Abstract
We assessed six-month progression-free survival (PFS) as an alternative primary efficacy endpoint to overall survival in newly diagnosed glioblastoma multiforme (GBM) patients receiving temozolomide (TMZ). A total of 183 patients with newly diagnosed GBM enrolled in 3 phase II protocols at the University of California–San Francisco were included. Patients were treated with interventions based on the Stupp regimen, each with the added component of a second oral agent given concurrently with radiotherapy and TMZ, followed by its coadministration with adjuvant TMZ. We examined whether progression status at 2, 4, and 6 months predicted subsequent survival using the landmark analysis. The hazard ratios of death as a function of progression status were estimated based on the Cox proportional hazards model after adjustment for putative prognostic factors. Progression status at 2, 4, and 6 months were all consistently found to be strong predictors of subsequent survival in all studies. The study-specific hazard ratios associated with progression status at 6 months ranged from 2.03 to 3.39. The hazard ratios associated with the earlier time points (2- and 4-month progression) all exceeded 2 in magnitude, ranging from 2.29 to 4.73. P-values were statistically significant for all time points. In this report, we demonstrated a strong association between the endpoints of PFS at 2, 4, and 6 months and survival. Patients who showed the signs of early progression were at significantly higher risk of earlier death. Our analysis suggests that 6-month PFS may be an appropriate primary endpoint in the context of phase II upfront GBM trials in the TMZ era.
Keywords: brain tumors, clinical trial endpoints, glioma, progression-free survival, temozolomide
Glioblastoma multiforme (GBM) represents the most aggressive form of primary malignant brain tumor. Despite encouraging results from the recent ground-breaking EORTC/NCIC trial by Stupp et al.,1 the typical median survival among patients with newly diagnosed GBM is 14.6 months with an estimated 2-year survival of 27%. Stupp's trial has changed the standard of care of these patients to include the combination of radiation therapy and temozolomide (TMZ) followed by adjuvant TMZ. However, the long-term disease outlook for these patients remains poor. Ultimately, most patients succumb to their cancer, irrespective of treatment intervention. Research on therapeutic approaches in recent years has been based on molecularly targeted strategies. While offering further hope to brain tumor patients, the results of these trials vary widely and none has become a new standard of care. With the increasing number of new molecularly targeted agents, it is imperative to develop more efficient clinical trials to evaluate these treatment strategies.
Phase II trials play an important role in the development process of oncologic drugs by serving as screens to select the most promising single agents or combinations of agents to be further tested in a phase III setting. Properly designed and executed, phase II trials can substantially increase the efficiency of drug discovery programs and reduce the costs associated with candidate drugs that offer little clinical benefit. Selecting appropriate endpoints is one key ingredient to the success of a clinical trial. A clinically meaningful timely endpoint allows the investigator to make decisions about the treatment efficacy efficiently and accurately. Historically, progression-free survival (PFS) and overall survival (OS) are the most commonly used primary endpoints in phase II neuro-oncology trials. In the University of California–San Francisco (UCSF) neuro-oncology program, we have routinely used OS as the primary endpoint to evaluate treatment efficacy in trials enrolling newly diagnosed GBM patients.
OS has the advantage of being the most objective measurement of efficacy. It is also easy to ascertain and offers a straightforward interpretation. However, the use of OS as the primary endpoint for neuro-oncology trials has several major limitations. For instance, since mortality generally takes longer to observe, a trial that uses OS as the primary endpoint requires a longer trial duration for a given number of patients, relative to other endpoints, to detect a statistically meaningful difference. Furthermore, the effect of the initial treatment on OS is likely to be diluted by the administration of subsequent anticancer treatments after patients go off the study in question, typically due to disease progression, toxicity, or completion of the trial treatment. This is a more pertinent issue now than in the past due to the increasing number of potentially effective salvage treatment options available for brain cancer patients at recurrence.
There is currently a paucity of literature addressing the relationship between PFS and OS in phase II trials of newly diagnosed GBM that takes into proper consideration the shift in the standard of care since publication of the above-mentioned trial by Stupp et al. In particular, few studies to date have included sufficient numbers of patients from trials based on the Stupp protocol, likely due to its relatively recent publication. In this study, we examined data from 3 phase II trials conducted at our institution in newly diagnosed GBM or gliosarcoma (GS) patients. In each trial, patients were treated with interventions based on the Stupp regimen, each with the added component of a second oral agent given concurrently with RT and TMZ, followed by its coadministration with TMZ in the adjuvant setting. Our aim was to evaluate PFS as a potential alternative primary endpoint to OS for neuro-oncology trials evaluating therapeutic effects in newly diagnosed GBM patients.
Materials and Methods
Patient Population
The primary goal of this study was to evaluate whether progression status was predictive of OS. This study was approved by the UCSF Committee on Human Research (CHR). Adult patients with newly diagnosed glioblastoma or GS who were enrolled between January 2000 and April 2007 in 1 of 3 single-arm open-label phase II protocols at UCSF were included in this analysis.2–4 Entry criteria across all protocols were similar and included KPS ≥60 and an estimated survival time of >8 weeks. All protocols mandated pathology review by an institutional pathologist. No prior anticancer treatment was allowed other than surgery. All patients were intended to have started radiotherapy (RT) within 5 weeks after surgery. Treatment plans in all protocols included conventional RT and TMZ plus an additional oral chemotherapy agent. After a 2-week break upon completion of RT, the added oral agent was to be coadministered with TMZ for at least 1 year, unless disease progression or unacceptable toxicity occurred.
Trial Endpoints
Patients were evaluated for disease progression, survival, and toxicity in all protocols. The primary endpoint for all trials was OS, defined as the time from histologic diagnosis until death due to any cause. Patients not known to have died were censored for survival as of the last date known to be alive. It is noteworthy that in Stupp's trial, although not explicitly stated, the clinical endpoints including OS and PFS were measured from the time of randomization, presumably soon before the start of RT. A median wait time of 5 weeks between surgical diagnosis and the start of radiation was indicated in Table 1 of their report. Hence, to ensure the consistency of our results with Stupp's trial and to more directly assess the effect of the chemoradiation regimen on disease prognosis, adjustment is made in our analysis to measure survival from the time of study registration, which was normally within 1 week prior to the initiation of RT.
Table 1.
Treatment plan for three studies included in the analysis
| Protocol | Number of patients | RT dose and schedule | Concurrent and adjuvant chemotherapy | Reference | Enrollment period |
|---|---|---|---|---|---|
| TTRT | 67 | 60 Gy at 2.0 Gy/d × 5 d × 6 wk | Temozolomide + thalidomide | Chang et al.2 | January 2000–April 2001 |
| RTRT | 61 | 60 Gy at 2.0 Gy/d × 5 d × 6 wk | Temozolomide + cis-retinoic acid (cRA) | Butowski et al.3 | April 2001–May 2002 |
| OTRT | 65 | 59.4–61 Gy at 1.8–2.0 Gy/d × 5 d × 6.5 wk | Temozolomide + erlotinib | Prados et al.4 | October 2004–April 2007 |
Similarly, PFS was defined in this analysis as time from study registration to the first event of disease progression or death. Patients not known to have progressed or died were censored for PFS as of the last date known to be progression-free and alive. Progression was determined by the local institutional investigator as per standard Macdonald criteria.5 Specifically, this was defined as a 25% increase in the sum of products of all measurable lesions (bidimensionally measurable lesions with clearly defined margins) over the smallest sum previously observed (over baseline if no decrease in tumor size), clear worsening of any evaluable disease (unidimensionally measurable lesions with margins not clearly defined), appearance of any new lesion, clear clinical worsening, or failure to return for evaluation due to death or deteriorating condition. Objective radiographic response was a secondary endpoint in two of the protocols. Evaluation of radiographic responses was also based on the standard Macdonald criteria as described previously, taking into account steroid dosing, with assessments performed after RT and every 8 weeks thereafter.
Statistical Methods
Summary statistics are provided as medians and ranges for continuous variables and frequencies and percentages for categorical variables. The Kaplan–Meier method was used to estimate PFS and survival. To allow for variability in the timing of MRI scans due to scheduling, progression status was analyzed based on 10, 18, and 26 weeks. We examined the primary question of whether progression status at these 3 time points, which are approximately 2, 4, and 6 months, predicted subsequent survival using the landmark analysis approach.6–8 Specifically, all patients alive with known progression status at the specified time point were included in the respective analysis. Survival was counted from that specific time point. Survival experience was compared between those deemed as having progressed by that time vs those who had not, again using the Kaplan–Meier method. The Cox proportional hazards (PH) model was used to adjust for known prognostic markers including age, KPS, and extent of resection. To examine whether the pattern of association between PFS and OS was consistent across protocols, we performed the Cox PH analysis separately for each trial. Analysis was also performed based on combined data across three studies using the Cox PH model, adjusting for treatment protocol, in addition to the other covariates. All P-values are 2-sided.
Results
Patient Characteristics
One hundred ninety-three patients with newly diagnosed GBM or GS (only 1 patient had GS) were included in this study. Table 1 summarizes the respective treatment plan, number of patients enrolled, and the enrollment period for all trials included in this analysis. Table 2 summarizes the patient characteristics with aggregated data across 3 trials. Median age was 54 years (range: 22–77). Sixty percent of the patients were male. Median KPS was 90 (range: 60–100). Sixteen percent of the patients had biopsy only prior to starting protocol treatment, 52% had subtotal resection, and the remaining 32% underwent gross total resection. The median time from initial definitive surgery to study registration was 4.1 weeks (range: 1.1–6.1 weeks).
Table 2.
Patient characteristics
| Characteristics | No. patients (%) |
|---|---|
| Sex | |
| Male | 115 (60) |
| Female | 78 (40) |
| Race | |
| White | 164 (85) |
| Other | 29 (15) |
| Age (yrs) | |
| Median | 54 |
| Range | 22–77 |
| KPS | |
| 60 | 5 (3) |
| 70 | 12 (6) |
| 80 | 48 (25) |
| 90 | 113 (59) |
| 100 | 15 (8) |
| Median | 90 |
| Range | 60–100 |
| Extent of Resection | |
| Biopsy | 32 (16) |
| Subtotal resection | 99 (52) |
| Gross total resection | 61 (32) |
| Time from diagnosis to enrollment (wks) | |
| Median | 4.1 |
| Range | 1.1–6.1 |
Progression-free and Overall Survival
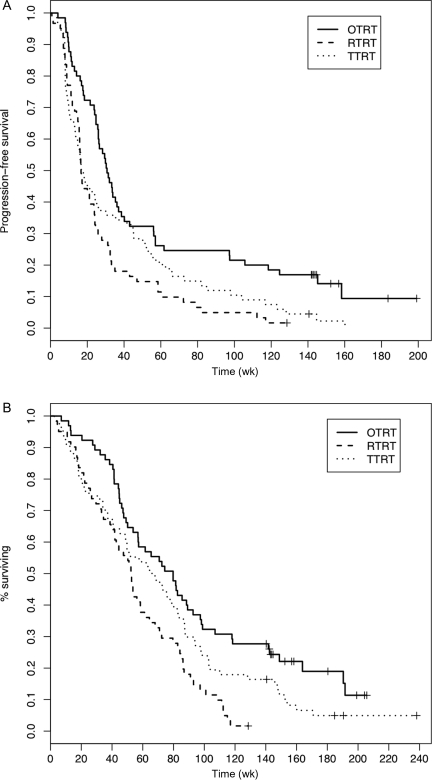
Table 3 presents the estimated PFS and OS at specified time points separately for each study. Figure 1A and B presents the Kaplan–Meier curves for PFS and survival by study protocol. PFS and OS were both measured from the time of enrollment into the trial. At the time of the analysis, no patient had been removed from study for reasons other than toxicities, progression, or death, and all patients who remained on treatment had been followed over 128 weeks. Two patients enrolled in the OTRT trial were still receiving protocol treatment (for 141 and 178 weeks, respectively). One patient each in TTRT and RTRT was censored for PFS at 141 and 129 weeks, respectively, whereas a total of 9 patients were censored in OTRT with a median follow-up time of 145 weeks (range: 142–199). In terms of OS, 1 patient in RTRT was censored at week 129, 4 patients were censored in TTRT with a median follow-up of 188 weeks (range: 141–238), and 12 patients were censored in OTRT with a median follow-up of 155 weeks (range: 142–206). The latest study, OTRT, which combined the use of TMZ with erlotinib, an orally active selective inhibitor of EGFR tyrosine kinase, during and following RT, was the only trial that successfully demonstrated prolonged survival when compared with its historical control, which consisted of TTRT and RTRT. In particular, this treatment combination was associated with the highest estimated PFS and OS at each time point we investigated with median PFS and OS of 31 and 80 weeks, respectively. The estimated median PFS was 17 weeks for both TTRT and RTRT. The median OS was 66 weeks for TTRT and 52 weeks for RTRT.
Table 3.
Patient outcome
| Outcome | TTRT | RTRT | OTRT |
|---|---|---|---|
| Progression-free survivala | |||
| No. patients (no. censored) | 67 (1) | 61 (1) | 65 (9) |
| % PFS (95% CI)b | |||
| 10 wks | 69 (58–81) | 77 (67–88) | 88 (80–96) |
| 18 wks | 48 (37–61) | 44 (33–59) | 74 (64–85) |
| 26 wks | 37 (27–51) | 30 (20–44) | 60 (49–73) |
| Median PFS in wks (95% CI) | 17 (14–30) | 17 (16–24) | 31 (26–39) |
| Overall survivala | |||
| No. patients (no. censored) | 67 (4) | 61 (1) | 65 (12) |
| % surviving (95% CI)b | |||
| 26 wks | 75 (65–86) | 75 (65–87) | 92 (86–99) |
| 52 wks | 55 (45–69) | 52 (41–67) | 65 (54–77) |
| 78 wks | 42 (32–55) | 30 (20–44) | 51 (40–65) |
| Median survival in wks (95% CI) | 66 (49–84) | 52 (42–64) | 80 (57–97) |
Abbreviation: PFS, progression-free survival.
The last three column headings represent the names of the three protocols included in the analysis.
aMeasured from the time of study registration.
bEstimated from the Kaplan–Meier curves.
Fig. 1.
Kaplan–Meier curves for (A) PFS and (B) OS by protocol.
Landmark Analysis
Table 4 presents the results of the landmark analyses characterizing survival as a function of progression status at 10, 18, and 26 weeks, both by study and with combined data. For each specified time point, the analysis only included patients whose progression status was known and who were known to be alive at that time. The number of patients who died before each specified time point is provided. OS was measured from that time. The status of survivorship and progression was unambiguous for all patients at each time point, that is, there was no undeterminable status due to loss of follow-up. For example, in the combined analysis of 6-month (26-week) progression status, 37 patients had died before 6 months and hence were excluded from that landmark analysis. Among the remaining patients, those known to be alive at 6 months after protocol registration, survival from that time onward was compared between the 74 patients who were known to have progressed vs the 82 patients who were known to be free of progression.
Table 4.
Survival as a function of progression status
| Study | Status at 10 wks |
Status at 18 wks |
Status at 26 wks |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Died | PD | Not PD | Died | PD | Not PD | Died | PD | Not PD | |
| TTRT | |||||||||
| No. patients | 6 | 15 | 46 | 11 | 24 | 32 | 17 | 25 | 25 |
| No. censored for survival | — | 0 | 4 | — | 2 | 2 | — | 2 | 2 |
| Median survival (wks) | — | 30 (8–61) | 75 (63–93) | — | 33 (22–61) | 69 (62–110) | — | 32 (22–61) | 73 (60–123) |
| % alive at 26 wks | — | 60 (40–91) | 83 (72–94) | — | 58 (42–82) | 91 (81–100) | — | 56 (40–79) | 96 (89–100) |
| % alive at 52 wks | — | 27 (12–62) | 70 (57–84) | — | 38 (22–63) | 72 (58–89) | — | 36 (21–61) | 76 (61–95) |
| P value | .0005 | .02 | .003 | ||||||
| HR (95% CI) | 3.52 (1.73–7.16) | 2.52 (1.15–5.53) | 3.39 (1.53–7.51) | ||||||
| RTRT | |||||||||
| No. patients | 3 | 11 | 47 | 9 | 25 | 27 | 15 | 28 | 18 |
| No. censored for survival | — | 0 | 1 | — | 0 | 1 | — | 0 | 1 |
| Median survival (wks) | — | 12 (10, inf) | 49 (42–74) | — | 32 (21–41) | 54 (40–75) | — | 27 (21–54) | 59 (38–86) |
| % alive at 26 wks | — | 46 (24–87) | 77 (65–90) | — | 52 (36–76) | 85 (73–99) | — | 57 (41–79) | 89 (75–100) |
| % alive at 52 wks | — | NA | 47 (35–64) | — | 20 (9–44) | 56 (40–78) | — | 29 (16–51) | 56 (37–84) |
| P value | 0.004 | 0.02 | 0.02 | ||||||
| HR (95% CI) | 3.16 (1.46–6.85) | 2.29 (1.15–4.54) | 2.14 (1.10–4.15) | ||||||
| OTRT | |||||||||
| No. patients | 1 | 7 | 57 | 4 | 13 | 48 | 5 | 21 | 39 |
| No. censored for survival | — | 0 | 12 | — | 1 | 11 | — | 2 | 10 |
| Median survival (wks) | — | 31 (19, inf) | 73 (61–97) | — | 29 (23, inf) | 71 (62–100) | — | 28 (18–73) | 67 (54–138) |
| % alive at 26 wks | — | 71 (45–100) | 90 (82–98) | — | 62 (40–95) | 90 (81–99) | — | 52 (35–79) | 80 (68–93) |
| % alive at 52 wks | — | NA | 65 (54–79) | — | 31 (14–70) | 67 (55–81) | — | 33 (18–61) | 67 (53–83) |
| P value | 0.001 | 0.02 | — | 0.02 | |||||
| HR (95% CI) | 4.73 (1.87–11.99) | 2.45 (1.18–5.08) | — | 2.03 (1.11–3.72) | |||||
| Combined | |||||||||
| No. patients | 10 | 33 | 150 | 24 | 62 | 107 | 37 | 74 | 82 |
| No. censored for survival | — | 0 | 17 | — | 3 | 14 | — | 4 | 13 |
| Median survival (wks) | — | 30 (12–38) | 70 (58–76) | — | 32 (24–39) | 68 (62–79) | — | 27 (23–42) | 66 (60–84) |
| % alive at 26 wks | — | 58 (43–77) | 83 (78–90) | — | 56 (45–70) | 89 (83–95) | — | 54 (44–67) | 87 (80–94) |
| % alive at 52 wks | — | 12 (5–30) | 61 (53–69) | — | 29 (20–43) | 65 (57–75) | — | 32 (23–45) | 67 (58–78) |
| P value | < 0.0001 | 0.0001 | 0.0002 | ||||||
| HR (95% CI) | 3.55 (2.28–5.52) | 2.06 (1.43–2.99) | 1.99 (1.38–2.85) | ||||||
Abbreviations: PD, progressive disease; PH, proportional hazards; HR, hazard ratio.
All HRs were estimated with Cox PH model adjusting for age (continuous), KPS (continuous), and extent of resection (categorical). The combined analysis also adjusted for study protocol.
Progression status at 2, 4, and 6 months after registration were all consistently found to be strong predictors of subsequent survival in all studies. The hazard ratios of death as a function of progression status at each time point were estimated based on the Cox PH model after adjustment for age, KPS, and extent of resection. These hazard ratios represent the hazard of death for those whose tumor had progressed by the specified time point vs those who were known to be progression-free at that time point. For example, in the combined analysis based on the progression status at 6 months, the estimated hazard ratio was 1.99 (95% CI = 1.38–2.85), suggesting a nearly 2-fold rate of death among those who had progressed by 6 months after study registration. The study-specific hazard ratios associated with progression status at 6 months ranged from 2.03 to 3.39. Similarly, hazard ratios associated with the earlier time points (2- and 4-month progression status) all exceeded 2 in magnitude, ranging from 2.29 to 4.73. P values were statistically significant for all time points within each study. As one would expect, a similar pattern of strong correlation between early progression status and subsequent survival was found in all analyses based on the aggregated data, as well. Figure 2 plots the Kaplan–Meier curves for survival from the 6-month time point, based on progression status at that time, for each trial and for the combined data. The graphs for the 2- and 4-month time points resemble those for the 6-month time point and are omitted from this report.
Fig. 2.
Kaplan–Meier curves for survival from 6 months after registration, comparing patients who had progressed vs those who had not by 6 months (dotted line, those who progressed by 6 months; solid line, those who did not progress by 6 months).
Discussion
OS is traditionally used to evaluate treatment efficacy in phase II clinical trials for newly diagnosed malignant glioma patients. Unquestionably, the ultimate goal of a treatment is to improve patient survival. As such, a treatment regimen that demonstrates the property of prolonging survival is more likely to lead to regulatory approval. However, the use of survival as the primary endpoint is limited by the need for a longer trial period, often measured in years before the final results are known and published. Beyond this, the potential for the dilution of treatment effect due to second- or third-line salvage treatments patients receive after going off study adds another layer of challenge. Alternative endpoints in oncology clinical trials have been evaluated in several disease areas in the literature including colorectal cancer and breast cancer.9–11 In brain cancer specifically, Ballman et al.12 assessed the relationship between PFS-6 and OS-12 by pooling data from a total of 27 phase II North Central Cancer Treatment Group (NCCTG) trials. In that study, the analysis of association between the two endpoints was done separately for newly diagnosed and recurrent GBM patients via a variety of approaches. The authors documented that progression status at the 6th month is highly predictive of survival in both patient cohorts, indicating that PFS-6 may be a useful alternative endpoint to survival. However, their analysis only included older trials (trial start years ranged from 1980 to 2001), and none of the upfront trials included TMZ as part of their protocol treatments. Therefore, their investigation does not address the question of whether PFS is a suitably reliable endpoint for upfront studies in place of OS in the framework of the current standard of care. Lamborn et al.13 analyzed data from 596 patients with high-grade gliomas treated in phase II North American Brain Tumor Consortium (NABTC) protocols. They reported that, in addition to progression status at 6 months, progressions at earlier time points (2 and 4 months) were also strong predictors of survivorship and hence may provide further insight into the evaluation of treatment success in the design of future clinical trials in neuro-oncology. However, their evaluation focused on endpoints for trials at recurrence and may not apply to upfront trials.
This study was conducted to answer a simple question: in the TMZ era, can a nonsurvival primary endpoint help us make decisions about the efficacy of a new treatment regimen more rapidly in first-line GBM trials? In this report, we present results from an analysis of 3 phase II trials conducted at our institution in newly diagnosed GBM patients: our primary objective was to examine whether progression status at an early time point may be predictive of survival. All trials included in this study reflect the current standard of care for newly diagnosed GBM patients, which comprises surgical resection followed by RT with concurrent TMZ, followed by adjuvant TMZ. Our analyses indicate that, in this setting, progression status at 2, 4, and 6 months are each highly predictive of subsequent survival on the patient level. This pattern of association was consistently found in all three trials. In addition, although our analysis only included 3 trials, our results seemed to provide encouraging insights into the correlation between the endpoints of early progression and survival on a study level. Specifically, Table 3 shows that a treatment regimen (OTRT) that demonstrates increased PFS-2, PFS-4, and PFS-6 is also likely to be associated with the higher likelihood of survival at later time points.
Although the use of progression status at a time point even earlier than 6 months may provide an additional reduction in the time required to complete a trial and to interpret its results, some questions remain as to whether these endpoints will prove to be reliable substitutes for survival. Clearly, there are potential problems that may limit the use of these early time points. First, many studies have suggested that patients with malignant gliomas who have undergone concurrent chemotherapy and RT are likely to manifest worrisome findings on their first post-RT MRI scan. Originally thought to universally indicate early tumor progression, this phenomenon has often been found to be due to a transient effect on imaging characteristics induced by chemoradiotherapy, referred to as “pseudoprogression” within the neuro-oncology community.14–17 In any treatment regimen modeled after the Stupp protocol, patients would receive RT 5 days per week for a total of 6 weeks in conjunction with daily TMZ, followed by a 1–2-week break before starting adjuvant TMZ. On the basis of this schedule, the evaluation of progression at 2 months would be 1–2 weeks after the completion of RT. Consequently, the reliability of evaluation of progression at 2 months may be confounded by pseudoprogression. In our analysis, the accrual period of the trials spanned from 2000 to 2007. During that time, increasing attention has been paid to the phenomenon of pseudoprogression, which may explain the decreasing incidence of early progression over time seen in our data. Specifically, by the chronological order with which these trials were conducted, the respective percentages of patients evaluated as having progressed at the first MRI scan (after completion of RT) were 28%, 26%, and 15%. It is difficult to determine whether the marked decrease in early progression at the 2-month time point in the most recent trial (OTRT) was influenced by the investigators' increased awareness of pseudoprogression, or if it was the actual result of treatment success. Regardless, we were able to establish a strong relationship between progression status at 2 months and survival not only in this trial, but in the other two trials as well.
Second, the trade-off in using an earlier time point to evaluate efficacy is a corresponding increase in sample size required. To illustrate, suppose in designing a new phase II single-arm upfront GBM trial, the criteria for a successful/unsuccessful trial is 75% vs 54% (consistent with Stupp et al.1) PFS-6. If we assume an exponential distribution, then a 54% vs 75% difference at 6 months would correspond to 82% vs 90% at 2 months and 66% vs 83% at 4 months. Whereas 33 patients are sufficient to have 90% power (with 1-sided α = 0.1) for the PFS-6 endpoint, 121 and 42 patients would be required to have similar power for the earlier PFS-2 and PFS-4 endpoints, respectively. For these reasons, there does not appear to be sufficiently compelling rationale to recommend the use of PFS at a very early time points such as 2 months as primary efficacy endpoints for the phase II upfront GBM trials. However, with increasing understanding of pseudoprogression, it may be appropriate to consider using these very early endpoints to devise early stopping rules for lack of efficacy in upfront trials, as a way to limit the investment of patients, time, and money in an ineffective trial.
A major appeal of using PFS-6 as the primary endpoint for upfront GBM trials is its potential to substantially shorten the time required to move forward an effective treatment regimen. In the example described in the previous paragraph, a trial using PFS-6 as the primary endpoint would need 33 patients to detect an improvement of PFS-6 from 54% to 75%. In addition to the accrual period, the trial would require a maximum 6 months of follow-up after the last subject has been enrolled. In contrast, assuming exponential distribution and 61% survival at 1 year under the null (consistent with Stupp et al.1), a trial that uses survival as the primary endpoint with the same effect size (54% vs 75%) would require a maximum 15 months of follow-up. Nevertheless, it should also be emphasized that our analysis does not represent a formal assessment of surrogacy of PFS for OS. Formal validation of surrogacy would call for analysis of patient data from large comparative phase III trials. In addition, the ability to generalize our results may be somewhat limited by the fact that all trials included in this analysis were based on a single-institutional experience.
In summary, in this analysis of 183 patients from 3 trials in the setting of a newly diagnosed GBM, we demonstrated that progression status at 2, 4, and 6 months were significant predictors of OS. Patients who showed the signs of early progression were at significantly higher risk of earlier death. Our analysis suggested that PFS at 6 months may be an appropriate primary endpoint in the context of phase II trials evaluating treatment regimen in newly diagnosed GBM patients based on the Stupp protocol. Future research is needed to validate our findings in a larger population, both to confirm our results and to ensure their generalizability.
Funding
This research was supported by National Health Institutes' Brain Tumor SPORE grant P50 CA097257.
Acknowledgments
We thank Ilona Garner, Department of Neurological Surgery, University of California–San Francisco, for editorial support.
Conflict of interest statement. None declared.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Eng J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Chang SM, Lamborn KR, Malec M, et al. Phase II study of temozolomide and thalidomide with radiation therapy for newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2004;60:353–357. doi: 10.1016/j.ijrobp.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 3.Butowski N, Prados MD, Lamborn KR, et al. A phase II study of concurrent temozolomide and cis-retinoic acid with radiation for adult patients with newly diagnosed supratentorial glioblastoma. Int J Radiat Oncol Biol Phys. 2005;61:1454–1459. doi: 10.1016/j.ijrobp.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Prados MD, Chang SM, Butowski N, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol. 2009;27:579–584. doi: 10.1200/JCO.2008.18.9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macdonald DR, Cascino TL, Schold SC, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 7.Mantel N, Byar DP. Evaluation of response-time data involving transient states: an illustration using heart-transplant data. J Am Stat Assoc. 2006;69:81–86. [Google Scholar]
- 8.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response and other comparisons of time-to-event by outcome variables. J Clin Oncol. 2008;26:3913–3915. doi: 10.1200/JCO.2008.16.1000. [DOI] [PubMed] [Google Scholar]
- 9.Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664–8670. doi: 10.1200/JCO.2005.01.6071. [DOI] [PubMed] [Google Scholar]
- 10.Buyse M, Burzykowski T, Carroll K. Progression-free survival is a surrogate for survival in advanced colorectal cancer. J Clin Oncol. 2007;25:5218–5224. doi: 10.1200/JCO.2007.11.8836. [DOI] [PubMed] [Google Scholar]
- 11.Burzykowski T, Buyse M, Piccart-Gebhart MJ, et al. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate endpoints in metastatic breast cancer. J Clin Oncol. 2008;26:1987–1992. doi: 10.1200/JCO.2007.10.8407. [DOI] [PubMed] [Google Scholar]
- 12.Ballman KV, Buckner JC, Brown PD, et al. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro-Oncology. 2006;9:29–38. doi: 10.1215/15228517-2006-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamborn KR, Alfred Yung WK, Chang SM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro-Oncology. 2008;10:162–170. doi: 10.1215/15228517-2007-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke JL, Chang SM. Pseudoprogression and pseudoresponse: challenges in brain tumor imaging. Curr Neurol Neurosci Rep. 2009 doi: 10.1007/s11910-009-0035-4. In press. [DOI] [PubMed] [Google Scholar]
- 15.Chamberlain M, Glantz M, Chalmers L, et al. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol. 2007;82:81–83. doi: 10.1007/s11060-006-9241-y. [DOI] [PubMed] [Google Scholar]
- 16.Taal W, Brandsma D, de Bruin H, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113:405–410. doi: 10.1002/cncr.23562. [DOI] [PubMed] [Google Scholar]
- 17.Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26:2192–2197. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]