.
Kaplan–Meier curves demonstrating tumor control rates for SRS-treated tumors. Progression-free survival at 2, 5, 8, 10, and 15 years was 91%, 83%, 70%, 61%, and 51%. Dashed lines represent 95% confidence intervals. Tick marks represent censored data.
Table 2.
Univariate analysis of variables that influence tumor progression
| Variable | Parameter | P value (test) |
|---|---|---|
| Dose | Higher | 0.56 (L) |
| Treatment modality | LINAC | 0.99 (F) |
| Tumor size | Larger | 0.59 (L) |
| Large (>2.0 cm) | 0.24 (F) | |
| Medium (1.0–2.0 cm) | 0.99 (F) | |
| Small (<1.0 cm) | 0.74 (F) | |
| Location | Brainstem | 0.99 (F) |
| Cyst at treatment | Present | 0.23 (F) |
| Length of follow-up | Longer | 0.35 (L) |
| Recurrent lesion | Yes | 0.16 (F) |
Abbreviations: L, logistic regression; F, Fisher's exact test; LINAC, linear accelerator.
Progressive Tumors
Among 14 progressive tumors, 4 (29%) required surgery to alleviate associated symptoms. Ten (71%) tumors remained asymptomatic despite radiographic enlargement. Mean time from SRS to resection was 2.9 ± 2.5 years (median: 2.7 years; range: 0.8–5.6 years). Nine of 27 (33%) small asymptomatic tumors progressed following treatment. Mean time to progression in this subset was 5.9 ± 2.9 years (median: 6.3 years; range: 0.3–10.3 years).
Peritumoral Cysts
Three tumors (2 cerebellar and 1 brainstem) had peritumoral cysts at treatment. Although 1 tumor and cyst remained stable through follow-up (4.2 years), the other 2 tumors or cysts (1 cerebellar [cyst and tumor progression], 1 brainstem [cyst progression]) progressed (0.3 and 5.6 years after SRS). Six cerebellar tumors (2 stable and 4 progressive; 14%) developed peritumoral cysts after SRS (mean: 6.1 ± 2.2 years; range: 4–9.4 years).
Effect of SRS on Tumor Volume
Eleven (25%) treated tumors had an initial volumetric response that was not indicative of final outcome. Two tumors (14%) shrank more than 50% before eventually becoming progressive. Mean time to smallest volume in this group was 2.0 ± 1.5 years (range: 0.9–3.1 years). Three tumors (21%) grew more than 50% before eventually undergoing regression. Mean time to maximal volume in this group was 1.2 ± 1.4 years (range: 0.3–2.8 years). Six tumors either grew (3 tumors; 19%) or shrank (3 tumors; 19%) initially over 50% before returning to meet stable criteria. These tumors reached maximum and minimum volumes at a mean of 1.5 ± 0.9 years (range: 0.6–2.3 years) and 3.1 ± 0.2 years (range: 2.9–3.3 years), respectively.
Untreated CNS Hemangioblastomas
Patients harbored 2.8 ± 2.8 untreated CNS hemangioblastomas (median: 2.5 hemangioblastomas; range: 0–10 hemangioblastomas) at the time of SRS. Thirty-three of these tumors (60%) were located in the posterior fossa and 22 (40%) were located within the spinal cord. Mean volume of these tumors at the time of SRS was 0.07 ± 0.1 cm3 (median: 0.03 cm3; range: 0.01–0.4 cm3). During the follow-up, patients developed a mean of 3.7 ± 4.1 new CNS hemangioblastomas (median: 3 hemangioblastomas; range: 0–17 hemangioblastomas; 0.4 hemangioblastomas/person/year). Fifty-five of the new tumors (75%) developed in the posterior fossa and 18 (25%) developed within the spinal cord.
Clinical Outcome
Generally, patients remained at their clinical baseline over the study period. Median KPS pre-SRS was 100 (range: 70–100) and at last follow-up was 100 (range: 60–100). Seven of 11 patients (64%) with pre-SRS symptoms experienced clinical improvement after treatment that corresponded with tumor volume reduction. The remaining 4 patients (36%) continued to have symptoms associated with progressive tumor growth that required surgical resection. These patients remained at their neurologic and KPS baseline after surgery.
SRS-related complications occurred in 4 patients (20%) and included headache (3 patients; 15%) or dizziness and diplopia (1 patient; 5%). One patient developed headache (duration, 1 month) responsive to nonsteroidal analgesics. Two patients developed headache that corresponded with increased peritumoral edema. Despite corticosteroid therapy, their headaches worsened contemporaneous with tumor enlargement, necessitating tumor resection for relief. One patient required a long-term course of corticosteroid therapy (8 months) for dizziness, hearing loss, and diplopia associated with increased cerebellar edema after SRS.
Histopathologic Findings
All 4 SRS-treated hemangioblastomas that required resection exhibited varying degrees of radiation effect characterized by necrosis, fibrosis, and vascular hyalinization intermixed with viable tumor (Fig. 3).
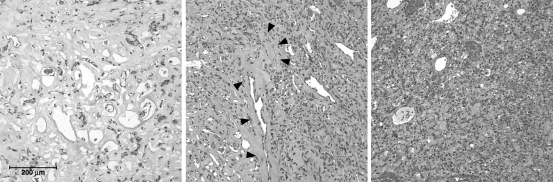
Fig. 3.
Hematoxylin and eosin stain of hemangioblastomas that required extirpation post-SRS for worsening symptoms. Photomicrographs (original magnification, ×20) from SRS-treated hemangioblastomas requiring resection reveal regions with dense radiation effect (left), transition zones with viable tumor stromal elements and hyalinized vasculature (middle), and areas maintaining typical hemangioblastoma architecture (right).
Discussion
Von Hippel–Lindau Disease
VHL is an autosomal dominant neoplasia syndrome (incidence, 1 in 39 000 live births)11 resulting from a germline mutation of the VHL tumor suppressor gene located on chromosome 3.12,13 VHL patients are predisposed to develop visceral and CNS lesions.1 Visceral lesions include renal cell carcinoma, renal cysts, pancreatic neuroendocrine tumors, pancreatic cysts, pheochromocytomas, and reproductive organ cystadenomas. CNS lesions include hemangioblastomas of the retina, cerebellum brainstem or spinal cord, and endolymphatic sac tumors. Despite surveillance and treatment advances, CNS hemangioblastomas remain a major cause of morbidity and mortality.
Complete surgical resection has been the mainstay of treatment for symptomatic hemangioblastomas in VHL. Meticulous extracapsular resection is associated with absolute local control and can be performed with minimal morbidity in the vast majority of the cases.14–17 Subtotal resection, with gross residual disease or microscopic surgical margins, results in predictable recurrence.18
Natural History of VHL-related Hemangioblastomas
Recently, Ammerman et al.4 studied CNS hemangioblastoma progression in VHL patients serially imaged and clinically evaluated for at least 10 years. Hemangioblastomas exhibited a saltatory growth pattern characterized by periods of rapid growth (mean: 1.1 ± 1.3 years) followed by periods of quiescence (2.1 ± 1.6 years) in nearly every case (94% of 143 tumors). Hemangioblastomas averaged 1.85 quiescent periods between growth periods before becoming symptomatic and requiring resection. Most hemangioblastomas (97%) demonstrated radiographic progression but only 50% required treatment for symptom formation. Moreover, nearly half (45%) of tumors that required resection (symptomatic) were not apparent on initial MRI.
Previous Reports
Previous reports have described the use of SRS for VHL-associated and sporadic CNS hemangioblastomas. Despite emerging evidence that indicates a better response for VHL-associated hemangioblastomas to SRS than sporadic hemangioblastomas, prior reports typically presented aggregate results.5–7,19–28 Three reports have focused exclusively on VHL-associated hemangioblastomas response to SRS, but are limited by the small number of patients and/or by short follow-up (3.6 years or less; Table 3).5–7
Table 3.
Previous studies of stereotactic radiosurgery treatment of central nervous system hemangioblastomas in von Hippel–Lindau disease patients
| Author | Modality | Total patients | Total tumors | Mean follow-up (years) | Control rate (%) |
|---|---|---|---|---|---|
| Page et al.5 | LINAC | 4 | 11 | >1.5 (0.6–2.5) | 100a |
| Chang et al.6 | LINAC | 13 | 29 | 3.6 (0.9–7) | 97 |
| Rajaraman et al.7b | GK | 13 | 27 | 2.8 (0.6–6.6) | 83 |
| Present study | LINAC/GK | 20 | 44 | 8.5 (3–17.6) | 68 |
Abbreviations: LINAC, linear accelerator; GK, gamma knife.
aOne patient required cyst-peritoneal shunt placement for persistent symptoms.
bExcluding one patient with no radiologic follow-up.
Clinical Implications
SRS for Hemangioblastomas in VHL
On the basis of the reports of high rates of local control (80%–90%) after SRS for hemangioblastomas, a number of centers have begun to treat asymptomatic tumors in VHL patients to potentially minimize the number of surgical resections and/or avoid resection of tumors that may be associated with increased operative risk. Previous reports (sporadic and VHL-associated) and the current study demonstrate SRS progression-free survival greater than 90% two years after treatment. Although this suggests that local control rates for SRS-treated tumors may be significantly better than the published natural history of untreated tumors, the long-term analysis performed in the current study reveals a diminishing margin of benefit (70%, 61%, and 51% local control rates at 8, 10, and 15 years after the treatment) at distant time points.
Early SRS Effect on Tumor Volume
SRS-treated hemangioblastomas demonstrated a variety of early volumetric changes. Although the majority of early changes predicted final outcome, in 25% of cases the initial response did not accurately reflect the final outcome. These changes are critical in determining long-term control and optimizing individual patient management. Specifically, tumors may have been incorrectly classified as regressing, progressing, or stable with shorter follow-up than patients received in this study. These transient volumetric changes all occurred within 3 years of SRS, emphasizing the need for lengthy follow-up, and the potential for conservative management in asymptomatic cases despite early radiographic progression.
Variables Affecting SRS Control
Although prior reports describing SRS treatment of CNS hemangioblastomas (sporadic and VHL-associated) have found the presence of a peritumoral cyst,27,28 lower prescribed marginal dose,20,28 and larger tumor size20,23,28 are associated with poorer control, analyses of treated tumors in this study did not identify variables associated with treatment failure. There are several reasons for these differences. First, the limited number of treated tumors with peritumoral cysts (3 tumors; 7%) at the time of SRS precludes identification of a significant association between SRS control rates and this biologic feature. The development and/or progression of edema and peritumoral cysts early after SRS treatment is likely due to radiation-induced increased tumor vessel permeability and underscores the need for the judicious use of SRS in tumors with associated symptomatic edema/cysts.29 Second, because the mean dose prescribed to the tumor margins used in this study (18.9 Gy) was higher than in reports that found dosing a significant factor (mean: 16 Gy or less), and the number of tumors receiving <16 Gy (6 tumors, 14%) was small, this study could not identify statistical differences in SRS control rates with varying treatment doses.20,28 Finally, the longer follow-up in this series may have contributed to the lack of correlation between tumor size and failure. Consistent with previous studies with shorter follow-up, this study identified a significant correlation between larger tumor size and failure at 3-year interim analysis (P = 0.049), but this association was not present at last follow-up (mean: 8.5 years). Moreover, although prior studies indicate that smaller tumors respond best to SRS, a large number of small asymptomatic hemangioblastomas in this study progressed several years (mean time to progression: 5.9 years) after treatment. Because of the long delay between treatment and progression, previous studies with shorter follow-up may have missed progression in comparable tumors.
Effects of Radiation in a Neoplasia Syndrome
Despite concerns that radiation to surrounding haploinsufficient tissues may result in additive mutational events, no increase in hemangioblastoma formation was identified during the posttreatment period.30 Previous studies in nonirradiated VHL patients report a similar de novo hemangioblastoma formation rate.2 Moreover, malignant transformation of a treated hemangioblastoma or development of a de novo malignancy was not seen.
Pathologic Features of Radiated Tumors
Consistent with prior irradiation, histologic evaluation of resected SRS-treated tumors demonstrated sclerotic regions characterized by coagulative necrosis, a paucicellular stromal component, and hyalinized vessels. Areas of viable-appearing stromal components with hyalinized vasculature were also present. These areas of normal appearing hemangioblastoma elements likely represent foci of tumor progression and cells refractory to radiotherapy.
Conclusions
The early age of hemangioblastoma-related symptom development (mean age: 33 years)1,2 necessitates that ideal treatment not only be safe but durable (often decades). Moreover, because of the inability to predict which tumors will cause symptoms in VHL, developing targeted preventative management paradigms is currently not possible. Since nearly half of VHL-associated hemangioblastomas that will become symptomatic are not visible on initial imaging studies and because the local control rate of SRS-treated hemangioblastomas diminishes with longitudinal follow-up, the prophylactic treatment of imaging-evident asymptomatic tumors (whether they are growing or quiescent) is unnecessary.4 Because complete surgical resection is associated with absolute local control and can be performed with minimal morbidity in the vast majority of cases,14–17 it should remain the treatment of choice for most VHL-associated CNS hemangioblastomas. SRS-treatment should be reserved for patients who cannot safely undergo surgery or harbor a tumor with specific features associated with unfavorable resection risks.
Conflict of interest statement. None declared.
Funding
This research was supported by the Intramural Research Program of the National Institute of Neurologic Disorders and Stroke at the National Institutes of Health.
References
- 1.Lonser RR, Glenn GM, Walther M, et al. Von Hippel–Lindau disease. Lancet. 2003;361(9374):2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 2.Wanebo JE, Lonser RR, Glenn GM, Oldfield EH. The natural history of hemangioblastomas of the central nervous system in patients with von Hippel–Lindau disease. J Neurosurg. 2003;98(1):82–94. doi: 10.3171/jns.2003.98.1.0082. [DOI] [PubMed] [Google Scholar]
- 3.Butman JA, Linehan WM, Lonser RR. Neurologic manifestations of von Hippel–Lindau disease. JAMA. 2008;300(11):1334–1342. doi: 10.1001/jama.300.11.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ammerman JM, Lonser RR, Dambrosia J, Butman JA, Oldfield EH. Long-term natural history of hemangioblastomas in patients with von Hippel–Lindau disease: implications for treatment. J Neurosurg. 2006;105(2):248–255. doi: 10.3171/jns.2006.105.2.248. [DOI] [PubMed] [Google Scholar]
- 5.Page KA, Wayson K, Steinberg GK, Adler JR., Jr. Stereotaxic radiosurgical ablation: an alternative treatment for recurrent and multifocal hemangioblastomas. A report of four cases. Surg Neurol. 1993;40(5):424–428. doi: 10.1016/0090-3019(93)90225-p. [DOI] [PubMed] [Google Scholar]
- 6.Chang SD, Meisel JA, Hancock SL, Martin DP, McManus M, Adler JR., Jr. Treatment of hemangioblastomas in von Hippel–Lindau disease with linear accelerator-based radiosurgery. Neurosurgery. 1998;43(1):28–34. doi: 10.1097/00006123-199807000-00018. discussion 28–35. [DOI] [PubMed] [Google Scholar]
- 7.Rajaraman C, Rowe JG, Walton L, Malik I, Radatz M, Kemeny AA. Treatment options for von Hippel–Lindau's haemangioblastomatosis: the role of gamma knife stereotactic radiosurgery. Br J Neurosurg. 2004;18(4):338–342. doi: 10.1080/02688690400004944. [DOI] [PubMed] [Google Scholar]
- 8.Melmon KL, Rosen SW. Lindau's disease. Am J Med. 1964;36:595–617. doi: 10.1016/0002-9343(64)90107-x. [DOI] [PubMed] [Google Scholar]
- 9.Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- 10.Lundin P, Pedersen F. Volume of pituitary macroadenomas: assessment by MRI. J Comput Assist Tomogr. 1992;16(4):519–528. doi: 10.1097/00004728-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Neumann HP, Wiestler OD. Clustering of features of von Hippel–Lindau syndrome: evidence for a complex genetic locus. Lancet. 1991;337(8749):1052–1054. doi: 10.1016/0140-6736(91)91705-y. [DOI] [PubMed] [Google Scholar]
- 12.Seizinger BR, Rouleau GA, Ozelius LJ, et al. Von Hippel–Lindau disease maps to the region of chromosome 3 associated with renal cell carcinoma. Nature. 1988;332(6161):268–269. doi: 10.1038/332268a0. [DOI] [PubMed] [Google Scholar]
- 13.Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel–Lindau disease tumor suppressor gene. Science. 1993;260(5112):1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 14.Jagannathan J, Lonser RR, Smith R, DeVroom HL, Oldfield EH. Surgical management of cerebellar hemangioblastomas in patients with von Hippel–Lindau disease. J Neurosurg. 2008;108(2):210–222. doi: 10.3171/JNS/2008/108/2/0210. [DOI] [PubMed] [Google Scholar]
- 15.Lonser RR, Wait SD, Butman JA, et al. Surgical management of lumbosacral nerve root hemangioblastomas in von Hippel–Lindau syndrome. J Neurosurg. 2003;99(1 suppl):64–69. doi: 10.3171/spi.2003.99.1.0064. [DOI] [PubMed] [Google Scholar]
- 16.Lonser RR, Weil RJ, Wanebo JE, DeVroom HL, Oldfield EH. Surgical management of spinal cord hemangioblastomas in patients with von Hippel–Lindau disease. J Neurosurg. 2003;98(1):106–116. doi: 10.3171/jns.2003.98.1.0106. [DOI] [PubMed] [Google Scholar]
- 17.Weil RJ, Lonser RR, DeVroom HL, Wanebo JE, Oldfield EH. Surgical management of brainstem hemangioblastomas in patients with von Hippel–Lindau disease. J Neurosurg. 2003;98(1):95–105. doi: 10.3171/jns.2003.98.1.0095. [DOI] [PubMed] [Google Scholar]
- 18.Smalley SR, Schomberg PJ, Earle JD, Laws ER, Jr, Scheithauer BW, O'Fallon JR. Radiotherapeutic considerations in the treatment of hemangioblastomas of the central nervous system. Int J Radiat Oncol Biol Phys. 1990;18(5):1165–1171. doi: 10.1016/0360-3016(90)90454-r. [DOI] [PubMed] [Google Scholar]
- 19.Niemela M, Lim YJ, Soderman M, Jaaskelainen J, Lindquist C. Gamma knife radiosurgery in 11 hemangioblastomas. J Neurosurg. 1996;85(4):591–596. doi: 10.3171/jns.1996.85.4.0591. [DOI] [PubMed] [Google Scholar]
- 20.Patrice SJ, Sneed PK, Flickinger JC, et al. Radiosurgery for hemangioblastoma: results of a multiinstitutional experience. Int J Radiat Oncol Biol Phys. 1996;35(3):493–499. doi: 10.1016/s0360-3016(96)80011-3. [DOI] [PubMed] [Google Scholar]
- 21.Chakraborti PR, Chakrabarti KB, Doughty D, Plowman PN. Stereotactic multiple are radiotherapy. IV—Haemangioblastoma. Br J Neurosurg. 1997;11(2):110–115. doi: 10.1080/02688699746447. [DOI] [PubMed] [Google Scholar]
- 22.Pan L, Wang EM, Wang BJ, et al. Gamma knife radiosurgery for hemangioblastomas. Stereotact Funct Neurosurg. 1998;70(suppl 1):179–186. doi: 10.1159/000056420. [DOI] [PubMed] [Google Scholar]
- 23.Jawahar A, Kondziolka D, Garces YI, Flickinger JC, Pollock BE, Lunsford LD. Stereotactic radiosurgery for hemangioblastomas of the brain. Acta Neurochir (Wien). 2000;142(6):641–644. doi: 10.1007/s007010070107. discussion 644–645. [DOI] [PubMed] [Google Scholar]
- 24.Park YS, Chang JH, Chang JW, Chung SS, Park YG. Gamma knife surgery for multiple hemangioblastomas. J Neurosurg. 2005;102(suppl):97–101. doi: 10.3171/jns.2005.102.s_supplement.0097. [DOI] [PubMed] [Google Scholar]
- 25.Tago M, Terahara A, Shin M, et al. Gamma knife surgery for hemangioblastomas. J Neurosurg. 2005;102(suppl):171–174. doi: 10.3171/jns.2005.102.s_supplement.0171. [DOI] [PubMed] [Google Scholar]
- 26.Wang EM, Pan L, Wang BJ, et al. The long-term results of gamma knife radiosurgery for hemangioblastomas of the brain. J Neurosurg. 2005;102(suppl):225–229. doi: 10.3171/jns.2005.102.s_supplement.0225. [DOI] [PubMed] [Google Scholar]
- 27.Matsunaga S, Shuto T, Inomori S, Fujino H, Yamamoto I. Gamma knife radiosurgery for intracranial haemangioblastomas. Acta Neurochir (Wien) 2007;149(10):1007–1013. doi: 10.1007/s00701-007-1274-2. discussion 1013. [DOI] [PubMed] [Google Scholar]
- 28.Kano H, Niranjan A, Mongia S, Kondziolka D, Flickinger JC, Lunsford LD. The role of stereotactic radiosurgery for intracranial hemangioblastomas. Neurosurgery. 2008;63(3):443–451. doi: 10.1227/01.NEU.0000313120.81565.D7. [DOI] [PubMed] [Google Scholar]
- 29.Lonser RR, Vortmeyer AO, Butman JA, et al. Edema is a precursor to central nervous system peritumoral cyst formation. Ann Neurol. 2005;58(3):392–399. doi: 10.1002/ana.20584. [DOI] [PubMed] [Google Scholar]
- 30.Evans DGR, Birch JM, Ramsden RT, Sharif S, Baser ME. Malignant transformation and new primary tumours after therapeutic radiation for benign disease: substantial risks in certain tumor prone syndromes. J Med Genet. 2006;43(4):289–294. doi: 10.1136/jmg.2005.036319. [DOI] [PMC free article] [PubMed] [Google Scholar]