Abstract
Enzastaurin, a potent inhibitor of protein kinase C-beta, inhibits angiogenesis and has direct cytotoxic activity against glioma cells in preclinical studies. Patients with recurrent high-grade gliomas were stratified by histology and use of enzyme-inducing antiepileptic drugs (EIAEDs). Patients on EIAED were treated on the phase I dose-escalation portion of the trial with evaluation of serum pharmacokinetics as the primary endpoint. Patients not on EIAED were treated on the phase II portion of the trial with radiographic response and progression-free survival (PFS) as primary objectives. Patients in phase I received enzastaurin 525–900 mg/d. Phase II patients received 500 or 525 mg/d. One hundred and eighteen patients were accrued to this trial. Therapy was well tolerated with thrombosis, thrombocytopenia, hemorrhage, and elevated alanine aminotransferase as the most commonly observed drug-associated grade 3 or higher toxicities. Patients on EIAED had serum enzastaurin exposure levels approximately 80% lower than those not on EIAED. Dose escalations up to 900 mg/d did not substantially increase serum exposure levels and a maximally tolerated dose was never reached. Twenty-one of 84 evaluable patients (25%) experienced an objective radiographic response. The 6-month PFS was 7% for patients with glioblastoma and 16% for patients with anaplastic glioma. Phosphorylation of glycogen synthase kinase-3 in peripheral blood mononuclear cells was identified as a potential biomarker of drug activity. Enzastaurin has anti-glioma activity in patients with recurrent high-grade glioma, but does not appear to have enough single-agent activity to be useful as monotherapy.
Keywords: angiogenesis, biomarker, glioblastoma, malignant glioma, targeted therapy
Malignant gliomas are a major cause of cancer-related morbidity and mortality in the United States. Even with aggressive surgical resection, radiation therapy, and chemotherapy, the median survival of patients with glioblastoma (GBM) is less than 15 months.1 Despite years of clinical research and numerous trials, there are very few drugs with significant activity against malignant gliomas. This experience has led many to believe that new therapeutic strategies against novel molecular targets are needed.
Angiogenesis-dependent tumor growth is a widely accepted concept in cancer therapeutic development. Efficacy of inhibiting vascular endothelial growth factor (VEGF) has been demonstrated in clinical trials of bevacizumab for advanced colon and lung carcinoma.2,3 Numerous preclinical studies have demonstrated the importance of VEGF as a primary mechanism for angiogenesis in glioma growth and progression.4,5 A recent clinical trial of bevacizumab in patients with malignant glioma demonstrated apparent clinical benefit.6 Thus, VEGF inhibition represents a potentially promising approach for glioma therapy.
Enzastaurin HCl (LY317615) is an acyclic bisindolylmaleimide, and is an adenosine triphosphatase-competitive small molecule inhibitor of protein kinase C-beta (PKC-β). Tumor-induced angiogenesis requires activation of PKC family enzymes, including PKC-β. In particular, activation of endothelial proliferation and migration requires VEGF receptor-mediated PKC activation. Inhibition of PKC-β by enzastaurin has been demonstrated to decrease VEGF-induced neovasculature in the rat corneal micropocket assay, and to decrease microvascular density and VEGF expression in human tumor xenografts.7,8 A recent phase II trial of enzastaurin in patients with relapsed or refractory diffuse large B-cell lymphoma suggests clinical antitumor activity.9 In addition to its antiangiogenic activity, enzastaurin has been shown to have direct antiglioma activity both in vitro and in vivo.10–12 We, therefore, chose to evaluate the clinical activity of enzastaurin in patients with recurrent high-grade gliomas.
Materials and Methods
Patients
Patients with a previously confirmed high-grade glioma that progressed following standard fractionated radiotherapy were eligible for this trial. Eligible histologies included: GBM, gliosarcoma, anaplastic astrocytoma, anaplastic oligoastrocytoma, anaplastic oligodendroglioma, and anaplastic glioma (AG) not otherwise specified. Patients were at least 18 years of age, had a Karnofsky Performance Status (KPS) of at least 60%, and a projected survival time of at least 2 months. There were no restrictions on the types or number of prior treatments, but patients could not have any other significant comorbid diseases or end-organ dysfunction that would obscure the results of treatment on this trial. The trial was amended approximately halfway through accrual to exclude patients on full anticoagulation with either warfarin, heparin, and/or low-molecular-weight heparin, when several patients experienced intratumoral hemorrhage on study while concomitantly treated with anticoagulation (see toxicity in Results section).
Study Design and Treatment
Phase I
Patients taking enzyme-inducing antiepileptic drugs (EIAEDs; Group B) were treated on the phase I portion of this trial based on a pharmacokinetic (PK) endpoint of achieving an enzastaurin concentration of >1400 nM. Given the IC90 of 70 nM and 95% protein binding of enzastaurin, the target mean steady-state concentration (parent and metabolites) for clinical efficacy was estimated to be 1400 nM. Three dose levels were explored: 525, 700, and 900 mg/d. Five patients were treated at each dose level and serum PKs were evaluated. If 2 or more patients did not achieve a steady-state serum concentration of >1400 nM, and there was no more than 1 drug-related dose-limiting toxicity within the group of 5 patients, then the next cohort of patients were treated at a higher dose level.
Phase II
Patients who were not taking EIAED (Group A) were treated on the phase II portion of this trial. Patients received a daily flat dose of 525 mg/d of enzastaurin, later changed to 500 mg/d when the formulation of the drug was changed from a pill to a tablet form. A treatment cycle was 6 weeks. Patients were instructed to take enzastaurin in the morning with a high-fat breakfast (approximately 40 g). No premedications were necessary. Treatment was continuous unless toxicity required termination of drug therapy. Complete blood counts, hepatic, renal, and metabolic blood chemistries were drawn every 3 weeks, or more often as medically indicated. EKGs were obtained at the end of each treatment cycle. Physical and neurological examinations were performed prior to patient enrollment, at 3 weeks, 6 weeks, and every 6 weeks thereafter. A baseline MRI and perfusion scan were obtained within 2 weeks of enrollment, then at the end of every 6-week cycle. Treatment was continued as long as there were no unacceptable toxicities and there were no signs of tumor progression. All patients were treated at the Clinical Center at the National Institutes of Health after providing informed consent for participation in this Institutional Review Board approved trial.
Statistical Design
Phase II patients were stratified based on whether they had GBM or AG. Given our expectation that enzastaurin would have cytostatic rather than cytotoxic activity, the trial was initially designed and powered to evaluate 6-month progression-free survival (PFS6) using historical control benchmarks for each histological subgroup. We saw encouraging radiographic responses early in the trial (6 of the first 17 evaluable patients) and amended the trial design to use radiographic response rate as the primary endpoint for the GBM cohort. Our revised statistical plan called for the enrollment of 35 additional GBM patients and was powered to see a 29% objective response rate when compared with the previously reported objective response rate of 9% when temozolomide was used in patients with recurrent GBM.13 Response criteria included the requirement for a stable dose of corticosteroids and clinical/neurological stability. Contrast-enhanced MRI was evaluated every 6 weeks where tumor response was assessed with standard criteria using largest cross-sectional diameters of measurable lesions, and scored evaluations of nonmeasurable, but evaluable, disease.14,15 All partial and complete responses were confirmed independently by 3 reviewers (HF, TK, JB). For the AG cohort, the study used a mini–max two-stage design16 to distinguish between a targeted 55% PFS6 and a historical control PFS6 of 35%. A futility analysis was planned for the first 31 patients, where accrual would be stopped if 10 or fewer patients had a PFS greater than 6 months.
Overall and PFS was estimated with Kaplan–Meier methodology, stratified by histology where the date of progression used was the earlier of either: date of radiographic progression, date of study for clinical decline or drug-associated toxicity, or start date of new therapy of study if that follow-up was known. All other patients were censored at date of last follow-up if they had not progressed. All patients were evaluable for survival; only those with a follow-up MRI scan were evaluable for radiographic response. Toward the end of enrollment to the efficacy portion of the trial, an additional 20 GBM patients were enrolled to assess the potential usefulness of a new biomarker (phosphorylation status of glycogen synthase kinase-3 [GSK3] in peripheral blood mononuclear cells [PBMCs]) for predicting clinical response and/or reflecting serum PKs.
PK Analysis
Plasma samples for characterizing PKs of enzastaurin and its metabolites were collected at specified time points on day 1 and within a 24-hour interval after 2 weeks of treatment in cycle 1 for all patients. Initial patients accrued to the phase II trial followed a sparse sampling schedule, but later patients followed the same schedule as phase I patients. Plasma samples were analyzed by turbo ion spray, liquid chromatography/tandem mass spectrometry (LC/MS/MS) in the positive ion mode (Advion BioSciences, Inc., Ithaca, New York). The assay demonstrated a lower limit of quantification of 0.5 ng/mL, whereas the upper limit of quantification was 150 ng/mL for all analytes. The intra- and inter-assay precision (RSD) results calculated from validation samples were ≤6.89% for all analytes at all concentrations.
PK parameters, such as Cmax, AUC 0–24, tmax following single dose and at steady-state, and apparent clearance were calculated using noncompartmental methods from the plasma concentration–time profiles of enzastaurin and its metabolites with WinNonlin Pro 3.1 (Pharsight, Mountain View, California). The metabolic ratio was calculated for the major metabolite, LY326020, as a ratio of the metabolite AUC to parent AUC at steady state.
Pharmacodynamic Determination of Phospho-S9-GSK3β in PBMCs
PBMCs were prepared from 3 to 5 mL of whole blood with BD vacutainer CPT tubes using the manufacturer's protocol. ELISA-based detection of the phopsho-S9-GSK3 was performed using a commercially available ELISA kit (BD kit Cat # 900-123 from Assay Design) according to the manufacturer's instructions.
Results
Between October 2002 and May 2005, a total of 15 patients were accrued to the phase I portion of this trial and 105 patients were accrued to the phase II portion. Two patients in Group A did not start treatment after registration and were excluded from the analysis. Results for a total of 118 patients are described. Patient characteristics by study arm and histology are displayed in Table 1. There were 83 patients with GBM: 11 enrolled in Phase I, 72 enrolled in Phase II (52 planned for response rate endpoint and additional 20 for study of correlative biomarker). There were 48 women and 70 men with a median age of 47 years (range 19–70). The patients were generally highly pretreated with more than half of the patients (55% GBM, 60% AG) having had 3 or more systemic chemotherapy regimens prior to enrollment. Nearly every patient had previously been treated with temozolomide either as part of his or her initial treatment regimen or for recurrent tumor (79 of 83 GBM; 34 of 35 AG). Despite the number of prior therapies, patients overall had a good performance status (median KPS 90). In the phase I portion of the trial, 5 patients were treated on each of the dose levels (525, 700, 900 mg/d). In the phase II portion of the trial, 34 patients were treated at 525 mg/d; 69 patients were treated at the 500 mg/d dose following the change of drug formulation from tablets to capsules.
Table 1.
Patient characteristics
| Phase I (Group B), n = 15 | |
|---|---|
| Men | 8 |
| Women | 7 |
| GBM | 11 |
| AG | 4 |
| Median age (y) | 53 |
| Phase II (Group A), n = 103 | |
| Men | 62 (60%) |
| Women | 41 (40%) |
| GBM | 72 (70%) |
| Median age (y) | 48 |
| Median KPS | 90 |
| Evaluable for response | 57 (79%) |
| AG | 31 (30%) |
| Median age (y) | 46 |
| Median KPS | 90 |
| Evaluable for response | 27 (87%) |
| Anaplastic oligoastrocytoma | 16 (50%) |
| Anaplastic astrocytoma | 16 (50%) |
PK Analysis
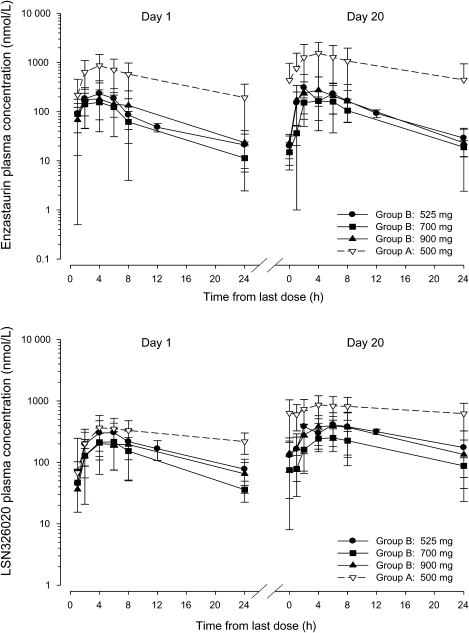
Plasma concentration–time data and dosing information (dose date and time) for PK evaluation based on intensive sampling were available from 79 patients. The PK parameters of enzastaurin and total analytes (enzastaurin + LY326020 + LY485912) following single and multiple dosing are summarized in Table 2. The maximum plasma concentration (Cmax) for enzastaurin was reached within 4 hours after dosing (Fig. 1). The steady state of enzastaurin is achieved within 2 weeks with daily oral dosing.17 Accumulation of enzastaurin and LY326020 was evident for Group A patients, and was similar in extent to that reported previously.17 In contrast, Group B patients had significantly lower exposures and showed little accumulation of enzastaurin. The targeted mean steady-state total concentration for clinical efficacy (1400 nM) was achieved in Group A patients. In contrast, the exposure (AUCs) of enzastaurin in Group B patients was 10%–15% of the exposures seen in Group A patients. Likewise, the LY326020 exposures observed in Group B patients were approximately 30% of those seen in Group A patients. Increasing the dose of enzastaurin in Group B patients from 525 to 900 mg did not result in increasing enzastaurin or LY326020 exposures. The exposures in Group A patients who did not have intensive PK sampling overlaid well with the Group A patients with intensive sampling (figure not shown) and can be expected to have similar values for the PK parameters as demonstrated in Table 2.
Table 2.
Summary of PK parameters, geometric means and (%CV) for enzastaurin and its metabolite, LY326020, following the first dose (day 1) and at steady state (day 20) for patients with (Group B) and without (Group A) concurrent EIAED administration
| Day 1 |
Day 20 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cmax (nmol/L) | tmaxa (h) | AUC0–24 (nmol h/L) | Cmax,ss (nmol/L) | tmax,ssa (h) | AUCτ,ss (nmol h/L) | Cav,ss (nmol/L) | CL/F (L/h) | MR (ratio) | |
| Enzastaurin | |||||||||
| Group A: 500 mg (n = 61) | 872 (64.6) | 4.00 (0.97–8.08) | 8700 (66.4) | 1440b (74.9) | 4.00b (1.08–8.08) | 15700c (89.5) | 655c (89.5) | 61.7c (89.5) | — |
| Group B: 525 mg (n = 5) | 306 (34.4) | 4.00 (2.08–6.02) | 1790 (38.4) | 313 (55.8) | 2.08 (2.00–6.05) | 2370 (58.4) | 98.6 (58.4) | 430 (58.4) | — |
| Group B: 700 mg (n = 5) | 181 (45.1) | 4.08 (2.00–6.00) | 1170 (65.4) | 181 (32.6) | 4.00 (2.00–6.00) | 1720 (29.3) | 71.8 (29.3) | 788 (29.3) | — |
| Group B: 900 mg (n = 5) | 169 (85.3) | 2.08 (2.00–6.03) | 1240d (96.9) | 250 (80.3) | 4.00 (1.00–6.00) | 1840e (104) | 76.7e (104) | 948e (104) | — |
| LY326020 | |||||||||
| Group A: 500 mg (n = 61) | 394 (49.3) | 4.05 (1.00–24.17) | 5960 (41.5) | 867b (44.6) | 4.00b (0.00–8.07) | 16300c (42.4) | 682c (42.4) | — | 1.04c (65.5) |
| Group B: 525 mg (n = 5) | 352 (30.5) | 4.00 (2.08–6.02) | 3480 (44.8) | 425 (57.5) | 2.08 (2.00–8.00) | 5700 (73.1) | 237 (73.1) | — | 2.41 (13.3) |
| Group B: 700 mg (n = 5) | 207 (70.2) | 4.08 (2.00–6.00) | 2190 (76.4) | 246 (48.9) | 6.00 (4.00–8.00) | 3590 (45.2) | 149 (45.2) | — | 2.08 (26.9) |
| Group B: 900 mg (n = 5) | 209 (51.8) | 4.00 (4.00–9.00) | 2540d (35.0) | 404 (53.3) | 5.97 (4.00–8.00) | 5390e (59.0) | 225e (59.0) | — | 2.93e (35.8) |
| Total analyte | |||||||||
| Group A: 500 mg (n = 51) | 1370 (55.7) | 4.00 (1.00–23.25) | 16600 (52.3) | 2540b (58.7) | 4.00b (1.00–8.08) | 36700c (62.2) | 1530c (62.2) | — | — |
EIAED, enzyme inducing antiepileptic drugs; CV, coefficient of variation, n, number of subjects used in the PK analysis.
aMedian (range)
bn = 51.
cn = 49.
dn = 3.
en = 4.
Fig. 1.
Plasma concentration–time profile of enzastaurin (upper panel) and LY326020 (lower panel) following the first dose and at steady state.
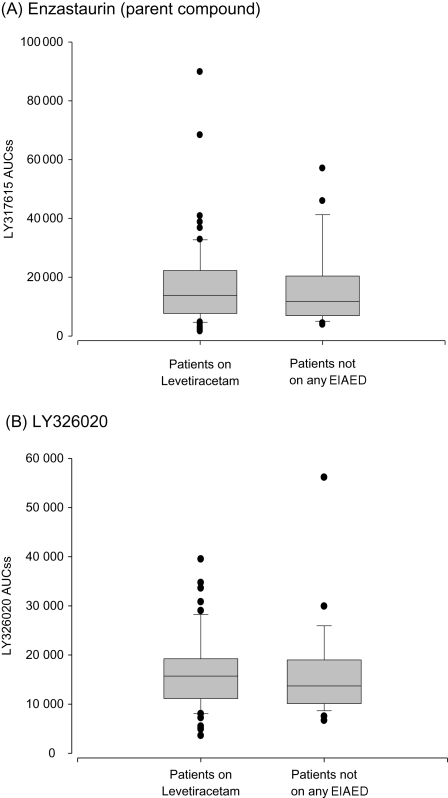
There were a number of patients treated with long-term EIAED therapy who were relatively quickly (2–4 weeks) converted to non-EIAED prior to receiving enzastaurin, raising the concern that these patients might still have induced CYP34A enzymes and thus altered enzastaurin PKs compared with other Group A patients. To evaluate this, we performed extensive enzastaurin PK analysis in 12 long-term phenytoin-treated patients who started enzastaurin 2 weeks after stopping phenytoin and converted to a non-EIAED (levetiracetam). As can be seen in Fig. 2, the enzastaurin exposure levels in these patients were similar to those seen in patients never treated with any antiepileptics and to those only treated with non-EIAEDs.
Fig. 2.
Plasma concentrations of enzastaurin at steady state in patients not on EIAED and those that were taken off of EIAED 2 weeks previously. Box plots are used to compare the distributions between the 2 groups. The center of the box denotes the median, while the lower and upper sides reflect the 1st and 3rd quartiles of the distribution. Error bars are drawn to the observation closest to the median which is not an outlier (defined as 1.5 times the inter-quartile range).
Treatment and Toxicity
Enzastaurin was well tolerated in this study (Table 3). Only 10% of patients with GBM and 17% patients with AG required dose reductions (12% total), only 4% of which were secondary to drug-related adverse events (most others were due to patient error). The most common grade 3 and higher adverse events related to the study drug were venous thrombo-embolic events, thrombocytopenia, hemorrhage, and asymptomatic elevation in alanine aminotransferase (ALT). Nine patients had deep venous thrombosis, and 3 patients had pulmonary emboli requiring intervention. Thrombo-embolic events observed in this study were not significantly increased above the baseline risk of the general malignant glioma patient population.18
Table 3.
Enzastaurin-related adverse events from 118 patients
| Toxicity | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total Grade ≥ 3 |
|---|---|---|---|---|---|
| Thrombosis/thrombus/embolism | 1 | 3 | 11 | 14 (12%) | |
| Platelets | 8 | 7 | 2 | 9 (7.6%) | |
| Hemorrhage, CNS | 6 | 2 | 8 (6.7%) | ||
| Hemorrhage, other | 2 | 1 | 3 (2.5%) | ||
| ALT, SGPT | 8 | 3 | 1 | 4 (3.4%) | |
| AST, SGOT | 3 | 1 | 1 | ||
| Prolonged QTc interval | 5 | 0 | |||
| Bilirubin (hyperbilirubinemia) | 4 | 0 | |||
| Diarrhea | 2 | 0 | |||
| Nausea | 1 | 0 | |||
| Alkaline phosphatase | 1 | 0 | |||
| Hypoalbuminemia | 1 | 0 | |||
| Cardiac ischemia | 1 | 0 |
Most patients who experienced a grade 3 or 4 thrombocytopenia never resumed treatment with enzastaurin since tumor progression usually occurred prior to resolution of the thrombocytopenia. Since thrombocytopenia was seen more frequently in this study than in other nonbrain tumor trials with enzastaurin, we evaluated potentially predisposing covariables. Neither the type nor number of prior chemotherapeutic agents used, the patient's age, sex or concurrent medications were associated with the likelihood of developing enzastaurin-induced thrombocytopenia. Furthermore, the PK profile of the patients who experienced thrombocytopenia was not significantly different from those patients who did not (data not shown).
Eight patients experienced intracranial hemorrhage (ICH), 6 of whom were on therapeutic anticoagulation (warfarin or low molecular weight heparin). Since only 12 patients in this trial were anticoagulated, the risk of bleeding in this subgroup of patients was 50%, prompting us to exclude enrollment of anticoagulated patients in the later part of the study. One of the 2 patients with ICH who was not anticoagulated bled in the setting of malignant hypertension. Thus, only 1 of 8 patients with ICH had no obvious predisposing cause other than the presence of tumor and treatment with enzastaurin. The 3 deaths on study were hemorrhagic events: 2 ICH, 1 upper gastrointestinal hemorrhage.
Efficacy
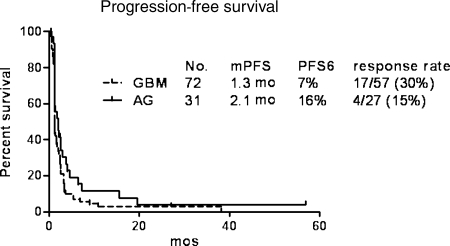
A total of 21 out of 84 (25%) patients with evaluable disease had objective radiographic responses to enzastaurin as demonstrated by decreased gadolinium enhancement and decreased cerebral edema: 4 of 27 (15%) AG, 17 of 57 (30%) GBM—one with a complete response. When excluding the first 17 GBM patients enrolled prior to the change in study primary endpoint, the response rate was 26% (11 of 42 evaluable). Both of the calculations for the GBM cohort (26% and 30%) are distinct from the 9% historical response rate for recurrent GBM (P < .001).19 Among the responders, 5 AG and 2 GBM patients had bidimensionally measurable disease that met the MacDonald criteria for partial response. The median PFS was 1.3 months for patients with GBM and 2.1 months for those with AG. The PFS6 was 7% for patients with GBM and 16% for patients with AG. (Fig. 3) Median overall survival for GBM patients was 4.6 months vs 6.8 months for AG patients. There was no significant difference in PFS or radiographic response rate between patients who were administered enzastaurin capsules versus those that received enzastaurin pills.
Fig. 3.
Clinical outcome of malignant glioma patients treated with enzastaurin.
Pharmocodynamics
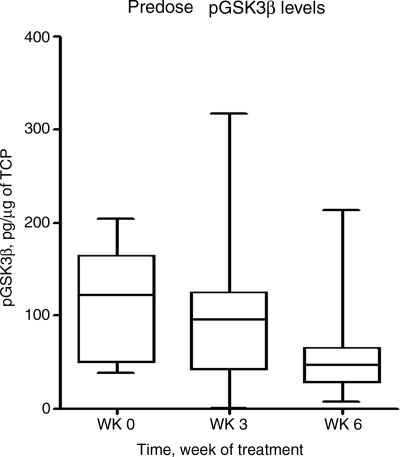
During the course of this trial, it was discovered that enzastaurin is a potent inhibitor of GSK3 in addition to being a potent inhibitor of PKC-β. Preclinical data suggest that some of the direct antiglioma activity of enzastaurin is secondary to its effects on GSK3.11 In an attempt to find a potentially useful clinical biomarker of drug activity, we modified the later part of the trial to allow evaluation of enzastaurin effects on GSK3 kinase activity in the PBMCs of treated patients. PBMCs of 17 GBM patients were successfully evaluated for GSK3 activity prior to treatment and then at 3 and 6 weeks after initiating treatment. As can be seen in Fig. 4, phospho-GSK3β levels decreased from baseline in a time-dependent manner (see also Supplementary Material, Fig. S1). The linear change using a linear mixed model to test whether there was a decrease in pGSK3β from 0–3 to 6 weeks was statistically significant (P = .01), confirming the observation that pGSK3β levels decrease with exposure time to enzastaurin. The maximum inhibition was observed between 4 and 8 hours after drug administration, coinciding with the Tmax for enzastaurin and its primary active metabolite (between 2–4 and 4–8 hours, respectively). Phamacodynamic profiles could not be correlated with patient outcomes, since only 3 out of the 20 patients enrolled for this portion of the study had a response to therapy.
Fig. 4.
Effects of enzastaurin on PBMC phosphorylated GSK3 levels.
Discussion
Enzastaurin, a PKC-β inhibitor, was developed as a novel inhibitor of angiogenesis based on the importance of PKC-β in endothelial cell VEGF-mediated proliferative signaling. Preclinical data support enzastaurin's ability to inhibit angiogenesis both in vitro and in vivo. Additionally, preclinical data suggest that the drug may have significant direct cytostatic/cytotoxic effects against a number of cancer cell types including gliomas.10,11 The results of this trial demonstrate that enzastaurin is well tolerated and possesses biological activity in a subpopulation of patients with recurrent malignant gliomas.
Enzastaurin, and its major metabolite, LY326020, is primarily metabolized by CYP3A that is known to have high variability in expression in the general population and even greater variability in cancer patients. As expected, there was a significant reduction in enzastaurin and LY326020 exposures in patients on EIAED. The extent of induction confirms that the primary route of elimination for enzastaurin and its metabolite is metabolism by CYP3A. High variability was seen in enzastaurin exposures (% CV of 89.5%), resulting in a 10-fold range of exposure even in the absence of enzyme inducers. Interestingly, increasing doses of enzastaurin in patients on EIAED did not result in increasing enzastaurin exposures. This suggests the possibility of autoinduction of enzastaurin metabolism, or that drug absorption and bioavailability are limited at higher doses. We have recently completed a dose escalation trial, starting at 500 mg, incorporating a multiple daily dosing strategy to address this question.
Given the profound effect that EIAED can have on drug metabolism, many clinical trials exclude the use of EIAED. Thus, it has become a common practice to rapidly convert selected patients to a non-EIAED in an attempt to make them eligible to the protocol when tumor progression is documented. Yet, there are little data relative to the amount of time it takes for P450 enzyme induction to revert to baseline following cessation of EIAED therapy. We addressed this question by performing extensive PKs on 14 patients who were administered enzastaurin 2 weeks after having stopped their long-term (>3 months) EIAED (either phenytoin and/or carbamazapine) and being converted to leviteracetam. As can be seen in Fig. 2, the serum exposure levels in all of these patients were identical to those in patients who were never treated with EIAED. We observed that the majority of the enzyme induction that occurs with EIAED resolves within 2 weeks of stopping the antiepileptic drug.
The patients in this trial tolerated treatment with enzastaurin very well. The most concerning toxicity was thrombocytopenia. Few patients required platelet transfusions, but 1 patient with a chronically hemorrhagic tumor-associated cyst bled into the cyst and died while being treated with enzastaurin. In contrast to standard cytotoxic-chemotherapy, enzastaurin-related thrombocytopenia seemed idiosyncratic and risk did not appear to be related to the amount of prior therapy, drug serum levels, or prior history of thrombocytopenia. Furthermore, the thrombocytopenia almost always occurred within the first 6 weeks of treatment and rarely if ever thereafter, so did not appear to be a cumulative effect of therapy. Interestingly, the prevalence and severity of thrombocytopenia was much higher in this trial than in other trials of enzastaurin for unclear reasons. No PK interaction with other coadministered drugs can explain this observation. Hemorrhagic complications were also observed, but were highly associated with concomitant anticoagulation, and enzastaurin should not be coadministered with these drugs.
Enzastaurin demonstrated a high response rate in GBM patients relative to historical controls (30% vs 9%). The radiographic responses were often profound and associated with decreased cerebral edema and clinical improvement. When responses occurred, they were reasonably well maintained with a PFS6 of 24%. Nevertheless, the majority of patients did not respond, and PFS was not significantly better than historical control.19 We could not find any clinical, pharmacological, or biological variable that correlated with the likelihood of response. This prompted exploration of a pharmacodynamic biomarker for response to enzastaurin. We demonstrated that patients treated with enzastaurin have a time and PK-associated decrease in GSK3 phosphorylation within their PBMCs. Unfortunately, by the time we had made this observation and worked out the assay, there were not enough patients remaining to be evaluated on trial to make any type of statistical correlation to response data.
The pattern of radiographic response observed was also interesting. Certainly, the early decrease in gadolinium enhancement and cerebral edema are consistent with an anti-VEGF vascular permeability effect, similar to that seen with other agents such as bevacizumab. We obtained perfusion data on a subset of such patients who show diminished blood flow to the treated tumors consistent with an anti-angiogenic effect, although we have seen similar decreases in blood volume and perfusion in gliomas following treatment with primarily cytotoxic agents (data not shown). Additionally, we did not observe the common toxicities associated with anti-VEGF therapy such as hypertension, arterial thrombosis, and proteinuria. Possibly, these toxicities were not observed either because enzastaurin is a relatively weak VEGF inhibitor in situ, PKC-β inhibition may not be associated with these other possibly “off-target” effects of anti-VEGF therapy, and/or because enzastaurin is not primarily working through an anti-VEGF effect in these patients. Whether or not the immediate radiographic changes seen in these patients was VEGF pathway mediated, the very prolonged PFS observed in a small subgroup of patients argues for an additional enzastaurin-mediated cytotoxic effect on glioma cells (Fig. 5).
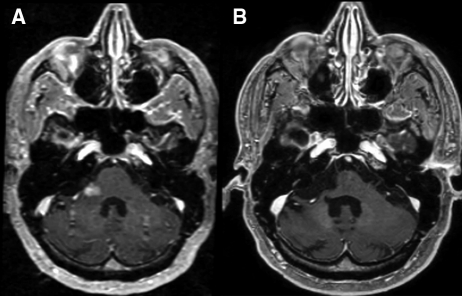
Fig. 5.
Complete response to enzastaurin therapy maintained almost 5 years since initiating treatment. (A) Baseline study. (B) Resolution of enhancing brainstem disease with volume loss indicating prolonged tumor response to therapy.
In conclusion, enzastaurin is very well tolerated and has antiglioma activity in a subset of patients with recurrent gliomas. Despite an encouraging objective response rate in GBM patients, lack of a significant impact on PFS6 and negative results from a subsequently completed phase III trial in recurrent GBM20 suggest that enzastaurin is unlikely to be a useful agent as monotherapy for the majority of patients with recurrent gliomas. Nevertheless, the paucity of significant drug-associated toxicities, the ease of administration, and provocative preclinical data make enzastaurin a promising new agent to evaluate in combination with other antiangiogenic and cytotoxic agents in patients with malignant glioma. Future studies will need to better delineate the mechanism of enzastaurin's antiglioma effects in situ and identifying biomarkers of drug activity for the purpose of rationally designing such combination trials.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Conflict of interest statement. None declared.
Funding
The study was financially supported by Eli Lilly and Company.
Supplementary Material
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 3.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 4.Pietsch T, Valter MM, Wolf HK, et al. Expression and distribution of vascular endothelial growth factor protein in human brain tumors. Acta Neuropathol. 1997;93(2):109–117. doi: 10.1007/s004010050591. [DOI] [PubMed] [Google Scholar]
- 5.Plate KH, Breier G, Weich HA, Mennel HD, Risau W. Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int J Cancer. 1994;59(4):520–529. doi: 10.1002/ijc.2910590415. [DOI] [PubMed] [Google Scholar]
- 6.Vredenburgh JJ, Desjardins A, Herndon JE, II, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 7.Keyes KA, Mann L, Sherman M, et al. LY317615 decreases plasma VEGF levels in human tumor xenograft-bearing mice. Cancer Chemother Pharmacol. 2004;53(2):133–140. doi: 10.1007/s00280-003-0713-x. [DOI] [PubMed] [Google Scholar]
- 8.Teicher BA, Alvarez E, Menon K, et al. Antiangiogenic effects of a protein kinase C beta-selective small molecule. Cancer Chemother Pharmacol. 2002;49(1):69–77. doi: 10.1007/s00280-001-0386-2. [DOI] [PubMed] [Google Scholar]
- 9.Robertson MJ, Kahl BS, Vose JM, et al. Phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2007;25(13):1741–1746. doi: 10.1200/JCO.2006.09.3146. [DOI] [PubMed] [Google Scholar]
- 10.Graff JR, McNulty AM, Hanna KR, et al. The protein kinase C beta-selective inhibitor, Enzastaurin (LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res. 2005;65(16):7462–7469. doi: 10.1158/0008-5472.CAN-05-0071. [DOI] [PubMed] [Google Scholar]
- 11.Kotliarova S, Pastorino S, Kovell LC, et al. Glycogen synthase kinase-3 inhibition induces glioma cell death through c-MYC, nuclear factor-kB, and glucose regulation. Cancer Res. 2008;68(16)):6643–6651. doi: 10.1158/0008-5472.CAN-08-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabatabai G, Frank B, Wick A, et al. Synergistic antiglioma activity of radiotherapy and enzastaurin. Ann Neurol. 2007;61(2):153–161. doi: 10.1002/ana.21057. [DOI] [PubMed] [Google Scholar]
- 13.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 14.Levin VA, Crafts DC, Norman DM, Hoffer PB, Spire JP, Wilson CB. Criteria for evaluating patients undergoing chemotherapy for malignant brain tumors. J Neurosurg. 1977;47(3):329–335. doi: 10.3171/jns.1977.47.3.0329. [DOI] [PubMed] [Google Scholar]
- 15.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 16.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 17.Carducci MA, Musib L, Kies MS, et al. Phase I dose escalation and pharmacokinetic study of enzastaurin, an oral protein kinase C beta inhibitor, in patients with advanced cancer. J Clin Oncol. 2006;24(25):4092–4099. doi: 10.1200/JCO.2005.05.3447. [DOI] [PubMed] [Google Scholar]
- 18.Dhami MS, Bona RD, Calogero JA, Hellman RM. Venous thromboembolism and high grade gliomas. Thromb Haemost. 1993;70(3):393–396. [PubMed] [Google Scholar]
- 19.Lamborn KR, Yung WK, Chang SM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro-Oncology. 2008;10(2):162–170. doi: 10.1215/15228517-2007-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fine HA, Puduvalli VK, Chamberlain MC, et al. Enzastaurin (ENZ) versus lomustine (CCNU) in the treatment of recurrent, intracranial glioblastoma multiforme (GBM): A phase III study. ASCO Meeting Abstracts; May 20, 2008. 2005;26(suppl 15) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.