Abstract
Patients with (a) recurrent malignant glioma (MG): glioblastoma (GBM) or recurrent anaplastic glioma (AG), and (b) nonprogressive (NP) GBM following radiation therapy (RT) were eligible. Primary objective for recurrent MG was progression-free survival at 6 months (PFS-6) and overall survival at 12 months for NP GBM post-RT. Secondary objectives for recurrent MGs were response, survival, assessment of toxicity, and pharmacokinetics (PKs). Treatment with enzyme-inducing antiepileptic drugs was not allowed. Patients received 150 mg/day erlotinib. Patients requiring surgery were treated 7 days prior to tumor removal for PK analysis and effects of erlotinib on epidermal growth factor receptor (EGFR) and intracellular signaling pathways. Ninety-six patients were evaluable (53 recurrent MG and 43 NP GBM); 5 patients were not evaluable for response. PFS-6 in recurrent GBM was 3% with a median PFS of 2 months; PFS-6 in recurrent AG was 27% with a median PFS of 2 months. Twelve-month survival was 57% in NP GBMs post-RT. Primary toxicity was dermatologic. The tissue-to-plasma ratio normalized to nanograms per gram dry weight for erlotinib and OSI-420 ranged from 25% to 44% and 30% to 59%, respectively, for pretreated surgical patients. No effect on EGFR or intratumoral signaling was seen. Patients with NP GBM post-RT who developed rash in cycle 1 had improved survival (P < .001). Single-agent activity of erlotinib is minimal for recurrent MGs and marginally beneficial following RT for NP GBM patients. Development of rash in cycle 1 correlates with survival in patients with NP GBM after RT.
Keywords: erlotinib, glioblastoma, glioma, meningioma, pharmacokinetics
The survival outcomes for glioblastoma (GBM) remain poor. Surgery and radiation therapy (RT) are the mainstay of therapy with survivals between 9 and 12 months.1 The current standard of care in newly diagnosed GBM consists of RT with concomitant temozolomide followed by at least 6 months of maintenance temozolomide.2 FDA-approved treatments for recurrent malignant gliomas (MGs) are limited to temozolomide for anaplastic astrocytoma and implantable carmustine wafers (Gliadel, MGI Pharmaceuticals). New agents are clearly needed. Agents that target specific cell surface receptors (i.e., receptor tyrosine kinases that work via the intracellular side) and intracellular signaling molecules or angiogenesis are currently being studied.
Epidermal growth factor receptor (EGFR) is over expressed in 40%–60% of GBM. Activation increases cell proliferation, migration, and invasiveness and decreases apoptosis by downstream signaling, especially via the Ras cascade.3,4 EGFR gene amplification is frequently associated with a mutant EGFR called variant 3 (EGFRvIII) in which deletion of exons 2–7 generates a constitutively active receptor, even in the absence of ligand-binding.3–6
In culture, inhibition of EGFR modulates GBM cell proliferation and invasion and affects differentiation.7–9 Erlotinib targets EGFR and EGFRvIII;10,11 EGFRvIII blockade inhibits constitutive EGFRvIII tyrosine kinase activity, the growth of EGFRvIII-transformed cells, and selectively down-regulates EGFRvIII-mediated induction of effector genes regulating tumor invasiveness.10,12
A phase II study of erlotinib in patients with recurrent MGs (anaplastic gliomas [AGs] [anaplastic astrocytomas or oligodendrogliomas] or GBMs) and nonprogressive (NP) GBM post-RT was initiated as North American Brain Tumor Consortium Trial 01-03. The primary endpoint for patients with recurrent MG was 6-month progression-free survival (PFS-6) and overall survival (OS) for NP GBM post-RT.
Patients and Methods
This protocol was IRB approved at all participating institutions. All patients signed an informed consent prior to enrollment. Major eligibility criteria included age >18 years, life expectancy >8 weeks, and Karnofsky performance status (KPS) ≥60 with histologically confirmed disease. Two groups of patients were studied: (a) recurrent GBM or AG, and (b) patients who had NP GBM post-RT. Patients with a previous diagnosis of a low-grade glioma were eligible if their tumor had histologically confirmed malignant transformation. All patients were required to have pretreatment brain CT or MRI within 14 days of starting therapy, on a stable steroid dosage for ≥5 days. Because erlotinib is metabolized by the cytochrome P450 isoenzyme 3A4 (70%) and CYP 1A2 (30%), patients taking enzyme-inducing anti-epileptic drugs (EIAEDs) were not eligible.
Patients with recurrent MG were limited to no more than 2 prior relapses and 2 prior treatments. Patients with NP GBM post-RT could not have prior chemotherapy (including temozolomide before, during or after RT, or Gliadel Wafers). All patients were required to have adequate bone marrow function (WB ≥3000/µL, ANC ≥1500/mm3, platelet count of ≥100 000/mm3, and hemoglobin ≥10 mg/dL), adequate liver function (SGOT and bilirubin <1.5 times ULN), and adequate renal function (creatinine <1.5 mg/dL) within 14 days prior to registration. Women of childbearing potential and men had to use adequate contraception for the duration of the study for 12 weeks after study completion and could not be pregnant or breast-feeding.
Treatment
Erlotinib was supplied by the NCI Division of Cancer Treatment and Diagnosis under a clinical trials agreement with OSI Pharmaceuticals. The tablets were taken either 1 h before or 2 h after food, in the morning. The dose was 150 mg/day on a continuous daily basis.
Patients with recurrent disease were treated in 4-week intervals (one cycle). Treatment continued indefinitely as long as there were no unacceptable toxicities or tumor progression. Patients with recurrent MGs who were candidates for surgery at the time of study entry were considered for an optional preoperative study to evaluate biological and tissue correlates. Erlotinib was administered for 7 days before surgery and then resumed 10–14 days postoperatively.
Patients with GBM with NP disease following RT started erlotinib no more than 6 weeks from the completion of radiation. Temozolomide or other adjuvant chemotherapy not allowed while on erlotinib.
Pretreatment and Treatment Evaluation
Prior to starting therapy, a complete history, physical examination, brain imaging, and blood work were required within 14 days. A CBC with differential and platelets and a comprehensive metabolic panel was performed every 2 weeks while on treatment. A physical and neurological examination was performed every 4 weeks and brain imaging every 8 weeks. All claimed radiographic responses were confirmed by central review (M.D.P.).
Pharmacokinetic Evaluation
Sample Collection
Whole blood (3 mL) was collected at the following times: baseline, 1, 2, 4, 6, 8, 12, and 24 h after the first dose in cycle 1. Trough levels were obtained on days 8 and 1 of cycles 2, 3, and 5. For the analysis of alpha-1-acid glycoprotein (AGP), 5 mL of blood was collected in a red top tube and allowed to clot prior to centrifugation.
For surgical patients, a baseline blood sample was drawn prior to the start of erlotinib and at the time of tumor resection. Tumor tissue (0.5–1.0 cm3) was flash frozen in liquid nitrogen. Prior to analysis, the tissue was weighed and homogenized in 1 mL of HPLC analytical grade methanol.
Plasma and serum samples were transferred to individually labeled tubes and stored at ≤ − 20°C until analysis. Flash-frozen tissue samples were stored at ≤ − 70°C until analysis.
Analytical Methods and Pharmacokinetic Analyses
Concentrations of erlotinib and its O-demethylated isomeric metabolites (OSI-420/OSI-413, collectively called OSI-420) in plasma and tumor tissue were analyzed as described previously.13 A radial immunodiffusion kit (Bindarid, Birmingham) was used for the measurement of AGP in serum. Erlotinib and OSI-420 plasma concentrations were analyzed by noncompartmental methods. Pharmacokinetic (PK) parameters are reported as mean ± SD.
Molecular Pathway Analysis
Protein and DNA extracts from flash-frozen tissue were used for the evaluation of the EGFR receptor and its signaling mechanisms as described,13 including EGFR gene sequencing, EGFR amplification or deletions by array-based comparative genomic hybridization, and total EGFR protein expression, and the analysis of effectors including phopho-EGFR, AKT, and ERK by Western blot.
Response and Toxicity
Radiographic responses were based on the Macdonald criteria.14 Adverse events were graded according to the NCI Common Toxicity Criteria, version 2.0.
Statistical Methods
The primary endpoint for recurrent MG patients was PFS-6. The planned sample size was 48 (32 GBM, 16 AG). For the GBM cohort, the goal was to discriminate between a 15% and 35% PFS-6 rate with α ≤ 0.1 and power of ≥0.9. With fewer AG patients, it was recognized that improvements of interest might not achieve statistical significance using the usual α-level and the emphasis was on estimation.
For the NP GBM cohort, the primary endpoint was OS at 12 months. The trial used a 12-month survival estimate of 65%. This was based on a historical database of 205 GBM patients treated on prospective phase-2 clinical trials at UCSF who had stable disease following XRT. The target accrual was 55 patients providing α ≤ 0.1 and power of 88% to detect a 12-month survival improvement from 65% to 80%. Ten percent over-accrual was permitted to assure sufficient number of eligible treated patients.
Analysis Methods
Response rate, PFS-6 (recurrent MG), and OS-12 (NP GBM post-RT) were based on the proportion of patients known to have achieved that endpoint using the concept of intent-to-treat. Median PFS and OS were calculated from the Kaplan–Meier curves. Time was measured from registration date except for patients who received therapy prior to surgery, when the date of first postsurgery erlotinib dose was used. All patients receiving protocol treatment were included in evaluation of safety.
For the analysis of rash as a predictor of outcome, only patients with PFS ≥4 weeks were included to prevent a bias for early failures who did not have time to develop a rash. The analysis used the Cox proportional hazards model to adjust for age and baseline KPS.
Results
Between August 15, 2002, and August 18, 2005, 104 patients were enrolled (Table 1). Eight patients were not included in the efficacy analyses due to ineligible histology (1), prior treatment history (4), and failure to initiate erlotinib therapy (3). Hence, 38 patients with recurrent GBM, 15 with recurrent AG, and 43 with NP GBM post-RT were included in the efficacy analyses. Pathology was unavailable for central review (by K.A.A.) in 3 recurrent MG and 8 NP GBM post-RT patients.
Table 1.
Demographics
| Patient cohorta | Number of patients | Patient cohorta | Number of patients |
|---|---|---|---|
| Recurrent MG | 59 | Newly diagnosed GBM post-RT | 45 |
| GBM | 42 | GBM | 44 |
| AA | 14 | AA | 1 |
| AO | 1 | ||
| OA | 1 | ||
| PXA | 1 | ||
| Gender | Gender | ||
| Men | 34 | Men | 35 |
| Women | 25 | Women | 10 |
| Age | Age | ||
| Median | 54 | Median | 52 |
| Range | 29–78 | Range | 19–72 |
| KPS | KPS | ||
| Median | 80 | Median | 90 |
| 100 | 5 | 100 | 10 |
| 90 | 20 | 90 | 25 |
| 80 | 16 | 80 | 6 |
| 70 | 16 | 70 | 3 |
| 60 | 2 | 60 | 1 |
| Prior RT | 100 | ||
| Prior chemotherapies | Extent of resection | ||
| 0 | 4 | Biopsy | 7 |
| 1 | 31 | Subtotal | 18 |
| 2 | 21 | Gross total | 20 |
| 3 | 3 |
aOn the basis of cohort patients were initially enrolled in.
Patient demographics for all cohorts are listed in Table 1. Seven GBM and 3 AG patients enrolled into the surgical arm of the trial. The median number of treatment cycles for recurrent tumors was 3 (range: 0–57 for recurrent MG and 1–24 for NP GBM).
Efficacy
Recurrent MG
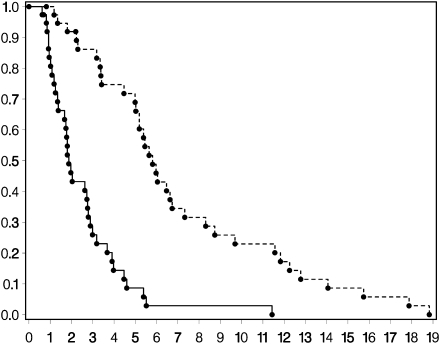
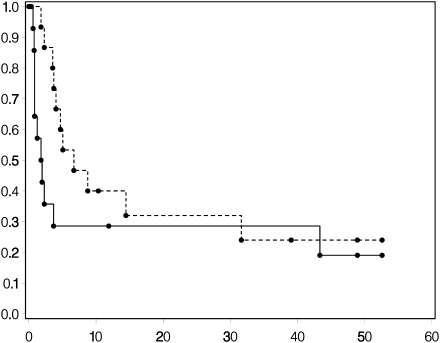
One patient with recurrent GBM and 4 patients with recurrent AG achieved PFS at 6 months for a PFS-6 of 3% and 27%, respectively (Figs 1 and 2). Median PFS was 2 months for both histologies. Two patients with AG remained progression-free beyond 1 year. Median OS was 6 months for the GBM patients and 7 months for AG patients (Figs 1 and 2). There was 1 CR (AG), 1 PR (AG), 5 SD (2 AG and 3 GBM), and 41 patients with PD (15 AG and 26 GBM).
Fig. 1.
Kaplan–Meier curves for recurrent GBMs for OS (upper curve) and PFS (lower curve).
Fig. 2.
Kaplan–Meier curves for recurrent AGs for OS (upper curve) and PFS (lower curve).
Among the 9 patients in the surgical arm who restarted erlotinib postoperatively (7 GBM and 2 AG), 1 patient with GBM-developed progressive disease at 11 months. Eight other patients developed disease progression in ≤3 months. One patient was censored due to postoperative complications and failure to resume treatment. Inclusion of these patients did not appear to bias assessment of the primary endpoint of PFS-6.
Nonprogressive GBM
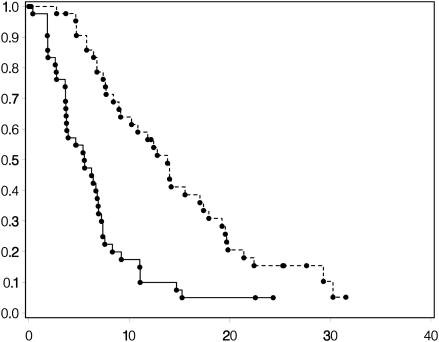
Among the stable GBM patients, estimated 1-year OS rate was 53%, estimated 1-year PFS was 9%, and the median OS was 14 months (95% CI 9–17) (Fig. 3).
Fig. 3.
Kaplan–Meier curves NP GBMs post-RT for OS (upper curve) and PFS (lower curve).
Rash
As a post hoc analysis, we assessed whether any grade of rash observed during the first 28 days of therapy predicted for either PFS or OS. If the early failures (PFS ≤4 weeks) are excluded, the number of AG patients were too few for analysis. When adjusted for age and KPS, for recurrent GBM patients, rash did not predict PFS or OS (P = .53 and .13, respectively). For patients with NP GBM post-RT, there was a statistically significant improvement in OS (hazard ratio = .19, P < .001). The 15 patients with no rash had a median survival of 8 months compared with a median of 18 months for the 26 patients who developed a rash in their first cycle. Rash was not a predictor of PFS in this patient group (P = .41).
Toxicity
There were 810 drug-related adverse events reported in 99 patients. Rash and diarrhea were the most common toxicities. Twenty-nine patients had 37 drug-related grade 3–5 adverse events (Table 2). The 2 patients who received no treatment were excluded from the toxicity assessment.
Table 2.
Drug-related grade 3–5 toxicitiesa
| Grade | Toxicity | Number |
|---|---|---|
| 3 | Hypocalcemia | 1 |
| Hypokalemia | 1 | |
| Elevated bilirubin | 1 | |
| Headache | 1 | |
| Thrombocytopenia | 1 | |
| Seizure | 1 | |
| Abdominal pain | 1 | |
| Myalgia | 1 | |
| Muscle weakness | 1 | |
| Dehydration | 1 | |
| Hypophosphatemia | 2 | |
| Diarrhea | 2 | |
| Weight loss | 2 | |
| Elevated serum glutamic pyruvic transaminase | 2 | |
| Fatigue | 3 | |
| Infection without neutropenia | 3 | |
| Rash | 11 | |
| 4 | Hypomagnesemia | 1 |
| 5 | Seizure | 1 |
aTwenty-nine patients had one or more grade 3–5 toxicities
PK Data and Tissue Analysis
The mean (±SD) PK parameters for erlotinib and OSI-420 are summarized in Table 3. Within 3–4 h of administration of drug, peak concentrations of erlotinib (872 ± 399 ng/mL) and OSI-420 (68 ± 45 ng/mL) were achieved. Trough steady-state levels were obtained by day 8 with accumulation ratios for erlotinib and OSI-420 of 2.5 and 3.5, respectively. Exposure to OSI-420 was minimal with a relative metabolic ratio (OSI-420 AUC0–24 to erlotinib AUC0–24) of 7%. AGP was overexpressed (n = 75); average was 102 (±39) mg/dL (normal 73 mg/dL). There was a significant (P < .05) albeit poor correlation (r = 0.1) between AGP levels and both erlotinib and OSI-420 Cpmax and AUC values (e.g., higher AGP, higher AUC, and Cpmax values).
Table 3.
PK parameters of erlotinib and OSI-420
| Number of patients | Dose (mg) | Cpmax (ng/mL) |
Tmax (h) |
AUC0–24 |
Erlotinib/OSI-420 trough levels (ng/mL) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Erlotinib | OSI-420 | erlotinib | OSI-420 | Erlotinib (µg h/mL) | OSI-420 (ng h/mL) | MR | C1D2 | C1D8 | C2D1 | C3D1 | C5D1 | ||
| 76 | 150 | 872 (±399) [76] | 68 (±45) [76] | 3.0 (±1.91) [76] | 3.6 (±3.05) [76] | 11.86 (±5.01) [74] | 835 (±479) [74] | 0.071 (±0.03) [74] | 385/25 (±213) (±18) [74] | 975/87 (±535) (±76) [61] | 1059/100 (±551) (±111)[50] | 1050/76 (±518) (±35) [30] | 980/85 (±368) (±38) [12] |
Abbreviations: MR, metabolic ratio (OSI-420 AUC/Erlotinib AUC); Cpmax, peak plasma concentrations; Tmax, time of Cpmax; AUC, area under the curve.
Values expressed as mean ± SD. Number of patients is given in square-brackets.
Tumor tissue and a plasma sample at the time of tumor resection were obtained from 6 patients (Table 4). The concentrations should have been reflective of trough steady-state levels as drug was administered for 7 days. The mean cycle 1, day 8 trough levels (erlotinib/OSI-420; 975 ± 535/87 ± 76 ng/mL) compare favorably with the mean trough levels (erlotinib/OSI-420; 761 ± 547/70 ± 68 ng/mL) obtained on day 8 at the time of surgical resection. Two patients with the highest tissue concentration of erlotinib and OSI-420 were likely contaminated by blood clots as their values were beyond what would be expected. For the remaining 4 patients, the tissue-to-plasma ratio (%) of erlotinib and the active metabolite OSI-420 ranged from 6% to 8% and 5% to 11% ng/mL or from 25% to 44% and 30% to 59% normalized to nanograms per gram dry weight, respectively.
Table 4.
Brain tumor concentrations of erlotinib and metabolite (OSI-420)
| Non-EIAED patients dose (150 mg) | Plasma concentration (ng/mL ; erlotinib/OSI-420) | Tumor tissue concentration (ng/g dry weight; erlotinib/OSI-420) | Tumor/plasma ratio (%) (ng/g; erlotinib/OSI-420) | Tumor/plasma ratio (%) (ng/mL; erlotinib/OSI-420) |
|---|---|---|---|---|
| 1 | 271/14 | 67/BLQa | 25/— | 6/— |
| 2 | 275/14 | 73/5 | 27/36 | 6/7 |
| 3 | 1345/105 | 581/31 | 43/30 | 7/5 |
| 4 | 1493/187 | 656/111 | 44/59 | 8/11 |
| 5b | 793/71 | 941/90 | 118/127 | 50/54 |
| 6b | 386/29 | 664/73 | 172/252 | 19/28 |
aBLQ, below limit of quantification.
bSuspected contamination with blood clot.
The effects of erlotinib on tumor tissue have been reported.13 The relevant findings suggested that erlotinib penetration into tumor was not high enough to consistently inhibit EGFR phosphorylation. There was no consistent effect on ERK or AKT phosphorylation over control samples; however, it was observed that AKT-activity status may represent a particularly important assay for EGFR inhibitor efficacy, as observed by others.15, 16
Discussion
Single-agent erlotinib had no efficacy in recurrent MG. The PFS-6 for patients with recurrent GBM was 3% and 27% for AG. The 12-month OS in patients with NP GBM post-RT was 57%. Neither group met our statistical goal for success.
Data from several trials using gefitinib or erlotinib in recurrent MG have been published or presented in abstract form.17,18 In these trials, primarily in recurrent GBM, the PFS-6 ranged from 0% to 33% with a median TTP of 1.7–4.7 months.17–21 Outcome did not appear to correlate with expression of wild-type or mutant EGFR or with gene amplification; diarrhea was predictive for OS, whereas skin rash was a borderline predictor for PFS in the gefitinib trials.13,17–19 Response and EGFR gene amplification had a minor correlation in only one erlotinib trial.20,21 A phase I trial of erlotinib ± temozolomide reported 8 of 57 patients responding; 6 of whom were only on erlotinib and had a more than 6-month PFS.22 One trial using a monoclonal antibody against EGFR (Cetuximab) as a single agent for patients with recurrent high-grade glioma had a PFS-6 of 7.3% and an OS of 5.1 months, which appears similar to the small molecules discussed earlier.23
For the NP GBM post-RT, there was a median OS of 14 months. Krishnan et al.24 treated 19 patients with erlotinib and RT with a median OS of 13 months and median TTP of 6.2 months. A phase II trial of gefitinib in NP GBM patients post-RT did not significantly improve overall (48.9% at 12 months) or PFS (13.3% at 12 months) over historical controls, except in patients who had diarrhea on a post hoc analysis.18 Three additional trials have evaluated erlotinib in the adjuvant setting either used in conjunction with maintenance temozolomide after RT + temozolomide (1 trial) or used with RT + temozolomide and then with maintenance temozolomide (2 trials). The median OS for those studies was 8.2 months, 20 months, and 14.5 months, respectively.25–27 A trial of Cetuximab with RT + temozolomide followed by standard temozolomide has also been presented with an OS at 12 months of 87%.28 Our data fall within the range of these other trials, and differences in survival are likely due to patient variability.
The most common toxicity seen was grade 1 and 2 rash. A relationship between rash and survival has been reported with EGFR inhibitors.29,30 Although our numbers were small, development of rash in cycle 1 did significantly increase OS in NP GBM post-RT, but the significance of this remains unclear.
The PK parameters in our trial are similar to values in patients not receiving anticonvulsants who had nonsmall cell lung cancer (NSCLC) (Table 5).31 Following 7 days of erlotinib treatment at 150 mg in the surgical group (n = 6), the tumor-to-plasma ratios of erlotinib and its active metabolite in tumor were 0.38 and 0.48, respectively. The data are limited by the number of patients and only one tissue specimen per patient representing a trough level 24 h after dosing. Not knowing erlotinib tissue distribution kinetics, it is possible that levels at earlier time-points could have been higher than what we observed at 24 h. Erlotinib and OSI-420 penetration into the CSF has been reported in two publications; CSF levels were about 1%–5% of plasma when given either IV or orally.32,33 The CSF levels are lower than what we measured in tissue and may be related to better brain penetration through a dysfunctional blood-brain barrier than into CSF. Gefitinib may have greater brain tissue penetration than erlotinib. Following treatment with gefitinib at 500 mg/day for 7 days in non-EIAED patients, gefitinib concentrations in brain tumor tissue were 221%–370% of the corresponding plasma concentrations (Lassman et al.13 and F.L., unpublished). Hofer and Frei34 were able to measure gefitinib in tumor tissue in 7 GBM patients evaluated, even with low to no plasma level of drug in 4 patients.
Table 5.
Study population groups and erlotinib dose
| Parameters | Non-EIAEDs (n = 76)a (150 mg/d) | Phase I/IIb (n = 18) (150 mg/d) |
|---|---|---|
| Cmax (ng/mL) | 872 (±399) | 983 (±500) |
| Tmax (h) | 3.0 (±1.9) | 4.1 (±3.1) |
| AUC0–24 (µg h/mL) | 11.86 (±5.01) | 12.94 (±4.56) |
| Css min (µg/mL) | 0.98 (±0.54) | 1.00 (±0.67) |
aCurrent study.
bHerbst et al.31.
How do we account for the limited activity seen with single-agent erlotinib? First, in studies in newly diagnosed GBM, balancing for known prognostic variables, EGFR fails to consistently hold-up the prognostic relevance in multivariate analysis so its relevance in gliomas is unclear.35,36 Second, the amount of drug present in tumor may not be sufficient for therapeutic benefit based on the surgical patients studied.13 There have been numerous EGFR ectodomain mutations (e.g., EGFRv111, R108K) identified in GBMs as discussed later. Many of these ectodomain mutants in cell cultures are sensitive to unbound erlotinib after 48 h exposure with IC50 concentrations between 50 and 150 nM (20–59 ng/mL).37 However, after a 2-week co-incubation of the R108K mutation with erlotinib (concentrations up to 10 µM [4 µg/mL]), an IC50 was never reached nor complete inhibition of autophosphorylation;38 suggesting that even at therapeutic concentrations activity may not occur. Also in a dose-escalation trial, the MTD of single-agent erlotinib was 200 and 250 mg/day when used with temozolomide.15 Third, EGFR inhibitors may be appropriate for only certain subpopulations.13,15,16,39 Haas-Kogan et al.16 found that, of the responders to erlotinib, a larger percentage had low levels of PKB/pAKT and high levels of EGFR expression. Mellinghoff et al.15 found that coexpression of EGFRvIII and wild-type PTEN (associated with low-pAKT) was significantly correlated with response. These results are similar to the results of Haas-Kogan et al. in that intact PTEN is associated with suppressed PI3K/AKT signaling, and constitutively activating mutations of EGFR such as EGFRvIII are generally found only among tumors with EGFR amplification. The findings by Mellinghoff et al.15 have not been uniformly confirmed in other trials where patients who have EGFRvIII and PTEN that are treated with EGFR inhibitors have in most cases not responded.21,27,40 The EGFR kinase domain mutation found in NSCLC that predict response to EGFR inhibitors is not found in gliomas;13,15,39 when present in patients with NSCLC response rates are approximately 75% compared with <10% if wild-type EGFR is present.41 A recent paper by Lee et al.37 reported novel missense mutations in the ectodomain of EGFR in approximately 14% of GBM; these mutations led to tumorigenicity of the cells tested and sensitivity to small-molecule EGFR inhibitors; hence, gliomas may have different mutations than those seen in NSCLC patients who are sensitive to EGFR inhibitors. These effects were independent of EGFRvIII, but had similar sensitivity. Two patterns of EGFR resistance have been proposed: the development of kinase-inhibitor resistant mutant clones, which could be overcome by kinase inhibitors that have a different mechanism of activity (acquired), or resistance, which is independent of EGFR and due to “bypass” intracellular pathways such as RAS or AKT (upfront).42 Finally, there are data that multiple receptor kinases are active at any one time and for this reason single agents may have limited activity.43
In conclusion, single-agent erlotinib has minimal activity in the settings studied and additional research should probably focus on multi-agent strategies, multi-targeting agents that may modulate EGFR pathways or enriching our patient selection by treating only patients with specific molecular profiles suggesting they would respond. This might be done in several ways, one might use an EGFR inhibitor only in patients with EGFRvIII and PTEN, since there is redundancy in active tyrosine kinases receptors one might inhibit the PDGF and the EGFR receptors or other combinations that are active in GBM as shown by Stommel et al.43
Funding
Grant Support: 5-U01CA62399-09 (J.J.R., L.E.A., A.B.L., and L.M.D.); NABTC # CA62399 and Member # CA62422, GCRC Grant # M01-RR00079 (S.M.C., K.R.L., and M.D.P.); CA62426 (J.G.K.); CA62412, GCRC Grant # CA16672 (W.K.A.Y. and M.R.G.); U01CA62407-08 (P.Y.W.); U01CA62421-08, GCRC Grant # M01 RR03186 (M.M. and H.I.R.); U01CA62405, GCRC Grant # M01-RR00056 (F.L.); U01 CA62399, GCRC Grant # M01-RR0865 (T.F.C.).
Acknowledgments
We thank Janelle Hibbert, Lisa Hughes, and Pamela Peterson for data management.
Conflict of interest statement. None declared.
References
- 1.DeAngelis LM. Chemotherapy for brain tumors—a new beginning. N Engl J Med. 2005;352:1036–1038. doi: 10.1056/NEJMe058010. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Nagane M, Coufal F, Lin H, et al. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996;56:5079–5086. [PubMed] [Google Scholar]
- 4.Nishikawa R, Ji XD, Harmon RC, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci USA. 1994;91:7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasheed BK, Wiltshire RN, Bigner SH, et al. Molecular pathogenesis of malignant gliomas. Curr Opin Oncol. 1999;11:162–167. doi: 10.1097/00001622-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Sehgal A. Molecular changes during the genesis of human gliomas. Semin Surg Oncol. 1998;14:3–12. doi: 10.1002/(sici)1098-2388(199801/02)14:1<3::aid-ssu2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 7.Han Y, Caday CG, Umezawa K, et al. Preferential inhibition of glioblastoma cells with wild-type epidermal growth factor receptors by a novel tyrosine kinase inhibitor ethyl-2,5-dihydroxycinnamate. Oncol Res. 1997;9:581–587. [PubMed] [Google Scholar]
- 8.Penar PL, Khoshyomn S, Bhushan A, et al. Inhibition of epidermal growth factor receptor-associated tyrosine kinase blocks glioblastoma invasion of the brain. Neurosurgery. 1997;40:141–151. doi: 10.1097/00006123-199701000-00032. [DOI] [PubMed] [Google Scholar]
- 9.Mishima K, Johns TG, Luwor RB, et al. Growth suppression of intracranial xenografted glioblastomas overexpressing mutant epidermal growth factor receptors by systemic administration of monoclonal antibody (mAb) 806, a novel monoclonal antibody directed to the receptor. Cancer Res. 2001;61:5349–5354. [PubMed] [Google Scholar]
- 10.Iwata KK, Provoncha K, Gibson N. Inhibition of mutant EGFRvIII transformed cells by tyrosine kinase inhibitor OSI-774 (Tarceva) Proc Am Soc Clin Oncol. 2002;21 abstract 79. [Google Scholar]
- 11.Vogelbaum MA, Goldlust S, Kanner A. The EGFR tyrosine kinase inhibitor TarcevaTM (OSI-774) shows activity against both wild-type and mutant EGFR. Neuro-Oncology. 2003;3 abstract ET-47. [Google Scholar]
- 12.Lal A, Glazer CA, Martinson HM, et al. Mutant epidermal growth factor receptor up-regulates molecular effectors of tumor invasion. Cancer Res. 2002;62:3335–3339. [PubMed] [Google Scholar]
- 13.Lassman AB, Rossi MR, Raizer JJ, et al. Molecular study of malignant gliomas treated with epidermal growth factor receptor inhibitors: tissue analysis from North American Brain Tumor Consortium Trials 01-03 and 00-01. Clin Cancer Res. 2005;11:7841–7850. doi: 10.1158/1078-0432.CCR-05-0421. [DOI] [PubMed] [Google Scholar]
- 14.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 15.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 16.Haas-Kogan DA, Prados MD, Tihan T, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97:880–887. doi: 10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- 17.Rich JN, Reardon DA, Peery T, et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22:133–142. doi: 10.1200/JCO.2004.08.110. [DOI] [PubMed] [Google Scholar]
- 18.Uhm JH, Ballman KV, Giannini C, et al. Phase II study of ZD1839 in patients with newly diagnosed grade 4 astrocytoma. J Clin Oncol. 2004;22 doi: 10.1016/j.ijrobp.2010.01.070. abstract 1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franceschi E, Cavallo G, Lonardi S, et al. Gefitinib in patients with progressive high-grade gliomas: a multicentre phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO) Br J Cancer. 2007;96:1047–1051. doi: 10.1038/sj.bjc.6603669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogelbaum MA, Peereboom D, Stevens G, et al. Phase II trial of the EGFR tyrosine kinase inhibitor erlotinib for single agent therapy of recurrent glioblastoma multiforme: interim results. J Clin Oncol. 2004;22 abstract 1558. [Google Scholar]
- 21.Van Den Bent MJ, Brandes A, Rampling R, et al. Randomized phase II trial of erlotinib (E) versus temozolomide (TMZ) or BCNU in recurrent glioblastoma multiforme (GBM): EORTC 26034. J Clin Oncol. 2007;25 doi: 10.1200/JCO.2008.17.5984. abstract 2005 (Meeting Abstracts) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prados MD, Lamborn KR, Chang S, et al. Phase 1 study of erlotinib HCl alone and combined with temozolomide in patients with stable or recurrent malignant glioma. Neuro-Oncology. 2006;8:67–78. doi: 10.1215/S1522851705000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neyns BSJ, Joosens E, Bouttens F, et al. A multicenter stratified phase II study of cetuximab for the treatment of patients with recurrent high-grade glioma. J Clin Oncol. 2008;26 abstract 2017. [Google Scholar]
- 24.Krishnan S, Brown PD, Ballman KV, et al. Phase I trial of erlotinib with radiation therapy in patients with glioblastoma multiforme: results of North Central Cancer Treatment Group protocol N0177. Int J Radiat Oncol Biol Phys. 2006;65:1192–1199. doi: 10.1016/j.ijrobp.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Brewer CJ, Suh JH, Stevens GHJ, et al. Phase II trial of erlotinib with temozolomide and concurrent radiation therapy in patients with newly-diagnosed glioblastoma multiforme. J Clin Oncol. 2005;23 abstract 1567 (Meeting Abstracts) [Google Scholar]
- 26.Prados M, DeBoer R, Chang S, et al. Phase II study of tarceva plus temodar during and following radiotherapy in patients newly diagnosed with glioblastoma or gliosarcoma. Neuro-Oncology. 2007;9:528. abstract MA-50. [Google Scholar]
- 27.Brown PD, Krishnan S, Sarkaria JN, et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol. 2008;26:5603–5609. doi: 10.1200/JCO.2008.18.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Combs SE, Schulz-Ertner D, Hartmann C, et al. Phase I/II study of cetuximab plus temozolomide as radiochemotherapy for primary glioblastoma (GERT) J Clin Oncol. 2008;26 abstract 2077. [Google Scholar]
- 29.Perez-Soler R, Chachoua A, Hammond LA, et al. Determinants of tumor response and survival with erlotinib in patients with nonsmall-cell lung cancer. J Clin Oncol. 2004;22:3238–3247. doi: 10.1200/JCO.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 30.Dudek AZ, Kmak KL, Koopmeiners J, et al. Skin rash and bronchoalveolar histology correlates with clinical benefit in patients treated with gefitinib as a therapy for previously treated advanced or metastatic nonsmall cell lung cancer. Lung Cancer. 2006;51:89–96. doi: 10.1016/j.lungcan.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Herbst RS, Johnson DH, Mininberg E, et al. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent nonsmall-cell lung cancer. J Clin Oncol. 2005;23:2544–2555. doi: 10.1200/JCO.2005.02.477. [DOI] [PubMed] [Google Scholar]
- 32.Meany HJ, Fox E, McCully C, et al. The plasma and cerebrospinal fluid pharmacokinetics of erlotinib and its active metabolite (OSI-420) after intravenous administration of erlotinib in nonhuman primates. Cancer Chemother Pharmacol. 2008;63(3)):387–392. doi: 10.1007/s00280-007-0616-3. [DOI] [PubMed] [Google Scholar]
- 33.Buie LW, Lindley C, Shih T, et al. Plasma pharmacokinetics and cerebrospinal fluid concentrations of erlotinib in high-grade gliomas: a novel, phase I, dose escalation study. J Clin Oncol. 2007;25 abstract 2054. [Google Scholar]
- 34.Hofer S, Frei K. Gefitinib concentrations in human glioblastoma tissue. J Neurooncol. 2007;82:175–176. doi: 10.1007/s11060-006-9257-3. [DOI] [PubMed] [Google Scholar]
- 35.Feldkamp MM, Lala P, Lau N, et al. Expression of activated epidermal growth factor receptors, Ras-guanosine triphosphate, and mitogen-activated protein kinase in human glioblastoma multiforme specimens. Neurosurgery. 1999;45:1442–1453. doi: 10.1097/00006123-199912000-00034. [DOI] [PubMed] [Google Scholar]
- 36.Chakravarti A, Seiferheld W, Tu X, et al. Immunohistochemically determined total epidermal growth factor receptor levels not of prognostic value in newly diagnosed glioblastoma multiforme: report from the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 2005;62:318–327. doi: 10.1016/j.ijrobp.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 37.Lee JC, Vivanco I, Beroukhim R, et al. Epidermal growth factor receptor activation in glioblastoma through novel missense mutations in the extracellular domain. PLoS Med. 2006;3:e485. doi: 10.1371/journal.pmed.0030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702–4711. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marie Y, Carpentier AF, Omuro AM, et al. EGFR tyrosine kinase domain mutations in human gliomas. Neurology. 2005;64:1444–1445. doi: 10.1212/01.WNL.0000158654.07080.B0. [DOI] [PubMed] [Google Scholar]
- 40.Preusser M, Gelpi E, Rottenfusser A, et al. Epithelial growth factor receptor inhibitors for treatment of recurrent or progressive high grade glioma: an exploratory study. J Neurooncol. 2008;89:211–218. doi: 10.1007/s11060-008-9608-3. [DOI] [PubMed] [Google Scholar]
- 41.Riely GJ, Politi KA, Miller VA, et al. Update on epidermal growth factor receptor mutations in nonsmall cell lung cancer. Clin Cancer Res. 2006;12:7232–7241. doi: 10.1158/1078-0432.CCR-06-0658. [DOI] [PubMed] [Google Scholar]
- 42.Mellinghoff IK, Cloughesy TF, Mischel PS. PTEN-mediated resistance to epidermal growth factor receptor kinase inhibitors. Clin Cancer Res. 2007;13:378–381. doi: 10.1158/1078-0432.CCR-06-1992. [DOI] [PubMed] [Google Scholar]
- 43.Stommel JM, Kimmelman AC, Ying H, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]