Abstract
There are few and conflicting studies on the optimal timing of initial cranial radiation in the treatment of glioblastoma multiforme (GBM) but none of them have addressed this issue in the elderly population. We used the linked Surveillance, Epidemiology, and End Results (SEER) Medicare database to investigate whether the time interval from surgery to initiation of radiation is a significant prognostic factor for survival in subjects aged ≥65 years with newly diagnosed GBM. Cox modeling was used to assess the effect of waiting time on overall survival. We identified a total of 1,375 patients, 296 with biopsies and 1,079 with resections. The median time to the initiation of radiotherapy was 15 days post operation (interquartile range 12–21). In the univariate Cox analysis of those who had debulking surgeries, a waiting time of >22 days showed a significant inverse relationship with survival (hazard ratio [HR] = 0.82, 95% CI 0.70–0.97, p = 0.02), but after adjustment for confounders, it was not a statistically significant factor in the final Cox model (HR = 0.99, 95% CI 0.97–1.01, p = 0.14). Therefore, waiting time was not a significant prognostic factor for subjects with biopsies in both the univariate and multivariate analyses. Although effort should be made to initiate radiotherapy as soon as possible after surgical resection/biopsy, a brief delay similar to that experienced by our cohort does not have a significant impact on survival.
Keywords: elderly, glioblastoma multiforme, SEER–Medicare, radiation delay, waiting time
A recent randomized controlled trial (RCT) established the efficacy of cranial radiation for newly diagnosed glioblastoma multiforme (GBM) in elderly patients aged more than 70.1 However, there is a paucity of studies on the appropriate timing of upfront radiation. Because of the aggressive nature of GBM, radiotherapy is often recommended to start approximately 2 weeks following surgery, but at some centers it was delayed more than 4 weeks post-operatively. For example, in one RCT comparing an abbreviated versus a full course of cranial radiation in elderly patients with GBM, the median time from diagnosis to the initiation of radiation was 33 days,2 and in another RCT involving radiotherapy for glioblastoma, the median time was 5.3 weeks.1 Many factors may affect the timing of radiation. Referral delay to a radiation oncologist, resource and staffing constraints, postoperative complications, time involved in seeking second opinions, and missed appointments are some of the factors that may result in the postponement of radiation.
A mathematical model that was developed to assess the effect of protracted waiting time found a mean tumor doubling time of 24 days, and thus a short delay would be expected to have an adverse effect on survival.3 Moreover, the model suggested that no patients could survive long-term after a 70-day delay. Although this study raises the theoretical concern that delay of the initiation of radiotherapy has an unfavorable effect on outcome, results among the three retrospective studies that addressed this issue have been conflicting, and none of them specifically evaluated it in the elderly population, for which the prognosis is worse than adult GBM.4 One analysis of 172 patients in a single hospital showed that every additional week (after a post-operative period of 2 weeks) of delay increased the risk of death by 8.9%.5 In contrast, an abstract published by the RTOG suggested that GBM patients who were irradiated beyond 4 weeks had a significant survival advantage over those who began radiation within 2 weeks of surgery.6 The third study on 179 malignant glioma patients found that the waiting time from surgery to radiotherapy had no impact on survival.7
Although an RCT is the best way to resolve these conflicting findings, ethical issues and barriers in conducting a trial targeted at elderly subjects would render such an attempt impossible.8 A representative population-based study is thus the most feasible method to resolve the issue of radiation timing in elderly patients with GBM. In this study, we used Medicare data from 1991 to 2002, linked to the Surveillance, Epidemiology, and End Results (SEER) cancer registries, to evaluate whether the time interval from surgery to the initiation of radiation is an important determinant for survival. An additional advantage of using the SEER–Medicare linked files from this time period is that the effect attributed to upfront radiotherapy will be unlikely confounded by concomitant temozolomide, as combined chemoradiation in the treatment of newly diagnosed GBM had not yet been a standard of care in this study cohort.
Patients and Methods
Data Sources
We used the merged SEER–Medicare database for patients aged 65 or older who were diagnosed with GBM from 1991 to 2002 in 13 SEER areas: Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco–Oakland, Seattle–Puget Sound, Utah, Los Angeles, San Jose-Monterey, Rural Georgia, and the Alaska Native Tumor Registry. These areas cover approximately 14% of the US population. The registries ascertain all newly diagnosed GBM cases from multiple reporting sources. Information derived from SEER includes GBM histological subtypes, tumor location in the brain, tumor size, age, sex, self-reported ethnicity, types of surgery, and survival information.
The Medicare program and database are maintained by the Health Care Finance Administration (HCFA), which covers hospital, physician, and other medical services for more than 97% of people aged ≥65 years. These data were available for all beneficiaries starting in 1991, and their claims are available through 2003. A previous study of persons aged ≥65 years appearing in the SEER registries established that Medicare eligibility could be verified for 94% of cases.9 In this study, we retrieved information related to socioeconomic status (SES), treatment hospital location (metropolitan versus nonmetropolitan), comorbidities, surgery dates, the timing of radiation, and chemotherapies from Medicare files (see details below).
The Selection of the Study Population
In the linked SEER–Medicare database from January 1991 to December 2002, we identified 2,018 newly diagnosed GBM subjects (age ≥65). To ensure that we have access to subjects' complete medical records, which are represented by billed items in Medicare files, we included only those who had both Medicare Parts A and B and were not enrolled in a health maintenance organization (HMO) during the period from 12 months before through 12 months after diagnosis. Subjects had no prior history of other malignancies. We excluded (1) subjects who did not have pathological confirmation (n = 148); (2) subjects who never got cranial radiation (n = 289); (3) those who did not initiate radiation within 90 days of surgery (n = 45); and (4) subjects who died within 90 days of diagnosis (n = 161). The exclusion of the last group was necessary as they might have died prior to completing upfront radiotherapy for a variety of unrelated reasons, and thus their inclusion could bias our survival estimates attributed to cranial radiation. Of the 161 patients who died within 90 days, 134 (83.2%) passed away within 30 days of diagnosis. Furthermore, we included only cases that started radiotherapy within 90 days of surgery, as the database does not allow us to ascertain whether radiation initiated beyond this period might have been indicated for recurrent disease. Therefore, we excluded a total of 643 of 2,018 subjects; the remaining 1,375 patients, 296 with biopsies and 1,079 with surgical resections, formed our study cohort.
Measurement of the Timing of Initiation of Upfront Cranial Radiation
The receipt and timing of cranial radiation after surgery were ascertained using Medicare files, using Current Procedural Terminology, Fourth Edition (CPT-4), codes for treatment (77403, 77413, 77417, 77418, and 77427).
We determined the time interval from surgery to cranial radiation (in days) using the start date of radiation subtracted by the last date of the surgical procedure (biopsy or craniotomy for surgical resection). For regression analyses, we categorized the variable time from surgery to radiation into quartiles based on the distribution of this variable.
Other Clinical Variables, Sociodemographic Measurements and Survival Estimates
The types of surgery were determined by SEER, and the surgical dates were confirmed by CPT-4. Surgery was classified as biopsy-only, subtotal resection, gross total resection, and natural orifice surgery if the degree of resection was not specified. We used the last surgical date if more than one surgery was performed. Adjuvant chemotherapy exposures, either after radiation or at recurrence, were ascertained from Medicare files using codes for ICD-9-CM diagnosis (V581, V662), HCPCS (Q0083, Q0084, J9000–J9999), and CPT (96401–96450; 96522–96549).
According to a method developed by Krieger,10 we generated an aggregate SES score by incorporating a hierarchy of income data from the 2000 census. The method was based on a formula that integrates census tract median income, ZIP code median income, census per-capita income, and ZIP code per-capita income. Patients were ranked on a 1–5 scale, with 1 as the lowest and 5 the highest values. Patients for whom all of these values were missing were assigned to the lowest SES category.
To assess the prevalence of comorbid disease in our cohort, we used the Klabunde adaptation of the Charlson comorbidity index.11,12 All relevant ICD-9 diagnosis codes, ICD-9 procedure codes, and HCPCS procedure codes within hospital and physician claim files were searched to identify selected comorbidities from 365 days before to 120 days after diagnosis of cancer. Each category was weighted based on the Charlson index.
We measured the overall survival time in months as the interval from the date of GBM diagnosis to the Medicare date of death. Our data set provided follow-up through December 31, 2003. Those who survived past this time were censored. As GBM is rapidly fatal, and we excluded those who were diagnosed with other cancers prior to their GBM diagnoses, it would be uncommon that the cause of death was due to reasons unrelated to GBM.
Statistical Analyses
The demographic and clinical characteristics of our cohort were illustrated by frequency and percentage. As the variable time from surgery to radiation was categorized in quartiles, its associations with other clinical variables were determined using ordinal logistic regression.
In Cox proportional hazard (PH) modeling, prognostic variables were first assessed using univariate analyses. Those variables that achieved a p-value of ≤0.25 were included in a multivariable model, which began with all potential covariates. Backward elimination was used to remove covariates with p > 0.05. This process was continued until covariates kept in the model were all significant. A preliminary main effect model was used after this stage. Subsequently, those variables that were not initially selected for model building (p-values of univariate analyses >0.25) were added back into the preliminary main effect model. This process would identify those covariates that were not significantly related to survival by themselves but could make an important contribution in the presence of other variables. Meaningful interaction terms based on prior knowledge were introduced one by one into the preliminary main effect model, and each was kept if p < 0.05. They were defined a priori as interactions between age and radiation, age and surgery, race and radiation, and race and surgery. After the derivation of the full model (main effect model plus interaction terms), PH assumption was tested by plotting Schoenfeld residuals against time for each covariate. Afterward, proportion hazard assumption tests based on the technique of Grambsch and Therneau were conducted globally as well as for each individual variable.13 STATA (version 9.2, College Station, TX, USA) was used for all analyses.
Results
Table 1 shows the socio-demographic and clinical characteristics of our entire study cohort, and separately for those who had surgical resections and biopsies. The median age of the entire study cohort was 72. More than 90% of the patients were Caucasian and resided in central and peripheral counties of metropolitan areas with populations greater than 250,000. Of the 1,375 subjects overall, 77% (1,079 patients) had a debulking surgery. Of the 1079 patients who had tumor resection, 53.2% had a gross total resection, 44.9% had a subtotal resection, and 1.9% had nonspecified resection. More than 1 in 4 patients received some form of intravenous chemotherapy. Because of the complexity in localizing these tumors, the variable tumor location provided only the broad regions or lobes of the primary lesions. Moreover, the variable tumor size was missing in more than 35% of patients; thus, because of imprecise characterization, these two clinical factors were not included in subsequent regression analyses.
Table 1.
Demographic and clinical characteristics of elderly patients with GBM in our SEER–Medicare cohort
| Clinical and Demographic Factors | Frequency (%) for the Entire Cohort, n = 1375 | Frequency (%) for those with Craniotomy, n = 1079 | Frequency (%) for those with Biopsy, n = 296 |
|---|---|---|---|
| Age at diagnosis | |||
| 65–69 | 398 (29.0) | 318 (29.5) | 80 (27.0) |
| 70–74 | 482 (35.1) | 385 (35.7) | 97 (32.8) |
| 75–79 | 336 (24.4) | 254 (23.5) | 82 (27.7) |
| 80 or above | 159 (11.6) | 122 (11.3) | 37 (12.5) |
| GBM histological subtypes | |||
| Classical GBM | 1331 (96.8) | 1038 (96.2) | 293 (99.0) |
| Giant cell GBM | 11 (0.80) | 9 (0.83) | <5 (<1.7)a |
| Gliosarcoma | 33 (2.40) | 32 (3.0) | <5 (<1.7)a |
| Racial groups | |||
| Caucasian | 1270 (92.4) | 1002 (92.9) | 268 (90.5) |
| African American | 35 (2.6) | 22 (2.0) | 13 (4.4) |
| Hispanic | 17 (1.2) | 16 (1.5) | <5 (<1.7)a |
| Other racial group | 53 (3.9) | 39 (3.6) | 14 (4.7) |
| Residence in metropolitan areas | 1245 (90.6) | 972 (90.1) | 273 (92.2) |
| Socio-economic status | |||
| Lowest quintile | 274 (19.9) | 207 (19.2) | 65 (22.0) |
| Second quintile | 266 (19.4) | 232 (21.5) | 54 (18.2) |
| Third quintile | 277 (20.2) | 212 (19.7) | 65 (22.0) |
| Fourth quintile | 286 (20.8) | 203 (18.8) | 63 (21.3) |
| Highest quintile | 272 (19.8) | 225 (20.9) | 49 (16.6) |
| Marital status | |||
| Not married | 403 (29.3) | 321 (29.8) | 82 (27.7) |
| Married | 946 (68.8) | 739 (68.5) | 207 (69.9) |
| Marital status unknown | 26 (1.9) | 19 (1.8) | 7 (2.4) |
| Comorbidity scores | |||
| 0 | 776 (56.4) | 615 (57) | 161 (54.4) |
| 1 | 405 (29.5) | 315 (29.2) | 90 (30.4) |
| 2 | 194 (14.1) | 149 (13.8) | 45 (15.2) |
| Tumor location | |||
| Frontal lobe | 286 (20.8) | 234 (21.7) | 52 (17.6) |
| Temporal lobe | 377 (27.4) | 334 (31.0) | 43 (14.5) |
| Parietal lobe | 249 (18.1) | 180 (16.7) | 69 (23.3) |
| Occipital lobe | 92 (6.7) | 77 (7.1) | 15 (5.1) |
| Ventricle, brainstem, or cerebellum | 14 (1.0) | 8 (0.8) | 6 (2.1) |
| Involvement of two lobes or bihemispheres | 273 (19.9) | 202 (18.7) | 71 (24.0) |
| Brain location not specified (NOS) | 84 (6.1) | 44 (4.1) | 40 (13.5) |
| Tumor size (mm) | |||
| < 30 | 237 (17.2) | 170 (15.8) | 67 (22.6) |
| 30–41 | 193 (14.0) | 151 (14.0) | 42 (14.2) |
| 42–53 | 231 (16.8) | 201 (18.6) | 30 (10.1) |
| ≥ 54 | 224 (16.3) | 192 (17.8) | 32 (10.8) |
| Size unknown | 490 (35.6) | 365 (33.8) | 125 (42.2) |
| Weeks from surgery to initiation of radiation | |||
| Within 1 week | 142 (10.3) | 61 (5.7) | 81 (27.4) |
| 1–2 weeks | 439 (31.9) | 322 (29.4) | 117 (39.5) |
| 2–3 weeks | 394 (28.7) | 341 (31.6) | 53 (17.9) |
| 3–4 weeks | 190 (13.8) | 177 (16.4) | 13 (4.4) |
| 4–5 weeks | 117 (8.5) | 102 (9.5) | 15 (5.1) |
| 5–6 weeks | 60 (4.4) | 49 (4.5) | 11 (3.7) |
| > 6 weeks | 33 (2.4) | 27 (2.5) | 6 (2.0) |
| Types of surgery | |||
| Biopsy | 296 (21.5) | N/A | N/A |
| Subtotal resection | 485 (35.3) | ||
| Gross total resection | 574 (41.8) | ||
| NOS | 20 (1.5) | ||
| Administration of chemotherapy | 370 (26.9) | 307 (28.5) | 63 (21.3) |
NOS, natural orifice surgery.
aPer SEER–Medicare regulation, counts with <5 patients must be stated as “< 5” instead of the actual numbers.
The median survival of those who had a gross total resection was 9.3 months, subtotal resection 8.0 months, surgery NOS 6.7 months, and biopsy 5.6 months. For the entire cohort, the median time to the initiation of radiation was 15 days (interquartile range [IQR] 12–22 days). For the group that had tumor resections, the median time to radiation was 16 days (IQR 12–22 days), whereas in those who had biopsies, the median was 10 days (IQR 6–16 days). The 95th percentile of those with biopsies was 37 days, and with tumor resections was 40 days. Therefore, to categorize the variable time interval to radiation, we divided it into quartiles: 0–12 days (reference), 13–16, 17–22, and ≥23 days. Since the sample size of the biopsy group was small, we dichotomized only the variable at its median (≤10 or >10 days).
Ordinal logistic regression revealed that only the types of surgery were associated with the timing of radiation (Table 2). When compared to biopsy subjects, those who had tumor resections were significantly more likely to start radiotherapy at a later time. Moreover, previous studies also supported that having tumor resection was a favorable prognostic factor for GBM.4,14 Therefore, we anticipated that the type of surgery would be a strong confounding factor for the variable time from surgery to radiation, and because residual confounding might be an issue even after statistical adjustment, it was necessary to separate the analyses into patients who had biopsies versus those who received surgical resections.
Table 2.
The associations between clinical/demographic variables and time from surgery to cranial radiation
| Clinical Variablesa | Days from Surgery to Radiation, Median (IQR) | Odds Ratio (OR) | p-Value |
|---|---|---|---|
| Age at diagnosis | |||
| 65–69 | 14 (11) | Reference | |
| 70–74 | 15 (11) | 1.25 (0.92–1.70) | 0.15 |
| 75–79 | 14 (12) | 1.08 (0.77–1.52) | 0.66 |
| 80 or above | 15 (12) | 1.18 (0.77–1.82) | 0.44 |
| GBM histological subtypes | |||
| Classical GBM | 15 (11) | Reference | |
| Giant cell GBM | 13 (14) | 0.80 (0.23–2.73) | 0.72 |
| Gliosarcoma | 16 (10) | 0.91 (0.41–2.00) | 0.81 |
| Racial groups | |||
| Caucasian | 15 (11) | Reference | |
| African American | 14 (13) | 1.16 (0.52–2.59) | 0.72 |
| Hispanic | 28 (21) | 1.20 (0.28–5.14) | 0.8 |
| Other racial group | 15 (15) | 0.76 (0.40–1.43) | 0.39 |
| Socio-economic status | |||
| Lowest quintile | 15 (13) | Reference | |
| Second quintile | 14 (12) | 1.10 (0.75–1.64) | 0.62 |
| Third quintile | 15 (11) | 1.11 (0.74–1.66) | 0.63 |
| Fourth quintile | 15 (11) | 0.97 (0.65–1.45) | 0.88 |
| Highest quintile | 14 (11) | 0.81 (0.54–1.21) | 0.3 |
| Residence | |||
| Metropolitan areas | 14.5 (11) | Reference | |
| Nonmetropolitan areas | 15 (11) | 0.88 (0.56–1.38) | 0.58 |
| Marital status | |||
| Not married | 15 (11) | Reference | |
| Married | 15 (11) | 1.13 (0.86–1.49) | 0.37 |
| Marital status unknown | 17 (18) | 1.44 (0.54–3.84) | 0.47 |
| Comorbidity scores | |||
| 0 | 14.5 (10) | Reference | |
| 1 | 15 (12) | 1.10 (0.83–1.44) | 0.51 |
| 2 | 15 (11) | 0.89 (0.61–1.28) | 0.52 |
| Types of surgeryb | |||
| Biopsy | 10 (10) | Reference | |
| Subtotal resection | 16 (10) | 4.55 (3.22–6.44) | 0.0001 |
| Gross total resection | 16 (10) | 3.95 (2.81–5.57) | 0.0001 |
| NOS | 14 (4) | 5.42 (2.03–14.47) | 0.001 |
| Chemotherapy | |||
| Not given | 15 (12) | Reference | |
| Given | 14 (11) | 0.81 (0.61–1.08) | 0.15 |
NOS, natural orifice surgery.
aResults were obtained from a multivariate logistic regression.
bIncreasing time interval from surgery to radiation is represented by OR >1.
Of those who had tumor resections, the univariate analysis revealed that beginning radiation after 22 days post craniotomy had a protective effect (hazard ratio [HR] = 0.82, 95% CI 0.70–0.97) on survival. In multivariable analyses, the variable time interval to radiation was no longer a significant prognostic factor (Table 3). The highest socioeconomic group, gross total resection, and the administration of chemotherapy were significant favorable factors, whereas age ≥70 was a poor determinant of survival. Subgroup analyses of patients with gross total and subtotal resections also did not show any association between the time interval from surgery to radiation and overall survival. However, those with gross total resection followed by cranial radiation after 22 days had a nonsignificant trend toward an improved survival (HR = 0.98, 95% CI 0.97–1.00, p = 0.08). This survival trend was not observed in those who had subtotal resection and subsequently initiated radiotherapy after 22 days (HR = 0.86, 95% CI 0.67–1.10, p = 0.23).
Table 3.
Multivariable Cox proportional hazard analysis of the relationship between time from surgery to radiation and overall survival in both patients who had surgical resections and those who only had biopsies
| Clinical Variables | HR (95% CI) for Surgical Resection, n = 1,079 | p-Value | HR (95% CI) for Biopsy, n = 296 | p-Value |
|---|---|---|---|---|
| Time from surgery to radiation (days) | ||||
| 0–12 | Reference | Reference | ||
| 13–16 | 1.00 (0.99–1.02) | 0.65 | 0.87 (0.69–1.11)a | 0.27 |
| 17–22 | 1.00 (0.99–1.02) | 0.9 | ||
| 23 and over | 0.99 (0.97–1.01) | 0.14 | ||
| Age at diagnosis (years) | ||||
| 65–69 | Reference | Reference | ||
| 70–74 | 1.27 (1.09–1.48) | 0.002 | 1.06 (0.78–1.44) | 0.72 |
| 75–79 | 1.29 (1.09–1.52) | 0.004 | 1.37 (0.98–1.90) | 0.06 |
| 80 or above | 1.73 (1.39–2.16) | 0.0001 | 1.16 (0.77–1.77) | 0.48 |
| GBM histological subtypes | ||||
| Classical GBM | Reference | Reference | ||
| Giant cell GBM | 0.89 (0.46–1.73) | 0.73 | 0.55 (0.13–2.29) | 0.41 |
| Gliosarcoma | 1.09 (0.76–1.57) | 0.63 | 0.86 (0.12–6.36) | 0.88 |
| Racial groups | ||||
| Caucasian | Reference | Reference | ||
| African American | 0.85 (0.55–1.31) | 0.47 | 1.72 (0.97–3.05) | 0.06 |
| Hispanic | 0.73 (0.38–1.15) | 0.1 | 1.43 (0.19–10.77) | 0.73 |
| Other racial group | 0.77 (0.55–1.08) | 0.13 | 1.21 (0.69–2.12) | 0.5 |
| Socio-economic status | ||||
| Lowest quintile | Reference | Reference | ||
| Second quintile | 0.96 (0.79–1.17) | 0.69 | 0.43 (0.29–0.63) | 0.0001 |
| Third quintile | 0.87 (0.71–1.07) | 0.18 | 0.49 (0.33–0.71) | 0.0001 |
| Fourth quintile | 0.83 (0.67–1.01) | 0.059 | 0.32 (0.22–0.46) | 0.0001 |
| Highest quintile | 0.79 (0.65–0.97) | 0.023 | 0.34 (0.23–0.46) | 0.0001 |
| Residence | ||||
| Metropolitan areas | Reference | Reference | ||
| Nonmetropolitan areas | 0.88 (0.70–1.09) | 0.25 | 1.02 (0.64–1.63) | 0.94 |
| Marital status | ||||
| Not married | Reference | Reference | ||
| Married | 0.99 (0.86–1.13) | 0.83 | 1.00 (0.76–1.33) | 0.98 |
| Unknown | 0.82 (0.51–1.32) | 0.41 | 0.73 (0.34–1.62) | 0.43 |
| Comorbidity scores | ||||
| 0 | Reference | Reference | ||
| 1 | 0.99 (0.86–1.13) | 0.84 | 0.92 (0.63–1.36) | 0.69 |
| 2 | 0.88 (0.73–1.05) | 0.16 | 0.98 (0.69–1.39) | 0.91 |
| Types of surgery | ||||
| Subtotal resection | Reference | N/A | ||
| Gross total resection | 0.74 (0.65–0.86) | 0.0001 | ||
| NOS | 0.81 (0.60–1.10) | 0.97 | ||
| Chemotherapy | ||||
| Not given | Reference | Reference | ||
| Given | 0.91 (0.86–0.97) | 0.003 | 0.88 (0.82–0.94) | 0.0001 |
aSince only 297 patients had biopsies, the variable time from surgery to radiation was dichotomized at its median at day 10. The group >10 days was compared to the reference group 0–10 days.
To test the robustness of our findings, we repeated the multivariate analysis of those with surgical resections using the 25th to 75th percentiles of the variable time interval from surgery to radiation as the reference, which corresponded to 13–22 days. Initiation of radiation >22 days post-surgery was not a significant factor for prognosis (HR = 0.99, 95% CI 0.96–1.01, p = 0.13), and the time interval from 0 to 12 days between surgery and radiation did not carry any impact on survival (HR = 1.07, 95% CI 0.92–1.25, p = 0.46). Moreover, as a sensitivity analysis, we excluded subjects who died within 30 days of diagnosis (instead of 90 days), but the conclusions remained the same for both surgical groups (data not shown).
In testing the PH assumption, the variable chemotherapy showed a significant deviation (ρ = 0.096, χ2 = 9.78, df = 1, p = 0.0018). This implied that the HR associated with chemotherapeutic use was changing through time. Since patients might have received chemotherapies more frequently during the latter period of the study cohort, and more effective therapy might have been prescribed as the study period advanced, statistical violation of the PH assumption by chemotherapy could have clinical correlates. Thus, we fitted a time-varying covariate, which took into account the natural log of time, to accompany the variable chemotherapy. The final adjusted HR of chemotherapy showed a significant 9% improvement in the overall survival (Table 3).
Of those who only had biopsies, radiation timing did not have an impact on overall survival in the univariate or multivariate analyses (Table 3). However, our sample size is small (n = 296), and the 95th percentile of patients had already started radiation at day 37 post-operatively. In the final multivariate analysis, the receipt of chemotherapy and higher socio-economic classes (compared with the lowest group) were significant protective factors in those who only had biopsies. Age group between 75 and 79 and African American race were poor and borderline significant risk factors. Similar to those who had debulking surgery of their tumors, the variable chemotherapy violated the PH assumption; therefore, a time-varying covariate was fitted to the model as before.
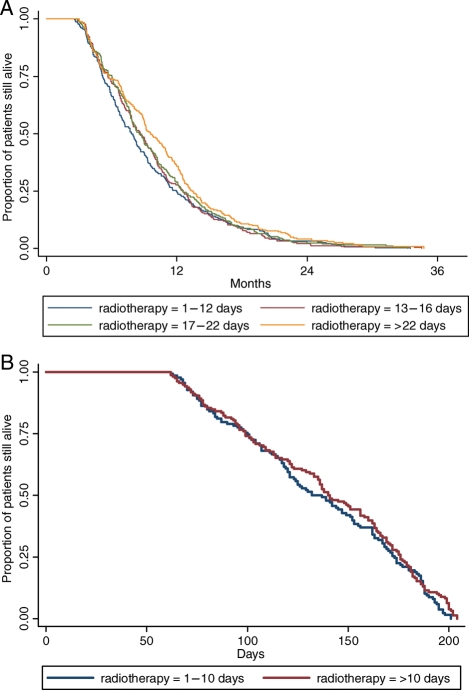
None of the a priori–declared interaction terms were significant factors in our modeling. Fig. 1A and B shows the Kaplan–Meier survival estimates of patients who had surgical resections and those who had biopsies, respectively.
Fig. 1.
(A) Kaplan–Meier survival curves of overall survival by time interval from surgical resection to the initiation of radiotherapy. (B) Kaplan–Meier curves of overall survival by time interval from biopsy to the initiation of radiotherapy.
Discussion
This population-based study found that initiation of cranial radiation within 6 weeks of surgery/biopsy had an equivalent survival effect in elderly patients diagnosed with GBM. Nevertheless, our results can only be generalized to those who began radiotherapy within 6 weeks of surgery, because the 95th percentile of our study cohort had already started radiation at Day 37 (biopsy) and Day 40 (tumor resection) post operation, even though our inclusion criteria allowed a time interval of up to 90 days.
Data from Do et al.7 also suggested no relationship between time interval from surgery to the start of radiotherapy and overall survival (HR = 1.00, 95% CI 0.99–1.01, p = 0.79). The investigators examined 182 malignant glioma patients diagnosed between 1979 and 1995 in the greater Sydney, Australia region. Their median time from surgery to the beginning of radiation was 26 days. However, they excluded 31 patients who had small tumors, had complete resections, and were treated with radiosurgery; they also eliminated those who had been treated with adjuvant chemotherapy. Perhaps, the removal of better prognostic groups from their data set also abolished an inverse survival trend similar to what we observed in our subgroup of patients with gross total resections.
While our results and others' showed no statistically significant relationship between waiting time and overall survival, a retrospective study conducted by the RTOG found that adult GBM patients radiated >4 weeks post-operatively had a statistically significant survival advantage over the group that started ≤2 weeks post-operatively (HR = 0.84, p < 0.0032).6 Their results were adjusted for age, performance status, extent of resection, and recursive partitioning analysis class. As a retrospective analysis, there might be unmeasured confounding factors that accounted for the inverse relationship.
In contrast, another retrospective study of 172 malignant glioma patients treated with radiotherapy in New Zealand showed that every additional week of delay 2 weeks post operation increased the risk of death by 8.9% (95% CI 2.0%–16.1%).5 Although these results parallel the findings of other cancers that delay in radiotherapy was associated with reduction in survival,15,16 comparison of their study with ours suggested several important differences that may partially explain the opposite findings. First, because of resource constraints, half of their patients initiated cranial radiation beyond 5 weeks and sometimes as late as 15 weeks post-operatively. Therefore, it is possible that a prolonged delay in starting radiotherapy is detrimental to survival as suggested by these authors, but our study was unable to verify this possibility as the wait experienced by our cohort was brief in comparison. Moreover, since a delay could be as long as 15 weeks, some patients might have died prior to the completion of radiotherapy. However, the paper did not seem to have excluded such subjects. Their inclusion may confound the study, because increased mortality could not be clearly attributed to delay in upfront radiation. In our analysis, we excluded patients who died within 90 days of surgery. Although this exclusion process had rendered our study cohort “less representative” of the elderly GBM population, it was a necessary step to ensure the validity of our conclusion. Finally, chemotherapy was rarely prescribed at recurrence in the New Zealand cohort and thus there would be no potential compensation for survival associated with a late start in radiation.
Confounding factors that explained an inverse association between waiting time and survival in the univariate analysis are multiple and may reflect triaging physicians' judgment to recommend early radiation for those judged to have worse outcome and to defer radiation for patients who were likely to have better prognoses. Although such a complex decision could be difficult to account for adequately via regression analyses, some factors that drove this decision include the extent of residual disease after surgery, size of tumors, comorbidities, post-operative complications, and Karnofsky performance status (KPS) scores. The lack of information on KPS scores in the SEER–Medicare linkage database is a shortcoming, as a previous study showed that those with higher KPS tended to begin radiotherapy later.5 However, this deficiency is at least partially compensated for by subjects' Charlson comorbidity scores, which incorporated many key medical conditions and symptoms, including hemiparesis and dementia. Nevertheless, these scores are not perfect substitutes for KPS in the assessment of brain tumor prognoses, as unmeasured neurological deficits such as gait imbalance, visual field deficit, and neglect also have an impact on the overall functioning of an individual. In other prognostic studies of GBM, KPS is an important predictor of survival.17 Therefore, it may be a residual unmeasured confounding factor in our analysis.
Chemotherapy is an important confounding factor, especially toward the later part of our study period when chemotherapeutic agents were used more frequently. However, we could estimate the prevalence of only intravenous, but not oral, chemotherapy use in this study, as temozolomide or other oral agents cannot be captured as billed items in Medicare files. During the late 1990s and early 2000, temozolomide was used either at the time of recurrence or after the completion of upfront cranial radiation.18 Nevertheless, our final result of 9% (resection group) and 12% (biopsy group) improvement in the hazard of dying due to chemotherapy was in accordance with that obtained by a previous meta-analysis, which showed a 15% reduction of mortality in GBM patients treated with chemotherapy.19 This meta-analysis did not include the randomized trial that established the efficacy of concomitant temozolomide and radiation in newly diagnosed GBM (the EORTC/NCIC trial).20 Similarly, our study cohort was diagnosed prior to the completion of this trial and thus confounding due to this regimen would be kept to a minimum.
The EORTC/NCIC trial will likely diminish the impact of short a delay in waiting time on the survival of GBM. Although this study excluded patients aged >70 years, and the worth of concomitant radiation with temozolomide in the elderly is still debatable, this combined therapy may add further benefit to survival if an elderly patient is able to tolerate it.21,22 Therefore, as this field moves forward, more effective treatment regimens may dilute the effects of a brief delay.
In summary, our results suggest that the initiation of radiotherapy up to approximately 37 days after a biopsy and 40 days following tumor resection will likely not have any impact on the overall survival, after accounting for the influence of confounding factors. Effort should be made to ensure the commencement of cranial radiation at the earliest possible time after surgery/biopsy. However, when delay is necessary, the aforementioned upper limit of 6 weeks may serve as the latest time point up to which radiotherapy could be started without incurring an adverse effect on survival.
Funding
This study was supported by a K07 Award (CA127468) to R.L., an American Society of Clinical Oncology Advanced Clinical Research Award to D.L.H., and an American Cancer Society grant (RSGT-01-02404-CPHPS) and a K05 Award (CA89155) to A.I.N.
References
- 1.Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356:1527–1535. doi: 10.1056/NEJMoa065901. [DOI] [PubMed] [Google Scholar]
- 2.Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22:1583–1588. doi: 10.1200/JCO.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 3.Burnet NG, Jena R, Jefferies SJ, et al. Mathematical modelling of survival of glioblastoma patients suggests a role for radiotherapy dose escalation and predicts poorer outcome after delay to start treatment. Clin Oncol (R Coll Radiol) 2006;18:93–103. doi: 10.1016/j.clon.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Mirimanoff RO, Gorlia T, Mason W, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 Phase III Randomized Trial. J Clin Oncol. 2006;24:2563–2569. doi: 10.1200/JCO.2005.04.5963. [DOI] [PubMed] [Google Scholar]
- 5.Irwin C, Hunn M, Purdie G, et al. Delay in radiotherapy shortens survival in patients with high-grade glioma. J Neurooncol. 2007;85:339–343. doi: 10.1007/s11060-007-9426-z. [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal DT, Berkey B, Nelson D, et al. “Short” delay in initiation of radiotherapy may not affect the outcome of patients with GBM: a secondary analysis from the RTOG database. Neuro-Oncology. 2006;8:483. [Google Scholar]
- 7.Do V, Gebski V, Barton MB. The effect of waiting for radiotherapy for grade III/IV gliomas. Radiother Oncol. 2000;57:131–136. doi: 10.1016/s0167-8140(00)00257-7. [DOI] [PubMed] [Google Scholar]
- 8.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 9.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer-related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 10.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 12.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: an overview. Med Care. 2002;40(8 Suppl):IV-26–IV-35. doi: 10.1097/00005650-200208001-00004. [DOI] [PubMed] [Google Scholar]
- 13.Grambsch P, Therneau T. Proportional hazard tests in diagnostic based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 14.Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 2004;6:227–235. doi: 10.1215/S1152851703000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.E C, Dahrouge S, Samant R, et al. Radical radiotherapy for cervix cancer: the effect of waiting time on outcome. Int J Radiat Oncol Biol Phys. 2005;61:1071–1077. doi: 10.1016/j.ijrobp.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 16.Hershman DL, Wang X, McBride R, et al. Delay in initiating adjuvant radiotherapy following breast conservation surgery and its impact on survival. Int J Radiat Oncol Biol Phys. 2006;65:1353–1360. doi: 10.1016/j.ijrobp.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 17.Gorlia T, van den Bent MJ, Hegi ME, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC Trial 26981-22981/CE.3. Lancet Oncol. 2008;9:29–38. doi: 10.1016/S1470-2045(07)70384-4. [DOI] [PubMed] [Google Scholar]
- 18.Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 20.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 21.Brandes AA, Vastola F, Basso U, et al. A prospective study on glioblastoma in the elderly. Cancer. 2003;97:657–662. doi: 10.1002/cncr.11097. [DOI] [PubMed] [Google Scholar]
- 22.Combs SE, Wagner J, Bischof M, et al. Postoperative treatment of primary glioblastoma multiforme with radiation and concomitant temozolomide in elderly patients. Int J Radiat Oncol Biol Phys. 2008;70:987–992. doi: 10.1016/j.ijrobp.2007.07.2368. [DOI] [PubMed] [Google Scholar]