Abstract
Glioblastoma (GB) is the most common malignant brain tumor in adults. It has limited treatment opportunities and is almost exclusively fatal. Owing to the central role the insulin-like growth factor-1 receptor (IGF-1R) plays in malignant cells, it has been suggested as a target for anticancer therapy including GB. The cyclolignan picropodophyllin (PPP) inhibits IGF-1R without affecting the highly homologous insulin receptor. Here, we show that PPP inhibits growth of human GB cell lines along with reduced phosphorylation of IGF-1R and AKT. In vivo, PPP-treatment causes dramatic tumor regression not only in subcutaneous xenografts but also in intracerebral xenografts, indicating passage of PPP across the blood-brain barrier.
Keywords: glioblastoma, glioma, IGF-1 receptor, picropodophyllin
Introduction
Approximately 60% of all diagnosed primary brain tumors are glioblastomas (GB). They comprise a heterogeneous group of neoplasms with respect to location within the central nervous system, age and sex distribution, growth potential, morphological features, tendency for progression, and response to treatment. Despite multimodality approaches including surgical resection, radiotherapy, and chemotherapy, the prognosis is still very poor. It has previously been suggested that the insulin-like growth factor-1 receptor (IGF-1R) is involved in tumors of cerebellum and in neuroblastomas; however, only a few studies have focused on its role in GB.1–3 The IGF-1R is a transmembrane heterotetramer whose cytoplasmic tyrosine kinase domain activates the PI3K/AKT and RAS-RAF-MAPK signaling pathways.4 Overexpression of IGF-1R is a common characteristic of many human cancers, implying that this receptor is a potential target for anticancer therapy.5 Owing to the high degree of homology between the tyrosine kinase domains of the IGF-1R and the insulin receptor, the design of small molecule inhibitors with strict selectivity for IGF-1R is exceedingly difficult. The cyclolignan picropodophyllin (PPP) has been shown to be a potent inhibitor of IGF-1R phosphorylation and downstream signaling.6–9 Picropodophyllin not only inhibits IGF-1R activity but also downregulates and degrades the receptor, effects that are achieved without downregulating phosphorylation and expression of the insulin receptor.6,10 Thus, treatment with PPP results in strong antitumor efficacy without compromising tolerability in vivo.6–9 Daily treatment with PPP in mice with multiple myeloma prolongs their survival by almost 3 months.9 An IGF-1R inhibitor of the cyclolignan family is currently under study in patients with advanced cancer (www.axelar.se). In this study, we assessed the effect of IGF-1R inhibition induced by PPP on GB in vitro and in vivo. Indeed, our results indicate that this strategy may constitute a novel treatment for this tumor.
Materials and Methods
Established Human GB Cell Lines
The GB cell lines U-178MG, U-251MG Sp (also known as U251MG), U-343MG, U-563MG, U-87MG, U-1242MG, and U-1242MG Cl 4 are GFAP−/fibronectin+, whereas U-343MGa Cl 2:3, U-343MGa Cl 2:6, U-343MGa 31L, and U-343MGa 5L are GFAP+/fibronectin−. This cell line panel represents all common GB cell line phenotypes.11 The U-343 cell lines are derived from the same patient, where U-343MG and U-343MGa and its four clonal derivatives U-343MGa Cl 2:3, U-343MGa Cl 2:6, U-343MGa 31L, and U-343MGa 5L exhibit different levels of platelet-derived growth factor (PDGF)/PDGFR expression.12 U-1242MG Cl 4 is a variant of U-1242MG with exogenously introduced transforming growth factor (TGF)-α using a tet-regulated system. Here, the U-1242MG Cl 4 cell line was cultured without tetracycline, resulting in TGF-α expression and phosphorylation of the epidermal growth factor receptor (EGFR), as described previously.13 The GB cell lines were routinely grown in 10-cm tissue culture plates (Becton Dickinson Labware, Franklin Lakes, New Jersey) using Eagle's minimal essential medium (MEM) supplemented with 10% fetal bovine serum (Sigma, St Louis, Missouri) and penicillin/streptomycin and were maintained in a humidified 5% CO2 in air atmosphere at 37°C.
Analysis of Cell Growth
The GB cell lines were harvested by trypsinization and seeded into 96-well tissue culture plates (Becton Dickinson Labware) at 5000 cells/well using Eagle's MEM supplemented with 10% fetal bovine serum and penicillin/streptomycin. The cells were allowed to adhere overnight before PPP, synthesized as described previously,6 was added at indicated concentrations followed by a 48-h incubation. Cell growth (ie, the net result of cell division − cell death) was analyzed by using resazurin (Sigma), which is the active compound of the commercially available growth indicator dye alamarBlue (AbD Serotec, Oxford, UK). In response to metabolically active cells, resazurin becomes increasingly fluorescent, and separate experiments show a linear correlation between the number of viable cells and the emitted light (data not shown). During harvest of the experiments, resazurin at 440 µM was added directly to the wells at a concentration of 10%. After incubation for an hour at 37°C in a humidified 5% CO2 in air atmosphere, analysis of fluorescence (excitation 550 nM, emission 590 nM) by using a Wallac Victor Multilabel Counter (Wallac, Turku) was performed. The relative number of viable cells was calculated from triplicates and expressed as a percentage of untreated control cells.
Analysis of Apoptosis and IGF-1R Surface Expression
Apoptosis was quantified at 48 hours of treatment with PPP using annexin V-Alexa Fluor 647 (Molecular Probes, Eugene, Oregon) and propidium iodide (PI)-staining, where only the annexin V-positive/PI-negative cell population was considered as truly apoptotic. The amount of apoptosis in the cells treated with 0.5 or 2.5 µM PPP was divided by the background apoptosis in the control (ranging between 0.8% and 2.8%) and expressed as a fold increase of apoptosis. The IGF-1R surface expression was estimated using phycoerythrin (PE)-conjugated antibody specific for the α-subunit of the IGF-1R and PE-conjugated isotype control antibody (BD Biosciences, San José, CA) resulting in mean fluorescence intensity (MFI). Relative MFI (rMFI) was calculated by dividing MFI obtained from staining with the IGF-1R antibody with MFI obtained from staining with the isotype control antibody.
Immunoprecipitation and Western Blotting
To determine tyrosine phosphorylation of the IGF-1R β-subunit and AKT, cells were cultured to subconfluency in 6-cm tissue culture plates, washed with phosphate-buffered saline (PBS), and then serum-starved for 24 hours before 1 hour treatment with PPP at 0.5 or 2.5 µM followed by 5 minutes stimulation with 50 ng/mL recombinant IGF-1 (Sigma) before lysis, centrifugation at 14 000 x g for 10 minutes at 4°C and immunoprecipitation and/or immunoblotting as described elsewhere.10 The developed films were scanned by Fluor-S MultiImager (Bio-Rad Laboratories AB, Sundbyberg) followed by quantification of the signals using the software Quantity One (Bio-Rad Laboratories AB). The ratios of phosphorylated (p)-IGF-1R/IGF-1R and p-AKT/AKT were calculated. Ratios derived from cultures stimulated with IGF-1 only were set to 100%.
In Vivo Experiments
For the subcutaneous xenograft model, 10-week-old, pathogen-free SCID mice were used. The GB cell line U-563MG was injected subcutaneously at 1 × 107 cells/mice in a volume of 200 µL PBS. Once the tumors were established, the mice were treated twice daily for 7 days with i.p. injections of 10 µL PPP dissolved in dimethyl sulfoxide (DMSO)/vegetable oil (10:1 v/v) corresponding to 10–20 mg/kg/12 h. Mice treated with vehicle served as the control. Three animals were treated in each group. Tumor size was determined every second day using vernier calipers, and the tumor volumes were calculated as a product of length × width × height.
For the intracranial xenograft model, 8–9-week-old male nude rats (Hsd: RH-rnu; Harlan) with an average weight of 200 g were used. Anesthesia of the animals was induced and maintained by isoflourane inhalation. Following fixation of the head in a stereotactic apparatus, a burr hole of 0.5 mm diameter was drilled in the skull over the right hemisphere. Under stereotactic guidance, 3 × 105 U-87MG cells in a volume of 3 µL PBS were implanted at a depth of 5 mm from the skull surface, reaching the area immediately lateral to the caudate nucleus.14 The burr hole was sealed with bone wax. Seven days after tumor cell implantation, treatment with PPP or vehicle was initiated as described above and maintained for 10 days. Three animals were treated in each group. All animals were observed daily for weight loss, abnormal behavior, or neurological symptoms. The animals were sacrificed by decapitation following deep anesthesia. Brains were dissected and immediately frozen in dry, ice-cold isopentane and transferred to a −70ºC freezer. The specimens were sectioned coronally at a thickness of 14 µm by using a cryostat and stained with hematoxylin and eosin. From the frozen sections containing the largest area of GB tissue, the tumor radius was measured using a caliper by two independent observers. Tumor volume was calculated using the formula 4/3 × r3 × π.
Samples of fresh-frozen tumors from mice and rats that had been treated with PPP or vehicle were cut into pieces and suspended in freshly prepared homogenization buffer.6 Analysis of protein levels of AKT and p-AKT was performed by Western blotting as described above. All animals were housed within plastic isolators in a sterile facility and experiments conducted according to the ethical guidelines for laboratory animal use and approved by the institutional ethical committee at Karolinska Institutet.
Frozen samples from 8 cases of human primary glioma tumors, including 7 World Health Organization (WHO) grade IV GBs and 1 WHO grade III anaplastic oligoastrocytoma, were retrieved from the Karolinska University Hospital Biobank and used according to the ethical guidelines after approval by the institutional ethical committee at Karolinska Institutet (KI Dnr 02-254). The tissue samples were disintegrated at −70ºC by ball mill grinding followed by extraction and solubilization in 2% sodium dodecyl sulfate (SDS) for 10 minutes in a volume corresponding to 10 times the tissue wet weight.15 After centrifugation, the samples were analyzed for the expression of IGF-1R by Western blotting as described above.
Statistical Analysis
For statistical analysis, the Student's t test was used.
Results
PPP Inhibits Growth and Induces Apoptosis in Human GB Cell Lines
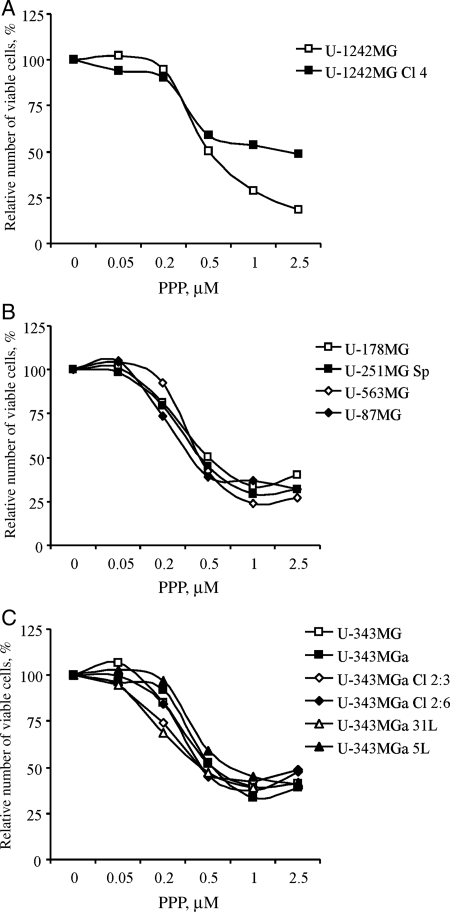
To assess the effect of PPP on cell growth, the different GB cell lines were incubated with PPP at indicated concentrations for 48 hours followed by analysis using the resazurin assay. All 12 cell lines were growth inhibited by addition of PPP (Fig. 1, Table 1). With the exception of U-1242MG Cl 4 (Fig. 1A), the cell lines exhibited IC50s from 0.36 µM for the U-87MG (Fig. 1B) to 0.70 µM for the U-343MG 5L (Fig. 1C). The U-1242MG Cl 4 cell line was least sensitive to PPP, with an IC50 of 1.93 µM (Fig. 1A).
Fig. 1.
Effect of PPP on growth of GB cell lines. Twelve glioma cell lines were treated with 0.05, 0.2, 0.5, 1.0, or 2.5 µM PPP for 48 hours followed by analysis of relative number of viable cells using the resazurin assay. Three independent experiments were performed for each cell line. The results were pooled followed by the calculation of mean values, which are depicted. Standard deviations were always less than 10%.
Table 1.
Picropodophyllin inhibits growth and induces apoptosis in human GB cell lines
| Apoptosis |
|||||
|---|---|---|---|---|---|
| GB Cell Line | IC50, μM | 0.5 µM PPP | 2.5 µM PPP | IGF-1R Expression | PTEN Status |
| U-1242MG | 0.50 | ++ + + | ++ + ++ + | + | WT |
| U-1242MG Cl 4 | 1.93 | ++ + ++ | ++ + ++ | ++ | N/A |
| U-178MG | 0.50 | + | ++ | ++ + | Frameshift |
| U-251MG Sp | 0.44 | + | ++ | ++ + + | Mutated |
| U-343MGa | 0.54 | ++ + + | ++ + + | ++ | Mutated |
| U-343MGaa | 0.53 | ++ | ++ + + | ++ + + | N/A |
| U-343MGa Cl 2:3a | 0.41 | + | ++ + | ++ + + | N/A |
| U-343MGa Cl 2:6a | 0.45 | + | ++ | ++ + | N/A |
| U-343MGa 31La | 0.44 | ++ + ++ | ++ + + | ++ | N/A |
| U-343MGa 5La | 0.70 | + | ++ + | ++ | N/A |
| U-563MG | 0.43 | ++ | ++ + + | ++ + ++ | N/A |
| U-87MG | 0.36 | ++ + + | ++ + ++ + | ++ | Mutated |
The GB cell lines were treated for 48 hours with 0.05, 0.2, 0.5, 1.0, and 2.5 µM PPP followed by analysis of cell growth using the resazurin assay followed by calculation of IC50 values by interpolation. Apoptosis was quantified by flow cytometry using annexin V-Alexa Fluor 647/PI staining. The relative amount of apoptosis is depicted by and is proportional to the number of (+), where (+) corresponds to fold increase of apoptosis >1 but <2.5; (++ ) to ≥2.5 but <5; (++ + ) to ≥5 but <7.5; (++ + +) to ≥7.5 but <10; (++ + ++ ) to ≥10 but <12.5; and (++ + ++ + ) to ≥12.5 but <15. Insulin-like growth factor-1 receptor surface expression was analyzed by flow cytometry using PE-conjugated IGF-1R-specific/isotypic control antibodies. The relative level of IGF-1R expression is proportional to the number of (+), where (+) corresponds to rMFI >1 but <2; (++ ) to ≥2 but <4; (++ + ) to ≥6 but <8; (++ + +) to ≥8 but <10; (++ + ++ ) to ≥10. At least three separate experiments were performed and the obtained results were pooled followed by the calculation of the means. The status of PTEN has been described.31,32 Abbreviations: PPP, picropodophyllin; GB, glioblastoma; IGF-1R, insulin-like growth factor-1 receptor;WT, wild type; N/A, not available.
aGB cell lines derived from the same patient.
The amount of apoptosis resulting from treatment with PPP at 0.5 and 2.5 µM was analysed at 48 hours by using annexin V-Alexa-Fluor 647/PI-staining and flow cytometry followed by the calculation of fold increase in apoptosis. All cell lines of the panel responded to PPP with increased apoptosis, however to varying degrees (Table 1). U-343MG, U-343MGa 31L, U-87MG, U-1242MG, and U-1242MG Cl 4 showed very prominent apoptotic responses even to the low PPP concentration, whereas U-178MG, U-251 Sp, U-343MGa Cl 2:3, U-343MGa Cl 2:6, and U-343MGa 5L only exhibited slightly increased apoptosis. Intermediate responses were demonstrated in the U-343MGa and U-563MG cell lines.
The Level of Membrane-Bound IGF-1R Differs Among GB Cell Lines
The IGF-1R surface expression in the GB cell lines was quantified by using PE-conjugated IGF-1R-specific/isotype control antibodies and flow cytometry followed by the calculation of rMFI. The IGF-1R was expressed at variable levels in the GB cell lines (Table 1). The U-251MG Sp, U-343MGa, U-343MGa Cl 2:3, and U-563MG expressed large amounts of IGF-1R, whereas the U-343MG, U-343MGa 31L, U-343MGa 5L, U-87MG, U-1242MG, and U-1242MG Cl 4 expressed low levels. Intermediate IGF-1R expression was found in the U-178MG and U-343MGa Cl 2:6 cell lines (Table 1).
PPP Inhibits Phosphorylation of IGF-1R and AKT in GB Cell Lines
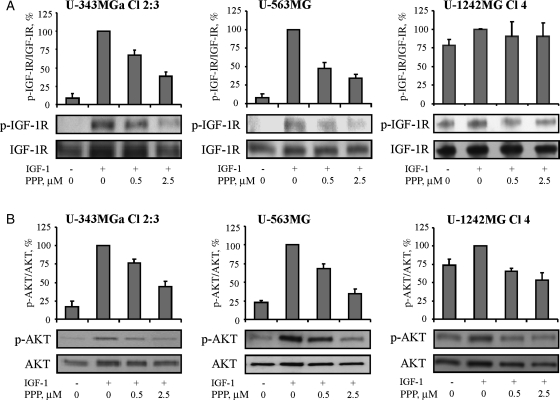
Serum-starved GB cell lines were pretreated for 1 hour with PPP at 0.5 or 2.5 µM before 5 minutes stimulation with IGF-1. Insulin-like growth factor-1 receptor was immunoprecipitated followed by Western blotting using antibodies against p-Tyr or IGF-1Rβ. Alternatively, Western blotting was used for analyzing the levels of p-AKT (Ser473) and AKT in whole cell lysates. The amounts of protein, as indicated by ECL-developed signals, were quantified using scanning densitometry. The results from the U-343MGa Cl 2:3, U-563MG, and the U-1242MG Cl 4 cell lines are depicted as representatives for GB cell lines showing high and low PPP sensitivity, respectively (Table 1, Fig. 2).
Fig. 2.
Effect of PPP on IGF-1R and AKT phosphorylation. U-343MGa Cl 2:3, U-563MG, and U-1242MG Cl 4 cells were serum-starved overnight and then treated for 1 hour with 0.5 or 2.5 µM PPP before 5 minutes of stimulation with 50 ng/mL IGF-1. (A) Lysates were subjected to IGF-1Rβ immunoprecipitation followed by Western blotting using p-Tyr or IGF-1Rβ antibodies. (B) The expression of p-AKT (Ser473) and AKT were analyzed by Western blotting. Mean ratios and SDs of triplicates are shown.
Without stimulation with IGF-1, serum-starved U-343MGa Cl 2:3 and U-563MG cells exhibited very low constitutive levels of p-IGF-1R (Fig. 2A). Addition of IGF-1 largely increased the level of p-IGF-1R, whereas PPP dose-dependently prevented it. Consistent with this, IGF-1-induced AKT-phosphorylation decreased dose-dependently (Fig. 2B). Similar responses to IGF-1 and PPP were seen in the other GB cell lines of the panel (data not shown). However, the U-1242MG Cl 4 cell line, which is a subclone of U-1242MG with exogenously introduced TGF-α,13 was found to exhibit a high constitutive level of p-IGF-1R. In contrast to the other cell lines of the panel, stimulation of U-1242MG Cl 4 cells with IGF-1 only led to a small increase in p-IGF-1R. Neither this increase nor the constitutive level of p-IGF-1R could be consistently counteracted by pretreatment with PPP (Fig. 2A). Similarly, the U-1242MG Cl 4 cell line also exhibited high p-AKT, which could be weakly increased by IGF-1 stimulation. However, although this effect was totally abrogated by PPP at 0.5 µM, a substantial level of constitutive p-AKT still remained despite treatment with PPP at 2.5 µM (Fig. 2B).
PPP Induces Strong Regression of Subcutaneous and Intracranial GB Xenografts
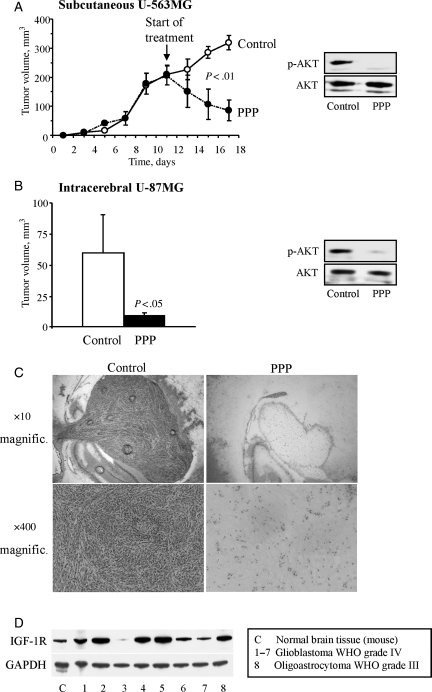
To investigate whether GB cells were sensitive to PPP in vivo as well, U-563MG cells were transplanted subcutaneously into severe combined immunodeficiency (SCID) mice. Once the tumors had become established, reaching sizes of 150–250 mm3, mice were treated twice daily with i.p. injections of PPP at 20 mg/kg or drug-free vehicle for 6 days according to a previous protocol.6,16 Tumors of the PPP-treated mice were strongly reduced in size (Fig. 3A). In contrast, control tumors continued growing steadily. The differences in tumor size were statistically significant even after only 4 days of treatment (P < .01). The expression of p-AKT in the tumor cells was greatly downregulated by PPP when compared with tumors from animals treated with vehicle only (Fig. 3A).
Fig. 3.
Effect of PPP on GB xenografts. (A) U-563MG cells were injected subcutaneously into SCID mice. When the tumors reached sizes of 150–250 mm3, treatment with i.p. injections twice daily with 20 mg/kg PPP or DMSO was initiated and continued for 6 days. Tumor volumes were determined every second day. At the end of the experiment, tumors were dissected, homogenized, and proteins extracted followed by analysis of the expression levels of p-AKT and AKT using Western blotting. (B) U-87MG cells were implanted into the right hemisphere of nude rats. After 7 days, treatment with i.p. injections twice daily with 20 mg/kg PPP or DMSO was initiated and continued for 10 days. Following anesthesia, the animals were sacrificed, the brains dissected, and the tumor sizes determined. Mean tumor volumes and SDs of triplicates are shown. As for the subcutaneous xenografts, the tumor tissues from the intracranial xenografts were analyzed for the expression levels of p-AKT and AKT using Western blotting. (C) Sections of fresh-frozen tissue from the intracerebral xenografts were stained with hematoxylin and eosin. (D) Fresh-frozen tumor tissue from 8 human primary gliomas as indicated was analyzed for the expression of the IGF-1R by Western blotting.
Since PPP caused such a strong regression of subcutaneous GB xenografts, we continued by investigating the effect of PPP in an intracerebral xenograft model. For this purpose, we used the well-established intracranial GB model where cells from the human U-87MG cell line were surgically implanted in the right brain hemisphere of nude rats.14 Empirically, it has been determined that such an implant produces an intracerebral, spherical tumor after 7 days. At this time, treatment was initiated with twice daily i.p. injections of 20 mg/kg PPP or drug-free vehicle and continued for 10 days. The tumor sizes of the PPP-treated animals were reduced by 90% (P < .05). Similar to the subcutaneous model, the level of p-AKT in the tumor tissue was strongly downregulated by PPP (Fig. 3B). No obvious side effects of PPP could be recorded. Staining of fresh-frozen tissue sections showed prominent tumor atrophy with only a few scattered tumor cells (Fig. 3C). As targeting of the IGF-1R by PPP in vivo resulted in such a dramatic antitumor response, we assessed the IGF-1R expression in tissues from 8 human high-grade glioma tumors. The majority of these samples were found to express high levels of IGF-1R (Fig. 3D).
Discussion
The IGF-1R is indisputably a pivotal player in cancer because of its involvement in the proliferation, progression, and protection against cell death of malignant cells. To this respect, the highly malignant brain tumor GB is no exception.3 However, the homeostasis of normal cells seems to lack an absolute dependency on the IGF-1R, making it a promising target for novel cancer treatment, not least for GB.
With the exception of U-1242MG Cl 4, all GB cell lines of our panel were highly sensitive to PPP. However, the extent of apoptosis varied substantially among the cell lines, suggesting that inhibition of cell cycle progression could be an important part of the PPP effect as demonstrated previously.8 In addition, other modes of cell death, for example necrosis or mitotic catastrophe, might contribute to the strong responses observed during treatment with PPP. Interestingly, low IGF-1R expression seemed to confer an increased tendency for responding to PPP at 0.5 µM with apoptosis, whereas a high level of IGF-1R expression instead might protect the cells against apoptosis induced by PPP at this concentration. The exception is the U-343MGa 5L cell line, which, despite a fairly low IGF-1R expression, only showed a weak apoptotic response when treated with 0.5 µM PPP.
We demonstrated that PPP reduces IGF-1R-dependent phosphorylation of AKT in GB cells, which is in accordance with previous studies.6,7,16 Since the PI3K/AKT pathway plays an important role in cell growth,17 it is conceivable that the observed attenuation of AKT phosphorylation might partly be responsible for the PPP-mediated inhibition of proliferation and/or induction of apoptosis in the GB cells. Intriguingly, in the U-1242MG Cl 4 cell line, we were unable to demonstrate a consistent inhibitory effect of PPP on ligand-stimulated p-IGF-1R increase. However, despite the lack of such an effect, PPP slowed down growth in this cell line and induced a substantial amount of apoptosis. It should, however, be remembered that the levels of p-IGF-1R and p-AKT were assayed after 1 hour of PPP treatment, whereas the effects on growth and apoptosis were determined after 48 hours. Therefore, it is reasonable to assume that the inhibitory effects on p-IGF-1R and p-AKT might be more prominent after longer treatment periods. Notably, the parental U-1242MG cell line, without the introduced TGF-α, exhibits an IC50 of 0.5 µM. Overexpression of TGF-α13 might affect the IGF-1R signaling pathway by causing heterodimerization of the EGFR and the IGF-1R, which, together with induction of survivin, has been shown to counteract the antitumor action of erlotinib.18 Such a mechanism could also provide a possible explanation for the reduced inhibitory effect of PPP on IGF-1R signaling and growth in the U-1242MG Cl 4 cell line.
Many of the cell lines used in this study exhibit mutated phosphatase and tensin homolog (PTEN), which may result in sustained activation of AKT and thus resistance to IGF-1R inhibition. However, inactivation of PTEN did not seem to protect GB cell lines against PPP. In line with this, studies using rat glioma C6 cells with mutant PTEN have shown that specific IGF-1R inhibition by antisense mRNA or truncated soluble receptors induces extensive apoptosis in vivo and inhibits tumorigenesis in syngeneic rats.1,19
As a continuation of the inhibitory effects on growth and IGF-1R/AKT phosphorylation detected in the GB cell lines, we investigated the efficacy of PPP in vivo. We showed that targeting the IGF-1R, using PPP at a dose previously used by our group,6 causes rapid regression of subcutaneous GB xenografts along with prominent downregulation of p-AKT. Encouraged by this excellent response, we continued by treating intracerebral GB xenografts with PPP. The growth of these tumors was also strongly inhibited, as was the expression of p-AKT, indicating an efficient passage of PPP across the blood-brain barrier. The aberrant upregulation/activation of various growth factor receptors and/or converging downstream signaling of the PI3K/AKT and RAS-RAF-MAPK pathways20,21 have been suggested to direct the progression of the malignant phenotype in GB.22 In the present study, we demonstrate substantial IGF-1R expression in tumor samples from GB patients suggesting the IGF-1R to be an important growth factor receptor for GB. In support of this, results obtained by others have shown a tendency toward elevated IGF-1R expression in high-grade astrocytomas when compared with low-grade tumors.22 Currently, there are several clinical cancer trials using humanized IGF-1R antibodies. Although these exhibit good antitumor effects in many solid cancers, their use in the treatment of brain tumors may be limited because of the presence of the blood-brain barrier.23 The EGFR has also been suggested as a target in GB therapy. However, results from clinical trials involving EGFR inhibitors have, thus far, been disappointing. The failure of these trials may be explained by pathways involving the activation of other, related tyrosine kinases, which compensate for the inhibition of the EGFR. A potential candidate for mediating such an escape mechanism in GB may be the IGF-1R. In support of this, the IGF-1R inhibitory tyrphostine AG1024 potentiated the effects of EGFR inhibition in an in vitro model of CD95L-induced GB cell death.2 Interestingly, contrary to expectations, inhibition of EGFR alone seems instead to protect GB cells from hypoxia-induced cell death.24 Another potential target in GB is the PDGFR, which can be efficiently inhibited by imatinib mesylate (Gleevec). However, because of this compound's limited ability to pass across the blood-brain barrier,25 it may not be ideal in a clinical setting. Intriguingly, despite the suggested difficulty for antibodies to penetrate through the blood-brain barrier,23 systemic infusion of antibodies targeting the hepatocyte growth factor conferred substantial antitumor effects in intracranial U-87MG xenografts.26
As previously mentioned, PPP may possess antitumor effects, unknown to us, that are unrelated to the IGF-1R. However, the fact that PPP induces strong antitumor activity in an IGF-1R-dependent multiple myeloma mouse model together with the fact that it is very well tolerated by the animals for up to 5 months of daily treatment9 suggest that the major effect occurs via the IGF-1R pathway. Furthermore, PPP essentially has no effect on established IGF-1R negative v-Src-transformed tumor cell xenografts6 and shows no inhibitory effects in other IGF-1R-independent cell systems either.27 When the phenotype of the cell lines used in these models was changed so that they became IGF-1R-dependent, they responded to PPP with extensive cell death.27
The most potent reversal of the pro-survival function of the IGF-1R seems to require downregulation of the receptor.28 In fact, PPP was recently shown by us to downregulate IGF-1R in several tumor cell types including the GB cell line U-343MG.10 Such a mechanism, together with the ability of PPP to permeate the blood-brain barrier, might explain the strong antitumor effects described herein. These findings along with the lack of serious side effects such as neurotoxicity in animals during long-term treatment9,29,30 allow us to suggest PPP, or other members of the cyclolignan family targeting the IGF-1R, as a promising treatment option for patients with GB.
Funding
This work was supported by the Swedish Cancer Society, the Cancer Society in Stockholm, the Swedish Research Council, the Swedish Childhood Cancer Foundation, Ingabritt and Arne Lundberg's Research Foundation, the King Gustaf V Research Foundation, Jeansson's Foundation and Karolinska Institutet.
Acknowledgments
Eric Trocmé is acknowledged for critically reading the manuscript.
Conflict of interest statement. Olle Larsson and Magnus Axelson are part-owners of Axelar AB developing cyclolignans for anti-cancer treatment.
References
- 1.Resnicoff M, Sell C, Rubini M, et al. Rat glioblastoma cells expressing an antisense RNA to the insulin-like growth factor-1 (IGF-1) receptor are nontumorigenic and induce regression of wild-type tumors. Cancer Res. 1994;54:2218–2222. [PubMed] [Google Scholar]
- 2.Steinbach JP, Eisenmann C, Klumpp A, Weller M. Co-inhibition of epidermal growth factor receptor and type 1 insulin-like growth factor receptor synergistically sensitizes human malignant glioma cells to CD95L-induced apoptosis. Biochem Biophys Res Commun. 2004;321:524–530. doi: 10.1016/j.bbrc.2004.06.175. [DOI] [PubMed] [Google Scholar]
- 3.Trojan J, Cloix JF, Ardourel MY, Chatel M, Anthony DD. Insulin-like growth factor type I biology and targeting in malignant gliomas. Neuroscience. 2007;145:795–811. doi: 10.1016/j.neuroscience.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 4.LeRoith D, Werner H, Beitner-Johnson D, Roberts CT., Jr. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- 5.Clemmons DR. Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. Nat Rev Drug Discov. 2007;6:821–833. doi: 10.1038/nrd2359. [DOI] [PubMed] [Google Scholar]
- 6.Girnita A, Girnita L, del Prete F, Bartolazzi A, Larsson O, Axelson M. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res. 2004;64:236–242. doi: 10.1158/0008-5472.can-03-2522. [DOI] [PubMed] [Google Scholar]
- 7.Vasilcanu D, Girnita A, Girnita L, Vasilcanu R, Axelson M, Larsson O. The cyclolignan PPP induces activation loop-specific inhibition of tyrosine phosphorylation of the insulin-like growth factor-1 receptor. Link to the phosphatidyl inositol-3 kinase/Akt apoptotic pathway. Oncogene. 2004;23:7854–7862. doi: 10.1038/sj.onc.1208065. [DOI] [PubMed] [Google Scholar]
- 8.Stromberg T, Ekman S, Girnita L, et al. IGF-1 receptor tyrosine kinase inhibition by the cyclolignan PPP induces G2/M-phase accumulation and apoptosis in multiple myeloma cells. Blood. 2006;107:669–678. doi: 10.1182/blood-2005-01-0306. [DOI] [PubMed] [Google Scholar]
- 9.Menu E, Jernberg-Wiklund H, De Raeve H, et al. Targeting the IGF-1R using picropodophyllin in the therapeutical 5T2MM mouse model of multiple myeloma: beneficial effects on tumor growth, angiogenesis, bone disease and survival. Int J Cancer. 2007;121:1857–1861. doi: 10.1002/ijc.22845. [DOI] [PubMed] [Google Scholar]
- 10.Vasilcanu R, Vasilcanu D, Rosengren L, et al. Picropodophyllin induces downregulation of the insulin-like growth factor 1 receptor: potential mechanistic involvement of Mdm2 and beta-arrestin1. Oncogene. 2008;27:1629–1638. doi: 10.1038/sj.onc.1210797. [DOI] [PubMed] [Google Scholar]
- 11.Nistér M, Westermark B. Human glioma cell lines. In: Hay RJ, Park JG, Gazdar A, editors. Atlas of Human Tumor Cell Lines. Academic Press Inc.; 1994. pp. 17–42. [Google Scholar]
- 12.Nister M, Heldin CH, Westermark B. Clonal variation in the production of a platelet-derived growth factor-like protein and expression of corresponding receptors in a human malignant glioma. Cancer Res. 1986;46:332–340. [PubMed] [Google Scholar]
- 13.El-Obeid A, Bongcam-Rudloff E, Sorby M, Ostman A, Nister M, Westermark B. Cell scattering and migration induced by autocrine transforming growth factor alpha in human glioma cells in vitro. Cancer Res. 1997;57:5598–5604. [PubMed] [Google Scholar]
- 14.Li H, Alonso-Vanegas M, Colicos MA, et al. Intracerebral adenovirus-mediated p53 tumor suppressor gene therapy for experimental human glioma. Clin Cancer Res. 1999;5:637–642. [PubMed] [Google Scholar]
- 15.Ericsson C, Peredo I, Nister M. Optimized protein extraction from cryopreserved brain tissue samples. Acta Oncol. 2007;46:10–20. doi: 10.1080/02841860600847061. [DOI] [PubMed] [Google Scholar]
- 16.Girnita A, All-Ericsson C, Economou MA, et al. The insulin-like growth factor-I receptor inhibitor picropodophyllin causes tumor regression and attenuates mechanisms involved in invasion of uveal melanoma cells. Clin Cancer Res. 2006;12:1383–1391. doi: 10.1158/1078-0432.CCR-05-1106. [DOI] [PubMed] [Google Scholar]
- 17.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 18.Morgillo F, Woo JK, Kim ES, Hong WK, Lee HY. Heterodimerization of insulin-like growth factor receptor/epidermal growth factor receptor and induction of survivin expression counteract the antitumor action of erlotinib. Cancer Res. 2006;66:10100–10111. doi: 10.1158/0008-5472.CAN-06-1684. [DOI] [PubMed] [Google Scholar]
- 19.D'Ambrosio C, Ferber A, Resnicoff M, Baserga R. A soluble insulin-like growth factor I receptor that induces apoptosis of tumor cells in vivo and inhibits tumorigenesis. Cancer Res. 1996;56:4013–4020. [PubMed] [Google Scholar]
- 20.Westermark B, Heldin CH, Nister M. Platelet-derived growth factor in human glioma. Glia. 1995;15:257–263. doi: 10.1002/glia.440150307. [DOI] [PubMed] [Google Scholar]
- 21.Yamada M, Ikeuchi T, Hatanaka H. The neurotrophic action and signalling of epidermal growth factor. Prog Neurobiol. 1997;51:19–37. doi: 10.1016/s0301-0082(96)00046-9. [DOI] [PubMed] [Google Scholar]
- 22.Hirano H, Lopes MB, Laws ER, Jr, et al. Insulin-like growth factor-1 content and pattern of expression correlates with histopathologic grade in diffusely infiltrating astrocytomas. Neuro Oncol. 1999;1:109–119. doi: 10.1093/neuonc/1.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rich JN, Bigner DD. Development of novel targeted therapies in the treatment of malignant glioma. Nat Rev Drug Discov. 2004;3:430–446. doi: 10.1038/nrd1380. [DOI] [PubMed] [Google Scholar]
- 24.Steinbach JP, Klumpp A, Wolburg H, Weller M. Inhibition of epidermal growth factor receptor signaling protects human malignant glioma cells from hypoxia-induced cell death. Cancer Res. 2004;64:1575–1578. doi: 10.1158/0008-5472.can-03-3775. [DOI] [PubMed] [Google Scholar]
- 25.Bihorel S, Camenisch G, Lemaire M, Scherrmann JM. Influence of breast cancer resistance protein (Abcg2) and p-glycoprotein (Abcb1a) on the transport of imatinib mesylate (Gleevec) across the mouse blood-brain barrier. J Neurochem. 2007;102:1749–1757. doi: 10.1111/j.1471-4159.2007.04808.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim KJ, Wang L, Su YC, et al. Systemic anti-hepatocyte growth factor monoclonal antibody therapy induces the regression of intracranial glioma xenografts. Clin Cancer Res. 2006;12:1292–1298. doi: 10.1158/1078-0432.CCR-05-1793. [DOI] [PubMed] [Google Scholar]
- 27.Guha M, Srinivasan S, Biswas G, Avadhani NG. Activation of a novel calcineurin-mediated insulin-like growth factor-1 receptor pathway, altered metabolism, and tumor cell invasion in cells subjected to mitochondrial respiratory stress. J Biol Chem. 2007;282:14536–14546. doi: 10.1074/jbc.M611693200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baserga R. Targeting the IGF-1 receptor: from rags to riches. Eur J Cancer. 2004;40:2013–2015. doi: 10.1016/j.ejca.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Davis RE, Schlumpf BE, Klinger PD. Comparative neurotoxicity of tubulin-binding drugs: inhibition of goldfish optic nerve regeneration. Toxicol Appl Pharmacol. 1985;80:308–315. doi: 10.1016/0041-008x(85)90088-2. [DOI] [PubMed] [Google Scholar]
- 30.Klinakis A, Szabolcs M, Chen G, Xuan S, Hibshoosh H, Efstratiadis A. Igf1r as a therapeutic target in a mouse model of basal-like breast cancer. Proc Natl Acad Sci USA. 2009;106:2359–2364. doi: 10.1073/pnas.0810221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Guessous F, Kwon S, et al. PTEN has tumor-promoting properties in the setting of gain-of-function p53 mutations. Cancer Res. 2008;68:1723–1731. doi: 10.1158/0008-5472.CAN-07-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishii N, Maier D, Merlo A, et al. Frequent co-alterations of TP53, p16/CDKN2A, p14ARF, PTEN tumor suppressor genes in human glioma cell lines. Brain Pathol. 1999;9:469–479. doi: 10.1111/j.1750-3639.1999.tb00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]