Abstract
Central nervous system (CNS) germ cell tumors (GCT) have not been epidemiologically well described. Our study describes 2 population-based series of nonpineal CNS GCT. Data on all primary (malignant and nonmalignant) CNS (ICD-O-3 sites: C70.0–C72.9, C75.1–C75.3) GCT diagnosed between 2000 and 2004 from the Central Brain Tumor Registry of the United States (CBTRUS) and on all malignant GCT diagnosed between 1992 and 2005 from the Surveillance, Epidemiology, and End Results (SEER) were analyzed. Of 234 nonpineal GCT in CBTRUS, the most common site was brain, NOS (31.6%). Males had a greater frequency (59.7%) than females (40.3%). However, by age group, the male-to-female incidence rate ratio (IRR) differed: children (0–14 years) had an IRR of 1.1, young adults (15–29 years) an IRR of 2.3, and adults (aged 30+) an IRR of 1.0. For children and young adults, most tumors were malignant (86.8% and 89.0%, respectively), whereas for adults, more than half were nonmalignant (56.8%). Germinoma was the most frequent diagnosis (61.5%). In SEER, the frequency of malignant GCT in the CNS (2.5%) was greater than that in the mediastinum (2.1%). Of 408 malignant CNS GCT, 216 (52.9%) were nonpineal. The male-to-female IRR was 1.5. Overall relative survival for nonpineal CNS malignant GCT was 85.3% at 2 years, 77.3% at 5 years, and 67.6% at 10 years. Previous studies of GCT that have not stratified by site have suggested greater gender disparity. Nonpineal CNS GCT show no significant gender preference, yet have outcomes similar to pineal GCT.
Keywords: brain tumor, epidemiology, germ cell tumors, germinoma, teratoma
Most germ cell cancers are gonadal in origin. Other primary sites are commonly in midline structures, including mediastinal, retroperitoneal, sacral areas, or the pineal gland. Germ cell tumors (GCT) in these extra-gonadal sites share histopathologic features with gonadal GCT, and clinically, the treatment goal of no residual tumor (ie, cure) remains the same regardless of the site.1,2
Intracranial GCT can be divided pathologically into two histologic patterns: germinoma (GGCT) and nongerminoma GCT (NGGCT).3 GGCT are histologically identical to the gonadal counterparts of seminoma (testes) and dysgerminoma (ovary) and are highly sensitive to radiotherapy and chemotherapy with high cure rates. NGGCT, however, have a poorer prognosis. GGCT account for 55%–65% of intracranial GCT and NGGCT account for the remaining 35%–45%. NGGCT comprises a heterogeneous group of tumors that include pure or mixed (more common) populations of germ cell elements including embryonal carcinoma, endodermal sinus tumor, choriocarcinoma, malignant teratoma, and/or mature or immature teratoma.
The descriptive epidemiology of malignant GCT in the pineal gland has been previously published by our group.4 Data on 1467 cases of malignant pineal GCT were obtained using 3 different databases: the Surveillance, Epidemiology, and End Results (SEER) database; the Central Brain Tumor Registry of the United States (CBTRUS); and the National Cancer Data Base (NCDB). This included a vastly greater number of cases than previously published literature, which was mostly case reviews from single institutions or larger series that did not separate pineal germ cell cancers within central nervous system (CNS) germ cell cancers.2,5 Notable in our study was an unexpectedly high male-to-female ratio of 15:1 compared with the literature of 4:1.1,6–8 This increased male-to-female ratio led us to our current epidemiological investigation of nonpineal CNS germ cell cancers, whose incidence is nearly the same as pineal region germ cell cancers.4
Methods
Data on all primary (malignant and nonmalignant) GCT located in a brain or CNS site (ICD-O-3 site codes: C70.0–C72.9 and C75.1–C75.3)9 from the Central Brain Tumor Registry of the United States10 for cases diagnosed between 2000 and 2004 and data from all malignant GCT located in a brain or CNS site from the SEER (13 registries limited-use data set, April 2008)11 for cases diagnosed between 1992 and 2005 were analyzed. CBTRUS compiled population-based incidence data on all primary brain and CNS tumors, regardless of biologic behavior, representing approximately 30% of the US population. Data were provided to CBTRUS from 16 state cancer registries (AZ, CO, CT, DE, ID, ME, MA, MN, MT, NM, NY, NC, RI, TX, UT, and VA). The SEER program is sponsored by the National Cancer Institute and collected population-based incidence and survival data on all primary malignant cancers prior to 2004 and on all primary brain tumors, regardless of behavior, beginning in 2004. Five states (CT, HI, IA, NM, and UT) and 8 population-based areas/groups (Atlanta, Detroit, San Francisco–Oakland, Seattle–Puget Sound, San Jose–Monterey, rural Georgia, Alaska Natives, and Los Angeles) were included, representing approximately 14% of the US population. These two databases are not mutually exclusive (17 cases were included in both data sets); however, data analysis and interpretation were conducted separately for each database. Cases diagnosed at autopsy were excluded from both data sets.
Germ cell tumors were selected by using ICD-O-3 histology codes 9060–9091 and 9100. The identified cases were then grouped into the following histologic subcategories: germinoma (9060, 9061, 9064, and 9065), teratoma (9080, 9082, and 9084), and mixed germ cell tumor (9081 and 9085). The “other” histologic subcategory (9062, 9063, 9070–9073, 9083, 9090–9100) had too few cases to report as a group. CNS tumors were defined by ICD-O-3 site codes C70.0–C72.9 and C75.1–C75.3. Pineal were identified by site code C75.3, whereas nonpineal brain were identified by C70.0–C72.9, C75.1, and C75.2. Mediastinum tumors were identified by site codes C38.1–C38.3, and sacrococcygeal region tumors were identified by site codes C44.5, C47.5, C49.5, and C76.3. The suprasellar region was defined as any tumor occurring in the following site codes: C71.5 (ventricle), C71.9 (brain, NOS), C72.9 (other CNS, NOS), and C75.1 (pituitary). Age groups were defined in the following way: children (0–14 years), young adults (15–29 years), and adults (30+ years).
Frequencies and incidence rates were estimated for both data sets using the SEER*Stat 6.4.4 software.12 Incidence rates were age adjusted to the 2000 US standard population. Survival rates were estimated from the SEER data set for those with malignant nonpineal CNS GCT. Relative survival was defined as the observed probability of survival adjusted for the expected survival rate of the US population for that age, sex, and race. Survival time was calculated from the date of diagnosis to the date of death or last contact. Patients who were alive were censored at the date of last contact. Survival rates were not estimated for categories with fewer than 10 cases. SEER*Stat version 6.4.4 was utilized to obtain relative survival rates in 1-year intervals for a period of up to 10 years using the life-table method to properly account for right censoring. The Kaplan–Meier product-limit method was used to estimate observed survival in 1-year intervals for a period of up to 10 years, and comparisons were made using the log-rank test.
Evaluation of first course of treatment was limited to cases identified from SEER (surgery and radiation therapy). Chemotherapy, hormonal, and immunotherapy treatment variables were not available for analyses. The variable for radiation therapy was categorized as “yes” (those patients who received any form of radiation therapy) or “no” (patients who received no radiation therapy). The variable for surgery was defined as “yes” (patients receiving any type of definitive or cancer-directed surgery) or “no” (patients who did not undergo definitive or cancer-directed surgery or who received exploratory or biopsy surgery only).
To capture the maximum number of article citations, we conducted a MEDLINE and PubMed search using keywords “CNS and Germ Cell Tumors”. This resulted in 2111 references. Relevant abstracts were identified for further review. Only 18 studies contained division of GCT by gender and location. Information was extracted regarding patient's gender, histology, and location of tumor.
Results
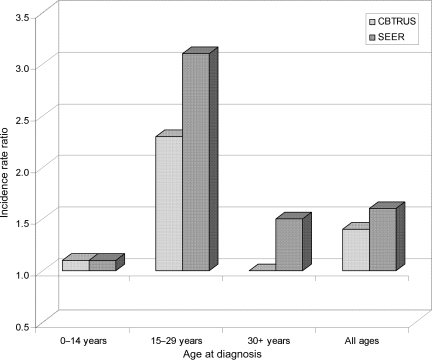
Three hundred and sixty-three malignant and nonmalignant GCT were identified in the CBTRUS data. Of these, 129 (35.5%) were located in the pineal region. Of the remaining nonpineal GCT (n = 234; Table 1), the most common site was the brain, NOS (31.6%), followed by the ventricles (17.1%), the pituitary (14.1%), and the cerebrum (9.8%). Sites comprising the suprasellar region accounted for 64.5% of the nonpineal GCT. The majority of nonpineal CNS GCT were found in those aged 0–14 years (45.3%), followed by those aged 15–29 years (38.9%), with adults over age 30 having the lowest frequency (15.8%). For both children and young adults, the majority of their tumors were malignant (86.8% and 89.0%, respectively), whereas for adults, more than half of the GCT were nonmalignant (56.8%). Germinoma (61.5%) was the most frequent diagnosis, followed by teratoma (27.8%) and mixed GCT (8.5%). The overall incidence rate for nonpineal GCT based on the CBTRUS data was 0.051/100 000 (95% CI: 0.045–0.058), which is significantly higher than the incidence of pineal GCT (0.028, 95% CI: 0.023–0.033). Overall, males had a greater frequency and a significantly higher incidence of nonpineal GCT than females (incidence rate ratio [IRR] = 1.40, P = .01; Table 1). However, the male-to-female IRR differed by age group, with children (0–14 years) having an IRR = 1.1 (P > .05), young adults (15–29 years) an IRR = 2.3 (P < .005), and adults (30+ years) an IRR = 1.0 (P > .05; Fig. 1). Male-to-female IRRs also differed by behavior, with males having a significantly higher incidence of malignant nonpineal GCT (IRR = 1.64; P = .001), but a lower incidence of nonmalignant tumors, all of which were teratomas (IRR = 0.73; P > .05).
Table 1.
Frequencies and incidence rates for nonpineal CNS germ cell tumors from CBTRUS (2000–2004) and SEER (1992–2005; 13 registries) data
| Characteristics | CBTRUS (malignant and nonmalignant) |
SEER (malignant only) |
||
|---|---|---|---|---|
| n (%) | Incidence (95% CI) | n (%) | Incidence (95% CI) | |
| Male | 139 (59.4) | 0.059 (0.050, 0.070) | 134 (62.0) | 0.048 (0.040, 0.057) |
| Female | 95 (40.6) | 0.042 (0.034, 0.052) | 82 (38.0) | 0.031 (0.024, 0.038) |
| 0–14 years | 106 (45.3) | 0.109 (0.089, 0.132) | 120 (55.5) | 0.103 (0.085, 0.123) |
| 15–29 years | 91 (38.9) | 0.096 (0.077, 0.117) | 74 (34.3) | 0.067 (0.052, 0.084) |
| 30+ years | 37 (15.8) | 0.014 (0.010, 0.019) | 22 (10.2) | 0.007 (0.004, 0.010) |
| White | 188 (80.3) | 0.051 (0.044, 0.059) | 153 (70.8) | 0.037 (0.032, 0.044) |
| Black | N/A | N/A | 18 (8.3) | 0.026 (0.016, 0.042) |
| Other | 31 (13.3) | 0.124 (0.084, 0.179) | 45 (20.8) | 0.066 (0.048, 0.089) |
| Malignant | 189 (80.8) | 0.041 (0.035, 0.047) | 216 (100) | 0.039 (0.034, 0.045) |
| Nonmalignant | 45 (19.2) | 0.010 (0.007, 0.013) | — | — |
| Selected primary sites | ||||
| Suprasellar regiona | 151 (64.5) | 0.033 (0.028, 0.039) | 130 (60.2) | 0.024 (0.020, 0.028) |
| Ventricle | 40 (17.1) | 0.009 (0.006, 0.012) | 24 (11.1) | 0.004 (0.003, 0.006) |
| Brain (NOS) | 74 (31.6) | 0.016 (0.013, 0.020) | 68 (31.5) | 0.013 (0.010, 0.016) |
| Pituitary gland | 33 (14.1) | 0.007 (0.005, 0.010) | 38 (17.6) | 0.007 (0.005, 0.010) |
| Cerebrum | 23 (9.8) | 0.005 (0.003, 0.007) | 34 (15.7) | 0.006 (0.004, 0.009) |
| Selected histologies | ||||
| Germinoma | 144 (61.5) | 0.031 (0.026, 0.037) | 154 (71.6) | 0.028 (0.024, 0.033) |
| Teratoma | 65 (27.8) | 0.014 (0.011, 0.018) | 31 (14.4) | 0.006 (0.004, 0.008) |
| Mixed germ cell tumor | 20 (8.5) | 0.004 (0.003, 0.007) | 22 (10.2) | 0.004 (0.003, 0.006) |
N/A, Numbers too small to report.
aIncludes all tumors in site codes C71.5, C71.9, C72.9, and C75.1.
Fig. 1.
Male-to-female IRRs by age at diagnosis for CBTRUS (2000–2004) and SEER (1992–2005) data.
Four hundred and eight malignant GCT were identified in CNS site codes in the SEER data. As a comparison, the number of cases identified in the mediastinum (n = 349) and the sacrococcygeal region (n = 99) are presented in Table 2. Of the CNS malignant GCT, 216 (52.9%) were nonpineal (Table 1), with the most common site being brain, NOS (31.5%), followed by pituitary (17.6%), cerebrum (15.7%), and ventricles (11.1%). Over a half of nonpineal CNS GCT were found in those aged 0–14 years (55.5%), and the incidence progressively decreased in the young adult and adult groups. Stratified by race, the highest incidence rates were found in the “Other” race category, followed by Whites and Blacks. As in the CBTRUS data, germinoma was the most frequently diagnosed histology. Sixty-two percent of nonpineal malignant CNS GCT occurred in males, with a male:female IRR of 1.6. A pattern similar to that found in the CBTRUS data was seen for male:female IRRs with the IRR in young adults statistically significantly higher than the IRR in children, and higher than the IRR in adults, although the difference was not statistically significant (Fig. 1).
Table 2.
Number of malignant germ cell tumor cases at select sites; SEER data (1992–2005; 13 registries)
| First or only primary (n [%]) | All primary tumors (n [%]) | |
|---|---|---|
| All germ cell tumors | 15 824 | 16 377 |
| CNS | 407 (2.6) | 408 (2.5) |
| Pineal | 192 (1.2) | 192 (1.2) |
| Nonpineal brain | 215 (1.4) | 216 (1.3) |
| Mediastinum | 339 (2.1) | 349 (2.1) |
| Sacrococcygeal region | 99 (0.6) | 99 (0.6) |
A literature review was performed to assess if a lower male:female frequency ratio had been present in the literature for nonpineal CNS GCT, but unidentified due to the limited number of cases in each study. Eighteen studies provided location of tumor, gender, and histology. A total of 123 cases were identified that did not involve the pineal region and resulted in a male:female frequency ratio of 1.0 (Table 3).
Table 3.
Summary of published literature on male-to-female ratios for nonpineal germ cell tumors of the CNS
| Author | Year | Nonpineal GCT | M:F cases |
|---|---|---|---|
| Allen et al.26 | 1987 | Suprasellar (6F) | 1:06 |
| Fourth ventricle (1M) | |||
| Chang et al.27 | 1989 | Suprasellar (7M/4F) | 9:04 |
| Parasellar (1M) | |||
| Basal ganglia (2M) | |||
| Nakagawa et al.28 | 1992 | Suprasellar (1M/1F) | 1:01 |
| Fuller et al.29 | 1994 | Third ventricle (1M) | 3:02 |
| Suprasellar (2M/2F) | |||
| Chang et al.30 | 1995 | Suprasellar (4F) | 0:06 |
| Thalamus (2F) | |||
| Robertson et al.31 | 1997 | Suprasellar (1M/4F) | 1:05 |
| Cerebral hemisphere (1F) | |||
| Baranzelli et al.32 | 1997 | Suprasellar (3M/7F) | 5:07 |
| Tentorial (2M) | |||
| Bamberg et al.33 | 1999 | Suprasellar (1M) | 1:01 |
| Third ventricle (1F) | |||
| Tada et al.34 | 1999 | Suprasellar (2M/2F) | 2:02 |
| Ushio et al.35 | 1999 | Suprasellar (4F) | 0:04 |
| Merchant et al.36 | 2000 | Suprasellar (3M/2F) | 3:02 |
| Sugiyama et al.37 | 2001 | Fourth ventricle (1M ) | 8:08 |
| Neurohypophesis (5M/7F) | |||
| Basal ganglia (2M/1F) | |||
| Janmohamed et al.38 | 2002 | Suprasellar (6M/5F) | 6:05 |
| Kellie et al.39 | 2004 | Nonpineal (6M/6F) | 1:01 |
| Kellie et al.40 | 2004 | Suprasellar/frontal lobe (1M) | 8:01 |
| Suprasellar (6M/1F) | |||
| Other (1M) | |||
| Phi et al.41 | 2005 | Foramen of monro (1F) | 2:01 |
| Temporal (1M) | |||
| T4–T12 (1M) | |||
| Crawford et al.42 | 2007 | Suprasellar (2M/5F) | 4:06 |
| Periventricule/mixed (2M/1F) | |||
| Wong et al.43 | 2008 | Thalamus (6M) | 6:00 |
| Total | 61:62 (ratio 0.98:1) | ||
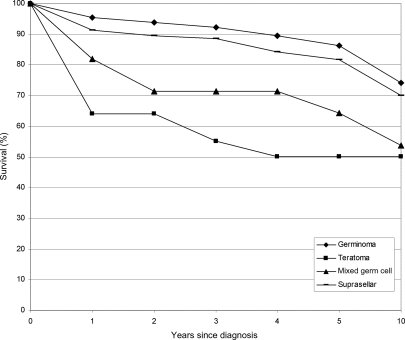
Survival data were available from the SEER (1992–2005) database for 207 malignant nonpineal CNS GCT. Overall relative survival was 87.9% at 1 year, 85.3% at 2 years, 77.3% at 5 years, and 67.6% at 10 years. At 5 years postdiagnosis, relative survival rates did not differ significantly from each other by site (location), gender, or race (data not shown). Survival rates did differ by histology, however, with germinomas having the highest 5-year survival (86.4%) and teratomas having the lowest 5-year survival (50.2%; Fig. 2).
Fig. 2.
Survival estimates in those with malignant nonpineal GCT by histology and, separately, for the suprasellar region using SEER (1992–2005) data.
Information on radiation therapy as a first course of treatment was available for 203 malignant nonpineal CNS GCT. Of these, 156 (76.8%) received some form of radiation treatment, whereas 47 received no radiation treatment. Five-year relative survival was significantly better for those who received radiation treatment (86.5%) than for those who did not (47.2%). Information on site-specific surgery was only available for 89 subjects. Of these, 52 (58.4%) received some form of surgery. Five-year relative survival estimates did not significantly differ between those who received some form of surgery and those who did not (71.3% vs 83.7%, respectively).
Discussion
CNS GCT are highly treatable and an understanding of the biology gives insight to the disease. Our epidemiology findings, with support from the review of the literature, demonstrate different gender incidence patterns dependent on site incidence. Why this is so within a complex structure as the brain is unknown, but with the young age of most patients, it may be likely rooted in CNS development.
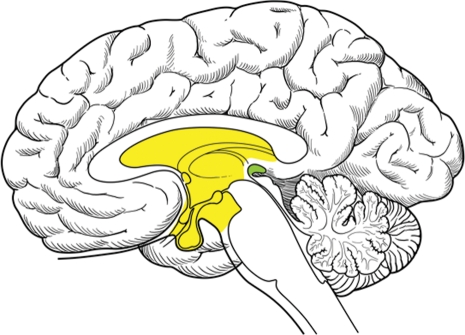
There are two prevailing theories on the development of CNS germ cells. The “germ cell theory” proposes that primordial germ cells (PGCs) are misplaced in migration and are the same both intracranially and extracranially.13 The PGCs appear in the yolk sac of the embryo during the 3–4th week of gestation and although their normal destination (via the dorsal mesentery of the hindgut) is the ovaries or testes, they may aberrantly migrate and rest mainly in midline sites, such as the mediastinum, sacrococcygeal region, and the third ventricle (Fig. 3).14–16 The other theory proposes a widespread distribution of germ cells during normal embyrogenesis in the brain, thymus, liver, and bone marrow, and that these cells provide important regulatory functions at these sites and are biologically distinct from PGC.17 Intracranial germ cell cancers, therefore, are part of an endogenous neural progenitor cell population, and distinct from extracranial PGCs. To investigate these possibilities, micro-RNA (miRNA) expression patterns have been utilized to identify tumor tissue of origin, as miRNA often have a critical role in cellular regulation.18 Murray et al. analyzed miRNA data on 48 samples that included 34 pediatric samples of malignant CNS GCT. When compared with gonadal germ cell cancers, a distinct miRNA expression (known as a heat map) for CNS germinomas was found (P < .001).19 However, previous analyses at the genomic-level have indicated CNS GCT are similar to gonadal germ cells,20,21 leaving the debate unresolved.
Fig. 3.
Sagittal view of brain, demonstrating common sites for primary CNS germ cell involvement with the pineal gland in green and the suprasellar and the third ventricular area in yellow.
As to why a large gender difference is present for malignant germ cell cancer in the pineal gland (15:1),4 but not the rest of the CNS is left for speculation. It is possible that germ cell progenitor cells are present at both sites (pineal and suprasellar) equally in both genders, but neoplastic transformation preferentially occurs in males in the pineal gland (eg, due to physiological/hormonal changes in puberty). Alternatively, the pineal region in males may have a unique developmental attraction for germ cell progenitor cells, leading to malignant transformation in a small percentage. Analysis of the male-to-female IRR for all primary (malignant and nonmalignant) CNS GCT from the CBTRUS data was 2.23, whereas the IRR for malignant CNS GCT from the SEER data was 3.27. These male-to-female ratios of all primary CNS GCT are more in line with other published accounts of a male-to-female ratio of 4:1.1,6–8
Gender differences are common in germ cell cancers: the overall male-to-female incidence ratio of malignant GCT in the SEER data was about 9 to 1. The mediastinum, a common extragonadal site for germ cell disease, also has clear gender differences. Similar to the higher incidence of malignant and lower incidence of nonmalignant nonpineal CNS GCT in males presented here, most (>90%) of malignant GCT in the mediastinum occur in males, but benign GCT (mature teratomas) occur in approximately equal frequency in males and females.15,22,23 In the SEER data presented here, the male-to-female IRR was 16.7 for malignant GCT in the mediastinum (data not shown). In gonadal GCT, there are also notable differences. In females, GCT are slightly more common, however, benign GCT (mature teratomas or dermoid tumors) predominate.24 Similarly, in the sacrococcygeal region, the incidence of malignant GCT predominates in females, with a male-to-female IRR of 0.63 in the SEER data presented here. Overall, malignant GCT are more common in males and are increasing in incidence, whereas in females the incidence is decreasing.24,25
Our findings from the SEER data demonstrate a slightly larger number of primary GCT in the CNS than in the mediastinum, which was unexpected, as the latter is considered to have the highest incidence of extragonadal GCT.15,23 However, our SEER analysis excluded benign GCT, which are mature/immature teratomas. Although the number of cases is small, the estimates are consistent with both sites being routinely involved.
The limitations regarding our data include a small degree of case overlap between registries (17 cases were included in both datasets). To prevent biasing the results, data from the two databases were not combined and each database was analyzed and results were presented and interpreted separately. We also recognize that registry information lacks centralized imaging review, for validation of site location. It is possible that inherent, although unknown to us, bias within the registry data has led to our current gender findings; for example, awareness of the anatomy and association with germ cell cancers and gender by the clinical team with translation to the tumor registrars. However, our findings are corroborated by findings on the literature review.
In summary, our findings of a smaller than previously reported gender difference for nonpineal CNS germ cell may indicate fundamental differences in the developmental biology of intracranial GCT, as the CNS is a common primary site for extragonadal involvement. Nonpineal CNS appears to be a site where GCT do not have large gender differences compared with pineal and mediastinum GCT.
Funding
This research was supported by the Valerie Landis Fund of the University of Illinois, Chicago, and by the Central Brain Tumor Registry of the United States.
Acknowledgments
The authors thank the participating registries of the Central Brain Tumor Registry of the United States for the use of these data: Arizona Cancer Registry; Colorado Central Cancer Registry; Connecticut Tumor Registry; Delaware Cancer Registry; Cancer Data Registry of Idaho; Maine Cancer Registry; Massachusetts Cancer Registry; Minnesota Cancer Surveillance System; Montana Central Tumor Registry; New Mexico Tumor Registry; New York State Cancer Registry; North Carolina Central Cancer Registry; Rhode Island Cancer Registry; South Dakota Cancer Registry; Texas Cancer Registry; Utah Cancer Registry; Virginia Cancer Registry; West Virginia Cancer Registry. We would also like to thank Christa Williams for her illustration and design work.
Conflict of interest statement. None declared.
References
- 1.Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg. 1985;63:155–167. doi: 10.3171/jns.1985.63.2.0155. [DOI] [PubMed] [Google Scholar]
- 2.Packer RJ, Cohen BH, Cooney K. Intracranial germ cell tumors. Oncologist. 2000;5:312–320. [PubMed] [Google Scholar]
- 3.Calaminus G, Bamberg M, Baranzelli MC, et al. Intracranial germ cell tumors: a comprehensive update of the European data. Neuropediatrics. 1994;25:26–32. doi: 10.1055/s-2008-1071577. [DOI] [PubMed] [Google Scholar]
- 4.Villano JL, Propp JM, Porter KR, et al. Malignant pineal germ-cell tumors: an analysis of cases from three tumor registries. Neuro-Oncology. 2008;10:121–130. doi: 10.1215/15228517-2007-054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsutani M, Sano K, Takakura K, et al. Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg. 1997;86:446–455. doi: 10.3171/jns.1997.86.3.0446. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman HJ, Otsubo H, Hendrick EB, et al. Intracranial germ-cell tumors in children. J Neurosurg. 1991;74:545–551. doi: 10.3171/jns.1991.74.4.0545. [DOI] [PubMed] [Google Scholar]
- 7.Kuratsu J, Takeshima H, Ushio Y. Trends in the incidence of primary intracranial tumors in Kumamoto, Japan. Int J Clin Oncol. 2001;6:183–191. doi: 10.1007/pl00023928. [DOI] [PubMed] [Google Scholar]
- 8.Drummond KJ, Rosenfeld JV. Pineal region tumours in childhood. A 30-year experience. Childs Nerv Syst. 1999;15:119–126. doi: 10.1007/s003810050347. discussion 127. [DOI] [PubMed] [Google Scholar]
- 9.Fritz AG World Health Organization. 3rd. Geneva: World Health Organization; 2000. International classification of diseases for oncology : ICD-O. [Google Scholar]
- 10.CBTRUS. Hinsdale, IL: Central Brain Tumor Registry of the United States; 2008. Statistical report: primary brain tumors in the United States, 2000–2004. [Google Scholar]
- 11.Surveillance Epidemiology, and End Results (SEER) Program. http://seer.cancer.gov , SEER*Stat Database: Incidence – SEER 13 Regs Limited-Use, Nov 2007 Sub (1992–2005) <Katrina/Rita Population Adjustment> – Linked To County Attributes – Total U.S., 1969–2005 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2008, based on the November 2007 submission; 2008. [Google Scholar]
- 12.Surveillance Research Program SEER*Stat software. 2008 www.seer.cancer.gov/seerstat. (ed version 6.4.4.). National Cancer Institute. [Google Scholar]
- 13.Willis RA. The borderland of embryology and pathology. Bull N Y Acad Med. 1950;26:440–460. [PMC free article] [PubMed] [Google Scholar]
- 14.Nichols CR, Fox EP. Extragonadal and pediatric germ cell tumors. Hematol Oncol Clin North Am. 1991;5:1189–1209. [PubMed] [Google Scholar]
- 15.Luna MA, Valenzuela-Tamariz J. Germ-cell tumors of the mediastinum, postmortem findings. Am J Clin Pathol. 1976;65:450–454. doi: 10.1093/ajcp/65.4.450. [DOI] [PubMed] [Google Scholar]
- 16.Malogolowkin MH, Mahour GH, Krailo M, et al. Germ cell tumors in infancy and childhood: a 45-year experience. Pediatr Pathol. 1990;10:231–241. doi: 10.3109/15513819009067110. [DOI] [PubMed] [Google Scholar]
- 17.Friedman NB. The function of the primordial germ cell in extragonadal tissues. Int J Androl. 1987;10:43–49. doi: 10.1111/j.1365-2605.1987.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 18.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 19.Murray M, Palmer R, Muralidhar B, et al. Micro-RNA profiling suggests intracranial malignant germ cell tumors (MGCTS) are biologically distinct from their extracranial counterparts. Neuro-Oncology. 2008;10 abstract ISPNO GCT 9. [Google Scholar]
- 20.Rickert CH, Simon R, Bergmann M, et al. Comparative genomic hybridization in pineal germ cell tumors. J Neuropathol Exp Neurol. 2000;59:815–821. doi: 10.1093/jnen/59.9.815. [DOI] [PubMed] [Google Scholar]
- 21.Schneider DT, Schuster AE, Fritsch MK, et al. Multipoint imprinting analysis indicates a common precursor cell for gonadal and nongonadal pediatric germ cell tumors. Cancer Res. 2001;61:7268–7276. [PubMed] [Google Scholar]
- 22.Moran CA, Suster S. Primary germ cell tumors of the mediastinum: I. Analysis of 322 cases with special emphasis on teratomatous lesions and a proposal for histopathologic classification and clinical staging. Cancer. 1997;80:681–690. [PubMed] [Google Scholar]
- 23.Nichols CR. Mediastinal germ cell tumors. Clinical features and biologic correlates. Chest. 1991;99:472–479. doi: 10.1378/chest.99.2.472. [DOI] [PubMed] [Google Scholar]
- 24.Smith HO, Berwick M, Verschraegen CF, et al. Incidence and survival rates for female malignant germ cell tumors. Obstet Gynecol. 2006;107:1075–1085. doi: 10.1097/01.AOG.0000216004.22588.ce. [DOI] [PubMed] [Google Scholar]
- 25.Walsh TJ, Grady RW, Porter MP, et al. Incidence of testicular germ cell cancers in U.S. children: SEER program experience 1973 to 2000. Urology. 2006;68:402–405. doi: 10.1016/j.urology.2006.02.045. discussion 405. [DOI] [PubMed] [Google Scholar]
- 26.Allen JC, Kim JH, Packer RJ. Neoadjuvant chemotherapy for newly diagnosed germ-cell tumors of the central nervous system. J Neurosurg. 1987;67:65–70. doi: 10.3171/jns.1987.67.1.0065. [DOI] [PubMed] [Google Scholar]
- 27.Chang T, Teng MM, Guo WY, et al. CT of pineal tumors and intracranial germ-cell tumors. AJNR Am J Neuroradiol. 1989;10:1039–1044. [PMC free article] [PubMed] [Google Scholar]
- 28.Nakagawa K, Aoki Y, Akanuma A, et al. Radiation therapy of intracranial germ cell tumors with radiosensitivity assessment. Radiat Med. 1992;10:55–61. [PubMed] [Google Scholar]
- 29.Fuller BG, Kapp DS, Cox R. Radiation therapy of pineal region tumors: 25 new cases and a review of 208 previously reported cases. Int J Radiat Oncol Biol Phys. 1994;28:229–245. doi: 10.1016/0360-3016(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 30.Chang TK, Wong TT, Hwang B. Combination chemotherapy with vinblastine, bleomycin, cisplatin, and etoposide (VBPE) in children with primary intracranial germ cell tumors. Med Pediatr Oncol. 1995;24:368–372. doi: 10.1002/mpo.2950240606. [DOI] [PubMed] [Google Scholar]
- 31.Robertson PL, DaRosso RC, Allen JC. Improved prognosis of intracranial non-germinoma germ cell tumors with multimodality therapy. J Neurooncol. 1997;32:71–80. doi: 10.1023/a:1005732105727. [DOI] [PubMed] [Google Scholar]
- 32.Baranzelli MC, Patte C, Bouffet E, et al. Nonmetastatic intracranial germinoma: the experience of the French Society of Pediatric Oncology. Cancer. 1997;80:1792–1797. [PubMed] [Google Scholar]
- 33.Bamberg M, Kortmann RD, Calaminus G, et al. Radiation therapy for intracranial germinoma: results of the German cooperative prospective trials MAKEI 83/86/89. J Clin Oncol. 1999;17:2585–2592. doi: 10.1200/JCO.1999.17.8.2585. [DOI] [PubMed] [Google Scholar]
- 34.Tada T, Takizawa T, Nakazato F, et al. Treatment of intracranial nongerminomatous germ-cell tumor by high-dose chemotherapy and autologous stem-cell rescue. J Neurooncol. 1999;44:71–76. doi: 10.1023/a:1006395719917. [DOI] [PubMed] [Google Scholar]
- 35.Ushio Y, Kochi M, Kuratsu J, et al. Preliminary observations for a new treatment in children with primary intracranial yolk sac tumor or embryonal carcinoma. Report of five cases. J Neurosurg. 1999;90:133–137. doi: 10.3171/jns.1999.90.1.0133. [DOI] [PubMed] [Google Scholar]
- 36.Merchant TE, Sherwood SH, Mulhern RK, et al. CNS germinoma: disease control and long-term functional outcome for 12 children treated with craniospinal irradiation. Int J Radiat Oncol Biol Phys. 2000;46:1171–1176. doi: 10.1016/s0360-3016(99)00375-2. [DOI] [PubMed] [Google Scholar]
- 37.Sugiyama K, Arita K, Tominaga A, et al. Morphologic features of human chorionic gonadotropin- or alpha-fetoprotein-producing germ cell tumors of the central nervous system: histological heterogeneity and surgical meaning. Brain Tumor Pathol. 2001;18:115–122. doi: 10.1007/BF02479424. [DOI] [PubMed] [Google Scholar]
- 38.Janmohamed S, Grossman AB, Metcalfe K, et al. Suprasellar germ cell tumours: specific problems and the evolution of optimal management with a combined chemoradiotherapy regimen. Clin Endocrinol (Oxf) 2002;57:487–500. doi: 10.1046/j.1365-2265.2002.01620.x. [DOI] [PubMed] [Google Scholar]
- 39.Kellie SJ, Boyce H, Dunkel IJ, et al. Intensive cisplatin and cyclophosphamide-based chemotherapy without radiotherapy for intracranial germinomas: failure of a primary chemotherapy approach. Pediatr Blood Cancer. 2004;43:126–133. doi: 10.1002/pbc.20026. [DOI] [PubMed] [Google Scholar]
- 40.Kellie SJ, Boyce H, Dunkel IJ, et al. Primary chemotherapy for intracranial nongerminomatous germ cell tumors: results of the second international CNS germ cell study group protocol. J Clin Oncol. 2004;22:846–853. doi: 10.1200/JCO.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Phi JH, Kim SK, Park SH, et al. Immature teratomas of the central nervous system: is adjuvant therapy mandatory? J Neurosurg. 2005;103:524–530. doi: 10.3171/ped.2005.103.6.0524. [DOI] [PubMed] [Google Scholar]
- 42.Crawford JR, Santi MR, Vezina G, et al. CNS germ cell tumor (CNSGCT) of childhood: presentation and delayed diagnosis. Neurology. 2007;68:1668–1673. doi: 10.1212/01.wnl.0000261908.36803.ac. [DOI] [PubMed] [Google Scholar]
- 43.Wong TT, Chen YW, Guo WY, et al. Germinoma involving the basal ganglia in children. Childs Nerv Syst. 2008;24:71–78. doi: 10.1007/s00381-007-0495-2. [DOI] [PubMed] [Google Scholar]