Abstract
Recurrent medulloblastoma is highly lethal in previously irradiated patients. Previously irradiated patients with M-0–M-3 recurrences who achieved a minimal disease state prior to protocol enrollment received carboplatin (Calvert formula with area under the curve = 7 mg/mL min, maximum 500 mg/m2/day) on days −8 to −6, and thiotepa (300 mg/m2/day) and etoposide (250 mg/m2/day) on days −5 to −3, followed by autologous stem cell rescue (ASCR) on day 0. Twenty-five patients, aged 7.6–44.7 years (median 13.8 years) at ASCR, were treated. Three (12%) died of treatment-related toxicities within 30 days of ASCR, due to multiorgan system failure (n = 2) and aspergillus infection with veno-occlusive disease (n = 1). Tumor recurred in 16 at a median of 8.5 months (range 2.3–58.5 months). Six are event-free survivors at a median of 151.2 months post-ASCR (range 127.2–201.6 months). The Kaplan–Meier estimate of median overall survival is 26.8 months (95% CI: 11.9–51.1 months) and of event-free survival (EFS) and overall survival are both 24% (95% CI: 9.8%–41.7%) at 10 years post-ASCR. M-0 (vs M-1 + ) recurrence prior to protocol, lack of tissue confirmation of relapse, and initial therapy of radiation therapy (RT) alone (vs RT + chemotherapy) were not significantly associated with better EFS (P = .33, .34, and .27, respectively). Trends toward better EFS were noted in patients (n = 5) who received additional RT as part of their retrieval therapy (P = .07) and whose recurrent disease was demonstrated to be sensitive to reinduction chemotherapy (P = .09). This retrieval strategy provides long-term EFS for some patients with previously irradiated recurrent medulloblastoma. The use of additional RT may be associated with better outcome.
Keywords: chemotherapy, hematopoietic stem cell transplantation, medulloblastoma
Children 3 years of age or older with medulloblastoma are currently treated with maximal safe neurosurgical resection, radiation therapy (RT), and chemotherapy. Approximately 80% of patients with standard-risk disease and 70% of patients with high-risk disease will achieve long-term event-free survival (EFS),1,2 but the 20%–30% of patients whose medulloblastoma recurs have a guarded prognosis.3,4 We have suggested that high-dose chemotherapy with autologous stem cell rescue (ASCR) may be beneficial for patients with recurrent disease,5 but this remains controversial.6 To help define whether high-dose chemotherapy should still be considered a therapeutic option for patients with recurrent medulloblastoma, we now report an expanded series with a long-term follow-up, including only patients with M-0–M-3 recurrences who were irradiated as part of their initial therapy.
Materials and Methods
Patients
Between November 1990 and June 1999, 25 patients with previously irradiated recurrent medulloblastoma were treated with this regimen. This report reflects the data as of the last patient contact up to October 11, 2007.
Characteristics of the patients are summarized in Table 1. There were 18 males and 7 females. The median age at the time of diagnosis was 11.5 years, with a range from 4.2 to 35.5 years. All patients received postoperative external beam RT, with (n = 15) or without (n = 10) chemotherapy.
Table 1.
Patient and clinical characteristics
| Patient | Age at Dx | Sex | M-stage at Dx | Rx prerelapse | Months from Dx to relapse | M-stage at relapse | Rx from relapse to protocol | Months from relapse to ASCR | Age at ASCR | Rx post-ASCR | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 28 | F | 0 | RT, Cx | 25 | 0 | S, Cx | 6 | 30 | None | DOD |
| 2 | 29 | M | 0 | RT | 29 | 2 | RT (focal), Cx | 3 | 31 | None | EFS |
| 3 | 6 | M | 0 | RT, Cx | 26 | 2 | S, Cx | 6 | 9 | None | EFS |
| 4 | 6 | M | 1 | RT, Cx | 17 | 3 | Cx | 5 | 8 | None | DOD |
| 5 | 35 | F | – | RT | 97 | 3 | Cx | 13 | 44 | RT (CS) | DOD |
| 6 | 5 | F | 0 | RT | 23 | 2 | S, Cx | 1 | 7 | None | DOD |
| 7 | 26 | M | 0 | RT | 114 | 0 | S, Cx | 3 | 35 | None | DOD |
| 8 | 4 | M | 3 | RT | 33 | 3 | S, Cx | 7 | 7 | None | EFS |
| 9 | 9 | M | 3 | RT, Cx | 29 | 3 | None | 0 | 12 | None | DOD |
| 10 | 19 | M | 3 | RT, Cx | 40 | 2 | S, Cx | 4 | 23 | None | DOT |
| 11 | 18 | M | 0? | RT, Cx | 38 | 2 | Cx | 4 | 21 | None | DOD |
| 12 | 6 | M | 0 | RT, Cx | 52 | 0 | S, Cx | 5 | 11 | RT (focal) | EFS |
| 13 | 11 | M | 0 | RT, Cx | 5 | 2 | Cx | 2 | 12 | None | EFS |
| 14 | 8 | F | 0 | RT | 11 | 3 | Cx | 4 | 9 | None | DOD |
| 15 | 11 | M | 2 | RT, Cx | 27 | 3 | S, Cx | 2 | 13 | None | DOD |
| 16 | 21 | M | 0 | RT, Cx | 63 | 0 | S, RT (focal), Cx | 13 | 27 | None | DOD |
| 17 | 7 | F | 0 | RT, Cx | 22 | 2 | S, Cx | 2 | 9 | None | DOD |
| 18 | 14 | M | 0 | RT, Cx | 39 | 3 | Cx | 4 | 17 | None | DOD |
| 19 | 15 | M | ? | RT | 48 | 1 | S | 1 | 20 | None | DOD |
| 20 | 6 | F | 0 | RT | 15 | 3 | Cx | 7 | 8 | None | DOD |
| 21 | 27 | F | 0 | RT | 105 | 0 | S, Cx | 4 | 37 | None | DOT |
| 22 | 8 | M | 0 | RT, Cx | 30 | 2 | S, Cx | 4 | 11 | None | DOD |
| 23 | 12 | M | 0 | RT | 91 | 3 | Cx | 6 | 21 | RT (CS) | EFS |
| 24 | 8 | M | 1 | RT, Cx | 11 | 0 | S, Cx | 3 | 9 | None | DOD |
| 25 | 15 | M | 0 | RT, Cx | 15 | 3 | Cx | 6 | 17 | None | DOT |
Abbreviations: Dx, diagnosis; Rx, treatment; M, male; F, female; RT, radiation therapy; CS, craniospinal; Cx, chemotherapy; S, surgery; EFS, event-free survivor; DOD, died of disease; DOT, died of toxicity; ASCR, autologous stem cell rescue.
The median time from diagnosis to relapse or disease progression was 29.8 months, with a range from 5.3 to 114.7 months. All of these patients had relapsed (or progressed on therapy) for the first time. At the time of relapse or progression, 21 had pathologic or cytologic confirmation of disease and the remaining 4 were enrolled in the study on the basis of radiographic evidence.
M-stage at the time of relapse was M-0 in 6 (24%), M-1 in 1 (4%), M-2 in 8 (32%), and M-3 in 10 (40%) cases. Of the 19 patients with M-1+ disease at recurrence, 6 patients had a single leptomeningeal focus whereas 13 patients had multifocal disease and/or CSF cytology positivity.
Patients were enrolled onto the protocol just prior to the initiation of the protocol-prescribed high-dose chemotherapy treatment. After recurrence, 24 patients had received therapy in addition to the study-prescribed therapy outlined below: neurosurgical resection in 14, conventional chemotherapy in 23, and external beam irradiation in 5 cases. The external beam irradiation was administered prior to the study-prescribed therapy in 2 patients and following the study-prescribed therapy in 3. Reirradiation was focal in 3 patients, and 2 other patients received a second course of craniospinal irradiation.
The median time from relapse to ASCR was 4.7 months, with a range from 0.8 to 13.3 months. The median age at the time of ASCR was 13.8 years, with a range from 7.6 to 44.7 years.
Chemotherapy Protocol
Five patients were enrolled on the CCG-9883 protocol, and the others were treated with an identical high-dose chemotherapy regimen on institutional protocols with comparable eligibility criteria. All patients, or their parents or legal guardians if they were minors, provided informed consent. The Institutional Review Board at each treating institution approved the relevant protocol.
Details of the chemotherapy protocol have been reported previously.5 Briefly, following harvesting and cryopreservation of autologous stem cells (either from bone marrow or from peripheral blood), high-dose chemotherapy was administered. On days −8, −7, and −6, the patients received carboplatin. Initially the dose was fixed at 500 mg/m2/dose, but subsequently the protocols were amended to dose the carboplatin according to the Calvert formula with a targeted predicted area under the curve of 7 mg/mL min/day.7,8 With the Calvert method, each day's dose of carboplatin was calculated based on a current daily estimate of the glomerular filtration rate, determined either by a renal technetium99-DTPA radionuclide study or by a timed urine collection for creatinine clearance. On days −5, −4, and −3, thiotepa (300 mg/m2/day) and etoposide (250 mg/m2/day) were administered. About 72 hours after completion of the chemotherapy, the cryopreserved stem cells were rapidly thawed and reinfused. Nine patients received bone marrow, 15 peripheral blood stem cells, and 1 both bone marrow and peripheral blood stem cells. Filgrastim was routinely used beginning on day +1 until engraftment of the neutrophils was established.
Statistics
Overall survival was calculated from the date of ASCR until the date of death, or last follow-up. EFS, was calculated from the date of ASCR until the date of disease progression, death, or last follow-up. Survival probabilities were estimated using the Kaplan–Meier method, and EFS curves were compared by exact log-rank test based on a complete permutation of log-rank scores.9
Typically, post-ASCR RT started 6 weeks after ASCR. A landmark analysis was used to compare the EFS curves among patients who did or did not receive pre- or post-ASCR RT conditioning on all patients who were alive at the 6-week landmark after ASCR.10 In consequence, 3 patients who died of toxicity within 1 month post-ASCR were excluded from the landmark analysis; none of them received pre- or post-ASCR RT. For 3 patients in which only the month and year of relapse, death, or last follow-up were known, the 15th day of the month was used to impute the unknown dates. All statistical analyses were performed using SAS (SAS Institution) or StatXact software (Cytel Software Corporation). A 2-sided P value of <0.05 was considered as significant.
Results
Toxicity
Toxicity was substantial in all patients. Pancytopenia, with the need for broad-spectrum antibiotics in the face of fever and neutropenia and for packed red blood cell and platelet transfusions, occurred in all patients, as did severe oropharyngeal mucositis, necessitating opioids, and total parenteral nutrition.
Toxic mortality occurred in 3 patients (12%) within 29 days of ASCR. This was due to multiorgan system failure in 2 patients and aspergillus infection/veno-occlusive disease in the other.
Survival
Tumor recurred in 16 patients at a median of 8.5 months post-ASCR (range 2.3–58.5 months). Median survival after recurrence was 12.3 months, with a range from 0.9 to 31.3 months.
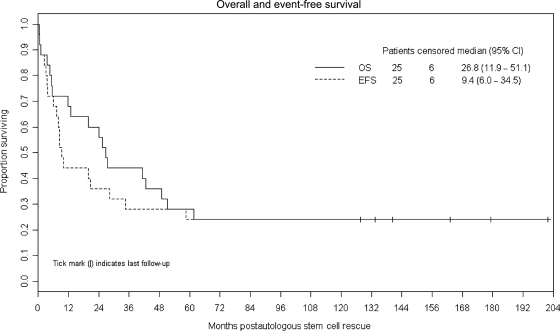
Six patients survive event-free with a median follow-up time of 151.2 months from ASCR (range 127.2–201.6 months). Figure 1 demonstrates that the Kaplan–Meier estimates of EFS and overall survival are both 24% at 10 years (95% CI: 9.8%–41.7%). The Kaplan–Meier estimate of median overall survival is 26.8 months (95% CI: 11.9–51.1 months).
Fig. 1.
Kaplan–Meier plots of survival and EFS after ASCR.
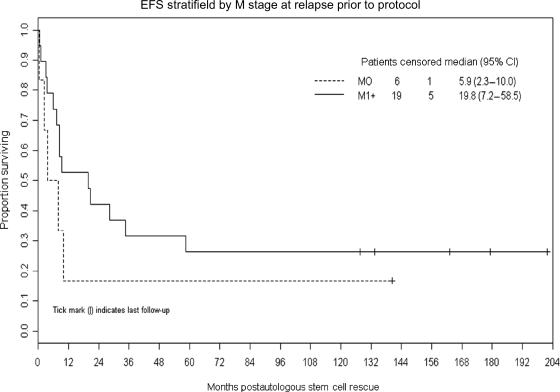
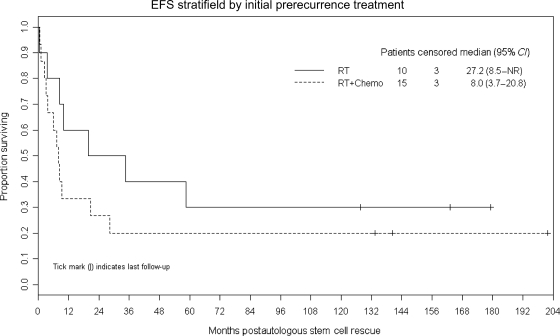
Figures 2 and 3 demonstrate that M-stage at the time of preprotocol relapse and initial therapy administered (RT only vs RT and chemotherapy) were not significantly associated with EFS (P = .33 and .27, respectively). Tissue confirmation of the preprotocol relapse (vs radiological diagnosis only) was also not associated with EFS (P = .34).
Fig. 2.
Kaplan–Meier plot of EFS after ASCR in patients with M-0 vs M-1+ disease at the time of relapse. There is no statistically significant difference (P = .33).
Fig. 3.
Kaplan–Meier plot of EFS after ASCR in patients whose initial treatment regimen consisted of RT only vs combined RT and chemotherapy. There is no statistically significant difference (P = .27).
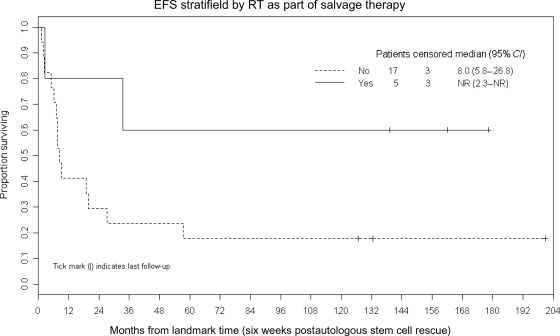
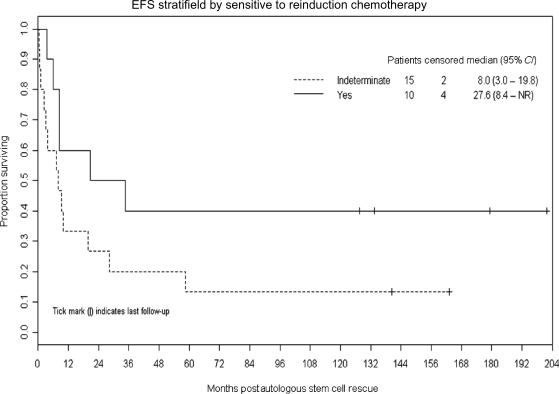
Figures 4 and 5 demonstrate that trends toward better EFS were noted in patients (n = 5) who received additional RT as part of their retrieval therapy (P = .07) and whose recurrent disease was demonstrated to be sensitive to reinduction chemotherapy (P = .09).
Fig. 4.
Kaplan–Meier plot demonstrating a trend toward better EFS after ASCR in patients (n = 5) who received additional RT as a part of their retrieval therapy (P = .07).
Fig. 5.
Kaplan–Meier plot demonstrating a trend toward better EFS after ASCR in patients whose recurrent disease was demonstrated to be sensitive to reinduction chemotherapy (P = .09).
A 2008 ISPNO meeting session moderator (Dr B. Pizer, Liverpool) suggested that patients with unifocal leptomeningeal disease at recurrence may have a better prognosis than patients with multifocal or diffuse leptomeningeal disease and we explored his hypothesis. In this series, 6 patients had M-0 recurrences (1 survives event-free), 6 others had unifocal leptomeningeal disease (1 survives event-free), and 13 had multifocal leptomeningeal disease and/or positive CSF cytology (4 survive event-free). Unifocal (vs multifocal) recurrence pre–high-dose chemotherapy was not associated with superior EFS (P = .43).
In all of these analyses, the power to appreciate a difference is limited by the small numbers of patients.
Quality of Life of Survivors
The 6 survivors have not been evaluated with formal quality of life assessments, but a review of the most recent progress notes was performed. Patients 3 and 12 were in college. Patient 13 was living independently with some assistance and working in a sheltered environment. Patients 2, 8, and 23 were unemployed; 2 due to motor issues and 1 due to cognitive issues.
Most of the patients had some chronic morbidities commonly seen in medulloblastoma survivors. Attribution of the problems is complicated, but it seems most likely that their neurocognitive issues and endocrinopathies were due to RT, whereas ototoxicity was likely due to treatment with platinum agents including the high-dose carboplatin used in the high-dose chemotherapy regimen.
Discussion
Although the prognosis for children 3 years of age or older with medulloblastoma has significantly improved, approximately 20%–30% will relapse and die of their disease. Children's Hospital of Philadelphia investigators described 23 patients treated for medulloblastoma between 1980 and 1991 whose tumor recurred and noted the median survival to be 5 months, with the longest survivor living for 38 months from recurrence.4
We previously reported that 23 patients with recurrent medulloblastoma treated with this high-dose carboplatin, thiotepa, and etoposide regimen achieved 36 month EFS of 34% and concluded that this strategy may provide long-term survival for some patients with recurrent medulloblastoma.5 Limitations of our prior work included the inclusion of younger patients originally treated with chemotherapy only who may have been curable with external beam RT without high-dose chemotherapy,11 and patients with extra-neural disease who are rarely encountered in contemporary clinical practice. This current manuscript addresses only the most currently clinically relevant patients, those whose original therapy included external beam RT who subsequently developed recurrent disease in the central nervous system (CNS), and we still note that a subset may achieve long-term EFS using this aggressive salvage strategy.
Several recent papers from other groups provide relevant data. A report from a Korean group included 6 patients with recurrent medulloblastoma whose original treatment had included external beam RT. Two of the 6 were event-free following tandem high-dose chemotherapy/ASCR treatments (melphalan–cyclophosphamide, carboplatin–thiotepa–etoposide), but their follow-up was short (9+ and 12+ months).12
A report from the Mayo Clinic included 10 previously irradiated patients with recurrent CNS embryonal tumors (8 medulloblastoma and 2 cerebral neuroblastoma) treated with high-dose chemotherapy and ASCR. Nine of the 10 received high-dose thiotepa-containing regimens (with a thiotepa dose of 200 mg/m2/day × 3 days) in addition to carmustine or carboplatin. Five of the patients were alive (4 without disease progression) with a median follow-up of 2.9 years. The authors concluded that high-dose chemotherapy with ASCR should be considered for adults with recurrent CNS embryonal tumors.13
High-dose chemotherapy regimens that do not include thiotepa appear to be less promising. St Jude investigators included 10 patients with recurrent medulloblastoma whose original treatment had included craniospinal RT in a larger series regarding a variety of high-dose chemotherapy regimens. Only 1 of their patients received a regimen that included thiotepa. Nine patients died and only 1 patient was free of disease (at 1319 days).14
POG investigators included 22 children with recurrent medulloblastoma in a report regarding the use of high-dose cyclophosphamide and melphalan with ASCR. Six were event-free at follow-up from 34 to 116 months.15 Four of the 18 patients whose initial therapy included external beam RT were event-free (R. Kadota, personal communication dated September 22, 2008).
Finally, Duke investigators included 12 previously irradiated patients in a report regarding the use of high-dose chemotherapy for recurrent medulloblastoma. Nine patients received high-dose cyclophosphamide and melphalan and 3 received high-dose busulfan and melphalan. Five patients were reirradiated as part of their salvage therapy (4 focal and 1 craniospinal). All 12 patients relapsed from 4 to 24 months post-ASCR and subsequently died of disease.16
In summary, these reports in conjunction with our data suggest that an aggressive retrieval regimen including the use of high-dose chemotherapy in conjunction with ASCR may provide long-term survival for some patients with recurrent medulloblastoma and that thiotepa may be an important component of the high-dose chemotherapy regimen. Additional RT may also be an important component of a salvage regimen (although the power to appreciate a difference was limited by the small numbers of patients), but it is noteworthy that 3 of the 6 event-free survivors in our series were not reirradiated. This supports the hypothesis that high-dose thiotepa-based chemotherapy is an important component of the retrieval strategy.
It is important to note a limitation of this study is that patients were enrolled onto the protocol just prior to the administration of high-dose chemotherapy, not at the time of first recurrence of their medulloblastoma. Therefore, the survival data that we describe are most relevant to patients who have achieved a minimal disease state following recurrence, not to the total population of patients with recurrent disease.
We continue to recommend that an aggressive salvage regimen should be considered for patients with previously irradiated recurrent medulloblastoma. We generally offer neurosurgical resection, irradiation, and/or conventional chemotherapy after detection of relapse in an attempt to achieve a very good partial or complete remission prior to high-dose chemotherapy with ASCR. Novel approaches such as intrathecal radioimmunotherapy17 or biologically targeted agents may also appropriate considerations for these patients, but since they may be most effective in the setting of minimal residual disease we recommend that they may be considered as supplemental approaches to high-dose chemotherapy rather than as mutually exclusive alternative strategies.
Conflict of interest statement. None declared.
Funding
This work was partially supported by the Witmer fund.
References
- 1.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 2.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 3.Belza MG, Donaldson SS, Steinberg GK, Cox RS, Cogen PH. Medulloblastoma: freedom from relapse longer than 8 years—a therapeutic cure? J Neurosurg. 1991;75:575–582. doi: 10.3171/jns.1991.75.4.0575. [DOI] [PubMed] [Google Scholar]
- 4.Torres CF, Rebsamen S, Silber JH, et al. Surveillance scanning of children with medulloblastoma. N Engl J Med. 1994;330:892–895. doi: 10.1056/NEJM199403313301303. [DOI] [PubMed] [Google Scholar]
- 5.Dunkel IJ, Boyett JM, Yates A, et al. High dose carboplatin, thiotepa and etoposide with autologous stem cell rescue for patients with recurrent medulloblastoma. J Clin Oncol. 1998;16:222–228. doi: 10.1200/JCO.1998.16.1.222. [DOI] [PubMed] [Google Scholar]
- 6.Gajjar A. High-dose chemotherapy for recurrent medulloblastoma: time for a reappraisal. Cancer. 2008;112:1643–1645. doi: 10.1002/cncr.23352. [DOI] [PubMed] [Google Scholar]
- 7.Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7:1748–1756. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- 8.Newell DR, Pearson AD, Balmanno K, et al. Carboplatin pharmacokinetics in children: the development of a pediatric dosing formula. J Clin Oncol. 1993;11:2314–2323. doi: 10.1200/JCO.1993.11.12.2314. [DOI] [PubMed] [Google Scholar]
- 9.Mehta C, Patel N. StatXact 7 User Manual 2005. Cambridge: Cytel Software Corporation; 2005. [Google Scholar]
- 10.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 11.Gajjar A, Mulhern RK, Heideman RL, et al. Medulloblastoma in very young children: outcome of definitive craniospinal irradiation following incomplete response to chemotherapy. J Clin Oncol. 1994;12:1212–1216. doi: 10.1200/JCO.1994.12.6.1212. [DOI] [PubMed] [Google Scholar]
- 12.Sung KW, Yoo KH, Cho EJ, et al. High-dose chemotherapy and autologous stem cell rescue in children with newly diagnosed high-risk or relapsed medulloblastoma or supratentorial primitive neuroectodermal tumor. Pediatr Blood Cancer. 2007;48:408–415. doi: 10.1002/pbc.21064. [DOI] [PubMed] [Google Scholar]
- 13.Gill P, Litzow M, Buckner J, et al. High-dose chemotherapy with autologous stem cell transplantation in adults with recurrent embryonal tumors of the central nervous system. Cancer. 2008;112:1805–1811. doi: 10.1002/cncr.23362. [DOI] [PubMed] [Google Scholar]
- 14.Shih CS, Hale GA, Gronewold L, et al. High-dose chemotherapy with autologous stem cell rescue for children with recurrent malignant brain tumors. Cancer. 2008;112:1345–1353. doi: 10.1002/cncr.23305. [DOI] [PubMed] [Google Scholar]
- 15.Kadota RP, Mahoney DH, Doyle J, et al. Dose intensive melphalan and cyclophosphamide with autologous hematopoietic stem cells for recurrent medulloblastoma or germinoma. Pediatr Blood Cancer. 2008;51:675–678. doi: 10.1002/pbc.21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gururangan S, Krauser J, Watral MA, et al. Efficacy of high-dose chemotherapy or standard salvage therapy in patients with recurrent medulloblastoma. Neuro-Oncology. 2008;10:745–751. doi: 10.1215/15228517-2008-044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer K, Humm J, Souweidane MM, et al. Targeted radioimmunotherapy for leptomeningeal cancers: results of a phase I study using intra-Ommaya 131-I-3F8. J Clin Oncol. 2007;25:5465–5470. doi: 10.1200/JCO.2007.11.1807. [DOI] [PubMed] [Google Scholar]