Abstract
Available data on genetic events in pediatric grade IV astrocytomas (glioblastoma [pGBM]) are scarce. This has traditionally been a major impediment in understanding the pathogenesis of this tumor and in developing ways for more effective management. Our aim is to chart DNA copy number aberrations (CNAs) and get insight into genetic pathways involved in pGBM. Using the Illumina Infinium Human-1 bead-chip-array (100K single-nucleotide polymorphisms [SNPs]), we genotyped 18 pediatric and 6 adult GBMs. Results were compared to BAC-array profiles harvested on 16 of the same pGBM, to an independent data set of 9 pediatric high-grade astrocytomas (HGAs) analyzed on Affymetrix 250K-SNP arrays, and to existing data sets on HGAs. CNAs were additionally validated by real-time qPCR in a set of genes in pGBM. Our results identify with nonrandom clustering of CNAs in several novel, previously not reported, genomic regions, suggesting that alterations in tumor suppressors and genes involved in the regulation of RNA processing and the cell cycle are major events in the pathogenesis of pGBM. Most regions were distinct from CNAs in aGBMs and show an unexpectedly low frequency of genetic amplification and homozygous deletions and a high frequency of loss of heterozygosity for a high-grade I rapidly dividing tumor. This first, complete, high-resolution profiling of the tumor cell genome fills an important gap in studies on pGBM. It ultimately guides the mapping of oncogenic networks unique to pGBM, identification of the related therapeutic predictors and targets, and development of more effective therapies. It further shows that, despite commonalities in a few CNAs, pGBM and aGBMs are two different diseases.
Keywords: pediatric high-grade astrocytomas, brain tumors, SNP arrays, LOH
Introduction
Brain tumors are the largest group of solid neoplasms in children and are currently the leading cause of cancer-related mortality and morbidity in the pediatric years. Pediatric high-grade astrocytomas (HGAs), including grade IV astrocytomas (glioblastoma [GBM]) account for 15% of all brain neoplasms in children,1 have a dismal prognosis despite aggressive management, and have a high morbidity linked to current treatments. Although their diagnosis still relies mainly on pathology, little is known about the molecular mechanisms underlying their development.
Physical changes in the DNA copy number of particular genomic regions, manifesting as loss of heterozygosity (LOH) or epigenetic changes such as loss of imprinting, have been shown to promote cancer formation and progression. In particular, LOH has been extensively used in the discovery of various tumor suppressor genes, including Rb1 and p53. A precise characterization of these genomic alterations in a given tumor may therefore increase our understanding of the oncogenic events promoting its growth and may provide more accurate means for its classification. However, most of the work done to date on nonrandom LOH in solid tumors has been based on searches for a small number of candidate loci. Comparative genomic hybridization (CGH), which identifies chromosomal segments with copy number changes (gain or loss),2–4 has an effective resolution that varies from ∼20 Mb to ∼100 kb and a far from optimal capacity in detecting chromosomal deletions, especially LOHs.5 More recently, a major breakthrough for the precise mapping of genomic aberrations in cancers has been made by the completion of the human genome sequence.6 Concurrently, high-density arrays for genotyping single-nucleotide polymorphisms (SNPs)6,7 have been made available (reviewed in Ref. 8). These arrays combine the genome-wide potential of hybridization arrays with higher-resolution (by an order of magnitude) detection of LOH, DNA copy number alterations, and other chromosomal aberrations compared to CGH.
Studies using these high-resolution arrays can be applied to unravel, with high precision, genomic imbalances in HGAs while providing an additional tool to classify these tumors. They have been used in adult HGAs (aHGAs, mainly high-resolution CGH arrays), providing more accurate tools for prognosis and the identification of therapeutic targets in this tumor.9–15 Genomic alterations involved in pHGAs are largely unknown, with only a few published studies using lower-resolution arrays.16–18 Moreover, pHGAs have distinct molecular profiles from aHGAs, indicating that results from adult studies cannot be applied to children.19,20 To identify genetic loci specifically involved in pHGAs, we analyzed 18 pediatric GBMs and 6 aHGAs using high-resolution Illumina 100K SNP arrays and an independent data set of 9 pHGAs using the Affymetrix 250K SNP arrays platform.
Materials and Methods
Sample Characteristics and Pathological Review
All samples were obtained under a protocol approved by the hospitals' institutional review boards and independently reviewed by senior pediatric neuropathologists (S.A. and C.H.) to ensure consistent classification based on contemporary guidelines from the World Health Organization. Eighteen pGBMs (average 10.3 ± 5.2 years) and 6 aGBMs (average 58.8 ± 18.2 years) were analyzed using the Illumina platform. Snap-frozen sections of areas immediately adjacent to the regions used for pathological diagnosis were provided and contained vascular tissue ranging from <10% to 30% of the full section. Normal tissue was not available for any of the samples. Tissues were obtained from the Pediatric Cooperative Human Tissue Network, the London/Ontario Tumor Bank, and from collaborators in Montreal and Toronto (Canada) and Hungary. Clinical findings of patients are provided in Table 1. Adult samples have previously been reported for gene expression analysis of pGBM.19
Table 1.
Clinical characteristics of GBM samples from adult and pediatric patients analyzed using the Illumina 100K SNP array
| GBM | Gender | Age at Diagnosis (Years) | Tumor Location |
|---|---|---|---|
| Pediatric | |||
| P1 | M | 9 | Infratentorial |
| P2 | F | 6 | Supratentorial (frontal) |
| P3 | F | 10 | Supratentorial (parietal) |
| P4 | F | 14 | Supratentorial (parietal) |
| P5 | M | 14 | Supratentorial (frontal) |
| P6 | M | 14 | Supratentorial (temporal) |
| P7 | F | 9 | Supratentorial (multilobar) |
| P8 | M | 3 | Supratentorial (temporal) |
| P9 | F | 11 | Supratentorial (temporal) |
| P10 | F | 13 | Supratentorial (frontal) |
| P11 | M | 7 | Supratentorial (thalamic) |
| P12 | F | 16 | Infratentorial |
| P13 | M | 16 | Infratentorial |
| P14 | M | 13 | Infratentorial |
| P15 | M | 15 | Infratentorial |
| P16 | F | 12 | Supratentorial (parietal) |
| P17 | F | 10 | Infratentorial |
| P18 | M | 13 | Mixed |
| Adult | |||
| Secondary | |||
| A1 | M | 52 | Supratentorial (multilobar) |
| A2 | F | 49 | Supratentorial (frontal) |
| A3 | M | 67 | Supratentorial (thalamic) |
| Primary | |||
| A4 | F | 30 | Supratentorial (parietal) |
| A5 | M | 70 | Supratentorial (temporal) |
| A6 | F | 82 | Supratentorial (frontal) |
DNA Extraction and Hybridization
DNA from frozen tumors was extracted as described previously.16 DNA (250 ng) from 25 samples was assayed with Infinium I whole genome genotyping, according to the recommendations of the manufacturer (Illumina, San Diego, CA, USA). The Illumina Sentrix Human-1 Genotyping BeadChip covers 109,365 gene-centric SNPs over the genome with a mean intermarker distance of 26 kb (13 kb median spacing). Image intensities were extracted using Illumina's BeadScan software. Data for each BeadChip were self-normalized using information contained within the array. For Affymetrix 250K SNP chips, 250 ng of tumor DNA was processed according to the manufacturer (Affymetrix, Inc., Santa Clara, CA, USA) and data were analyzed as described elsewhere.16 Genome profiles were created using the Illumina Genome Viewer and Chromosome Browser, which allow viewing, identifying, and manually annotating the chromosomal aberrations. For the initial analysis, the output by BeadStudio software was used to interpret the nature of the aberration. These variables include the normalized intensity of hybridization, expressed as its base-2 logarithm (log2 R), and the allelic ratio, expressed as the ratio of one allele over the sum of both alleles.21 Visualization of copy number and LOH in normal tissues is performed by plotting the log2R and the allele ratios across the genome. This algorithm, however, assumes single-lineage DNA and could not be used alone with these tumor tissue samples, which are unavoidably mixed with normal vascular tissue. Amplifications were therefore detected if there was an increase in the log2 R value (≥1, corresponding to tetraploid copy number ie ≥4). The SNPs with log2 R ≤ 2 in tumor tissues, but with normal log2 R in all CEU (CEPH [Centre de l'Etude du Polymorphisme Humain] European) DNA samples analyzed in the HapMap project were taken as homozygous deletions.21 If log2 R ≤ 2 in the CEU samples, the marker was discarded as a failed assay. To identify heterozygous deletions, we estimated the LOH score using the genotypes of all SNPs in a sequence window of 1 Mb, around any marker. This score is the base-10 logarithm of the ratio of the probability of observing the genotypes of all SNPs in the presence of LOH over the probability of observing the same genotype in the absence of LOH. It is based on the allele frequencies observed in the European-ancestry subjects used in the HapMap project (CEU set) and assigned to the marker in the middle of the window.22 We considered that a score of more than 10 is diagnostic of LOH. Four DNA samples from the CEU set were used as the normal control in the same assay.
BAC Arrays
The CGH-array analysis of tumor samples was performed as described previously.23 The V5S2 genomic array uses 3913 BAC markers from across the human genome with a mean resolution of 1 Mb. Normalization and data analyses were done with VAMP software (Bioinformatics Department, Curie Institute, Paris, France).23 Fluorescence ratios exceeding 1.2 were considered indicative of gains of chromosomal material, whereas losses were indicated by ratios lower than 0.8.
Validation of Copy Number Changes by Quantitative Real-Time PCR
Quantitative real-time PCR was done on an ABI-Prism 7000 sequence detector (Applied Biosystems) using a SYBR Green kit (Applied Biosystems). The target locus from each tumor DNA was normalized to the reference, Line-1 as previously described.16
Statistical Analysis
To be considered causative, a somatic copy-number change must be non-random, that is, recur at the same locus in different tumors more frequently than expected by chance alone. To assess the statistical significance of the occurrence of such overlaps in different tumors, their number was compared to a distribution generated by cyclically permuting the position of the LOH regions on each chromosome of each tumor 10,000 times. The false-detection rate (FDR) was calculated as the proportion of overlaps in any given number of tumors that would be expected to occur by chance alone (see Supplementary Material, Fig. S1).
Results
Genomic Alterations Identified in BAC and in SNP Arrays in 16 pGBM Samples
We used a previously validated BAC-array platform, which has provided numerous results in tumor LOH studies,23 and investigated the concordance of copy number aberration (CNA) detection between this platform and the Illumina 100K SNP arrays in 16 pHGAs (P1–P16; Table 1). BAC-array results validated data obtained by SNP arrays (see Supplementary Material, Table S1). However, because of the higher resolution of the SNP arrays, a higher number of alterations, left undetected by the BAC arrays, were uncovered. This is in keeping with previous findings on the higher sensitivity of SNP technology for the detection of CNAs.24
CNAs in pGBM and aGBMs Detected Using Illumina SNP Arrays
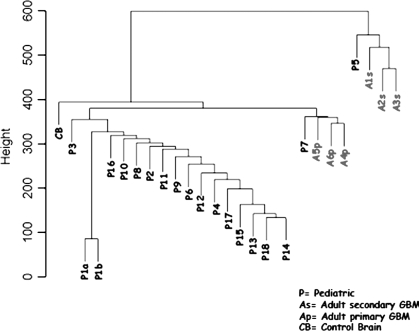
Analysis of the data set of 18 pGBMs and 6 aGBMs using the Illumina SNP arrays showed that heterozygous deletions, detected as LOH, are common phenomena mostly in pGBM and were seen for each chromosome in a number of samples (Tables 1–4; see also Supplementary Material, Table S1). Despite common LOH regions between pGBM and aGBM such as 9p24.3-9p13.1 and 17p13.3, there was little concordance between the CNAs detected in both settings, with most LOHs (Tables 3 and 4), amplifications (Table 5), and homozygous deletions (Table 6) found in children being different from the ones encountered in adults (Tables 2–6). Unsupervised hierarchical clustering analysis, using as input data regions with CNAs in at least one sample, clustered the aGBM samples separately from most pGBMs and the normal brain, confirming the presence of distinct molecular imbalances specific to pGBM.19 Interestingly, it also separated primary from secondary aGBMs (Fig. 1).
Table 2.
CNAs identified in 18 pGBMs and aGBMs using the 100K SNP Illumina array
| Tumors | No of CNAs | CNAs |
|---|---|---|
| P1 | 1 | (−)22q |
| P2 | 12 | (−)2q11-13, (−)9p24-21, (−)10p12-11, (−)10q, (−)14q23-32, (−)15q15-14, (−)16q, (−)17p, (+)17q21-25, del17q11, (−)19q, del21q11-21 |
| P3 | 2 | (−)12q24, (−)22q |
| P4 | 38 | (−)2p25-11, (−)3q21-29, (−)4p16-13, (−)4p12, (+)4p13, (+)4p12, (−)4q13-35,(+)4q12, (+)5p33-15, (+)5p15-12 (−)5p15, (−)5q11-21, (−)5q31-32, (del)6q24, (−)7p, (−)7q, (+)7q31, (−)9p22-21, (+)9p24, (+)9p23-22, (+)9p21-13, (+)10p12, (−)10q11-23, (+)10q23-24, (−)10q24-26,del11q11-22, del11q22-23, (−)12q21-23, (−)13q, (−)14q, del16q23, (−)17p13-17q11, (−)18p, (−)18q, (−)19p13, (+)19p13, (−)20q, (−)22q |
| P5 | 4 | (+)2p24, (+)11p, (−)11q13-25, (+)17q21-24 |
| P6 | 6 | (+)1q, (−)8p23, (−)9p, (+)9q, (+)21q21, (−)22q12-13 |
| P7 | 23 | (−)3p, (−)3q, (−)4p, (−)4q, (−)5p, (−)5q, (−)6p, (−)6q, (+)7q21, (−)8p, (−)8q, (+)9q33-34, (−)10p, (+)10p12, (−)10q, (+)13q14, (+)13q31, (+)13q33-34, (−)15q, (−)17p, (−)17q, (−)19p13, (−)22q |
| P8 | 33 | (+)1q43-44, (−)2p24-23, (+)2p25, (+)2p24.3, (+)2p24.1, (+)2p23.1, (−)3p, (−)3q,(+)3q26, (−)4p,(−)4q, (−)5p, (−)5q, (−)6p, (−)6q, (+)7p11, (+)7q21, (+)7q21, (+)7q31, (+)7q33, (−)8p, (−)8q, (−)10p, (−)10q, (−)11p15, (−)11q13-23 |
| (−)14q, (−)15q, (−)17p, (−)17q, (−)18p, (−)18q, (−)22q | ||
| P9 | 33 | (−)1p, (−)1q, (−)2p, (−)2q, del2q21-22, (−)3p, (−)3q, (−)4q32-35, (−)5q15-35, (−)6p, (−)6q, (−)8q11-24, (−)8q24, (−)9p, del9p23, del9p21, (−)9q, (−)10p, (−)10q, (+)10q26, (−)11p, (−)11q, (−)13q, (−)14q, (−)16p, (−)16q, (−)17p, (−)17q, (−)18p, (−)18q, (−)20p, (−)20q, (−)21q |
| P10 | 17 | (−)2p, (−)3q12-27, (+)3q26-29, (−)4q31, (+)7p, (+)7q, (−)8p21-12, (−)8p12-11, (−)9p21-13, 9q31-33, (−)10p, del 10q21-22, (−)10q25-26, (−)11q22-24, (−)14q31-32, (−)17p13, (−)19q13 |
| P11 | 4 | (−)1p, (−)6q12-13, (+)6q14, (−)6q14-27 |
| P12 | 4 | (−)10q21-26, (−)15q12-26, (−)16p, (+)17p13 |
| P13 | 15 | del1q24-31, (+)5p15, (+)7p22, (+)7p21, (+)7p21, (−)9p24-22, (−)9p21, (−)10q23-26, (+)10q22, (+)10q23, (−)16q, (−)17p, (−)18q12-23, (−)20p, (−)21q |
| P14 | 1 | (−)22q |
| P14 | 4 | (+)2p24, (+)4q12, (+)7p, (+)7q |
| P16 | 1 | (+)9p21-13 |
| P17 | 1 | (+)7q11 |
| P18 | 0 | |
| A1 | 10 | (+)3p11-12, (−)3p26-24, (−)3q27-28, (−)3q29, (−)9p, del9p22-21, (−)9q12-21, (−)10p, (−)10q, (−)22q12-13 |
| A2 | 14 | (−)3p26, (−)9p24-21, (−)9p21, (−)9p13, (−)9q22, (−)11p15, 12p13-12, (−)13q12-21, del 14q23, del14q24, (−)10p, (−)10q, (−)17p13, (−)19q13, (−)21q, (−)22q13 |
| A3 | 3 | (−)4q13-35, (−)9p24-21 |
| A4 | 4 | (+)7p11-14; del 9p22-21, (−)10q, (+)12q13-15, (−)19p12 |
| A5 | 9 | (−)5q, (−)7q11-21, (−)11p15-11, (−)12p, (−)13q, (−)14q, (−)17p13-11, (−)18p11, (−)21q22 |
| A6 | 2 | (+)7p14-15, (+)7p11-15, (−)10q |
A, adult glioblastoma; P, pediatric glioblastoma. (−) indicates an LOH; (+) indicates an amplification or gain; “del” indicates a homozygous deletion.
Table 3.
LOH analysis showing distinct imbalances between pGBM and aGBM
| Cytogenetic Band | Locus (Mb) |
Minimal Region | Genes | Number of Tumors | Known or Novel | Interesting Genes | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Start SNP | Position | End SNP | Position | ||||||||
| LOH regions in 19 pGBMs | |||||||||||
| 2p25.3-2p11.2 | rs876724 | 104974 | rs4832054 | 86970238 | 2p24.1-2p23.1 | 100 | 4 | Novel | |||
| 3q12.1-3q29 | rs7614366 | 100979041 | rs7374380 | 199195232 | 3q21.1-3q26 | 361 | 5 | Novel | |||
| 4q13.1-4q35.2 | rs1449043 | 59562234 | rs1317423 | 191081281 | 4q32.3-4q35.2 | 137 | 5 | Novel | |||
| 5q11.1-5q21.3 | rs4865676 | 49610934 | rs7701086 | 109419588 | 5q11.1-5q21.3 | 348 | 4 | Novel | |||
| 6q12-6q27 | rs9363741 | 68085762 | rs2021899 | 170823609 | 6q12-6q27 | 630 | 4 | Novel | |||
| 8p23.3-8p11.21 | rs888580 | 204809 | rs907561 | 40930754 | 8p23.3-8p11.21 | 373 | 4 | Novel | |||
| 9p24.3-9p13.1a | rs493348 | 202421 | rs7848575 | 38708308 | 9p24.3-9p22.1 | 92 | 5 | Known | ELAVL2 | CDKN2A | CDKN2B |
| 10p | 10p12.31-10p11.1 | 133 | 5 | Novel | |||||||
| 10q22.1-10q26.3 | rs10762360 | 71729512 | rs2803990 | 134410330 | 10q22.1 | 1 | 7 | Novel | CBARA1 | ||
| 11q13.2-11q25 | rs3133269 | 67560732 | rs4300405 | 133977871 | 11q22.1-11q23.1 | 107 | 4 | Novel | |||
| 14q23.3-14q32.33 | rs1290900 | 66770921 | rs7492357 | 105033704 | 14q31.3-14q32.31 | 189 | 5 | Novel | |||
| 15q15.1-15q23 | rs2289218 | 38853018 | rs10220851 | 66092261 | 15q15.2-15q15.3 | 13 | 10 | Novel | CCNDBP1 | TP53BP1 | H76p |
| 16q | 16q | 4 | Known | ||||||||
| 17p13-17p11.2 | rs2750007 | 623976 | rs4924750 | 18201478 | 17p13.3a | 3 | 7 | Novel | SMYD4 | RPA1 | |
| 17p13.1 | 2 | 7 | Novel | CLEC10A | ASGR2 | ||||||
| 17p13.1 | 79 | 7 | Novel | GPS2 | |||||||
| 18q12.2-18q23 | rs1484095 | 33971576 | rs4798947 | 76064957 | 18q23 | 19 | 4 | Novel | KCNG2 | ||
| 22q11.1-22q13 | rs3788277 | 16054103 | rs6520165 | 49144461 | 22q12.2 | 1 | 7 | Novel | NF2 | ||
| 22q12.3 | 7 | 7 | Novel | KCTD17 | |||||||
| 22q13.1 | 18 | 7 | Novel | RAC2 | CDC42EP1 | ||||||
| 22q13.1 | 9 | 7 | Novel | LOC400927 | TPTE, PTEN homologs | ||||||
| LOH regions in 6 aGBMs | |||||||||||
| 3p26.3-3p24.2 | rs6803398 | 982443 | rs892940 | 24513842 | 3p26.3 | 4 | 2 | Novel | CHL1 | ||
| 9p24.3-9p13.1a | rs6477419 | 979943 | rs7036799 | 38747881 | 9p24.3-9p21.1 | 146 | 3 | Known | ELAVL2 | CDKN2A | CDKN2B |
| 11p15.5-11p11.2 | rs2280544 | 194062 | rs6485999 | 49615562 | 11p15.4 | 1 | 3 | Known | |||
| 17p13.3-17p11.2 | rs4985615 | 172350 | rs9783820 | 18601046 | 17p13.3a | 21 | 2 | Novel | TUSC5 | ||
| 22q12.2-22q13.33 | rs7289095 | 28983276 | rs1001469 | 49428897 | 22q13.2-22q13.3 | 122 | 2 | Known | |||
aCommon alterations between pGBM and aGBM.
Table 4.
Identification of statistically overrepresented gene ontologies (GOs) in the genes harbored in LOH regions analyzed using the GOstat tool (http://gostat.wehi.edu.au/cgi-bin/goStat.pl)
| Gene Ontology Term | p | Number of Genes | Total |
|---|---|---|---|
| Negative regulation of biological process | 3.80E−11 | 119 | 1187 |
| Cell-cycle process | 5.08E−11 | 89 | 809 |
| System development | 5.83E−11 | 149 | 1623 |
| Negative regulation of cellular process | 2.40E−10 | 109 | 1095 |
| Cellular developmental process | 3.22E−10 | 198 | 2389 |
| Cell differentiation | 3.22E−10 | 198 | 2389 |
| Regulation of cell proliferation | 3.21E−09 | 55 | 446 |
| Transcription from RNA polymerase II promoter | 4.01E−09 | 71 | 642 |
| Regulation of cellular process | 6.90E−09 | 393 | 5660 |
| Organ development | 6.90E−09 | 111 | 1183 |
| Positive regulation of cellular process | 3.34E−08 | 89 | 905 |
| Cell development | 5.44E−08 | 155 | 1865 |
| Regulation of cell cycle | 5.71E−08 | 65 | 599 |
| Regulation of progression through cell cycle | 1.09E−07 | 64 | 594 |
| Positive regulation of biological process | 3.01E−07 | 104 | 1152 |
| Defense response to bacterium | 2.14E−06 | 18 | 99 |
| Negative regulation of cell proliferation | 9.98E−06 | 29 | 216 |
| Cell death | 1.12E−05 | 79 | 862 |
| Death | 1.12E−05 | 79 | 862 |
| Protein kinase cascade | 1.12E−05 | 42 | 370 |
| Apoptosis | 1.12E−05 | 75 | 807 |
| Response to bacterium | 1.43E−05 | 18 | 107 |
| Programmed cell death | 1.48E−05 | 75 | 813 |
| Enzyme-linked receptor protein signaling pathway | 1.57E−05 | 35 | 290 |
| Cell migration | 1.64E−05 | 30 | 233 |
The complete database of annotated human genes was used as a control set. Results were filtered to include only GOs with a minimum path length of 5 and that fall under the biological process segment of the GO hierarchy.
Table 5.
Amplifications in pGBM and aGBM
| Cytogenetic Band | Locus (Mb) |
Minimal region | Genes | Number of Tumors | Known or Novel | Interesting Genes | |||
|---|---|---|---|---|---|---|---|---|---|
| Start SNP | Position | End SNP | Position | ||||||
| Amplifications in pGBM | |||||||||
| 1q | 1q43-1q44 | 26 | 2 | Novel | AKT3 | ||||
| 2p24.3 | rs12616227 | 14424553 | rs300168 | 17884078 | 2p24.3 | 15 | 3 | Known | MYCN |
| 3q26-3q29 | rs7638716 | 174896221 | rs6770002 | 199198093 | 3q26.31-3q26.33 | 34 | 2 | Novel | PIK3CA, NGL1 |
| 4q12 | rs11734039 | 54105356 | rs7692791 | 55821167 | 4q12 | 11 | 2 | Known | PDGFRa, KIT |
| 5p33-5p15 | rs4956990 | 236134 | rs2578553 | 5502367 | 5p15.33-5p15.2 | 62 | 2 | Novel | TPPP |
| 7p22.3-7p11.2a | rs7786069 | 160449 | rs4947882 | 54032344 | 7p22.3-7p11.2 | 417 | 2 | Known | EGFR |
| 7q11.21-7q33b | rs2198391 | 61491258 | rs3735019 | 137020053 | 7q21.13-7q21.3 | 23 | 5 | Novel | CDK6 |
| 7q11.21-7q33 | rs2198391 | 61491258 | rs3735019 | 137020053 | 7q31.2-7q33 | 163 | 4 | Novel | |
| 9p24.3-9p13 | rs999924 | 1322257 | rs2799738 | 38468670 | 9p13.3-9p13.1 | 122 | 3 | Novel | |
| 9q | 9q33.1-9q34.3 | 265 | 2 | Novel | |||||
| 10p12.31-10p12 | rs1055340 | 16595534 | rs751006 | 26145481 | 10p12.2-10p12.1 | 3 | 2 | Novel | OTUD1 |
| 10q22.2-10q26.3 | rs4746308 | 76999278 | rs2515641 | 135240243 | 10q22.2-10q26.3 | 516 | 3 | Novel | PTEN |
| 17q21-17q25.3 | rs8074034 | 44287297 | rs599314 | 78321361 | 17q21.33-17q22 | 73 | 2 | Novel | |
| Amplifications in aGBMs | |||||||||
| 3p12.3-3p11.2 | rs4258921 | 81083700 | rs1521800 | 90084278 | 3p12.3-3p11.2 | 21 | 1 | Novel | EPHA3 |
| 7p15.2-7p11.1 | rs10259620 | 26975529 | rs6974091 | 57689510 | 7p15.2-7p14.1 | 38 | 2 | Novel | CREB5 |
| 7p12a | rs1454520 | 54455112 | rs7796351 | 55230615 | 7p11 | 6 | 2 | Known | EGFR |
| 12q14.3-12q15 | rs1797404 | 64866345 | rs7973221 | 69380480 | 12q14.3-12q15 | 41 | 1 | Novel | NGFR |
Seventeen (94.4%) of the 18 pediatric patients and all the 6 adult patients have at least one amplification. All the samples have at least 2 loci with amplification or homozygous deletion.
aCommon alterations between pGBM and aGBM.
bKnown CNA with a much higher incidence than the one found in the normal population.
Table 6.
Homozygous deletions in pGBM and aGBM
| Cytogenetic Band | Locus (Mb) |
Minimal Region | Genes | Number of Tumors | Known or Novel | Interesting Genes | |||
|---|---|---|---|---|---|---|---|---|---|
| Start SNP | Position | End SNP | Position | ||||||
| Deletions in pGBM | |||||||||
| 1q24.2-1q31.1 | rs2901029 | 165831827 | rs1011726 | 187652124 | 1q24.2-1q31.1 | 195 | 1 | Novel | FASLG |
| 2q21.1-2q22.2 | rs1918615 | 140631475 | rs1465839 | 143468685 | 2q21.1-2q22.2 | 3 | 1 | Novel | LOC730114 |
| 5q14.2-5q22.1 | rs2656989 | 82226475 | rs6881837 | 111305383 | 5q14.2-5q22.1 | 111 | 1 | Novel | |
| 6q24.3 | rs1832378 | 145733157 | rs1039085 | 147677240 | 6q24.3 | 13 | 1 | Novel | RAB32 |
| 9p23 | rs419387 | 10050049 | rs10809546 | 11642121 | 9p23 | 3 | 1 | Novel | |
| 9p21.2a | rs1475660 | 20746547 | rs1197944 | 27991944 | 9p21.2 | 48 | 1 | Known | ELAVL2, CDKN2A-2B |
| 10q21.3-10q22.1 | rs747942 | 71006472 | rs10823922 | 73852923 | 10q21.3-10q22.1 | 35 | 1 | Novel | CBARA1 |
| 11q11-11q22.3 | rs9666583 | 54865587 | rs7112835 | 108318814 | 11q11-11q22.3 | 858 | 1 | Novel | |
| 11q22.3-11q23.1 | rs7112835 | 108318814 | rs1293344 | 111542594 | 11q22.3-11q23.1 | 37 | 1 | Novel | ARHGAP20 |
| 16q23.2-16q23.3 | rs7204972 | 79379066 | rs9922131 | 81700946 | 16q23.2-16q23.3 | 16 | 1 | Novel | PLCG2 |
| 17q11.2 | rs1061346 | 26138355 | rs9915569 | 27490232 | 17q11.2 | 24 | 1 | Novel | OMG |
| 18q23 | rs2196736 | 74449768 | rs4798947 | 76064957 | 18q23 | 19 | 1 | Novel | KNCG2 |
| 21q11.2-21q21.3 | rs2742158 | 13560787 | rs3737413 | 26270243 | 21q11.2-21q21.3 | 64 | 1 | Novel | BIC |
| Deletions in aGBMs | |||||||||
| 9p21.3 | rs1556475 | 20811287 | rs2939 | 21156004 | 9p21 | 5 | 3 | Novel | INFB1, INFA21 |
| 14q23.2 | rs1695770 | 64284919 | rs8015408 | 66216433 | 14q23.2 | 19 | 1 | Novel | MAX, YBX1P1 |
| 14q24.1-14q24.2 | rs8016781 | 68963945 | rs6574074 | 71945439 | 14q24.1-14q24.2 | 26 | 1 | Novel | SIPA1L1 |
Fifteen (83.3%) of the 18 pediatric patients and all the 6 adult patients have at least one homozygous deletion. All the samples have at least 2 loci with amplification or homozygous deletion. Homozygous deletion in 10q including PTEN was found in 4 of 6 adult tumors and is not included as it is a known alteration.
aCommon alterations between pGBM and aGBM.
Fig. 1.
Unsupervised hierarchical clustering of 24 GBMs (18 pediatric and 6 adult) and 1 control normal brain (CB). We used statistically significant CNAs present in at least one sample as data input (Welsch t-test, p < 0.001) and separated our data set accordingly. Pediatric (P) samples clustered separately from adult samples (A) and from the CB. DNA from one pediatric sample P1 was extracted from two separate parts of the same tissue sample. DNA from both extractions was independently subjected to whole-genome amplification prior to analysis on the 100K SNP array platform. P1a and P1b clustered similarly showing the reproducibility of the analysis of genome profiling. Primary adult glioblastoma (Ap) clustered separately from secondary adult glioblastoma (As).
Analysis of Recurrent Regional LOH in GBMs
Several overlapping regions of LOH in tumors were identified in pGBM samples (Tables 3 and 4). By permutation analysis, the 95% confidence interval of the FDR for seven tumor-overlaps is 0–0.096, which makes it unlikely that more than one of these overlaps is the result of chance alone (see Supplementary Material, Fig. S1). Some of these LOHs found in more than seven samples have been previously described, but most are novel (Tables 2–4). The highest LOH frequency peak in pGBM was from a 103-kb cluster located in Chr15q15 (10/18) (Tables 3 and 4). Novel genes, including protein p53 binding protein 1 (TP53BP1) and Cyclin D-type binding-protein 1 (CCNDBP1), and two potential tumor suppressor genes that regulate the p53 and the Rb pathways are within this LOH peak.
Analysis of Regional Chromosomal Amplification and Homozygous Deletions in GBMs
Only a small number of amplifications (defined as 4 copies or more) and homozygous deletions were found in the pGBM samples. These were mainly in regions close to the centromeres of Chrs 5, 7, 11, 19, and 20, where typically no genes are found (Table 3). In contrast, chromosomal amplifications were more frequent in aGBMs (Tables 1 and 5). For example, all aGBMs had amplification of 4p16, 5p11, 11q11, and 20p11.2, and five of six had amplification in 8p23 and 19p12-13.3. Further, as expected and previously shown, epidermal growth factor receptor (EGFR) maps to the largest SNP cluster of amplification in Chr7p12 in two-thirds of the primary aGBMs included in this study (Table 5).25,26 In pGBM, a previously described amplification of 7p12 including EGFR was observed with a similar incidence to what is reported in the literature (2 of 18; 10.5%). However, a new amplification in 7q21-22 including CDK6 (Cyclin D Kinase 6) was seen in 5 of 18 pGBMs. All tumors were supratentorial. Interestingly, the latter is a known copy number variant with a seemingly higher incidence in pGBM than in the general population (5 of 18 versus 7 of 270; 26% versus 3%, p < 0.001, Fisher exact test). For regional homozygous chromosomal deletion, the most significant finding was the deletion of an SNP cluster in aGBMs containing interferon genes (IFNB1, IFNW1, IFNA21) that are important in the induction of p53 gene expression (Table 6).27 No homozygous deletions were observed in pGBM.
Validation of CNAs Identified in This Study
In the absence of control DNA from the same individual allowing confirmation that the CNAs we observed are tumor-derived and not a normal variant, we cross-referenced our data with the Database of Genomic Variants and the CEU study.28,29 We additionally cross-referenced our data for the most relevant CNAs with data from a set of 1,363 control DNA analyzed with the Illumina Human-Hap 300K platform.30 This allowed us to identify some previously reported regions of common structural polymorphisms in the general population, further validating the other regions as tumor-specific LOHs (Tables 2–6; see also Supplementary Material, Tables S1 and S2). We also compared our data with other available genotyping studies on pHGAs16–18 and on aHGAs9,31,32 as the number of adult cases used for comparison in this study is too few to make a meaningful conclusion. We also compared our data set to two publicly available 100K SNP array data sets on aHGAs (NCBI GEO DataSets as GSE9635 and GSE6109 Records).13,33 Cross-referencing our results with these data sets on aHGAs showed several commonalities with previously published studies, mainly for the adult patients included in our study (Pearson correlation, r: 0.83 and Tables 1–6; see also Supplementary Material, Table S1); however, only few previously published CNAs were common with pGBM (Pearson correlation, r: 0.21). We further validated the data set against an independent data set of 9 pGBMs analyzed using another SNP platform, the Affymetrix 250K SNP array that interrogates more than 200,000 loci and has a similar average resolution of 15 kb,34 and obtained concordant results for several CNAs, further validating data obtained using the Illumina SNP arrays (see Supplementary Material, Fig. S2).
We also arbitrarily chose a set of genes involved in regions identified as homozygous deletions, LOH, or DNA amplification in our data set and validated these CNAs using quantitative real-time (qRT)-PCR on DNA extracted from the same tumors (Tables 5 and 6). ELAVL2 (embryonic lethal, abnormal vision, Drosophila-like 2), a gene located on 9p21, was within a region of LOH in 5 of 18 pGBMs and potentially deleted in 1 of 18. We confirmed these results using qRT-PCR (Table 7). Chromosomal amplification in pGBMs on 7q21-22, which contains CDK6, was identified in 5 of 18 tumors. CDK6 amplification was also confirmed using qRT-PCR (Table 7).
Table 7.
Validation of Illumina Infinium 1 SNP-array data using qRT-PCR on selected genes
| Illumina data | Normalized ratio | Normalized STD | |
|---|---|---|---|
| CDK6 | |||
| P1 | Normal copy number | 1.24 | 0.52 |
| P2 | Amplification | 114.45 | 7.63 |
| P3 | Amplification | 42.55 | 7.26 |
| P5 | Amplification | 3.80 | 0.86 |
| P7 | Normal copy number | 1.34 | 0.36 |
| P11 | Amplification | 2.63 | 0.38 |
| P13 | Normal copy number | 1.27 | 0.28 |
| P15 | Normal copy number | 1.20 | 0.31 |
| P16 | Amplification | 7.82 | 0.27 |
| ELAVL2 | |||
| P13 | LOH | 0.40 | 0.13 |
| P15 | LOH | 0.50 | 0.07 |
| P12 | LOH | 0.42 | 0.14 |
| P11 | Homozygous deletion | 0.19 | 0.04 |
| P1 | LOH | 0.46 | 0.11 |
| P7 | LOH | 0.48 | 0.16 |
| P2 | Normal copy number | 1.20 | 0.17 |
| P4 | Normal copy number | 0.80 | 0.23 |
| P6 | Normal copy number | 1.10 | 0.32 |
To validate results we obtained following the analysis of data from SNP arrays, we selected previously identified genes in high-grade astrocytomas that were included in regions showing chromosomal amplification (CDK6, amplification of Ch 7q21 in 5 tumors P2–P3–P5–P11–P16) or LOH or homozygous deletion (ELAVL2, LOH in P1–P7–P13–P15–P12, complete deletion in P11) and performed qRT-PCR on DNA extracted from the same tumors and from other tumors included in this study and showing no copy number change for these regions. Normalized ratio obtained using qRT-PCR was interpreted as follows: <0.2 homozygous deletion; 0.4 < X < 0.6 LOH; >2 amplification. Pediatric high-grade astrocytomas were numbered from 1 to 18 for clarity issues with the suffix P for pediatric.
CDK6, cyclin D kinase 6; ELAVL2, embryonic lethal, abnormal vision, drosophila-like 2; STD, standard deviation.
Discussion
Understanding the molecular pathogenesis of pHGAs requires a detailed cataloguing of all the genetic lesions in the somatic lineage of this cancer. This study includes the largest cohort of pGBMs analyzed for genomic imbalances in tumor DNA and is the first to use high-resolution SNP arrays. While further confirming that aGBM and pGBM are genetically distinct cancers, we provide a comprehensive whole-genome map of CNAs for pGBM and identify, within regions of CNAs, altered genes and pathways for further analysis (Tables 1–7).
Our data show findings in common with available array-based genomic studies on pGBM, including ELAVL2, CDKN2A, and CDKN2B deletions that have been reported in both pGBM and aGBM, supporting the validity of our data set. However, previous studies on pHGAs used arrays that had a lower resolution of at least one log than the 100K and 250K arrays used in this study.16 In these former studies, as for BAC arrays, this resulted in a vast number of genetic abnormalities going undetected. We uncover herein several previously unidentified CNAs specific to pediatric tumors that help provide insight into molecular pathways involved in the genesis of pGBM. These CNAs include LOH in 15q15.1-15q23 and 17p13-17p11.2 and amplification of 7q21-22 and 1q43-44. Even if some CNAs are common to the pediatric and adult setting, their overall incidence will differ whether they occur in children or in adults. When we reviewed published data sets on aHGAs, commonalities with our findings on pGBM included LOH of 22q17p and 9p, whereas LOH of 3p21 and 10q21 and amplification of 7q, 2p, 9p, and 10q seemed specific to pGBM (see Supplementary Material, Table S2). As an added example, amplification of 7p12 including EGFR is a frequent event in primary aGBMs (∼45%).9,35 We and others16,36 show amplification of 7p12 including EGFR in only rare cases of pediatric GBMs (less than 10%). Conversely, amplification of 7q21-22 including CDK6 is a rare event in aHGAs (one case report), whereas it was present in 5 of 18 pGBMs. In addition, as a consequence of the higher resolution of the arrays we used, the genetic interval subject to alteration in tumors will differ in pediatric and adult samples in many cases. For example, 10q LOH/deletion will include the PTEN gene (10q23.31) in adults whereas the genetic interval will be different in children and LOH on 10q will not include it (10q21.3-10q22.1).
Cell-cycle abnormalities, including alterations of the p53 and the RB1 pathways, play an important role in the genesis of HGAs, including pHGAs.20,26 As shown in the analysis of genes included in the CNA regions (Tables 3–6) and by the gene ontology analysis (Table 4), genes associated with the cell-cycle checkpoints and genes involved in the regulation of cell-cycle and cell-death pathways are the major targets of LOHs in pGBM. For example, we identified recurrent LOHs in several tumors that encompass several genes including H76p, TP53BP1, and CCNDBP1 on 15p (Table 3). H76p is associated with the γ-tubulin complexes and may participate in the nucleation process.37 TP53BP1 is a conserved checkpoint protein with properties of a DNA double-strand break sensor. It binds to the central domain of p53, enhancing transactivation, and its deletion may play an important role by impairing function of tumor protein p53 (TP53).38 CCNDBP1 is a helix–loop–helix leucine zipper protein, recently identified as a novel tumor suppressor in epithelial cancers, showing LOH and/or deletions on Chr15.39 It decreases the levels of Cyclin D expression, reducing the phosphorylation of RB1, thus regulating the RB1 pathway and cell growth.39
The rare gene containing regions showing genomic amplification in pGBM included 7q21-22 in 5 pGBMs (Table 5). This region is interesting from two standpoints. It includes CDK6, which encodes for a kinase also regulating the RB1 pathway. Amplification of CDK6 in this subset of pGBM was validated by qRT-PCR (Table 7) and has not been previously reported in pHGAs. In aHGAs, amplification of CDK6 has been reported in only one patient, whereas its over-expression, without genetic amplification, is more common (∼40%). Secondly, a CNA found with statistically higher frequency in a given tumor is likely a germline predisposition to the tumor. 7q21-22 seems to be amplified in a small percentage of the normal population, and the higher incidence of its amplification in pGBM, when further confirmed, may indicate an association of a common variant with susceptibility to these tumors (Table 5).
Little is known on the genetics of pGBM and our study fills an important gap in understanding this pediatric brain tumor. Moreover, the considerable wealth of information on the molecular genetics of aHGA is often projected onto the pediatric population without critical comparison between these two different disease contexts. Our data document how profound these differences are. We propose that analyses such as the one presented in this study may ultimately guide mapping of oncogenic signaling networks unique to pGBM, identification of the related therapeutic predictors and targets, and development of more effective therapies. Alteration of copy number for genes involved in p53 and the RB pathways including, for example, amplification of CDK6, or LOH of CCNDBP1 or TP53BP1, are some of the interesting findings unraveled in our data set that will help shed light on the unique molecular pathogenesis of this disease, providing hope of developing new therapeutic strategies to improve survival in a devastating cancer.
Supplementary Material
Supplementary material is available at Neuro-Oncology Journal online.
Supplementary Material
Acknowledgments
This work was supported by the Canadian Institutes of Health Research, the Cole Foundation, One Day at a Time, and the Vinchiaturese Association (N.J.), the Hungarian Scientific Research Fund (OTKA) Contract No. T-04639, and the National Research and Development Fund (NKFP) Contract No. 1A/002/2004 (P.H., M.G., L.B., and Z.H.). H.Q. and K.J. are the recipients of a fellowship and a studentship, respectively, from the Canadian Institutes of Health Research. N.J. is the recipient of a Chercheur Boursier Award from Fonds de la Recherche en Sante du Quebec.
Conflict of interest statement. None declared.
References
- 1.Broniscer A. Past, present, and future strategies in the treatment of high-grade glioma in children. Cancer Invest. 2006;24:77–81. doi: 10.1080/07357900500449702. [DOI] [PubMed] [Google Scholar]
- 2.Vissers LE, Veltman JA, van Kessel AG, et al. Identification of disease genes by whole genome CGH arrays. Hum Mol Genet. 2005;14(2):R215–R223. doi: 10.1093/hmg/ddi268. [DOI] [PubMed] [Google Scholar]
- 3.Pinkel D, Albertson DG. Comparative genomic hybridization. Annu Rev Genomics Hum Genet. 2005;6:331–354. doi: 10.1146/annurev.genom.6.080604.162140. [DOI] [PubMed] [Google Scholar]
- 4.He M, Rosen J, Mangiameli D, et al. Cancer development and progression. Adv Exp Med Biol. 2007;593:117–133. doi: 10.1007/978-0-387-39978-2_12. [DOI] [PubMed] [Google Scholar]
- 5.Thomas R, Scott A, Langford CF, et al. Construction of a 2-Mb resolution BAC microarray for CGH analysis of canine tumors. Genome Res. 2005;15:1831–1837. doi: 10.1101/gr.3825705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altshuler D, Brooks LD, Chakravarti A, et al. A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunderson KL, Steemers FJ, Lee G, et al. A genome-wide scalable SNP genotyping assay using microarray technology. Nat Genet. 2005;37:549–554. doi: 10.1038/ng1547. [DOI] [PubMed] [Google Scholar]
- 8.Dutt A, Beroukhim R. Single-nucleotide polymorphism array analysis of cancer. Curr Opin Oncol. 2007;19:43–49. doi: 10.1097/CCO.0b013e328011a8c1. [DOI] [PubMed] [Google Scholar]
- 9.Maher EA, Brennan C, Wen PY, et al. Marked genomic differences characterize primary and secondary glioblastoma subtypes and identify two distinct molecular and clinical secondary glioblastoma entities. Cancer Res. 2006;66:11502–11513. doi: 10.1158/0008-5472.CAN-06-2072. [DOI] [PubMed] [Google Scholar]
- 10.Nigro JM, Misra A, Zhang L, et al. Integrated array-comparative genomic hybridization and expression array profiles identify clinically relevant molecular subtypes of glioblastoma. Cancer Res. 2005;65:1678–1686. doi: 10.1158/0008-5472.CAN-04-2921. [DOI] [PubMed] [Google Scholar]
- 11.Rich JN, Hans C, Jones B, et al. Gene expression profiling and genetic markers in glioblastoma survival. Cancer Res. 2005;65:4051–4058. doi: 10.1158/0008-5472.CAN-04-3936. [DOI] [PubMed] [Google Scholar]
- 12.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Kotliarov Y, Steed ME, Christopher N, et al. High-resolution global genomic survey of 178 gliomas reveals novel regions of copy number alteration and allelic imbalances. Cancer Res. 2006;66:9428–9436. doi: 10.1158/0008-5472.CAN-06-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horvath S, Zhang B, Carlson M, et al. Analysis of oncogenic signaling networks in glioblastoma identifies ASPM as a molecular target. Proc Natl Acad Sci USA. 2006;103:17402–17407. doi: 10.1073/pnas.0608396103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLendon R, Friedman A, Bigner D, et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong KK, Tsang YT, Chang YM, et al. Genome-wide allelic imbalance analysis of pediatric gliomas by single nucleotide polymorphic allele array. Cancer Res. 2006;66:11172–11178. doi: 10.1158/0008-5472.CAN-06-2438. [DOI] [PubMed] [Google Scholar]
- 17.Warr T, Ward S, Burrows J, et al. Identification of extensive genomic loss and gain by comparative genomic hybridisation in malignant astrocytoma in children and young adults. Genes Chromosomes Cancer. 2001;31:15–22. doi: 10.1002/gcc.1113. [DOI] [PubMed] [Google Scholar]
- 18.Rickert CH, Strater R, Kaatsch P, et al. Pediatric high-grade astrocytomas show chromosomal imbalances distinct from adult cases. Am J Pathol. 2001;158:1525–1532. doi: 10.1016/S0002-9440(10)64103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faury D, Nantel A, Dunn SE, et al. Molecular profiling identifies prognostic subgroups of pediatric glioblastoma and shows increased YB-1 expression in tumors. J Clin Oncol. 2007;25:1196–1208. doi: 10.1200/JCO.2006.07.8626. [DOI] [PubMed] [Google Scholar]
- 20.Rood BR, Macdonald TJ. Pediatric high-grade glioma: molecular genetic clues for innovative therapeutic approaches. J Neurooncol. 2005;75(3):267–272. doi: 10.1007/s11060-005-6749-5. [DOI] [PubMed] [Google Scholar]
- 21.Steemers FJ, Gunderson KL. Illumina, Inc. Pharmacogenomics. 2005;6:777–782. doi: 10.2217/14622416.6.7.777. [DOI] [PubMed] [Google Scholar]
- 22.Peiffer DA, Le JM, Steemers FJ, et al. High-resolution genomic profiling of chromosomal aberrations using Infinium whole-genome genotyping. Genome Res. 2006;16:1136–1148. doi: 10.1101/gr.5402306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.La Rosa P, Viara E, Hupe P, et al. VAMP: visualization and analysis of array-CGH, transcriptome and other molecular profiles. Bioinformatics. 2006;22:2066–2073. doi: 10.1093/bioinformatics/btl359. [DOI] [PubMed] [Google Scholar]
- 24.Greshock J, Feng B, Nogueira C, et al. A comparison of DNA copy number profiling platforms. Cancer Res. 2007;67:10173–10180. doi: 10.1158/0008-5472.CAN-07-2102. [DOI] [PubMed] [Google Scholar]
- 25.Wong AJ, Bigner SH, Bigner DD, et al. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci USA. 1987;84:6899–6903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Y, Parada LF. The molecular and genetic basis of neurological tumours. Nat Rev Cancer. 2002;2:616–626. doi: 10.1038/nrc866. [DOI] [PubMed] [Google Scholar]
- 27.Takaoka A, Hayakawa S, Yanai H, et al. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424:516–523. doi: 10.1038/nature01850. [DOI] [PubMed] [Google Scholar]
- 28.Conrad DF, Andrews TD, Carter NP, et al. A high-resolution survey of deletion polymorphism in the human genome. Nat Genet. 2006;38:75–81. doi: 10.1038/ng1697. [DOI] [PubMed] [Google Scholar]
- 29.Hinds DA, Kloek AP, Jen M, et al. Common deletions and SNPs are in linkage disequilibrium in the human genome. Nat Genet. 2006;38:82–85. doi: 10.1038/ng1695. [DOI] [PubMed] [Google Scholar]
- 30.Hakonarson H, Grant SF, Bradfield JP, et al. A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature. 2007;448:591–594. doi: 10.1038/nature06010. [DOI] [PubMed] [Google Scholar]
- 31.Bredel M, Bredel C, Juric D, et al. High-resolution genome-wide mapping of genetic alterations in human glial brain tumors. Cancer Res. 2005;65:4088–4096. doi: 10.1158/0008-5472.CAN-04-4229. [DOI] [PubMed] [Google Scholar]
- 32.Liu F, Park PJ, Lai W, et al. A genome-wide screen reveals functional gene clusters in the cancer genome and identifies EphA2 as a mitogen in glioblastoma. Cancer Res. 2006;66:10815–10823. doi: 10.1158/0008-5472.CAN-06-1408. [DOI] [PubMed] [Google Scholar]
- 33.Beroukhim R, Getz G, Nghiemphu L, et al. Assessing the significance of chromosomal aberrations in cancer: methodology and application to glioma. Proc Natl Acad Sci USA. 2007;104:20007–20012. doi: 10.1073/pnas.0710052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mullighan CG, Goorha S, Radtke I, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- 35.Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 36.Pollack IF, Hamilton RL, James CD, et al. Rarity of PTEN deletions and EGFR amplification in malignant gliomas of childhood: results from the Children's Cancer Group 945 cohort. J Neurosurg. 2006;105:418–424. doi: 10.3171/ped.2006.105.5.418. [DOI] [PubMed] [Google Scholar]
- 37.Fava F, Raynaud-Messina B, Leung-Tack J, et al. Human 76p: a new member of the gamma-tubulin-associated protein family. J Cell Biol. 1999;147:857–868. doi: 10.1083/jcb.147.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang B, Matsuoka S, Carpenter PB, et al. 53BP1, a mediator of the DNA damage checkpoint. Science. 2002;298:1435–1438. doi: 10.1126/science.1076182. [DOI] [PubMed] [Google Scholar]
- 39.Ma W, Stafford LJ, Li D, et al. GCIP/CCNDBP1, a helix–loop–helix protein, suppresses tumorigenesis. J Cell Biochem. 2007;100:1376–1386. doi: 10.1002/jcb.21140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.