Abstract
Akt, one of the major downstream effectors of phosphatidylinositol 3-kinase, is hyper-expressed and activated in a variety of cancers including glioblastoma. However, the expression profiles of the Akt isoforms Akt1/PKBα, Akt2/PKBβ, and Akt3/PKBγ and their functional roles in malignant glioma are not well understood. Therefore, we examined the protein and mRNA expression patterns of Akt isoforms in tissues from human astrocytomas, glioblastomas, and non-neoplastic regions. We also explored the biological role of each Akt isoform in malignant glioma cells using RNA interference-mediated knock-down and the over-expression of plasmid DNA of each isoform. The expression of Akt1 protein and mRNA was similar in glioma and normal control tissues. Although the protein and mRNA level of Akt2 increased with the pathological grade of malignancy, the expression of Akt3 mRNA and protein decreased as the malignancy grade increased. In U87MG, T98G, and TGB cells, the down-regulation of Akt2 or Akt3 by RNA interference reduced the expression of the phosphorylated form of Bad, resulting in the induction of caspase-dependent apoptosis. Akt1 knock-down did not affect cell growth or survival. We first demonstrate that the over-expression of Akt2 or Akt3 down-regulated the expression of the other protein and that endogenous Akt3 protein showed high kinase activity in U87MG cells. Our data suggest that Akt2 and Akt3 play an important role in the viability of human malignant glioma cells. Targeting Akt2 and Akt3 may hold promise for the treatment of patients with gliomas.
Keywords: Akt2, Akt3, apoptosis, glioma, protein and gene expression profile
Malignant glioma, the most common primary brain tumor in adults, carries a poor prognosis. The median survival of patients with high-grade glioma (World Health Organization [WHO] grade III or IV) is 10–30 months despite multimodal treatment with surgery, radiation, and chemotherapy.1 Malignant gliomas develop as a result of the stepwise accumulation of alterations in genes regulating cell proliferation, differentiation, and apoptosis. The amplification and mutation of epidermal growth factor receptor, a transmembrane receptor with intrinsic tyrosine kinase activity, have been documented. Tyrosine kinase receptors activate several signaling pathways including mitogen-activated protein kinase and phosphatidylinositol 3-kinase (PI3K). The activation of tyrosine kinase receptor-mediated signaling pathways plays an important role in the tumorigenesis and maintenance of malignant human gliomas.2,3 The constitutively activated PI3K/Akt pathway delivers major survival signals to glioblastoma multiforme (GBM) and other cancer cells.4–7 Akt, with its serine–threonine protein kinase activity, is a major downstream effector of the PI3K pathway, and the ectopic expression of Akt induces cell survival and malignant transformation. On the other hand, the inhibition of Akt activity stimulates apoptosis in a range of mammalian cells.8–10 Akt is activated in 70% of gliomas and is usually associated with PTEN mutations.11–13 These observations suggest Akt as a potential target for therapy.
The Akt family consists of 3 members, Akt1, Akt2, and Akt3. It has been suggested that these 3 Akt isoforms have distinct functions. For example, in knock-out mice, Akt2 and Akt1 were implicated in glucose hemostasis and growth regulation, respectively.14,15 Akt1 activation suppressed cell migration and invasion, whereas Akt2 promoted cell invasion, an issue of particular relevance in metastasis.16,17 The particularly high homology between the catalytic domain of Akt1, Akt2, and Akt3 suggests that their functional differences may not depend on their substrate specificity.18 Although optimal therapeutic efficacy at acceptable toxicity may require Akt isoform-specific inhibitors, it remains unknown which Akt isoform is the most appropriate candidate for inhibition.
Using human malignant glioma tissues and cells, we studied Akt isoform protein and Akt gene-expression patterns and the role of each Akt isoform. We show that Akt2 and Akt3 isoform-specific knock-down effectively inhibited cell growth and induced mitochondrial apoptosis in malignant glioma cells.
Materials and Methods
Sample Collection
All donors provided prior written consent to use their brain tissue material and additional clinical data for research purposes. Our study was approved by our ethics committee. Between March 2002 and June 2008, we obtained human tissue samples from the Department of Neurosurgery of the University of Tokushima Graduate School. All tissue samples were prepared as described by our laboratory.19,20 We used normal tissues from patients without brain tumors who underwent lobectomy and tissues from non-neoplastic regions (NNR) containing no obvious tumor cells from patients undergoing surgery for astrocytic tumors. All samples (normal, n = 6; NNR, n = 16; grade I, n = 5; grade II, n = 5; grade III, n = 11; grade IV, n = 16) were diagnosed by neuropathologists according to the WHO classification. Oligodendrogiomas and mixed tumors (eg, oligo-astrocytoma) were not included in this study.
Cell Culture and Culture Conditions
The human glioblastoma cell lines U87MG and T98G were from American Type Culture Collection (Virginia), and U251MG and GB1 cells were from the Health Science Research Resources Bank (Osaka). TGB cells derived from primary GBM cells from a patient who granted prior informed consent for their use in this study. Normal human astrocytes (NHA) were from Cambrex (New Jersey). All malignant glioma cell lines were cultured in the RPMI-1640 medium (Invitrogen, New Jersey) with 10% fetal bovine serum (FBS) (Gibco-BRL, New York) at 37°C in an atmosphere of 5% CO2 and air. NHA were cultured in astrocyte basal medium (AGMTM) (Cambrex, Maryland) with 3% FBS at 37°C in 5% CO2 and air.
Western Blot Analysis
Tissue samples and harvested cells were homogenized in RIPA lysis buffer that contained protease (Santa Cruz, California) and phospatase inhibitors (PIERCE, Rockford, Illinois). The protein concentration in supernatants was assayed using a BCA kit (PIERCE) to allow the acquisition of a consistent amount of protein from different samples. After reducing in 10 mM Tris–HCl (pH 7.5), 2 mM EDTA, 1% SDS, and DTT, 20–50 µg of protein were separated by SDS–PAGE and transferred to nitrocellulose membranes (trans-blot transfer medium, BIO-RAD, California) by electroblotting. The membranes were immersed for 1 hour in blocking buffer (5% nonfat dry milk or BSA in TBS) and then incubated with primary antibodies recognizing Akt1, Akt2, Akt3, the phosphorylated form of Akt at Ser473 (pAkt), PTEN, BAD, the phosphorylated form of BAD (pBAD), cleaved caspase-3 antibody (Asp175), cleaved caspase-8 antibody (Asp384), cleaved caspase-9 antibody (Asp330) (all at 1:1000, Cell Signaling Technology, California), GSK-3β, and the phosphorylated form of GSK-3β (pGSK-3β) (all at 1:2000, BD Biosciences, California) diluted in T-TBS or Canget signal solution I (TOYOBO, Osaka). β-Actin antibody (Sigma, Missouri) was diluted 1:5000 in 5% nonfat dry milk. After 1 hour incubation with horseradish peroxidase-conjugated secondary antibody, protein–antibody complexes were detected with Amersham ECL plus Western blotting detection reagents (GE Healthcare, UK) using an LAS-3000 Lumino image analyzer (Fuji Film, Tokyo). To calculate the protein levels obtained by Western blot analysis, we used Image-J software.
Quantitative Real-time PCR
Total RNA isolated from tumor samples and glioma cell lines was purified with the RNeasy lipid tissue mini kit (QIAGEN, Valencia, California) according to the manufacturer's protocol and reverse-transcribed with the QuantiTect reverse transcription kit (QIAGEN). Quantitative real-time PCR (qRT-PCR) was under the conditions recommended by the manufacturer on a Light Cycler rapid thermal cycler (Roche Diagnostics, Lewes). The forward and reverse primer sequences for Akt1 were 5′-CGTGCATTTGAGAGAAGCCA-3′ and 5′-CACCCGGAGAACAAACTG-3′. For Akt2, they were 5′-AGGAGGTCATGGAGCACA-3′ and 5′-AAGCCCAGGCYGTCATAG-3′; for Akt3, 5′-AAGGGAAGAATGGACAGA-3′ and 5′-ATGGGTTGTAGAGGCATC-3′; and for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), they were 5′-GGGTGTGAACCATGAGAAGTATGA-3′ and 5′-TGCTAAGCAGTTGGTGGTGC-3′. The primers were optimized at Nihon Gene Research Laboratories, Inc. (Sendai). Akt1, Akt2, and Akt3 mRNA expression was normalized with the GAPDH mRNA content.
Immunohistochemistry
Tissue samples fixed with 4% paraformaldehyde in PBS were cut into 5-μm sections. These were subjected to 60 minutes blocking of nonspecific protein binding with serum-free protein block (DakoCytomation), and incubated (4°C, overnight) with the primary antibodies recognizing Akt1 (at 1:100), Akt2 (at 1:100), Akt3 (at 1:50) (Cell Signaling Technology), GFAP (at 1:50, Santa Cruz), or NeuN (at 1:100, Chemicon, California) in a 1% BSA/PBS solution. After washing in PBS, the sections were incubated (room temperature, 1 hour) with the secondary antibody (A11005, Alexa Fluoro® 594 goat antimouse IgG; dilution 1:400, 1% BSA/PBS solution) and analyzed under a fluorescence microscope (OLYMPUS, IX71). To identify the cell nuclei, we performed 15 minutes staining with 4′,6′-diamino-2-phenylindole (DAPI; Dojindo, Kumamoto).
Plasmids and Transfection
Human full-length Akt2 and Akt3 cDNAs in a pcDNA3.1-FLAG vector were obtained from CycLex Co., Ltd (Nagano). U87MG, T98G, and TGB cells were plated (3 × 105 cells/well) in 6-well tissue culture plates. After 24 hours culture, they were transfected with Akt2 or Akt3 using FuGENE HD (Roche, Basel, Switzerland) according to the manufacturer's protocol. Control cells were transfected with an empty pcDNA3 vector. The viability of cells was evaluated by counting adherent cells at 24-, 48-, and 72-hour post-transfection.
siRNA Protein Knock-down
Individual siRNA duplexes for Akt1, Akt2, and Akt3 and a nontargeting control were purchased from QIAGEN. The siRNA sequences were: Akt1, AATCACACCACCTGACCAAGA; Akt2, ACGGGCTAAAGTGACCATGAA; Akt3, CAGCAGGCACGTTAACTCGAA; and an All Stars Negative Control (Catalog No. 1027281). The vendor (QIAGEN) validated a >80% knock-down in the mRNA level of each siRNA by qRT-PCR. U87MG and TGB cells were transfected with siRNA to suppress the human Akt gene using the HiPerFect transfection reagent (QIAGEN) according to the manufacturer's instructions. Briefly, before transfection, the cells were seeded at 0.5–1.0 × 106 cells/well in 60 mm dishes containing serum and appropriate growth medium; 256 ng of siRNA was diluted in 100 µL culture medium without serum, and then 12 µL of HiPerFect transfection reagent was added to the diluted siRNA and mixed by vortexing. After the drop-wise addition of the transfection complexes to the cells, they were incubated under their normal growth conditions.
Cell Proliferation Assay
Cells were seeded in 96-well culture plates (1 × 104/well) and transfected with Akt isoform-specific siRNA or cDNA. The number of viable cells was determined with WST-8 reagent (Dojindo) at 48-, 72-, and 96-hour post-transfection. The conversion of WST8 to formazan by metabolically active cells was measured on a microplate reader (model 550, BIO-RAD) at an absorbance of 450 nm. The percent viability was calculated considering the controls as 100%.
Colony-formation Assay
Cells (1 × 106) were cultured for 24 hours after the introduction of siRNA. Thereafter, 5 × 103 cells were replated in 10 cm tissue plates containing culture medium. Colony growth was assessed by the size and number of colonies after 7 days. Colonies exceeding the minimum diameter of 80 µm were counted in triplicate wells. Individual experiments were done in triplicate.
Apoptosis Analysis
Flow Cytometry
The distribution of various cell-cycle phases was examined by flow cytometry. Adherent and floating cells were harvested 72 hours after exposure to siAkt2 or siAkt3, fixed for at least 3 hours in 70% ethanol at 4°C, washed twice with PBS, resuspended in PBS containing 50 µg/mL propidium iodide (PI) (Dojindo) and 20 µg/mL RnaseA (QIAGEN), and analyzed with an EPICS XL-MCL FACScan instrument (Coulter Corp., Hialeah, Florida) using the Coulter cytological program. All experiments were performed in triplicate.
Annexin V/PI Assay
Annexin V staining of exposed membrane phospholipid phosphatidylserine was carried out using the Annexin V assay kit (BioVision, California) following the manufacturer's protocol. U87MG and TGB cells transfected with siAkt were exposed or not exposed to 40 µm of the caspase inhibitor Z-VAD-FMK (MBL, Nagoya) for 12 hours. They were then harvested and stained with Annexin V-FITC and PI. Apoptotic cells were labeled with FITC, necrotic cells with PI. The percent of Annexin V and PI positive cells was analyzed by FACS.
TUNEL Assay
DNA fragmentation was assessed in siAkt-treated cells by TUNEL staining using an in situ apoptosis detection kit (TaKaRa, Tokyo). Briefly, cells were fixed in 4% paraformaldehyde at room temperature and washed with permeabilization buffer for 5 minutes on ice. They were incubated (90 minutes, 37°C) in a TUNEL reaction mixture (labeling-safe buffer and TdT enzyme) to label 3′-OH end DNA strand-breaks with fluorescein-dUTP. Using a fluorescent microscope, we randomly counted 6 fields with >100 cells/field.
Immunoprecipitation and Akt Kinase Activity
All immunoprecipitations were with the immunoprecipitation kit Catch and Release® v2.0 (Upstate Signaling Solutions, California) according to the manufacturer's instructions. Briefly, 500 µg of wild U87MG whole-cell lysates, antibodies against Akt1 (2 µg), Akt2 (10 µg), or Akt3 (10 µg) and 10 µL of antibody capture affinity ligand were mixed and placed in a Catch and Release v2.0 spin column containing 0.5 mL of prepacked immunoprecipitates (IP) capture resin. After 12 hours' end-over-end shaking, the column was centrifuged, washed 3 times, and then eluted with 70 µL elution buffer. The IP were analyzed by immunoblotting using the antibody pAkt at serine-473. Next, IP-Akt1, IP-Akt2, and IP-Akt3 elution were monitored for Akt kinase activity using a CycLex Akt/PKB kinase assay screening kit and the manufacturer's instructions.
Statistical Analysis
Statistical significance was calculated with Stat-View 5.0 software (SAS Institute Inc., Cary, North Carolina). All data are presented as the mean ± SD. We used the Tukey–Kramer test to compare the difference between means. P-values of <.05 were considered statistically significant.
Results
Protein and mRNA Expression of Akt Isoforms in Malignant Glioma Tissues
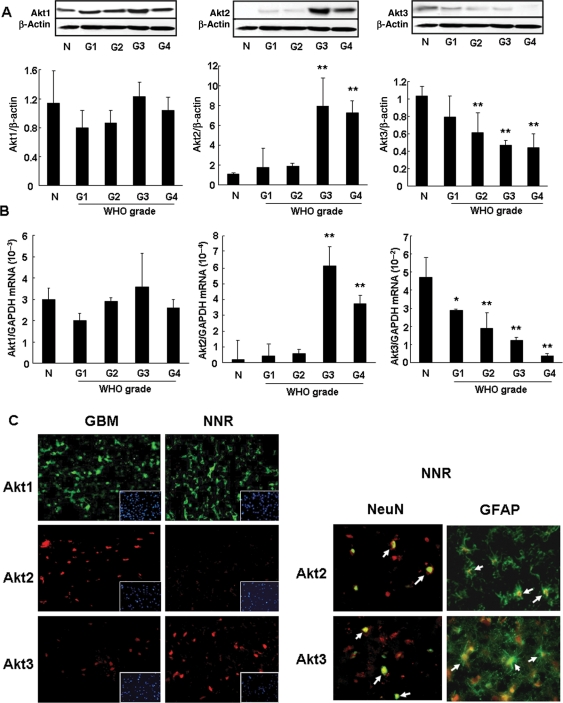
On the basis of the WHO classification of astrocytoma, the tissue samples were classified into grades I, II, III, and IV. The expression of Akt1 protein was not associated with the pathological glioma grade (Fig. 1A). The expression of Akt2 protein was markedly higher in tissues from grade III and IV gliomas than tissues from grade I and II gliomas and normal tissue (Fig. 1A). In contrast, the expression of Akt3 protein decreased with increasing glioma grades (P < .01) (Fig. 1A).
Fig. 1.
Protein and mRNA expression of Akt isoforms in malignant glioma tissues. (A) Western blot analysis. The tissue samples were separated into 5 tissue groups, that is, normal tissues (n = 9) or NNR (n = 10) and grade I (n = 4), II (n = 4), III (n = 9), and IV (n = 14) gliomas. The band density of Western blots was quantified using Image-J software. The expression of Akt2 protein was significantly higher in glioma tissues of grades III and IV than grades I and II and the normal control. The Akt3 protein level was low in parallel with the glioma grade. **P < .01, vs normal tissue. (B) mRNA level of Akt isoforms. The mRNA level of Akt isoforms in 6 normal, 14 NNR, 4 grade I, 5 grade II, 10 grade III, and 15 grade IV tissues was determined and normalized with GAPDH mRNA. Akt2 mRNA was remarkably increased in grade III and IV gliomas. Akt3 mRNA decreased with the malignancy grade; it was significantly lower than in normal control tissues. **P < .01, *P < .05 vs normal tissue. (C) Immunohistochemistry of tissues from NNR and areas with GBM. Akt isoform antigen (Akt1, green; Akt2 and Akt3, red) was detected immunohistochemically. The nuclei were counterstained with DAPI (blue). In GBM tissue, the expression of Akt2 protein was high; Akt3 expression was lower. Costaining of NNR tissues with Akt isoforms and NeuN (green) or GFAP (green) showed that Akt2 and Akt3 proteins were expressed in both glial and neural cells (white arrows).
The mRNA and protein expression profiles of Akt isoforms exhibited similar tendencies. The Akt1 gene was expressed at similar levels in each type of tissue (Fig. 1B). On the other hand, expression of the Akt2 gene was remarkably increased in high-grade gliomas (Fig. 1B), whereas Akt3 gene expression decreased in parallel with the grade of malignancy and was significantly lower than in the normal control tissues (Fig. 1B) (P < .01).
Immunohistochemically, the expression patterns of Akt isoforms were similar in tissues from NNR and gliomas, this finding coincided with the results of Western blot analysis. Costaining of NNR tissue samples with antibodies for the Akt isoforms (Akt1, green; Akt2 and Akt3, red) and the neuronal marker NeuN (green) or the glial marker GFAP (green) showed Akt2 and Akt3 protein in both glial and neural cells (arrows) (Fig. 1C).
Effect of Akt Isoform-specific Knock-down and Over-expression on Cell Growth
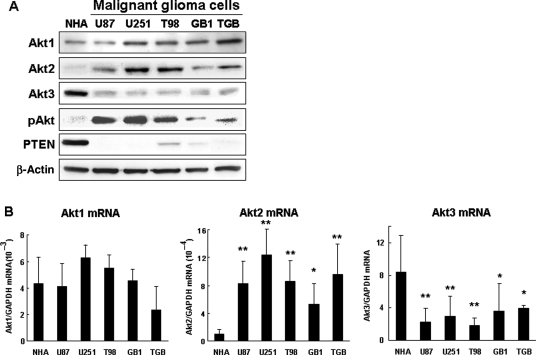
We used malignant glioma cell lines to investigate the effect of Akt isoforms on cell growth. Akt isoform expression was similar in the cell lines and in cells derived from human tissues. There was no difference between the glioma cell lines and NHA with respect to the gene and protein expression of Akt1 (Fig. 2A and B). The expression of Akt2 was higher and that of Akt3 was lower in glioma cells than NHA (Fig. 2A and B). We posit that the protein expression of Akt isoforms in glioma tissues and cells is regulated by gene and transcriptional mechanisms.
Fig. 2.
Protein and mRNA expression of Akt isoforms in glioma cell lines. (A) Representative Western blot analysis of malignant glioma cell lines (U87MG, U251MG, T98G, GB1, and TGB) and NHA. Compared with NHA, the Akt2 protein level was increased while the level of Akt3 protein was decreased in malignant glioma cell lines. (B) The mRNA expression of Akt isoforms in malignant glioma cell lines (U87MG, U251MG, T98G, GB1, and TGB) and NHA. Akt1, Akt2, and Akt3 mRNA expression was normalized with the GAPDH mRNA content. Compared with NHA, the expression of Akt2 mRNA in all cell lines was significantly higher and the expression of Akt3 mRNA was significantly lower. **P < .01, *P < .05 vs NHA.
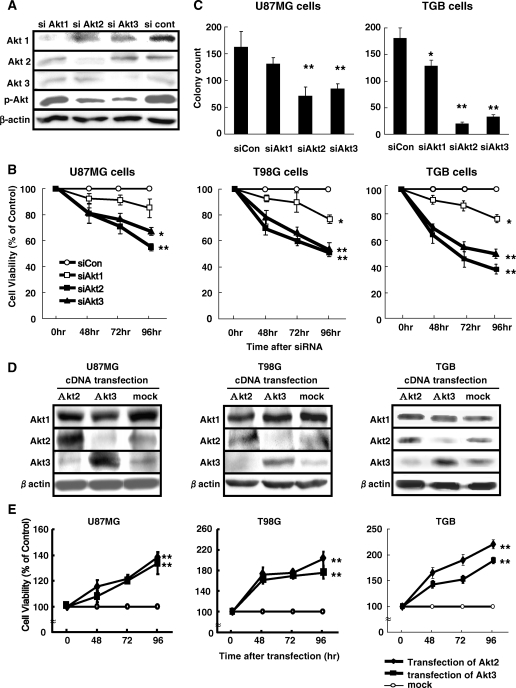
To examine the effect on cell viability, we introduced siRNA against each Akt isoform into U87MG and T98G cell lines and TGB cells derived from a primary GBM. As shown in Fig. 3A, the introduction of siRNA targeting each Akt isoform reduced selectively the expression of each Akt isoform protein. At 48–96 hours after the introduction of siRNA, Akt1 knock-down had little effect on cell growth. Akt2 and Akt3 knock-down significantly inhibited the growth of U87MG, T98G, and TGB cells (Fig. 3B). On the basis of these observations, we focused on the role of Akt2 and Akt3 on cell growth. To further confirm the effects of Akt2 and Akt3 knock-down on cell growth, U87MG and TGB cells were transfected with each siAkt and siCon and then subjected to clonogenic cell survival assay. The knock-down of Akt2 and Akt3 significantly suppressed colony formation in both cell lines (Fig. 3C). To examine the role of Akt2 and Akt3 in cell proliferation, U87MG, T98G, and TGB cells were transfected with plasmids encoding Akt2 or Akt3. In these cells, Akt2 or Akt3 was highly expressed (Fig. 3D). Endogenous Akt2 and Akt3 protein was reduced in cells over-expressing Akt3 and Akt2, respectively. On the other hand, the expression of Akt1 protein was not affected by the ectopic expression of Akt2 or Akt3. These data suggest an interaction between the protein expression of Akt2 and Akt3. Compared with mock-transfected cells, in cells, over-expressing Akt2 or Akt3 cell growth was promoted (Fig. 3E).
Fig. 3.
Effect of the specific knock-down of Akt isoforms and Akt over-expression on cell growth. (A) Representative Western blots of cells transfected with siRNA. U87MG cells were transfected with siRNA-targeting Akt1 (siAkt1), Akt2 (siAkt2), or Akt3 (siAkt3) and a nontargeting control (siCon). After 72 hours, cell lysates were subjected to Western blot analysis. Akt isoform protein expression was reduced selectively. siAkt2 and siAkt3 produced a greater reduction in the expression of pAkt than siAkt1. (B) Effect of siAkt on cell viability. At 48–96 hours after the introduction of siAkt1, siAkt2, or siAkt3 or a siCon, viable cells were identified with WST-8 reagent. Akt2 or Akt3 knock-down inhibited the growth of U87MG, T98G, and TGB cells. Akt1 knock-down had little effect. *P < .05, **P < .01 vs siCon. (C) Inhibition of colony-forming ability by Akt2 or Akt3 knock-down. U87MG and TGB cells transfected with siAkt or siCon were cultured for 7 days. The efficiency of colony formation was determined. *P < .05, **P < .01 vs siCon. (D) Representative Western blots of malignant glioma cells over-expressing Akt2 or Akt3. U87MG, T98G, and TGB cells were transfected with plasmid encoding Akt2 and Akt3. After 48 hours, cell lysates were analyzed for Akt isoform protein levels. Akt2 and Akt3 were selectively hyper-expressed compared with mock-transfected cells. Akt2 protein was reduced in cells over-expressing Akt3 and Akt3 protein was decreased in cells over-expressing Akt2. (E) Cell growth after the induction of Akt2 or Akt3 protein over-expression. At 48–96 hours post-Akt2 or -Akt3 plasmid transfection, viable cells were identified with WST-8 reagent. The over-expression of Akt2 or Akt3 promoted the growth of U87MG, T98G, and TGB cells compared with mock-transfected cells. **P < .01 vs mock plasmid-transfected cells.
Induction of Apoptosis by Akt2 and Akt3 Knock-down
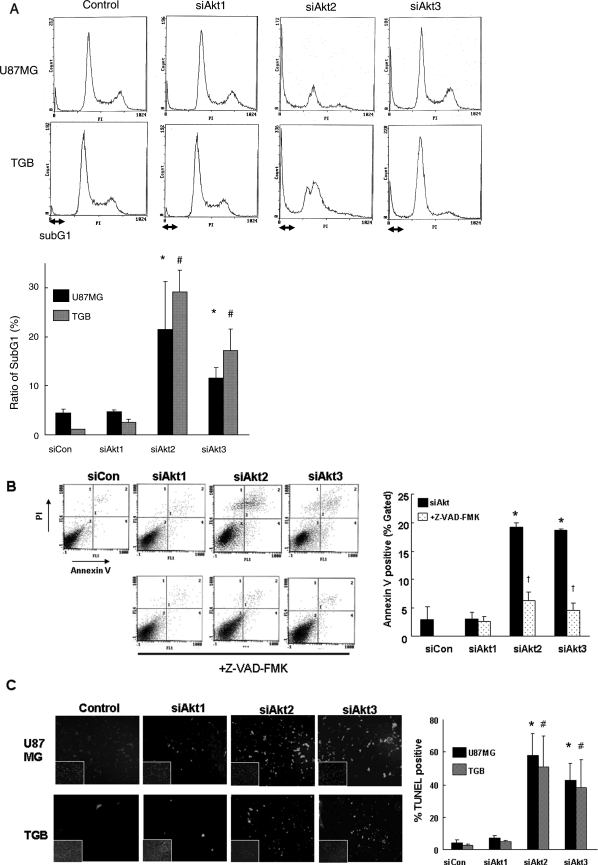
To examine the molecular mechanisms underlying the cell growth inhibition induced by Akt2 or Akt3 knock-down, we analyzed the cell-cycle profiles of U87MG, T98G, and TGB cells flow cytometrically 72 hours after their exposure to siAkt2 or siAkt3. We found that the subG1 population was significantly increased in U87MG and TGB cells treated with siAkt2 or siAkt3 compared with those treated with siAkt1 and siCon. There was no significant difference in the G1 or G2M population (Fig. 4A). Apoptotic cell death was assessed by both Annexin V/PI and TUNEL staining. No significant apoptosis (<10%) was observed in cells transfected with siAkt1 or siCon (Fig. 4B and C). However, when cells were transfected with siAkt2 or siAkt3, a significant increase (20%–60%) in Annexin V/PI and TUNEL positivity was observed (Fig. 4B and C). Similar results were obtained in T98G cells (data not shown). Furthermore, treatment with Z-VAD-FMK reversed the effect of Akt2 or Akt3 knock-down as indicated by Annexin V assay (Fig. 4B). The mean percent of apoptotic cells in siAkt2 or siAkt3 transfectants was 19.7 ± 0.7% and 18.4 ± 0.3%, respectively; in Z-VAD-FMK-treated cells, it was 6.2 ± 1.5% and 4.5 ± 1.3%. Similar results were obtained in U87MG cells (data not shown). These observations showed that Z-VAD-FMK had a major effect on Annexin V binding to apoptotic TGB and U87MG cells and that Akt2 or Akt3 knock-down induced caspase-dependent apoptosis in malignant glioma cells.
Fig. 4.
Induction of apoptosis by Akt2 and Akt3 knock-down. (A) Flow cytometry. At 72 hours after the introduction of siAkt2 or siAkt3 or siCont into U87MG and TGB cells, the cell-cycle profile was analyzed by flow cytometry. All experiments were performed in triplicate. The ratio of subG1 cells was significantly higher in U87MG cells treated with siAkt2 or siAkt3 than those treated with negative control siRNA. *P < .01 vs siControl or siAkt1 in U87MG and #P < .01 vs siControl or siAkt1 in TGB. (B) Annexin V/PI assay. TGB cells were transfected with each Akt isoform, specific siRNA, and negative control, collected 12 hours later, stained with Annexin V/PI, and analyzed by flow cytometry. In siAkt2- or siAkt3-transfected cells, there was a 5-fold increase in the Annexin V positive rate vs the control and siAkt1-treated cells. Z-VAD-FMK and FBS were added 4 hours after transfection. Z-VAD-FMK treatment reversed the effect of siAkt2 or siAkt3 as indicated by Annexin V assay. *P < .01 vs siControl or siAkt1 and †P < .01 vs siAkt2 or siAkt3. (C) Representative TUNEL staining. TUNEL positivity was analyzed 72 hours after the introduction of siAkt2, siAkt3, or siCon into U87MG and TGB cells. All experiments were performed in triplicate. The ratio of TUNEL-positive (green) to DAPI-positive (blue) cells was higher in cells treated with siAkt2 or siAkt3 than those treated with negative-control siRNA. *P < .01 vs siControl in U87MG cells and #P < .01 vs siControl in TGB cells.
Knock-down of Akt2 and Akt3 Activates Caspase-9 and Caspase-3
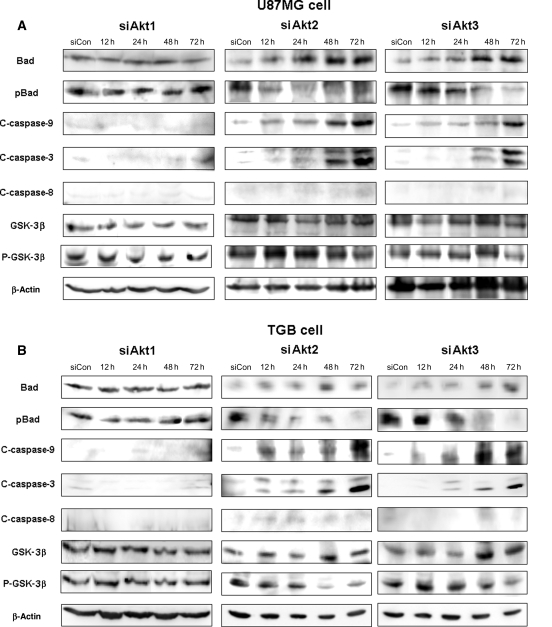
To further clarify the molecular mechanisms underlying the proapoptotic effect of siAkt, we assessed several apoptosis-related proteins in U87MG and TGB cells. At 12–72 hours post-transfection with siAkt2 and siAkt3, respectively, pBad was decreased and cleaved caspase-9 and caspase-3 were increased in a time-dependent manner (Fig. 5A and B). However, the expression of cleaved caspase-8 was not affected by siAkt2 or siAkt3. The pGSK-3β was reduced at 48–72 hours post-transfection with siAkt2 or siAkt3 after the increase in cleaved caspase-9 and caspase-3. In siAkt1-treated cells, there was no dephosphorylation of BAD, nor was there an increase in cleaved caspase-8, caspase-9, and caspase-3 (Fig. 5A and B). These results indicate that Akt2 and Akt3 knock-down activated the intrinsic apoptotic pathway mediated by mitochondria.
Fig. 5.
Akt2 and Akt3 knock-down activates caspase-9 and -3. Western blots of U87MG cells treated from 12 to 72 hours with siRNA targeting Akt1, Akt2, Akt3, or nontargeting control (siCon). In cells treated with siAkt2 or siAkt3 but not with siAkt1, the pBad was decreased and the level of cleaved caspase-9 and -3 was increased in a time-dependent manner. The expression of cleaved caspase-8 was not affected by siAkt2 or siAkt3.
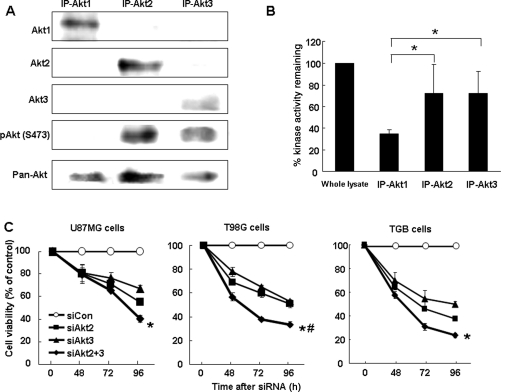
Akt3 Manifests High Kinase Activity in U87MG Cells
Our observations raised the question of why the knock-down of endogenous Akt3 inhibited the growth of glioma cells in view of the low expression level of Akt3 in malignant glioma. To evaluate the functional contribution of Akt3 in U87MG cells, we performed Akt isoform-specific immunoprecipitation and Akt kinase activity assays. As shown in Fig. 6A, the level of Akt phosphorylation at serine-473 was similar in Akt3 and Akt2 immunoprecipitated protein. pAkt was undetectable in Akt1 IP. In the IP-Akt3 eluent, Akt kinase activity was high and equal to the kinase activity in the IP-Akt2 eluent (Fig. 6B). On the basis of the high phosphorylation level and the high Akt kinase activity in IP-Akt3 lysates of U87MG cells, we posit that Akt3 has a highly functional role even if it is down-regulated and its level is low in glioma tissues.
Fig. 6.
Phosphorylation status and kinase activity of Akt isoforms. (A) Akt isoform-specific IP. Lysates from U87MG cells were immunoprecipitated with antibodies against each Akt isoform. Resultant IP were subjected to Western blot analysis with antibodies with the pAkt at serine-473. The pAkt was detected at similar levels in IP-Akt3 and IP-Akt2. The pAkt was not detected in IP-Akt1. (B) Akt kinase activity. IP with antibodies against each Akt isoform were monitored for kinase activity. The kinase activity of Akt3 was equal to that of Akt2. Akt2 and Akt3 kinase activity was much higher than that of Akt1. All experiments were performed in triplicate. *P < .05 vs IP-Akt1. (C) Cell viability after double knock-down of Akt2 and Akt3. At 48–96 hours after the introduction of either siCon, siAkt2, or siAkt3 alone or with siAkt2, and siAkt3 into U87MG, T98G, and TGB cells, cell viability was analyzed. The combination of siAkt2 and siAkt3 enhanced cell death 30%–40% compared with death observed in Akt2 or Akt3 single knock-down cells. *P < .05 vs siAkt3 alone and #P < .05 vs siAkt2 alone.
As our results suggested that both Akt2 and Akt3 play a pivotal role in the growth of malignant glioma cells, we subjected U87MG, T98G, and TGB cells to Akt2 and Akt3 double knock-down. In combination, siAkt2 and siAkt3 enhanced cell death by 30%–40% compared with cells subjected to single Akt2 or Akt3 knock-down (Fig. 6C).
Discussion
We showed that the protein and mRNA expression of Akt2 was higher and that of Akt3 was lower in malignant glioma than normal and NNR tissue. Akt3 was down-regulated in malignant glioma cell lines over-expressing Akt2. This phenomenon may reflect the expression pattern of Akt isoforms in glioma tissue. In the glioma cell lines exposed to Akt2 or Akt3 knock-down, we observed the induction of apoptosis mediated by mitochondrial apoptosis via the dephosphorylation of BAD and the activation of caspase-9 and caspase-3. Although the expression of Akt3 was low in malignant glioma cells, the remaining kinase activity was high. Based on these observations, we posit that Akt2 and Akt3 double knock-down may effectively inhibit the growth of malignant glioma cells.
In cancer patients, several components and regulators of the PI3K/Akt pathway manifest a higher rate of amplification, mutation, and translocation than other pathways.9,21–24 Furthermore, in GBM, Akt is formed from genetically modified neural progenitors and NHA, suggesting that the activation of Akt plays an important role in the formation and progression of glioma.11,12,25 These observations suggest Akt as an attractive target in the treatment of cancers including malignant glioma.
In some types of cancer, the Akt1 protein level is elevated despite the rare amplification of the gene.21 It has been suggested that Akt1 plays a dual role in tumorigenesis; it acts not only pro-oncogenically by suppressing apoptosis but also exerts antioncogenic activity via the suppression of cell invasion and metastasis.16,26–28 Akt2 is amplified and over-expressed in ovarian, breast, and pancreatic cancer, and Akt3 is over-expressed in these cancers and in malignant melanoma.29–36 We found that Akt1 was expressed at similar levels in NNR and glioma tissues and that Akt2 was highly expressed in high-grade gliomas (WHO grade III or IV) and glioma cell lines (Fig. 1). In contrast, the expression of Akt3 was decreased as the WHO brain tumor grade increased (Fig. 1). In addition, Akt2 and Akt3, but not Akt1, were activated and exhibited kinase activity (Fig. 6). The Akt2 expression pattern we identified was similar to that reported in other studies.29–31 However, the reduction in Akt3 in human cancer has not been reported previously. In neuronal and glial cells from NNR tissues, Akt3 protein was expressed at comparable levels (Fig. 1C), suggesting that the decrease in Akt3 in malignant gliomas is not attributable to a reduction in neurocytes with aberrant glial cell proliferation. Strikingly, in established glioma cell lines (U87MG and T98G) and TGB cells from a primary GBM over-expressing Akt2, the Akt3 protein level was decreased compared with mock-transfected cells (Fig. 3B). Our findings support the hypothesis of Taniyama et al.37 that Akt protein is affected by the negative feedback regulation of Akt signaling. They reported that in mice, endogenous Akt1 and Akt2 protein and phosphorylation levels were down-regulated in Akt3 over-expressed hearts. Thus, the low-expression of Akt3 in malignant glioma tissues may be attributable to the over-whelming expression of Akt2.
Whereas Akt3 was down-regulated in malignant glioma cells, it was activated and exhibited high kinase activity in these cells (Fig. 6). This suggests that although the level of endogenous Akt3 was reduced, it retained the functional capacity as an oncoprotein and that its down-regulation may inhibit the growth of malignant glioma cells. Moreover, the knock-down of Akt2 and of Akt3 resulted in stronger cell growth inhibition than exposure to siAkt2 or siAkt3 alone, indicating that the co-introduction of siAkt2 and siAkt3 inhibited cell growth in an additive manner. Our results suggest that Akt2 and Akt3 function redundantly in the regulation of cell survival.
Tissue microarray analysis of biopsies from glioblastoma patients showed that the loss of PTEN was inversely correlated with Akt activation.38 PTEN is expressed in T98G but not in U87MG or TGB cells (Fig. 2A). Akt2 and/or Akt3 knock-down inhibited the growth of U87MG, T98G, and TGB cells (Figs 3A and 6C). Bad was dephosphorylated by siAkt2 and siAkt3, but not by siAkt1. We observed similar cell-growth-inhibiting effects in U87MG, T98G, and TGB cells. Therefore, Akt2 and/or Akt3 knock-down may effectively inhibit the growth of both PTEN-harboring and PTEN-deficient malignant glioma cells.
The inhibition of Akt activation can be achieved by inhibiting PI3K with inhibitors such as LY294002 and wortmannin.39–42 However, the inhibition of PI3K indiscriminately affects not only all 3 Akt isoforms but also other PH domain-containing signaling molecules that depend on phosphatidylinositol-3,4,5-trisphosphate lipids. In cancer, Akt isoforms have been shown to perform specific functions. Therefore, isoform-specific inhibitors may be necessary to achieve optimal therapeutic efficacy at acceptable levels of toxicity. Experiments are underway in our laboratory to determine whether siAkt2 or siAkt3 is the best in vivo therapeutic agent. To identify the mechanisms underlying Akt2 up-regulation, further studies are required.
Our study first demonstrates that Akt2 and Akt3 play a pivotal role in the biology of human glioblastoma. The expression status of Akt2 and Akt3 may hold promise as a candidate for molecular target therapy and as biological markers to predict the treatment outcome in patients with malignant glioma.
Funding
This work was supported in part by the Taiho Cancer Research Foundation to the University of Tokushima Graduate School.
Acknowledgments
This study was a collaboration between Taiho Co. Ltd and The University of Tokushima Graduate School, Japan. We are indebted to Dr Y. Sugimoto and Dr K. Kitazato for useful advice and to Mrs Rika Okabe for technical assistance.
Conflict of interest statement. None declared.
References
- 1.Davis FG, McCarthy BJ. Current epidemiological trends and surveillance issues in brain tumors. Expert Rev Anticancer Ther. 2001;1:395–401. doi: 10.1586/14737140.1.3.395. [DOI] [PubMed] [Google Scholar]
- 2.Schwechheimer K, Huang S, Cavenee WK. EGFR gene amplification—rearrangement in human glioblastomas. Int J Cancer. 1995;62:145–148. doi: 10.1002/ijc.2910620206. [DOI] [PubMed] [Google Scholar]
- 3.Omuro AM, Faivre S, Raymond E. Lessons learned in the development of targeted therapy for malignant gliomas. Mol Cancer Ther. 2007;6:1909–1919. doi: 10.1158/1535-7163.MCT-07-0047. [DOI] [PubMed] [Google Scholar]
- 4.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 5.Holland EC. Glioblastoma multiforme: the terminator. Proc Natl Acad Sci USA. 2000;97:6242–6244. doi: 10.1073/pnas.97.12.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maher EA, Furnari FB, Bachoo RM, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 7.Wechsler-Reya R, Scott MP. The developmental biology of brain tumors. Annu Rev Neurosci. 2001;24:385–428. doi: 10.1146/annurev.neuro.24.1.385. [DOI] [PubMed] [Google Scholar]
- 8.Sun M, Wang G, Paciga JE, et al. AKT1/PKB alpha kinase is frequently elevated in human cancers and its constitutive activation is required for oncogenic transformation in NIH3T3 cells. Am J Pathol. 2001;159:431–437. doi: 10.1016/s0002-9440(10)61714-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng JQ, Lindsley CW, Cheng GZ, et al. The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene. 2005;24:7482–7492. doi: 10.1038/sj.onc.1209088. [DOI] [PubMed] [Google Scholar]
- 10.Ahn JY, Hu Y, Kroll TG, et al. PIKE-A is amplified in human cancers and prevents apoptosis by up-regulating Akt. Proc Natl Acad Sci USA. 2004;101:6993–6998. doi: 10.1073/pnas.0400921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas-Kogan D, Shalev N, Wong M, et al. Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr Biol. 1998;8:1195–1198. doi: 10.1016/s0960-9822(07)00493-9. [DOI] [PubMed] [Google Scholar]
- 12.Holland EC, Celestino J, Dai C, et al. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25:55–57. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- 13.Rajasekhar VK, Viale A, Socci ND, et al. Oncogenic Ras and Akt signaling contribute to glioblastoma formation by differential recruitment of existing mRNAs to polysomes. Mol Cell. 2003;12:889–901. doi: 10.1016/s1097-2765(03)00395-2. [DOI] [PubMed] [Google Scholar]
- 14.Cho H, Mu J, Kim JK, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 15.Cho H, Thorvaldsen JL, Chu Q, et al. Akt1/PKB alpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 16.Irie HY, Pearline RV, Grueneberg D, et al. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol. 2005;171:1023–1034. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoeli-Lerner M, Yiu GK, Rabinovitz I, et al. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol Cell. 2005;20:539–550. doi: 10.1016/j.molcel.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 18.McKenna LB, Zhou GL, Field J. Isoform-specific functions of Akt in cell motility. Cell Mol Life Sci. 2007;64:2723–2725. doi: 10.1007/s00018-007-7247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizobuchi Y, Matsuzaki K, Kuwayama K, et al. REIC/Dkk-3 induces cell death in human malignant glioma. Neuro-Oncology. 2008;10:244–253. doi: 10.1215/15228517-2008-016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuwayama K, Matsuzaki K, Mizobuchi Y, et al. Promyelocytic leukemia protein induces apoptosis due to caspase-8 activation via the repression of NF{kappa}B activation in glioblastoma. Neuro-Oncology. 2009;11:132–141. doi: 10.1215/15228517-2008-083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 23.Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci USA. 2005;102:802–807. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Testa JR, Bellacosa A. AKT plays a central role in tumorigenesis. Proc Natl Acad Sci USA. 2001;98:10983–10985. doi: 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonoda Y, Ozawa T, Aldape KD, et al. Akt pathway activation converts anaplastic astrocytoma to glioblastoma multiforme in a human astrocyte model of glioma. Cancer Res. 2001;61:6674–6678. [PubMed] [Google Scholar]
- 26.Reif K, Lucas S, Cantrell D. A negative role for phosphoinositide 3-kinase in T-cell antigen receptor function. Curr Biol. 1997;7:285–293. doi: 10.1016/s0960-9822(06)00151-5. [DOI] [PubMed] [Google Scholar]
- 27.Jauliac S, Lopez-Rodriguez C, Shaw LM, et al. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat Cell Biol. 2002;4:540–544. doi: 10.1038/ncb816. [DOI] [PubMed] [Google Scholar]
- 28.Toker A, Yoeli-Lerner M. Akt signaling and cancer: surviving but not moving on. Cancer Res. 2006;66:3963–3966. doi: 10.1158/0008-5472.CAN-06-0743. [DOI] [PubMed] [Google Scholar]
- 29.Bellacosa A, de Feo D, Godwin AK, et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995;64:280–285. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- 30.Cheng JQ, Ruggeri B, Klein WM, et al. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci USA. 1996;93:3636–3641. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miwa W, Yasuda J, Murakami Y, et al. Isolation of DNA sequences amplified at chromosome 19q13.1–q13.2 including the AKT2 locus in human pancreatic cancer. Biochem Biophys Res Commun. 1996;225:968–974. doi: 10.1006/bbrc.1996.1280. [DOI] [PubMed] [Google Scholar]
- 32.Nakatani K, Thompson DA, Barthel A, et al. Up-regulation of Akt3 in estrogen receptor-deficient breast cancers and androgen-independent prostate cancer lines. J Biol Chem. 1999;274:21528–21532. doi: 10.1074/jbc.274.31.21528. [DOI] [PubMed] [Google Scholar]
- 33.Ruggeri BA, Huang L, Wood M, et al. Amplification and over expression of the AKT2 oncogene in a subset of human pancreatic ductal adenocarcinomas. Mol Carcinog. 1998;21:81–86. [PubMed] [Google Scholar]
- 34.Stahl JM, Sharma A, Cheung M, et al. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–7010. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- 35.Cristiano BE, Chan JC, Hannan KM, et al. A specific role for AKT3 in the genesis of ovarian cancer through modulation of G(2)-M phase transition. Cancer Res. 2006;66:11718–11725. doi: 10.1158/0008-5472.CAN-06-1968. [DOI] [PubMed] [Google Scholar]
- 36.Arboleda MJ, Lyons JF, Kabbinavar FF, et al. Overexpression of AKT2/protein kinase B beta leads to up-regulation of beta-1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 2003;63:196–206. [PubMed] [Google Scholar]
- 37.Taniyama Y, Ito M, Sato K, et al. Akt3 over expression in the heart results in progression from adaptive to maladaptive hypertrophy. J Mol Cell Cardiol. 2005;38:375–385. doi: 10.1016/j.yjmcc.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Choe G, Horvath S, Cloughesy TF, et al. Analysis of the phosphatidylinositol 3′-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63:2742–2746. [PubMed] [Google Scholar]
- 39.Rosenzweig KE, Youmell MB, Palayoor ST, et al. Radiosensitization of human tumor cells by the phosphatidylinositol3-kinase inhibitors wortmannin and LY294002 correlates with inhibition of DNA-dependent protein kinase and prolonged G2-M delay. Clin Cancer Res. 1997;3:1149–1156. [PubMed] [Google Scholar]
- 40.Ng SS, Tsao MS, Nicklee T, et al. Wortmannin inhibits pkb/akt phosphorylation and promotes gemcitabine antitumor activity in orthotopic human pancreatic cancer xenografts in immunodeficient mice. Clin Cancer Res. 2001;7:3269–3275. [PubMed] [Google Scholar]
- 41.Hu L, Zaloudek C, Mills GB, et al. In vivo and in vitro ovarian carcinoma growth inhibition by a phosphatidylinositol 3-kinase inhibitor (LY294002) Clin Cancer Res. 2000;6:880–886. [PubMed] [Google Scholar]
- 42.Newton HB. Molecular neuro-oncology and development of targeted therapeutic strategies for brain tumors. Part 2: PI3K/Akt/PTEN, mTOR, SHH/PTCH and angiogenesis. Expert Rev Anticancer Ther. 2004;4:105–128. doi: 10.1586/14737140.4.1.105. [DOI] [PubMed] [Google Scholar]