Abstract
The prognosis for patients with recurrent glioblastomas (GBMs) is dismal, with a median survival of 3–6 months. We performed a phase II trial of low-dose continuous (metronomic) treatment using temozolomide (TMZ) for recurrent GBMs. TMZ-refractory patients with GBM who experienced disease recurrence or progression during or after the cyclic treatment schedule of TMZ after surgery and standard radiotherapy were eligible. This phase II trial included 2 cohorts of patients. The initial cohort, comprising 10 patients, received TMZ at 40 mg/m2 everyday. After this regimen seemed safe and effective, the metronomic schedule was changed to 50 mg/m2 everyday. The second cohort, comprising 28 patients, received TMZ at 50 mg/m2 everyday. The 6-month progression-free survival in all 38 patients was 32.5% (95% CI: 29.3%–35.8%) and the 6-month overall survival was 56.0% (95% CI: 36.2%–75.8%). One patient developed a grade III neutropenia, grade II thrombocytopenia in 3 patients, and grade II increase of liver enzyme (GOT/GPT) in 3 patients. Of all patients included in this study, 4 patients were withdrawn from this study because of side effects including sustained hematological disorders, cryptococcal infection, and cellulitis. In a response group, quality of life measured with short form-36 was well preserved, when compared with the pretreatment status. Metronomic treatment of TMZ is an effective treatment for recurrent GBM that is even refractory to conventional treatment of TMZ and has acceptable toxicity.
Keywords: glioblastoma, metronomic, recurrent
Despite multidisciplinary treatment approaches, glioblastomas (GBMs) are very aggressive tumors and their prognosis remains extremely poor. A recent phase III randomized study for newly diagnosed GBMs demonstrated that concurrent chemoradiotherapy using temozolomide (TMZ) followed by cyclic TMZ chemotherapy significantly prolonged the survival in patients with GBMs, when compared with the radiotherapy alone.1 However, despite optimal treatment for malignant gliomas, recurrence is common within the first 2 years. Since the majority of recurrent GBMs are no longer candidates for further irradiation or surgical intervention, most patients with recurrent GBMs do not survive for 1 year after the diagnosis of recurrence.2–10
Angiogenesis has recently been identified as an important hallmark of malignant glioma pathology. Malignant gliomas including GBMs are among the most vascularized of tumors in humans.11,12 Recent studies suggest that tumor angiogenesis may be a therapeutic target in GBMs.9,10,13 Although several antiangiogenic compounds have recently become important, conventional administration of chemotherapeutic agents typically requires a treatment-free period for the recovery of normal host cells. However, during the resting period, endothelial cells may have enough chance to repair the damage inflicted by the chemotherapy and resume tumor regrowth during this treatment-free interval.3 Recently, it has been suggested that this repair process could be compromised by continuous administration of chemotherapeutic drugs with lower doses.3,12,14 This frequent regular administration of chemotherapeutic agents, known as “metronomic chemotherapy,” increases the antiangiogenic activity of the drugs.4,8,15–17 This hypothesis has been confirmed in several experimental tumor models that have led us to the concept of “antiangiogenic scheduling.”3,18–20 The metronomic scheduling for chemotherapy targets both proliferating tumor cells and endothelial cells and minimizes toxicity.4,8,21
Our preclinical study indicated that metronomic treatment was effective to control the glioma in the orthotopic model of TMZ resistant C6 rat glioma.18 We also found the efficacy of metronomic treatment for recurrent GBMs in the pilot study.22 The objective of this phase II study is to evaluate the efficacy and safety of metronomic treatment of TMZ for recurrent GBMs that were refractory to conventional TMZ administration.
Patients and Methods
Eligibility
Adult patients (>18 years) with a Karnofsky performance status score of ≥60 who had histologically proven GBM were eligible for this phase II study. Patients were required to receive chemotherapy using TMZ following radiation therapy or concomitant chemoradiotherapy for GBMs and unequivocal evidence of tumor recurrence or progression by MRI. To exclude pseudoprogression, tumor recurrence or progression should be developed at least 3 months after the initiation of radiotherapy. All patients were required to have normal hematologic, liver, and renal function and did not undergo redo surgery for the recurrence.
Previous or current malignancies at other sites were excluded in this study. Additional exclusion criteria included the presence of uncontrolled systemic disease, serious comorbid disease, or allergy to TMZ or the presence of any psychological, chronic alcohol addiction, and central nervous system disorders that could result in noncompliance with the study protocol or the follow-up schedule. All patients provided written informed consent prior to the study participation. The study protocol was reviewed and approved by a local Institutional Review Board/Ethics Committee.
Study Design
This open-label, single-center Phase II study was composed of 2 cohorts of recurrent GBM patients. The first 10 patients received treatment with TMZ at a dose of 40 mg/m2 everyday. After this regimen was proved safe and effective, the second cohort, comprising 28 patients, received TMZ at 50 mg/m2 everyday. The regimen was given on an outpatient basis. Treatment continued until progressive disease occurred. At a regular interval of 1 month, continuance of treatment was determined based on toxicity. If grade II or higher toxic effects as measured by the National Cancer Institute Common Toxicity Criteria version 3.0 (NCI-CTC) were observed, treatment was held up for at least 2 weeks and subsequently restarted at the same dose level. Doses missed during a treatment period were not to be replaced. All toxicities should recover to normal levels before retreating. In the case of persistent grade II or higher toxicity despite discontinuation of treatment, further protocol treatment was stopped. The primary endpoint was progression-free survival at 6 months (PFS-6) from the time of study inclusion. Secondary endpoints included safety and tolerability of treatment, response rates, and quality of life (QOL).
Evaluation of Treatment Response
Disease progression was radiologically confirmed by baseline contrast-enhanced MRI. MRI was followed up at a regular interval of 2 months (Figs 1 and 2). Pretreatment tests included the assessment of complete blood count with differential, platelet counts, and serum chemistry profiles, which was repeated every 4 weeks. The criteria of MacDonald et al.24 were used to determine treatment response. A complete response (CR) required disappearance of all contrast-enhancing disease and no evidence of new lesions. A partial response (PR) was defined as a decrease of >50% of the baseline sum of measurable contrast-enhancing lesions with no progression of evaluable disease and no new lesions. Patients with stable disease (SD) were those who had neither CR nor PR but no disease progression. A retrospective analysis was made to correlate O6-methylguanine-DNA-methyltransferase (MGMT) promoter methylation status and disease progression.
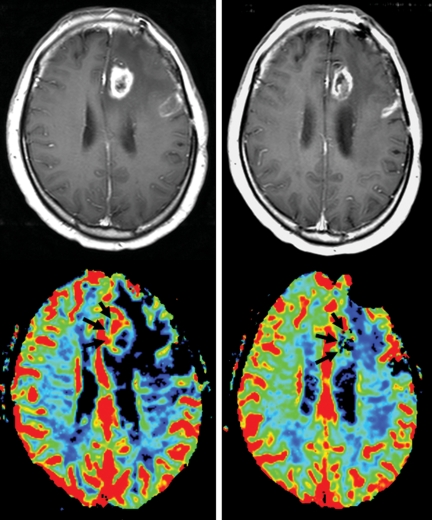
Fig. 1.
Left, before treatment. On the gadolinium-enhanced T1-weighted magnetic resonance scans, the enhancing lesion on the left frontal lobe shows increased cerebral blood perfusion (arrows). Right, after follow-up MRI 8 months after treatment. The size of the enhancing lesion on contrast T1-weighted image was not definitely changed and cerebral blood perfusion (arrows) was partially reduced.
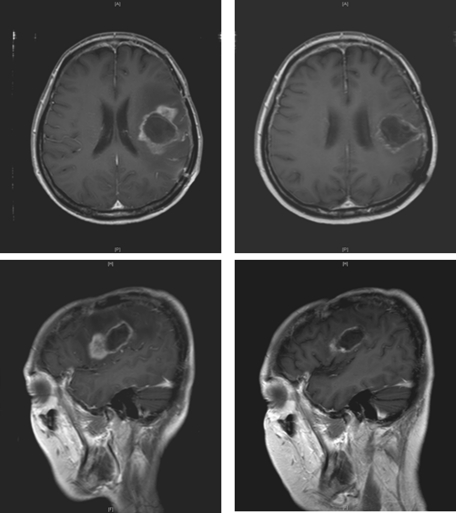
Fig. 2.
This 48-year-old woman underwent metronomic treatment after tumor recurrence. Left; before treatment; right, after follow-up MRI 4 months after treatment.
Bisulfite Modification and Methylation-specific Polymerase Chain Reaction
DNA from both the primary tumor and the renal carcinoma cell lines was subjected to bisulfite treatment. Briefly, 1 µg of DNA was denatured by sodium hydroxide and modified by sodium bisulfite. DNA samples were then purified using the Wizard purification resin (Promega, Madison, WI), treated again with sodium hydroxide, precipitated with ethanol, and resuspended in water. DNA methylation patterns in the CpG islands of the MGMT gene were determined by chemical treatment with sodium bisulfite and subsequent methylation-specific polymerase chain reaction (PCR). Primer pairs specific for methylated, 5′-tttcgacgttcgtt cgtaggttttcgc-3′ (sense) and 5′-gcactcttcc gaaaacgaaacg-3′ (antisense), and unmethylated, 5′-tttgtg ttttgatgtttgtaggttttt gt-3′ (sense) and 5′-aactccacactcttccaaa aacaaaaca-3′ (antisense), were prepared. PCR conditions were 95° for 5 minutes, and 95° for 40 seconds, 60° for 40 seconds, and 72° for 40 seconds for 35 cycles, and 72° for 5 minutes. Each PCR product was directly loaded on an 8% acrylamide gel, stained with ethidium bromide, and observed under ultraviolet illumination.
Evaluation of QOL
Self-reported physical functioning measures were administered at baseline, 3 months, and 6 months from the beginning of treatment. All patients completed the physical and mental component scores from the short form-36 (SF-36),23 which is a general health QOL measure that is well-validated and commonly used. Higher scores indicated better health status for these measures.
Statistical Considerations
Sample size was calculated to reject a 10% PFS-6 in favor of a target PFS-6 of 30%, with a significance level of 0.05 and a power of 80%. The Kaplan–Meier method was used to estimate OS and PFS in the intent-to-treat (ITT) population and the log-rank test was retrospectively performed to identify the association between the MGMT methylation status and PFS.
Results
Patient Characteristics
Between January 2006 and February 2008, 38 patients with recurrent GBM were registered in this study. The patient characteristics are listed in Table 1. There were 22 men and 16 women, with a median age of 51 years (22–69 years). Sixteen patients had received prior concomitant chemoradiotherapy using TMZ followed by adjuvant TMZ treatment and 22 other patients had also undergone adjuvant TMZ treatment after radiation therapy alone. All patients had tumor progression or recurrence at a median 41.6 weeks (95% CI: 28.6–54.6 weeks) and 13 of 38 patients developed recurrence within 8 months from the initial diagnosis. At the initial diagnosis, the methylation status of the MGMT promoter was evaluated in a total of 32 patients, because tumor specimens in 6 other patients were not available for the reason of referral from other institutions. The MGMT promoter was unmethylated in 23 (71.9%) of the 32 patients, whereas 9 patients revealed hypermethylation of MGMT (Table 1).
Table 1.
Patient characteristics
| Characteristic | Value |
|---|---|
| No | 38 |
| Age (yr) | |
| Median | 51 |
| Range | 27–69 |
| Sex | |
| Male | 22 |
| Female | 16 |
| Karnofsky performance status (%) | |
| 60 | 2 |
| 70–80 | 22 |
| 90–100 | 14 |
| Extent of resection | |
| Gross total resection | 32 |
| Partial resection | 3 |
| Biopsy only | 2 |
| Previous chemotherapy of TMZ | |
| Concomitant TMZ with RT | 16 |
| Subsequent TMZ after RT | 22 |
| MGMT methylation status | |
| Unmethylated | 23 |
| Methylated | 8 |
| Time from diagnosis (mo) | |
| Median | 9.7 |
| Range | 3.1–38.6 |
Abbreviations: TMZ, temozolomide; RT, radiotherapy; MGMT, O6-methylguanine-DNA methyltransferase.
Treatment Efficacy
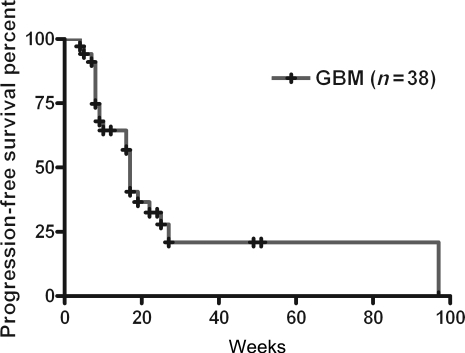
On the basis of the ITT analysis, PFS-6 was 32.5% (95% CI: 29.3%–35.8%) and the median PFS was 17 weeks (95% CI: 15.8–18.2 weeks) (Fig. 3). There was no statistical difference between the two cohorts of patients. No significance was shown between groups who underwent CCRT or radiotherapy alone followed by adjuvant TMZ treatment (22 vs 16 weeks, respectively, P = .156). Two patients had PR and 21 patients showed SD. Twenty-three of 38 patients (60.5%) had SD or PR to continuous metronomic treatment with TMZ. In the hypermethylated MGMT group, a median time to progression was 17 weeks (95% CI: 5.9%–28.1%), and in the unmethylated group, 23 of 38 patients also showed a median time to progression of 17 weeks (95% CI: 8.8–25.1 weeks). Consequently, response to the metronomic treatment was not closely associated with the MGMT methylation status (log-rank test, P > .05).
Fig. 3.
Kaplan–Meier PFS.
Overall Survival
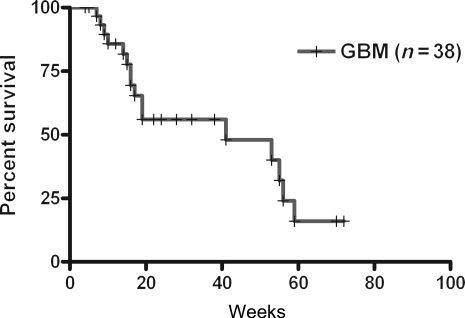
The median 6 months overall survival (OS-6) from the initiation of this study was 56.0% (95% CI: 36.2%–75.8%) (Fig. 4). The median OS was 41.0 weeks (95% CI: 4.4–77.6 weeks).
Fig. 4.
Kaplan–Meier OS from the recurrence.
Toxicities
All patients experienced treatment-related toxicities; however, most toxicities were limited to grade I or II events. The most common toxicities in this study included nausea sensation, thrombocytopenia, lymphopenia, anemia, vomiting, elevation of liver enzyme, and neutropenia. Three patients underwent grade III lymphopenia, but it did not require drug discontinuation. Treatment-related toxicities required 4 patients to discontinue therapy in this study. Two patients experienced grade III neutropenia and had to discontinue therapy. For another patient, grade 2 thrombocytopenia and frequent seizure attacks required the patient stop therapy, even though follow-up MRI showed SD. Two other patients had to discontinue therapy because of cellulitis and cryptococcal pulmonary infection (Tables 2 and 3).
Table 2.
Toxicities related to the treatment
| Toxicities (NCI-CTC) | Number |
||
|---|---|---|---|
| Grade I | Grade II | Grade III | |
| Neutropenia | 4 | 0 | 1 |
| Thrombocytopenia | 17 | 2 | 0 |
| Lymphopenia | 3 | 5 | 3 |
| Anemia | 16 | 1 | 0 |
| Nausea | 23 | 2 | 0 |
| Vomiting | 13 | 0 | 0 |
| Elevated liver enzyme | 15 | 3 | 0 |
| Cellulitis | 1 | ||
| Cryptococcal pulmonary infection | 1 | ||
Abbreviations: NCI-CTC, National Cancer Institute Common Terminology Criteria (version 3.0).
Table 3.
Reasons for patient discontinuation from the study and number of patients
| 40 mg/m2 daily | 50 mg/m2 daily | Total | |
|---|---|---|---|
| Disease progression | 5 | 22 | 27 |
| Withdrew consent | 0 | 5 | 5 |
| Follow-up loss | 3 | 1 | 4 |
| Side effects | 2 | 2 | 4 |
Assessment of QOL
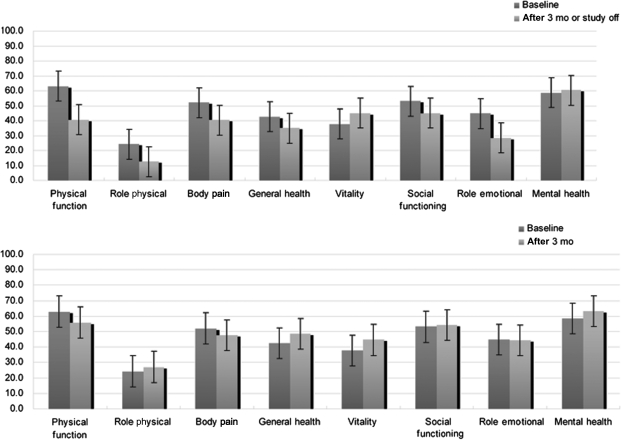
On the basis of a baseline SF-36, QOL scores are described in Fig. 4. For all patients, follow-up SF-36 at 3 months or study-off time revealed a significant decrease in QOL score in physical health status, whereas no significant difference was shown in mental health status. In contrast, for 23 patients showing response to the treatment, SF-36 at 3 months demonstrated that there was no significant difference in physical and mental health status, when compared with the baseline SF-36 (Fig. 5).
Fig. 5.
Assessment of QOL using SF-36 of all patients (upper) in this study and good responders among them (lower).
Discussion
Although chemoradiothrapy followed by adjuvant TMZ treatment is known to be effective for newly diagnosed GBMs, no definitive treatment for TMZ-intractable GBMs, recurrent tumors, or disease that has progressed has been determined yet. Most recurrent gliomas are found during the period of adjuvant therapeutic schedule with TMZ. Like this, the treatment-free interval may afford tumor-associated endothelial cells the opportunity to recover, which allows tumor cells to resume growth.23 To overcome these limits of conventional schedules, continuous administration of certain cytotoxic agents at low doses, known as “metronomic chemotherapy,” has been reported to be more effective in targeting tumor endothelium.2–4,8,23,24 For performing metronomic treatment, TMZ itself may be an optimal drug because it can be easily taken everyday by oneself without requiring further hospitalization. TMZ can be reasonably rechallenged in this heavily pretreated population. Perry et al.25 supported that metronomic treatment by rechallenging TMZ was effective for controlling recurrent malignant gliomas. Recent review by Wick et al.26 also reported a similar outcome of alternative schedules of TMZ for recurrent gliomas, including 1-week on and 1-week off. Their results suggest that most of the recurrences in gliomas are not caused by only the intractability of the tumor to TMZ. Although this phase II study had too small a sample size to prove the efficacy of metronomic treatment for recurrent GBMs, the result can be added to the evidence supporting the therapeutic effect of a rechallenge schedule of TMZ. Recently, Perry et al. (unpublished) performed a phase II RESCUE study. According to their data on recurrent GBMs and anaplastic glioma, they reported the various treatment outcomes (PFS-6, 7%–52%) based on the period of recurrence and the tumor type (grade III or IV).
In this study, the ethylation status of the MGMT promoter did not affect the response rate of metronomic treatment. It was notable that metronomic treatment using TMZ could control the tumor progression in even tumors with unmethylated MGMT status, which were more resistant to the conventional chemotherapy and even lead to poor survival. This finding was consistent with another rechallenge of TMZ study.25,26 Little is known, to our knowledge, about the result of long-term use of TMZ for MGMT methylation status. However, Tolcher et al.27 suggested that a protracted schedule of TMZ markedly inactivates the activity of MGMT. According to their report, low-dose continuous use of TMZ may overcome the resistance to chemotherapy in unmethylated GBM by inhibiting tumor endothelial proliferation. This study supports the concept of metronomic use of TMZ regardless of MGMT promoter methylation status. However, this concept had an important drawback that MGMT methylation status was evaluated at the initial presentation, not at the time of recurrence. At the recurrent presentation, it could not be identified whether methylation status had changed or not, compared with at the initial presentation. As another limitation of this study, preselection bias by a small sample size should be considered when interpreting these data.
Metronomic treatment has some advantages over other salvage chemotherapies for recurrent GBMs. First, it can provide better compliance of application for recurrent GBM patients. Second, although treatment-related toxicities were present in all accrued patients, most of them were included in the grade I or II events and only 4 patients discontinued the treatment protocol because of the adverse effects (Table 2). Third, it can provide a relatively good performance status in terms of health-related QOL, leading to preserved QOL in GBM patients. Finally, it is likely that the combination of metronomic chemotherapy with more potent inhibitors of angiogenesis or more effective agents inhibiting invasion will lead to greater antitumor activity.
In summary, metronomic treatment using TMZ was fairly well tolerated and proved to be a feasible and safe chemotherapeutic modality for patients with recurrent GBMs. This antitumor and antiangiogenic therapeutic schedule warrants further combination treatment with other various agents. In addition, a phase II clinical trial of a larger series and a controlled randomized study should also be conducted to provide a differential impact on recurrent GBMs.
Funding
This study was supported in part by Samsung Biochemical Research Institute (CRS 106633) and Schering-Plough Company (P05035 Temodal IIS).
Acknowledgments
We thank Dr Andrew Chi, Dr Groves, and Mrs Eugean Oh for providing her valuable contribution to this work.
Conflict of interest statement. None declared.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Brada M, Hoang-Xuan K, Rampling R, et al. Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol. 2001;12:259–266. doi: 10.1023/a:1008382516636. [DOI] [PubMed] [Google Scholar]
- 3.Browder T, Butterfield CE, Kraling BM, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 4.Gasparini G. Metronomic scheduling: the future of chemotherapy? Lancet Oncol. 2001;2:733–740. doi: 10.1016/S1470-2045(01)00587-3. [DOI] [PubMed] [Google Scholar]
- 5.Jaeckle KA, Hess KR, Yung WK, et al. Phase II evaluation of temozolomide and 13-cis-retinoic acid for the treatment of recurrent and progressive malignant glioma: a North American Brain Tumor Consortium Study. J Clin Oncol. 2003;21:2305–2311. doi: 10.1200/JCO.2003.12.097. [DOI] [PubMed] [Google Scholar]
- 6.Chang SM, Prados MD, Yung WK, et al. Phase II study of neoadjuvant 1, 3-bis (2-chloroethyl)-1-nitrosourea and temozolomide for newly diagnosed anaplastic glioma: a North American Brain Tumor Consortium Trial. Cancer. 2004;100:1712–1716. doi: 10.1002/cncr.20157. [DOI] [PubMed] [Google Scholar]
- 7.Reardon DA, Friedman HS, Powell JB, Jr, Gilbert M, Yung WK. Irinotecan: promising activity in the treatment of malignant glioma. Oncology (Williston Park) 2003;17:9–14. [PubMed] [Google Scholar]
- 8.Silvani A, Eoli M, Salmaggi A, et al. Phase II trial of cisplatin plus temozolomide, in recurrent and progressive malignant glioma patients. J Neurooncol. 2004;66:203–208. doi: 10.1023/b:neon.0000013479.64348.69. [DOI] [PubMed] [Google Scholar]
- 9.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd,, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 10.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd,, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 11.Klement G, Huang P, Mayer B, et al. Differences in therapeutic indexes of combination metronomic chemotherapy and an anti-VEGFR-2 antibody in multidrug-resistant human breast cancer xenografts. Clin Cancer Res. 2002;8:221–232. [PubMed] [Google Scholar]
- 12.Plate KH, Risau W. Angiogenesis in malignant gliomas. Glia. 1995;15:339–347. doi: 10.1002/glia.440150313. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J, Browder T, Palmblad J. Angiogenesis research: guidelines for translation to clinical application. Thromb Haemost. 2001;86:23–33. [PubMed] [Google Scholar]
- 14.Kerbel RS, Klement G, Pritchard KI, Kamen B. Continuous low-dose anti-angiogenic/metronomic chemotherapy: from the research laboratory into the oncology clinic. Ann Oncol. 2002;13:12–15. doi: 10.1093/annonc/mdf093. [DOI] [PubMed] [Google Scholar]
- 15.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 16.Kieran MW, Turner CD, Rubin JB, et al. A feasibility trial of antiangiogenic (metronomic) chemotherapy in pediatric patients with recurrent or progressive cancer. J Pediatr Hematol Oncol. 2005;27:573–581. doi: 10.1097/01.mph.0000183863.10792.d4. [DOI] [PubMed] [Google Scholar]
- 17.Tuettenberg J, Grobholz R, Korn T, Wenz F, Erber R, Vajkoczy P. Continuous low-dose chemotherapy plus inhibition of cyclooxygenase-2 as an antiangiogenic therapy of glioblastoma multiforme. J Cancer Res Clin Oncol. 2005;131:31–40. doi: 10.1007/s00432-004-0620-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J, Kim J, Ko K, et al. Metronomic treatment of temozolomide inhibits tumor cell growth through reduction of angiogenesis and augmentation of apoptosis in orthotopic models of gliomas. Oncol Rep. 2006;16:33–39. [PubMed] [Google Scholar]
- 19.Zhao D, Jiang L, Hahn EW, Mason RP. Continuous low-dose (metronomic) chemotherapy on rat prostate tumors evaluated using MRI in vivo and comparison with histology. Neoplasia. 2005;7:678–687. doi: 10.1593/neo.04757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bello L, Carrabba G, Giussani C, et al. Low-dose chemotherapy combined with an antiangiogenic drug reduces human glioma growth in vivo. Cancer Res. 2001;61:7501–7506. [PubMed] [Google Scholar]
- 21.Kesari S, Schiff D, Doherty L, et al. Phase II study of metronomic chemotherapy for recurrent malignant gliomas in adults. Neuro-Oncology. 2007;9:354–363. doi: 10.1215/15228517-2007-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong DS, Lee JI, Kim WS, et al. A pilot study of metronomic temozolomide treatment in patients with recurrent temozolomide-refractory glioblastoma. Oncol Rep. 2006;16:1117–1121. [PubMed] [Google Scholar]
- 23.Keller SD, Majkut TC, Kosinski M, Ware JE., Jr. Monitoring health outcomes among patients with arthritis using the SF-36 Health Survey: overview. Med Care. 1999;37:MS1–MS9. doi: 10.1097/00005650-199905001-00001. [DOI] [PubMed] [Google Scholar]
- 24.MacDonald DR, Cascino TL, Schold SC, Jr,, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 25.Perry JR, Rizek P, Cashman R, Morrison M, Morrison T. Temozolomide rechallenge in recurrent malignant glioma by using a continuous temozolomide schedule: the “rescue” approach. Cancer. 2008;113:2152–2157. doi: 10.1002/cncr.23813. [DOI] [PubMed] [Google Scholar]
- 26.Wick W, Platten M, Weller M. New (alternative) temozolomide regimens for the treatment of glioma. Neuro-Oncology. 2009;11:69–79. doi: 10.1215/15228517-2008-078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tolcher AW, Gerson SL, Denis L, et al. Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br J Cancer. 2003;88:1004–1011. doi: 10.1038/sj.bjc.6600827. [DOI] [PMC free article] [PubMed] [Google Scholar]