Abstract
The activating receptor NKG2D, expressed by natural killer (NK) cells and CD8+ T cells, has a role in the specific killing of transformed cells. We examined NKG2D expression in patients with glioblastoma multiforme and found that NKG2D was downregulated on NK cells and CD8+ T cells. Expression of NKG2D on lymphocytes significantly increased following tumor resection and correlated with an increased ability to kill NKG2D ligand-positive tumor targets. Despite the presence of soluble NKG2D ligands in the sera of glioblastoma patients, NKG2D downregulation was primarily caused by tumor-derived tumor growth factor-β, suggesting that blocking of this cytokine may have therapeutic benefit.
Keywords: GBM, immune escape NKG2D, NK cell, TGF-β
Introduction
Studies using mice deficient in T cells and natural killer (NK) cells or the proinflammatory cytokines these cells produce have demonstrated the importance of an intact immune system in tumor surveillance.1–3 As a result, much of the focus has been on determining the effects of different tumor types on the activation of cytolytic NK cells and CD8+ T cells and the surface receptors that govern their activation. NKG2D is an activating receptor expressed on the surface of all human NK cells and CD8+ T cells,4 which can induce cytotoxic function mediated by NK cells,4 and augments TcR-mediated activation in CD8+ T cells.5 NK cells expressing NKG2D prevent the in vivo growth of tumors expressing NKG2D ligands6 and blockade of NKG2D results in impaired ability of effectors to lyse transformed targets.7 Ligands for NKG2D include major histocompatibility complex (MHC) class I–like molecules MICA and MICB, as well as a family of proteins designated ULBP1-4 that are expressed by cells under stress, including those that are virally infected or transformed.5,8 Here, we show that tumor burden in glioma patients is associated with decreased NKG2D expression on the surface of NK cells and CD8+ T cells, rendering them less efficient at tumor cell killing. Tumor growth factor (TGF)-β secreted by glioma in the sera of patients appears to play a predominant role in converting NKG2D+ active cells to the inactive NKG2D− phenotype.
Materials and Methods
Peripheral Blood and Tumor-Infiltrating Lymphocyte Isolation
Peripheral blood lymphocytes (PBLs) were isolated by Ficoll centrifugation. Tumor-infiltrating lymphocytes (TILs) were isolated with a three-step density gradient as described previously.9
Patients
Peripheral blood lymphocytes were isolated from recurrent glioblastoma multiforme (GBM) patients immediately prior to tumor resection and again 34.8 (SD 5.6) days following gross total resection. No patients had tumor recurrence at the time of the postsurgical blood draw. Patients were not on steroids at the time of blood draws.
Flow Cytometry
Fc receptors (FcRs) were blocked using FcR blocking reagent (Miltenyi Biotec) and stained with the indicated antibodies or isotype-matched control antibodies (BD, eBioscience, or R&D Systems). Samples were acquired using a FACSCalibur (BD) and analyzed using FlowJo software (TreeStar).
Immunofluorescence
Frozen tissues of 10-μm sections were incubated with monoclonal antibodies to MICA (clone M673) and MICB (clone M362) or ULBP1 (clone M295), ULBP-2 (clone M310), or ULBP-3 (clone M551). Antibodies were generously provided by Amgen, Inc. Antibody binding was detected by using fluorescently conjugated anti-mouse Immunoglobulin G + Immunoglobulin M antibodies (Abcam).
Cytotoxicity
Natural killer cells were isolated with an NK cell selection kit (StemCell Technologies, Inc.) and activated overnight with 1000 U/mL recombinant Interleukin-2 (NIH). Natural killer cells were cultured at a 1:1 ratio with SF767 or U87 glioma cells in the presence of PE-conjugated anti-CD107 for 3 h.
Enzyme-Linked Immunosorbent Assay
Amounts of soluble MICB in sera were determined using the MICB ELISA Duoset (R&D Systems) according to the manufacturer's instructions.
Reverse Transcribed-Quantitative Polymerase Chain Reaction
Whole cell mRNA was reverse transcribed, and quantitative polymerase chain reaction was performed using the following primers: forward NKG2D, 5′-CAC AGC TGG GAG ATG AGT GA-3′, reverse NKG2D 5′-CTA CAG CGA TGA AGC AGC AG-3′; forward MICA, 5′-ACA ATG CCC CAG TCC TCC AGA-3′, reverse MICA, 5′-ATT TTA GAT ATC GCC GTA GTT CCT-3′; forward MICB 5′-TGA GCC CCA CAG TCT TCG TTA C-3′, reverse MICB, 5′-TGC CCT GCG TTT CTG CCT GTC ATA-3 ; forward ULBP1, 5′-TGC AGG CCA GGA TGT CTT GT-3′, reverse ULBP1, 5′-CAT CCC TGT TCT TCT CCC ACT TC-3′; forward ULBP2, 5′-CCC TGG GGA AGA AAC TAA ATG TC-3′, reverse ULBP2, 5′-ACT GAA CTG CCA AGA TCC ACT GCT-3′; forward ULBP3 5′-AGA TGC CTG GGG AAA ACA ACT-3′, reverse ULBP3 5′-GTA TCC ATC GCC TTC ACA CTC ACA-3′; forward ULBP4 5′-TAT GTC GAC CTC CAC AGT ATG CGA AGA-3′, reverse ULBP4 5′-GTA TCC ATC GGC TTC ACA CTC ACA-3′; and forward HPRT 5′-GAC CAG TCA ACA GGG GAC AT-3′, reverse HPRT 5′-CTT GCG ACC TTG ACC ATC TT-3′. Data were collected and analyzed using the iQ5 Real Time System (BioRad).
Statistical Analysis
A two-tailed Student t test was used to compare GBM and meningioma (MNG) groups of patients. Statistical analyses were done using Prism software (GraphPad Software, Inc.).
Results and Discussion
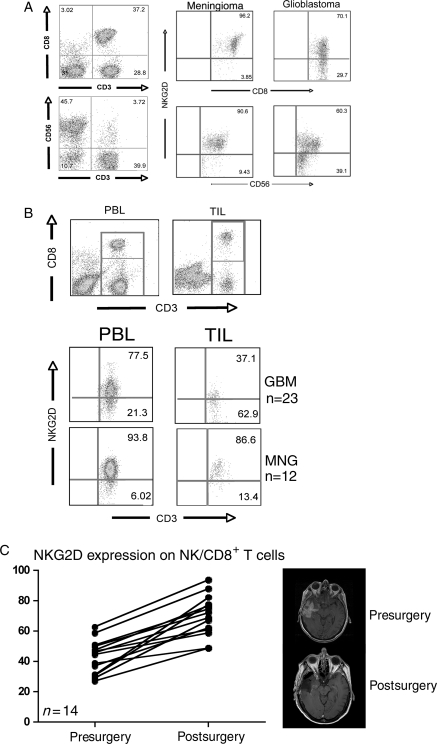
Successful immunotherapy is predicated upon effective activation, trafficking, and tumor cell recognition followed by tumor cell lysis mediated by immune effector cells. In patients with GBM, it is especially important to avoid nonspecific tissue damage and autoimmune responses that can be associated with other types of immunotherapy.10 We speculated that in patients with GBM, the functions of immune effector cells could be impaired by the tumor through modulation of activating receptors expressed by lymphocytes. While monitoring patients before and after surgical resection of a recurrent GBM, we made the repeated observation that expression of the activating receptor NKG2D was diminished on the surface of both CD8+ T cells and NK cells (n = 23) when compared with those isolated from patients with meningioma or other benign tumors (n = 12) (Fig. 1A), suggesting a systemic impact on these lymphocyte populations by tumor burden. We found that this impaired expression was more pronounced in tumor-infiltrating CD8+ T cells when compared with circulating CD8+ T cells isolated from the same patient (Fig. 1B). Following tumor resection, the frequency of circulating NK cells and CD8+ T cells expressing NKG2D in the GBM patients increased from an average of 43.0% to 70.1% (P < .01), which correlated with decreased tumor burden (Fig. 1C). Together, these data suggested that in patients with GBM, tumor cells and/or the factors that they secrete impair expression of NKG2D on the surface of effector lymphocytes. Cell surface levels of NKG2D can be modulated by soluble NKG2D ligands11 or through TGF-β inhibition of NKG2D transcription.12,13
Fig. 1.
Expression of the activating receptor NKG2D is decreased on NK and CD8+ T cells in patients with glioblastoma multiforme (GBM). (A) Peripheral blood lymphocytes (PBLs) isolated from patients with either GBM or MNG were isolated prior to tumor resection. CD8+ T lymphocytes were defined as CD3+, CD8+ cells: NK cells were defined as CD3−, CD56+ (left panels). After gating, cells were analyzed for the expression of NKG2D relative to the expression levels in patients with MNG. (B) PBL and tumor-infiltrating lymphocytes (TILs) were isolated from GBM patients (n = 23) and analyzed for the expression of NKG2D on CD3+, CD8+ cells, gated as shown in the top panels, and compared with those isolated from patients with MNG (n = 12). (C) PBL from GBM patients were analyzed for expression levels of NKG2D on NK and CD8+ T cells before and after a significant surgical reduction in tumor burden as confirmed with MRI. Each point represents one patient.
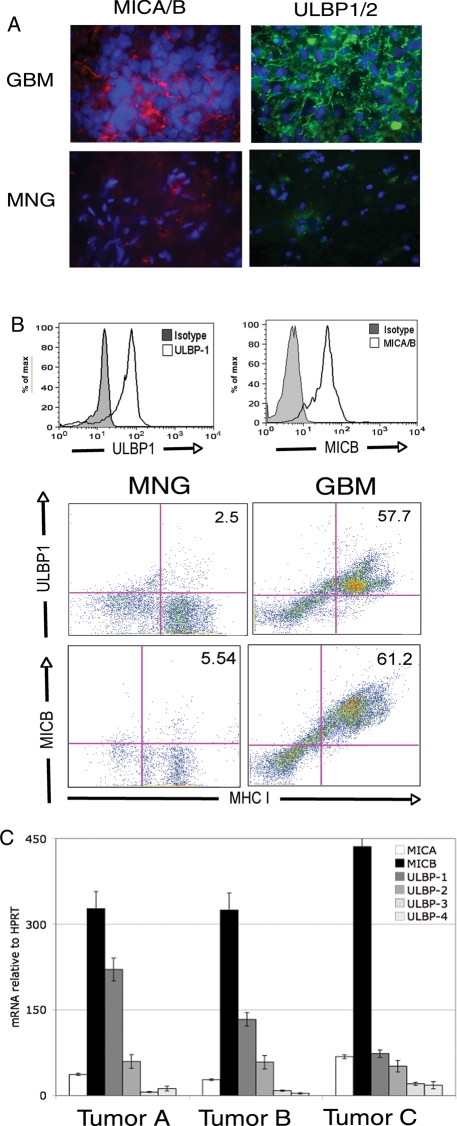
Studies using several different animal tumor models demonstrate the importance of active immunity against transformed cells.14 In humans, tumor outgrowth has been demonstrated in patients who are immunosuppressed recipients of transplanted organs received from donors with a history of cancer.15 The precipitating events for tumor outgrowth in patients with a normal immune system have not been well defined, although evidence exists to suggest that mechanisms described in animal models also occur in humans.16 To determine if the downregulation of NKG2D on the surface of NK cells and CD8+ T cells might affect the ability of these cells to recognize and respond to tumor cells, we investigated whether GBM-derived tumor cells expressed NKG2D ligands. The known ligands are the MHC class I–like molecules MICA and MICB and the ULBP1, ULBP2, ULBP3, and ULBP4 proteins. Despite their structural diversity, ligation of NKG2D with any one of the ligands is sufficient to activate NK cells and facilitate target cell lysis.8,17 NKG2D ligand expression functions as an indication of cellular stress to the innate and adaptive immune systems, including expression by transformed or virally infected cells, but they are not expressed in healthy, nonmalignant tissues of adults. We found that 10 of 11 GBM specimens expressed MICA or MICB and ULBP1 or 2 by immunofluorescence using cocktails containing monoclonal antibodies to either MICA (clone M637) and MICB (clone M362) or ULBP-1 (clone M295) and ULBP-2 (clone M310) (Fig. 2A), whereas tissues isolated from meningioma patients did not (0 of 11). ULBP-3 (clone M551) was not detected in either GBM tissues or MNG tissues (data not shown). To independently confirm the expression of the NKG2D ligands, we analyzed single-cell suspensions of GBM tissues by flow cytometry and found MICA/B (clone 6D4, reactive to both MICA and MICB) and ULBP-1 (clone 170818) expressed by MHC class I-positive, CD45-negative tumor cells (Fig. 2B). A majority of GBM patients expressed both MICA/B and ULBP-1 (88.9%, n = 18), whereas very few MNG specimens expressed either MICA or MICB, but no ULBP-1 (8.3%, n = 12). A small subset (23.5%, n = 17) of GBM patient tumor cells also expressed ULBP-2 by flow cytometry (data not shown). Neither GBM nor MNG cells had detectable expression of ULBP-3 or ULBP-4. To ensure that the expression we detected was not because of nonspecific antibody binding, we analyzed tumor cell mRNA and detected MICA, MICB, ULBP1, and ULBP2 transcripts by primary tumor cells (Fig. 2C). Thus, the expression at the mRNA level confirmed the expression of MIC and ULBP-1 in tumor cells isolated from GBM patients.
Fig. 2.
GBM tumor cells express NKG2D ligands on the surface. (A) Immunofluorescence staining of 10-µm frozen tissue sections with monoclonal antibody cocktails with either anti-MICA (clone M673) and anti-MICB (clone M362) (left panels, red) or anti-ULBP1 (clone M295) and anti-ULBP-2 (M310) (right panels, green) in GBM or MNG tumor sections. Nuclei were stained with DAPI (blue). Images are representative of 11 GBM samples analyzed. (B) Histograms: single-cell suspensions of tumor tissue from either GBM patients were stained PE-conjugated anti-MICA/B (clone 6D4) or FITC-conjugated anti-ULBP-1 (clone 170818) or the relevant isotype controls. Histograms represent gating of CD45−, MHC class I+ cells (tumor cells). Dot plots: GBM or MNG patient single-cell suspensions were stained with anti-MICA/B or anti-ULBP-1 and gated on CD45− cells. Data are representative of 18 GBM samples and 11 MNG samples analyzed. (C), mRNA expression levels of NKG2D ligands in CD45−, MHC class I+ tumor cells. mRNA was analyzed for the presence of NKG2D ligands and expressed as mRNA units relative to the expression of the housekeeping gene HPRT.
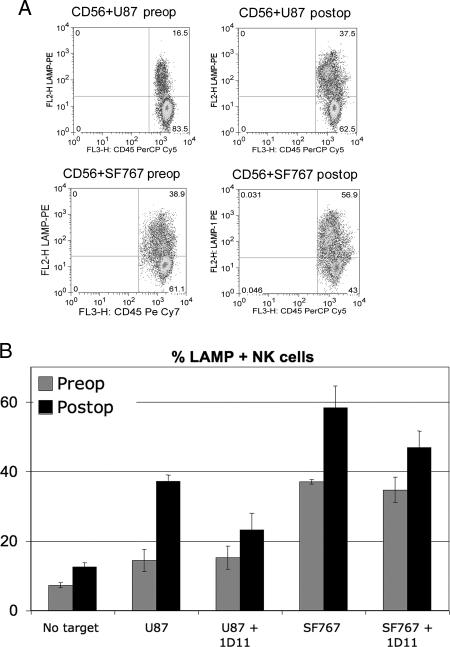
Surface expression of NKG2D ligands is sufficient to activate NK cells through ligation of NKG2D on the surface of NK cells. Because we observed expression of activating ligands on tumor cells, we examined whether NK cell function was impaired prior to and after tumor resection. Patients' NK cells were better able to kill, as measured by NK cell degranulation, two NKG2D ligand-positive tumor cell lines, U87 (preoperatively vs postoperatively, P = .009) and SF767 (preoperatively vs post-operatively, P = .008), following tumor resection that correlated with the observed increase in NKG2D expression on NK cells postsurgery (Fig. 3A). To determine if this recognition was NKG2D dependent, we added NKG2D-blocking antibody (clone 1D11) to the coculture and found that NK cell killing of tumor cell targets was significantly reduced (Fig. 3B) in U87 (P = .004) and SF767 (P = .041) cultures, suggesting that preventing the interaction of NKG2D with its cognate ligands on GBM tumor cells can impair effector cell recognition and lysis of tumor targets. NKG2D downregulation on the surface of effector lymphocytes by the presence of a GBM may therefore contribute to the survival of these tumor cells in vivo despite the expression of NKG2D ligands on the tumor cell surface.
Fig. 3.
NK cells lyse tumor cell targets in an NKG2D-dependent manner more efficiently after tumor resection. (A) NK cells were isolated from PBLs and activated overnight with IL-2. NKG2D ligand-positive tumor cell lines U87 (top panels) and SF767 (bottom panels) were cocultured for 3 h with NK cells isolated from patients before and after tumor resection (preoperatively and post-operatively). Target cell recognition was measured by expression of LAMP-1 (CD107), a marker for NK cell degranulation, and lymphocytes (CD45+) were distinguished from tumor cells based on CD45 expression. (B) CD107 staining of NK cells following coculture. Anti-NKG2D (clone 1D11) was added to inhibit the interaction between NKG2D and its ligands. Data shown are from a representative experiment (of three) performed in triplicate.
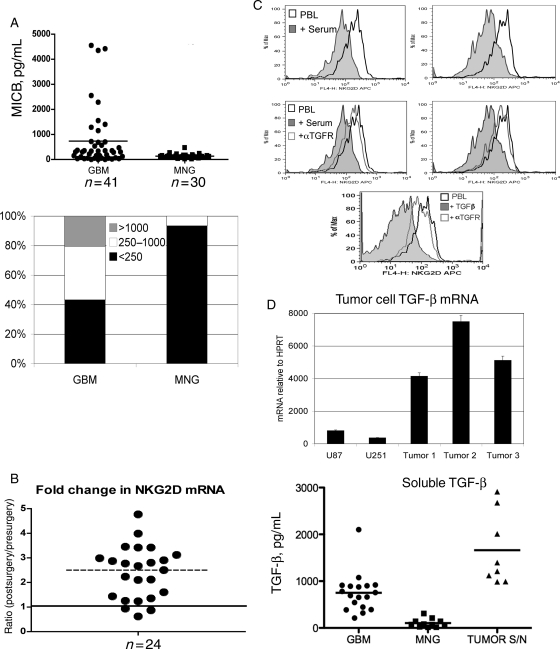
In many types of cancer, NKG2D expression can be decreased on the surface of NK cells and CD8+ T cells through shedding of one or more NKG2D ligands by tumor cells.11 In a manner similar to decoy cytokines that bind their receptor but fail to initiate a productive signal, these soluble ligands can bind NKG2D and cause receptor internalization. The resulting decrease in surface expression of NKG2D impairs tumor cell lysis, and likely serves as a mechanism for immune escape and tumor cell survival. Recent studies have also demonstrated that constitutive signaling through NKG2D can desensitize NKG2D receptor function, rendering the cells incapable of activation through NKG2D-ligand interactions,18 sensitizing them to their targets regardless of expression levels. To determine if soluble NKG2D ligands were responsible for the decreased NKG2D expression that we observed in patients with GBM, we tested the sera of GBM patients as compared to non-GBM tumor patients. We found that sera from GBM patients had greater (n = 41, P < .01) amounts of soluble MICB than patients with MNG (n = 30) (Fig. 4A, top panel). Fifty-eight percent of the patients examined had soluble MICB present in their sera at levels greater than those observed in MNG patients. In a subset of patients, we found concentrations greater than 1 ng/mL, whereas a larger subset of patients also contained a group that expressed soluble NKG2D ligands at intermediate levels (Fig. 4A, bottom panel). Sera from patients with MNG contained low or barely detectable amounts of soluble MICB, suggesting that the shedding and/or secretion of soluble NKG2D ligands may be specific to GBM and not a feature of all patients with a CNS tumor. Patients with soluble MICB in the sera also had detectable soluble MICA, although all sera had less than 1 ng/mL of soluble MICA (data not shown).
Fig. 4.
GBM patient sera contain soluble NKG2D ligands but decreased expression of NKG2D is at the level of transcription and mediated by TGF-β. (A) Patient sera from 41 GBM patients and 30 MNG patients were analyzed by ELISA for soluble MICB. Each point represents one patient. The bottom panel represents the percentage of GBM or MNG patients with soluble MICB levels within a given range. (B) NK cells and CD8+ T cells were isolated using CD56 or CD8 cell selection and whole-cell mRNA reverse transcribed before and after tumor resection. Amount of NKG2D mRNA was determined relative to HPRT and expressed as a ratio of detected levels after surgery: levels before surgery. A ratio of >1 represents an increase in NKG2D levels following tumor resection. (C) NK cells from an MNG patient were cocultured with patient sera containing 4.8 ng/mL soluble MICB for 48 hours in the presence of 10 µg/mL of TGF-β–receptor blocking antibody. As a positive control, 1 ng/mL recombinant TGF-β was added to PBLs and blocked using TGF-β receptor antibody. Surface expression of NKG2D was then analyzed by flow cytometry.
TGF-β modulates NKG2D expression at the level of transcription. In some cancers, including GBM, where high amounts of TGF-β can be detected in the sera of patients, it is possible that active TGF-β can modulate NK and CD8+ T cell activation by actively decreasing mRNA levels of NKG2D. To determine if downregulation of NKG2D in GBM patients was occurring at the transcript level, we evaluated NKG2D mRNA in GBM patient NK cells and CD8+ T cells before and after tumor resection. We found that in 21 of 24 patients (87.5%), NKG2D mRNA levels significantly increased following tumor resection (P = .02), supporting the premise that TGF-β impairs the expression of NKG2D on lymphocytes from GBM patients (Fig. 4B). To distinguish between soluble ligand and TGFβ-mediated effects on GBM patient NK and CD8+ T cells, we tested whether patient sera containing the greatest detected amount of soluble MICB (4.8 ng/mL) was sufficient to decrease surface NKG2D expression. Soluble MIC containing sera was able to decrease NKG2D expression (Fig. 4C); however, NKG2D expression was recovered using a blocking antibody to TGF-β receptor, indicating that TGF-β, not soluble NKG2D ligands, is responsible for decreased NKG2D expression on the GBM patients' lymphocytes. TGF-β is produced by tumor cells (Fig. 4D) and found in the sera of these patients. However, the source of TGF-β in the sera of these patients may be either tumor cells or an elevated population of regulatory T cells in GBM patients.19 Recent work demonstrates that inhibition of regulatory T cell function, including TGFβ secretion, can enhance cytotoxic T cell anti-tumor responses.20 Collectively, these data demonstrate that TGF-β–mediated downregulation of NKG2D may allow tumor cells in GBM patients to escape recognition by cytolytic effector cells of the immune system, resulting in tumor outgrowth. Accordingly, patients with significant tumor burden may not be ideal candidates for immunotherapy protocols predicated upon T-cell mediated target cell killing. In summary, we provide evidence for a mechanism of TGF-β–mediated immunosuppression, not previously described for patients with GBM.
Funding
L.L.L. is an American Cancer Society Research Professor and is supported by NIH grant AI066897. A.T.P. is supported by 2 P50 CA097257-06 Brain Tumor SPORE Grant Project 5.
Acknowledgements
Immunofluorescence antibodies for MICA, MICB, ULBP-1, ULBP-2, and ULBP-3 were gifts from Amgen, Inc.
Conflict of interest statement. None declared.
References
- 1.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Stutman O. Tumor development after 3-methylcholanthrene in immunologically deficient athymic-nude mice. Science. 1974;183:534–536. doi: 10.1126/science.183.4124.534. [DOI] [PubMed] [Google Scholar]
- 3.Dighe AS, Richards E, Old LJ, et al. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447–456. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 4.Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. [PubMed] [Google Scholar]
- 5.Groh V, Rhinehart R, Randolph-Habecker J, et al. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 6.Arina A, Murillo O, Hervas-Stubbs S, et al. The combined actions of NK and T lymphocytes are necessary to reject an EGFP+ mesenchymal tumor through mechanisms dependent on NKG2D and IFN gamma. Int J Cancer. 2007;121:1282–1295. doi: 10.1002/ijc.22795. [DOI] [PubMed] [Google Scholar]
- 7.Strid J, Roberts SJ, Filler RB, et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9:146–154. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- 8.Cosman D, Mullberg J, Sutherland CL, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 9.Ford AL, Goodsall AL, Hickey WF, et al. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol. 1995;154:4309–4321. [PubMed] [Google Scholar]
- 10.Hodi FS. Cytotoxic T-lymphocyte-associated antigen-4. Clin Cancer Res. 2007;13:5238–5242. doi: 10.1158/1078-0432.CCR-07-0813. (Pt 1) [DOI] [PubMed] [Google Scholar]
- 11.Groh V, Wu J, Yee C, et al. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 12.Castriconi R, Cantoni C, Della Chiesa M, et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci USA. 2003;100:4120–4125. doi: 10.1073/pnas.0730640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friese MA, Wischhusen J, Wick W, et al. RNA interference targeting transforming growth factor-beta enhances NKG2D-mediated antiglioma immune response, inhibits glioma cell migration and invasiveness, and abrogates tumorigenicity in vivo. Cancer Res. 2004;64:7596–7603. doi: 10.1158/0008-5472.CAN-04-1627. [DOI] [PubMed] [Google Scholar]
- 14.Teague RM, Sather BD, Sacks JA, et al. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med. 2006;12:335–341. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- 15.Stratta P, Morellini V, Musetti C, et al. Malignancy after kidney transplantation: results of 400 patients from a single center. Clin Transplant. 2008;22:424–427. doi: 10.1111/j.1399-0012.2008.00802.x. [DOI] [PubMed] [Google Scholar]
- 16.Epling-Burnette PK, Bai F, Painter JS, et al. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood. 2007;109:4816–4824. doi: 10.1182/blood-2006-07-035519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinle A, Li P, Morris DL, et al. Interactions of human NKG2D with its ligands MICA, MICB, and homologs of the mouse RAE-1 protein family. Immunogenetics. 2001;53:279–287. doi: 10.1007/s002510100325. [DOI] [PubMed] [Google Scholar]
- 18.Oppenheim DE, Roberts SJ, Clarke SL, et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 19.Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008;14:5166–5172. doi: 10.1158/1078-0432.CCR-08-0320. [DOI] [PubMed] [Google Scholar]
- 20.Kong LY, Abou-Ghazal MK, Wei J, et al. A novel inhibitor of signal transducers and activators of transcription 3 activation is efficacious against established central nervous system melanoma and inhibits regulatory T cells. Clin Cancer Res. 2008;14:5759–5768. doi: 10.1158/1078-0432.CCR-08-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]