Abstract
Glioblastoma is the most common malignant brain tumor in adults. The currently available treatments offer only a palliative survival advantage and the need for effective treatments remains an urgent priority. Activation of the p53 growth suppression/apoptotic pathway is one of the promising strategies in targeting glioma cells. We show that the quinoline derivative chloroquine activates the p53 pathway and suppresses growth of glioma cells in vitro and in vivo in an orthotopic (U87MG) human glioblastoma mouse model. Induction of apoptosis is one of the mechanisms underlying the effects of chloroquine on suppressing glioma cell growth and viability. siRNA-mediated downregulation of p53 in wild-type but not mutant p53 glioblastoma cells substantially impaired chloroquine-induced apoptosis. In addition to its p53-activating effects, chloroquine may also inhibit glioma cell growth via p53-independent mechanisms. Our results clarify the mechanistic basis underlying the antineoplastic effect of chloroquine and reveal its therapeutic potential as an adjunct to glioma chemotherapy.
Keywords: apoptosis, chloroquine, glioma, p53, transcription
Glioblastoma is the most common malignant brain tumor in adults. Owing to its notorious radiation- and chemoresistance, recurrence of glioblastomas following surgical resection and adjuvant radiation- and chemotherapy is inevitable, with an invariably lethal outcome. The currently available treatments offer only a palliative survival advantage, underscoring the need for effective treatments being an urgent priority.1,2 Targeting intracellular signaling pathways involved in the regulation of the growth and viability of glioma cells is the molecular basis of several experimental therapeutic strategies.3–5 In the past decade, activation of the endogenous p53 growth inhibitory pathway or restoration of p53 functions in glioma cells by introduction of exogenous wild-type p53 (wtp53) has been an intensively explored strategy to suppress glioma progression.6–10 The tumor suppressor p53 can potently inhibit cell growth by inducing a transient or permanent block of proliferation or by activating cell death programs in response to different types of cellular stress, which has provided a rationale for p53-based anticancer therapies.11–13 Further, the relevance of p53-based therapies for glioma treatment is highlighted by the fact that p53—in contrast to most other solid tumors—is infrequently mutated in primary or de novo glioblastomas (less than 30% mutations in the TP53 gene,14 the most frequently occurring form of this tumor).15 A phase I trial provided compelling evidence that re-establishment of wtp53 functions by introduction of exogenous wtp53 is a feasible approach.7,10 However, expression of recombinant wtp53 in glioma cells effectively activates the p53-dependent cell cycle checkpoints, but fails to induce apoptosis,10 which from a therapeutic point of view would be the most desired outcome.16 An alternative approach to activate the p53-dependent apoptotic response is based on the ability of some agents to activate the endogenous p53 pathway either by DNA-damaging agents or by the agents that can stabilize p53 protein in the absense of DNA damage.17 In this context, the potential antitumor effects of quinolines have recently attracted considerable interest.18–20 Chloroquine is an aminoquinolinic membrane-penetratable agent capable of intercalating into double-stranded DNA without causing physical damage to the DNA.21 Owing to its weak base properties, chloroquine also accumulates in lysosomes and may trigger apoptosis via the inhibition of autophagic protein degradation.22–26 Widely known as an antimalarial and antirheumatoid drug, chloroquine has recently emerged as a potential anticancer agent. The cytotoxic effects of chloroquine have been demonstrated for tumor cells derived from different types of human cancers.22,23,27,28 The effects of chloroquine on glioma cells have not been systematically investigated previously, but there is empirical evidence that chloroquine may suppress clinical glioma progression by unknown mechanisms.29,30 Prompted by these findings, we have examined the effects of chloroquine on the growth and viability of glioma cells in vitro and in vivo. In this study, we demonstrate that chloroquine induces apoptosis in glioma cells in vitro and suppresses the growth of experimental gliomas in vivo. Our results demonstrate that chloroquine treatment results in a sustained stabilization of the p53 protein and induces the transcriptional activity of p53 in glioma cells. Further, we show that chloroquine shows cytotoxic activity independent of activation of the p53 pathway in cells with deficient p53 function, although less efficiently compared with glioma cells with functional wtp53.
Materials and Methods
Cells and Antibodies
The human glioma cell lines used in the study have been previously characterized with respect to their p53 functional status.31 Cells were propagated in minimal essential medium (Biochem) supplemented with 10% fetal calf serum. A concentrated chloroquine solution was prepared for each experiment by dissolving the sodium salt of chloroquine in PBS, filter-sterilized, and diluted to the desired concentration in cell culture medium. Cells were harvested at the indicated time points after chloroquine treatment, washed in ice-cold PBS, and lysed in SDS cell lysis buffer (50 mmol/L Tris–HCl, pH 8.0, 150 mmol/L NaCl, and 1% SDS) containing protease inhibitors (Roche). Human p53 was detected by the antibody DO-7 (BD Pharmingen) or the phosphorylation-sensitive antibody 16G8 recognizing p53 protein phosphorylated at Ser15 (Cell Signaling Technology, Inc.). Other antibodies used in the study included those against p21, mdm2, TBP (Santa Cruz Biotechnology), pig3 (Calbiochem), α-tubulin (Oncogene), bax (Upstate), or cleaved caspase-3 (Cell Signaling Technology, Inc.). For Western blot analyses, cells were lysed in SDS-containing cell lysis buffer supplemented with protease inhibitors. The protein concentration was determined using the Bradford reagent (Sigma-Aldrich) and equalized by using SDS lysis buffer.
Assessment of Cell Growth, Cell Death, and Apoptosis
To assess the effects of chloroquine on cell growth, cells were seeded in 96-well plates at a density of 2.5 × 103 cells/well 1 day before treatment. After 24 hours of incubation, treatment with chloroquine was started by addition of chloroquine at the desired concentration to the medium. After 24 hours of incubation with chloroquine, cells were washed with sterile PBS and replenished with fresh medium. Cells in 6 replicate wells were fixed with 3% glutaraldehyde at 24-hour intervals. After 8 consecutive days, fixed cells were stained with the DNA dye crystal violet, washed with PBS, and the dye was solubilized in buffer containing 1% SDS. Absorbance was measured at 560 nm and plotted versus incubation time. To assess cell death, the percentage of nonviable cells was determined by the trypan blue exclusion assay. To estimate rates of apoptosis, the percentage of apoptotic cells was determined by counting the number of immunostained cells positive for activated caspase-3. Apoptotic DNA fragmentation was assessed by immunofluorescence detection of TdT-mediated dUTP nick-end labeling (TUNEL)-positive cells (ApoAlert™ DNA Fragmentation Assay Kit, Clontech, TAKARA Bio). To evaluate the effects of chloroquine on the integrity of the mitochondrial membrane function, untreated or chloroquine-treated cells were stained with the fluorescent cationic dye (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide JC-1; Mitochondrial Membrane Potential Detection Kit, Stratagene), which forms red fluorescent aggregates in the mitochondria of healthy cells but not in the apoptotic cells.32 Red (excitation 550 nm, emission 600 nm) and green (excitation 485 nm, emission 535 nm) fluorescence were measured using a Spectrafluor plate reader (TECAN) to determine the red/green fluorescence ratios.
siRNA Transfections and Immunofluorescence Staining
Cells were seeded on cover slips in 24-well tissue cuture plates at a density of 0.6–1.0 × 105 cells/mL for at least 24 hours prior to transfection. Cells were transfected with commercially validated p53-siRNA (TP53 Validated Stealth, Invitrogen) or unspecific scrambled siRNA (Stealth Negative Control, Invitrogen) using the Lipofectamin 2000 reagent (Invitrogen) according to the supplier's recommendations. For immunofluorescence staining, cells were washed with PBS, fixed with a 4% paraformaldehyde in PBS, and permeabilized in cold acetone/methanol (1:1) mixture overnight. Paraformaldehyde-fixed and permeabilized cells were washed in 0.5% BSA in PBS, blocked in the same buffer, and incubated with anticleaved caspase-3 antibody at +4°C overnight. Washed cells were then incubated with Alexa Fluor 555-conjugated goat antirabbit antibody (Molecular Probes Inc.) for 30 min at room temperature followed by three PBS washes. Finally, washed cells were counterstained with DAPI.
Orthotopic Glioma Model and Chloroquine Treatment
All procedures were performed in accordance with the institutional guidelines for animal welfare and experimental conduct. For intracranial implantation, U87MG cells were harvested from monolayer culture, washed twice, and resuspended in PBS at a concentration of 0.5 × 105/µL. Prior to implantation, nude mice (NMRI, Taconic Europe) were anesthetized by a peritoneal injection of a ketamine/xylazine mixture (120 mg ketamine and 16 mg xylazine in 10 mL of PBS) at 0.1 mL/10 g of body weight. For implantation, the cranium was fixed in a stereotactic frame (TSE Systems). Cells were injected into the caudato-putamen of the right-brain hemisphere using the following stereotactic coordinates in reference to the bregma: 1 mm (anteroposterior axis), 3 mm (lateromedial axis), and 2.5 mm (vertical axis). A threshold dose of chloroquine with respect to acute brain toxicity was established by injection of 5 µL of 0.7, 7.0, 30, or 70 mM chloroquine solutions into the internal capsule of the right-brain hemisphere of ketamine-anesthesized mice. At 70 mM, repetitive injections of chloroquine were found to be toxic, as they provoked seizures in two out of three mice. At 30 mM, repetitive injections of chloroquine were well tolerated by all recipient animals and caused no neurological symptoms. Therefore, a 30 mM concentration of chloroquine was used for the treatment of intracranial glioma xenografts. At day 10 postimplantation, 5 µL of PBS or chloroquine was administered into the site used for injection of tumor cells by means of a screw bold-guided injection as described33 for 17 days. Twenty-six days postimplantation, animals were sacrificed following a lethal intraperitoneal injection of a ketamine/xylazine mixture (50 mg xylazine and 350 mg ketamine per 1 kg body weight). The tumor-bearing brains were explanted, fixed in 4% formalin, cut in coronal sections, and embedded in paraffin. One- to three-micrometer thick sections were placed on glass slides and stained with hematoxylin and eosin. The largest tumor areas were determined by microscopic examination of consecutive histological sections and measured by using Cell-A-Image software (Olympus Soft Imaging Systems). Paraffin-embedded sections were scored for apoptosis by TUNEL assay and mitoses. For calculating the apoptotic as well as the mitotic indexes, up to three sections of the entire tumor were scored by screening the tumor on adjacent high-power fields. The number of apoptoses or mitoses counted was divided by the number of high-power fields (up to 49) used for screening the tumor.
Statistical Analyses
Each experimental point has been performed in triplicate per experiment unless stated otherwise; the data shown represent means (SEM). Statistical analyses were performed using the GraphPad Prism 5 software (GraphPad Software).
Results
Chloroquine Inhibits Glioma Cell Growth
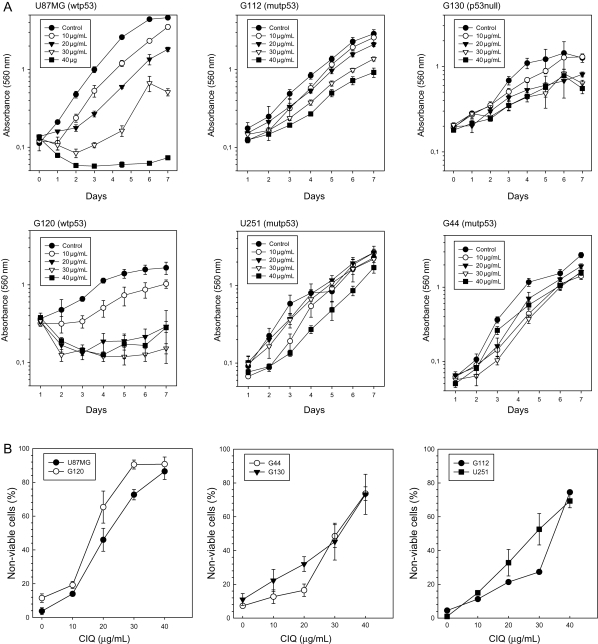
To assess the effect of chloroquine on the growth of glioma cells, the growth of a panel of glioma cell lines with different functional p53 status was analyzed in the presence of increasing concentrations of chloroquine. Glioma cell lines U87MG and G120 express wtp53. G130 and G44 lines express no or a truncated p53 due to gross chromosomal aberrations (G130) or a nonsense mutation in the TP53 gene,31 respectively. G112 and U251 harbor a TP53 mutation within the hot-spot codon 273 and express transcriptionally inactive mutant p53. The results showed that chloroquine strongly suppressed glioma cell growth in a dose-dependent manner (Fig. 1A). Although the growth-suppressing effect of chloroquine was observed in all cell lines tested, cell lines with wtp53 (U87MG and G120) appeared more sensitive to all doses of chloroquine tested compared with cell lines that are null for p53 (G130), express truncated p53 (G44), or harbour inactivating TP53 mutations (U251 and G112). To examine whether the growth inhibition by chloroquine was a consequence of affected cell viability, we analyzed the percentage of nonviable cells in untreated or chloroquine-treated cultures using a trypan blue exclusion assay. The results summarized in Fig. 1B show that chloroquine affects glioma cell viability in a dose-dependent manner. Within the range of the tested chloroquine treatment doses, cell viability was significantly lower in cell lines with wtp53 compared with mutp53-expressing cell lines, consistent with the notion that the p53 status may be an important factor determining the sensitivity of glioma cells to chloroquine.
Fig. 1.
Chloroquine inhibits glioma cell growth and viability in culture. (A) Assessment of cell growth rates in glioma cells with known functional status of p53.31 Cells were treated with a range of chloroquine concentrations indicated in the legends. (B) Assessment of cell death rates in glioma cells lines with wtp53 (left panel) or deficient p53 function (middle and right panels). Values represent the mean of 6 replicates.
Apoptosis Contributes to Chloroquine-Induced Death in Glioma Cells
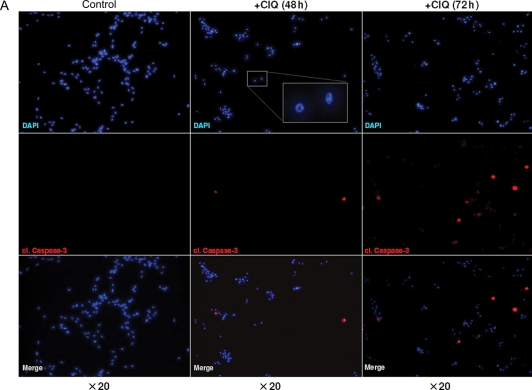
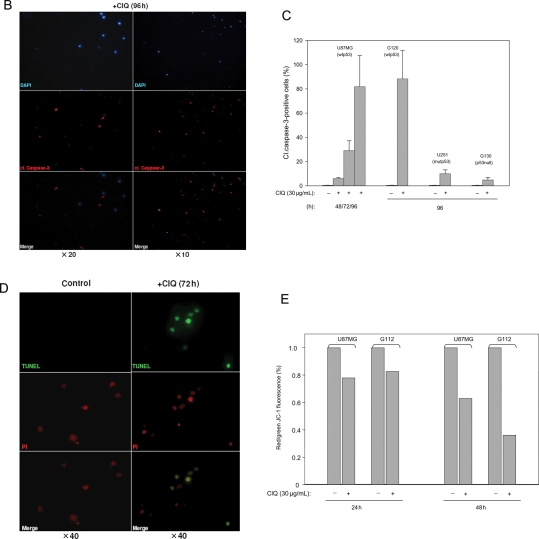
Chloroquine can induce cell death by distinct mechanisms. A caspase-independent mechanism involving lysosomal targeting has been described for some types of nontumorigenic cells.26,34 In contrast, caspase-dependent apoptosis has been implicated in chloroquine-induced cell death in different types of malignant tumor cells22,35 and in some types of neurons.25 To analyze the mechanisms of chloroquine-induced death in glioma cells, we assessed hallmarks of the apoptotic cascade, including the activation of caspase-3 and fragmentation of genomic DNA. Glioma cells were treated with chloroquine at a dose of 30 µg/mL, a concentration which lies within the window of chloroquine concentrations (20–40 µg/mL) found to be cytotoxic for all of the tested glioma cell lines (Fig. 1) and which was sufficient to induce apoptosis in other tumor cell types.22,23,27 Immunofluorescence staining for the cleaved form of caspase-3 revealed that chloroquine treatment led to the activation of caspase-3, which is indicative of induction of apoptosis (Fig. 2A–C). Notably, chloroquine-treated cells generally showed a characteristic change in nuclear morphology (see inset, Fig. 2A), which, however, did not coincide with caspase-3 activation. Chloroquine-induced activation of caspase-3 occurred in a time-dependent manner and was first observed after 48 hours of chloroquine treatment (Fig. 2A, shown for the U87MG line). After 96 hours of treatment, the majority of the cells were positive for cleaved caspase-3 (Fig. 2B) indicating a robust apoptotic response. The assessment of caspase-3 activation by chloroquine in different cell lines is summarized in Fig. 2C. The activation of caspase-3 was considerably more profound in glioma cells with wtp53 compared with those with mutant p53 (Fig. 2C), suggesting a contribution of wtp53 activities to chloroquine-induced apoptosis. Further supporting the impact of apoptosis in chloroquine-mediated cytotoxicity, glioma cells treated with chloroquine showed disintegration of genomic DNA as demonstrated by TUNEL (Fig. 2D). One of the early events induced by chloroquine in glioma cells was a decrease in the mitochondrial aggregation of the fluorescent dye JC-1 indicative of a distortion of the mitochondrial membrane potential integrity, which preceded activation of caspase-3 and occurred at a much earlier time, as early as 24 hours after treatment (Fig. 2E). Interestingly, a collapse of the mitochondrial membrane potential caused by chloroquine occurred with comparable efficacy in cells with wtp53 or mutp53 (Fig. 2E), indicating that mitochondrial dysfunction caused by chloroquine may be a p53-independent effect.
Fig. 2.
Chloroquine induces apoptosis in cultured glioma cells. (A and B) Time-dependent activation of caspase-3 by chloroquine in U87MG cells. Untreated or chloroquine-treated cells were stained for the cleaved form of caspase-3 and counterstained by DAPI. The inset in (A) shows the characteristic nuclear morphology of chloroquine-treated cells. (C) Summary of the caspase-3 activation assessment in glioma cells lines with different status of p53. The percentage of cells positive for cleaved caspase-3 was determined by counting a minimum of 500 cells in 5–10 microscopic fields in replicates of 3 for each condition. (D) Assessment of apoptosis in U87MG cells by TUNEL. A propidium iodide (PI) counterstain was used. (E) The effects of chloroquine on the mitochondrial membrane potential integrity assessed by measurements of the mitochondrial accumulation of fluorescent JC-1 in glioma cells with wtp53 (U87MG) or mutp53 (G112). The ratio of red/green JC-1 fluorescence was determined in cells untreated or treated with chloroquine for 24 or 48 hours.
Chloroquine Leads to Stabilization of the p53 Protein and Induces p53 Transcriptional Targets in Glioma Cell Lines with wtp53
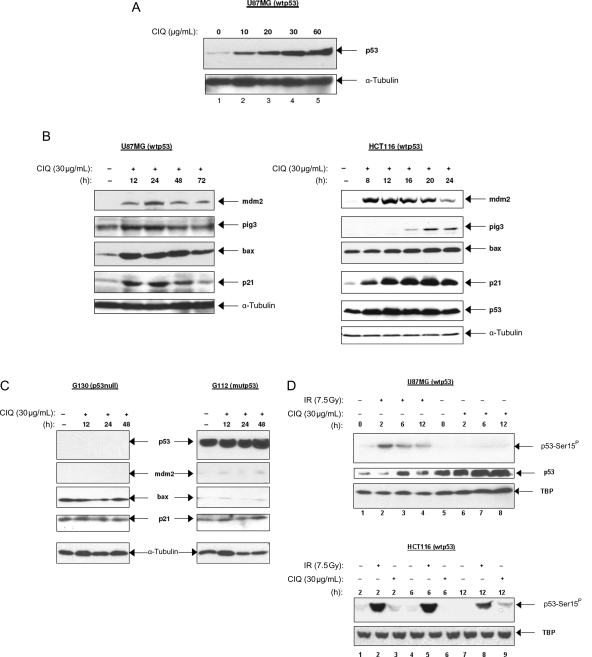
The increased sensitivity to chloroquine in glioma lines expressing endogenous wtp53 compared with cell lines expressing mutp53 (Figs 1 and 2) suggested an involvement of the p53 pathway in the cytotoxicity of chloroquine. Therefore, we assessed the levels of p53 protein and its transcriptional targets in glioma cell lines with known functional status of p53 by Western blot analyses. The results showed that chloroquine treatment caused a marked stabilization of the p53 protein, which was chloroquine dose-dependent (Fig. 3A, shown for the U87MG line). The chloroquine-induced stabilization of the p53 protein was considered functionally relevant because it was accompanied by an increased expression of genes regulated by p53 (Fig. 3B, left panel). Importantly, the chloroquine-activated p53 transcriptional response was effective not only in inducing genes involved in p53 regulation and cell cycle control/DNA repair (mdm2 and p21) but also of apoptotic targets of p53 (pig3 and bax). The chloroquine-induced increase in the expression levels of p53 target genes was also observed in other cell lines expressing wtp53 such as HCT116 and G168 (Fig. 3B, right panel and data not shown). In contrast, chloroquine treatment did not induce p53 target genes in glioma lines lacking functional p53 (Fig. 3C). These results demonstrate that chloroquine leads to an increase in the p53 protein level and induces a p53-dependent transcriptional response in glioma cells with wtp53. Since p53 activity is regulated mostly at the posttranslational level36,37 and as chloroquine does not influence the levels of p53 transcription,38 we next examined the possibility that chloroquine may induce p53 posttranslational modifications known to promote p53 stabilization in response to different types of cellular stress. In particular, we were interested in assessing p53 phosphorylation at a serine residue at position 15 (p53-Ser15), a posttranslational modification mediated by the ATM/ATR kinases39 and essential for p53 stabilization during the cell's response to DNA damage.
Fig. 3.
The p53 pathway is responsive to chloroquine. p53 protein and products of the known p53 targets p21, mdm2, bax1, or pig3 were assessed in glioma cell lines with different p53 status (A–C) and in the human colon carcinoma cell line HCT116 expressing wtp53 (B) by Western blot. Cells were treated with varying concentrations of chloroquine for 24 hours (A) or with a constant dose of chloroquine (30 µg/mL) for the indicated time periods (B and C). (D) Assessment of the p53 phosphorylation status at a serine residue Ser15 in U87MG (upper panel) and HCT116 (bottom panel) cells treated with chloroquine or ionizing radiation. The phosphorylated form of p53 (p53-Ser15P) was detected using a phosphorylation-sensitive antibody 16G8, which recognizes only the phosphorylated p53-Ser15P isoform. Total p53 detection was by antibody DO-7. The ubiquitously expressed cytoskeleton component α-tubulin or the basal transcription factor TBP was assessed to assure equal protein loading.
The phosphorylation status of p53-Ser15 was assessed in U87MG cells treated by chloroquine or by γ-irradiation. As expected, γ-irradiation induced a rapid phosphorylation of p53-Ser15, which preceded stabilization of the p53 protein (Fig. 3D, upper panel, compare the levels of p53-Ser15P with the total p53 levels in lanes 1–4). These results indicate that DNA damage-dependent signaling converging on p53 is intact in U87MG cells. In contrast, p53 stabilization following chloroquine treatment, apparent already 6 hours after treatment (Fig. 3D, upper panel, compare the levels of total p53 in lanes 5 and 7), was not accompanied by appreciable phosphorylation at p53-Ser15 over baseline levels (compare the levels of p53-Ser15P with the total p53 levels in lanes 1–4 and 5–8). This lack of correlation between chloroquine-induced stabilization of p53 and its phosphorylation at Ser15 was observed not only in glioma cells but also in the different cellular context of an HCT116 colon carcinoma cell line, a widely used experimental paradigm of the p53 pathway.40 Similar to the pattern observed in glioma cells, HCT116 cells respond to chloroquine by a marked stabilization of the p53 protein and induction of p53 target genes (Fig. 3D, right panel). In contrast to the robust and rapid phosphorylation of p53-Ser15 induced by γ-radiation (Fig. 3D, bottom panel, compare the levels of p53-Ser15P in lanes 2, 5, and 8 with the basal p53-Ser15P levels shown in lane 1), chloroquine treatment did not cause a considerable increase in p53-Ser15 phosphorylation (compare the levels of p53-Ser15P in lanes 3, 6, and 9 with the basal p53-Ser15P levels shown in lane 1). These observations strongly suggest that p53 stabilization and activation of p53 transcriptional response by chloroquine may depend on mechanisms distinct from signaling induced by DNA damage.
Induction of Apoptosis by Chloroquine Requires p53
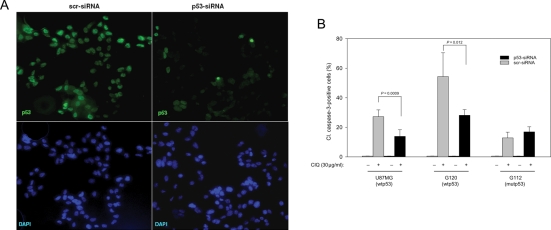
The findings that chloroquine induces apoptosis and activates the p53 pathway raised the question of a causal link between the two phenomena. To address this question, we assessed the effects of p53 knockdown on the efficacy of apoptosis induced by chloroquine. Glioma cell lines were transfected with commercially available p53-siRNA to inhibit endogenous p53. Cells transfected with unspecific siRNA (scr-siRNA) were used as a control. The inhibition of p53 by siRNA-p53 was confirmed by immunofluorescence staining for p53 protein (Fig. 4A) and by Western blot analysis (data not shown). Glioma cell lines transfected with p53-siRNA or with scr-siRNA were treated with chloroquine for 72 hours and assessed for activated caspase-3. To score the rate of apoptotic cells, the percentage of cells positive for cleaved caspase-3 was determined. The results showed that inhibition of wtp53 by p53-siRNA led to a significant reduction in the number of cells positive for activated caspase-3 in chloroquine-treated U87MG and G120 cells expressing wtp53 (P = .0009 and .012, respectively, Fig. 4B). In contrast, inhibition of mutp53 in the G112 line had no effect on the chloroquine-induced apoptosis rates in the p53-siRNA or scr-siRNA-transfected cells (Fig. 4B). These results demonstrate that wtp53 function is essential for apoptosis induction by chloroquine.
Fig. 4.
Inhibition of p53 diminishes apoptotic response to chloroquine in glioma cell lines with wtp53. (A) Assessment of the efficacy of endogenous p53 inhibition by transfection with unspecific scr-siRNA or p53-siRNA. U251 cells, which express high levels of endogenous mutp53 were transfected with scr-siRNA or p53-siRNA and stained with antibody DO-7 to ascertain the effect of siRNAs. (B) p53 inhibition by p53-siRNA diminished significantly (P < .02) the activation of caspase-3 in glioma cell lines with wtp53 but not in mutp53 lines. The percentage of cells with activated caspase-3 was determined by counting at least 500 cells in 5–10 microscopic fields from three replicate cover slips. The results shown represent the mean of two experiments.
Chloroquine Inhibits Growth of Experimental Glioma
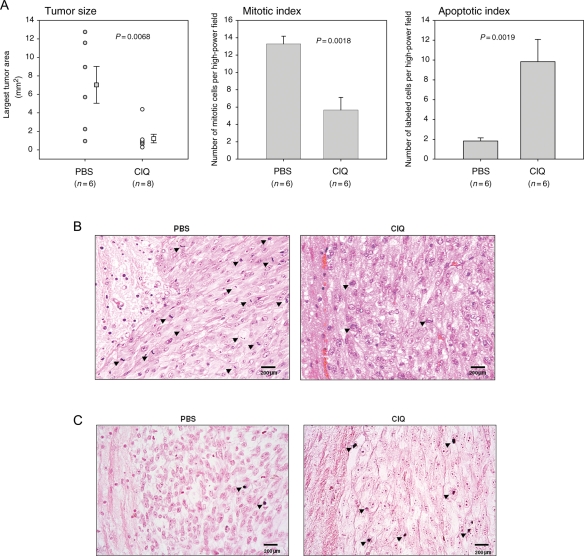
Our findings that chloroquine induces the death of cultured glioma cells prompted us to examine the effects of chloroquine in an orthotopic glioma mouse model.41 Nude mice were intracranially implanted with U87MG cells and randomized to chloroquine or placebo treatment. Ten days postimplantation (approximately one-third of the average survival of U87MG implanted mice in this xenograft model), one group received a daily injection of intratumoral chloroquine in PBS and the second group was administered PBS alone. Twenty-six days postimplantation, animals were sacrificed and tumor-bearing brains were processed for histology. The histomorphometric measurements revealed a significant reduction in the average tumor size in the chloroquine group compared with the control group (P = .0068) (Fig. 5A, left panel). Chloroquine-treated tumors showed a significantly lower number of mitotic cells compared with the control group (P = .0018) (Fig. 5A, middle panel and Fig. 5B), whereas the number of apoptotic cells by TUNEL was significantly higher in chloroquine-treated tumors compared with placebo-treated group (P = .0019) (Fig. 5A, right panel and Fig. 5C). These data confirm our in vitro data and demonstrate that chloroquine is effective in suppressing growth and inducing apoptosis of experimental glioma in vivo.
Fig. 5.
Treatment of experimental glioma with chloroquine in vivo. Tumor volumes and mitotic and apoptotic indexes were determined in chloroquine- or PBS-treated U87MG tumors as described in the Materials and Methods section. (A) Chloroquine-treated tumors show a significant decrease in the average tumor volume and a lower mitotic index, but significantly elevated rates of apoptotic cells. The data analysis was performed using a one-way analysis of variance. (B) Representative hematoxylin/eosin-stained histological sections. The arrowheads indicate mitotic cells. (C) Representative TUNEL-stained histological sections. The arrowheads indicate TUNEL-positive cells.
Discussion
This study demonstrates the effects of the quinoline derivative chloroquine on the growth and viability of cultured glioma cells and on experimental glioma in nude mice. The results show that induction of p53-mediated apoptosis is one of the mechanisms underlying the growth-suppressing effects of chloroquine.
Although there is some preliminary and empiric evidence that chloroquine may retard glioma progression and improve outcome in glioblastoma patients,30 the molecular basis of the chloroquine-induced effects in glioma cells remain poorly characterized. We show that chloroquine cause a sustained stabilization of the p53 protein and activates p53 transcriptional response in glioma cells. This suggests that the p53 pathway plays an essential role in the chloroquine-mediated suppression of cell growth and apoptosis. Supporting this conclusion, the apoptotic response to chloroquine is diminished in glioma cells when wtp53 is experimentally inhibited by p53-siRNA. Although the precise mechanisms by which chloroquine activates the apoptotic arm of the p53 response needs further elucidation, our data suggest that p53-dependent transcription of proapoptotic genes induced in response to chloroquine may be involved. Another nonexcluding possibility is that chloroquine may also promote a nontranscriptional pathway to apoptosis mediated by the mitochondria-associated fraction of p53.42 The latter does not preclude a p53-dependent transcription of proapoptotic genes by chloroquine.
The tumor-suppressing function of p53 relies on its abilities to act as a surveillance factor responsible for the maintenance of an error-free genome or as a potent inducer of cell death. The prevalence of survival- or death-promoting activities of wtp53 appears to be dependent on the cellular context and the specific mechanism of action of a particular stress factor or drug. For cytotoxic treatments inducing DNA damage, a switch between survival- and apoptosis-promoting activities of p53 is thought to occur when the extent of DNA damage exceeds the DNA repair capacity of the cell. On the other side, a successful repair of DNA lesions serves as a feed-back signal to re-enter the cell cycle, which requires the reduction of the p53 protein to base levels. Because radioresistant glioma cells are equipped with extraordinarily efficient mechanisms of DNA repair and the ability to activate DNA damage checkpoints,43 a switch to death-promoting activities of p53 may only occur at doses that may be difficult to achieve in the setting of clinically relevant DNA-damaging agents. Furthermore, some activities of wtp53 may even contribute to the increased DNA repair in glioma cells in response to some DNA-damaging agents.44–47 The mechanisms of chloroquine-dependent activation of p53 appear versatile and may or may not involve the DNA damage response depending on the cell type and experimental system. In mammary gland epithelial cells, chloroquine-mediated activation of p53 relies on ATM signaling.38,48 However, several ATM-independent mechanisms of p53 activation by chloroquine have also been revealed, indicating that in certain cell types, an ATM-dependent DNA damage signal might be dispensable for p53 activation by chloroquine.26 Our results are concordant with such a conclusion and indicate that chloroquine-induced activation of p53 in glioma cells may occur by by-passing ATM driven DNA damage signaling. The lack of a need for DNA damage signaling in p53 activation by chloroquine would also be concordant with the mechanism of DNA interaction of chloroquine, which intercalates into, but does not damage DNA.21 A scenario could be envisioned whereby DNA intercalation by chloroquine, while unrecognized as DNA damage by the genome surveillance maschinery, may have a considerable and durable impact on p53 activities associated with DNA binding, particularly those relevant for the regulation of transcription. In this context, it should be mentioned that DNA-associated activities of p53 are sensitive to structural DNA alterations,49 and chloroquine may enhance transcription by intercalating and changing the structure of DNA.50
In addition to its p53-activating effects, chloroquine also suppresses the growth of glioma cells with mutant p53, although less efficiently compared with glioma cells with wtp53. p53-independent cytotoxic effects of chloroquine are well known and related to the ability of chloroquine to cause mitochondrial dysfunction as a consequence of inhibition of lysosomal autophagy.22–24 Our data demonstrating that chloroquine is capable of reducing the mitochondrial membrane potential in glioma cells regardless of p53 status suggest that this mechanism may also contribute to the overall cytotoxicity of chloroquine against glioma cells (Fig. 1C). An important question is whether there is a link between the p53-independent effects of chloroquine (mitochondrial depolarization) and its ability to induce p53-dependent apoptosis. In this regard, previous findings demonstrating that chloroquine-mediated mitochondrial dysfunction per se is not sufficient for induction of apoptosis but requires bax,22,23 a p53-regulated mediator of mitochondrial apoptosis, might provide important clues. It may be envisioned that activation of the p53 response by chloroquine may be essential for the later phases of chloroquine-induced apoptosis triggered initially through a p53-independent distortion of the mitochondrial function. Such a composite mechanism relying on the synergistic impact of the p53-independent and -dependent actions of chloroquine would also be consistent with the previous findings that apoptosis and nonapoptotic mechanisms contribute to cell death induced by chloroquine23,24,26 and explain why wtp53-expressing glioma cells generally show a higher susceptibility to chloroquine compared with mutp53-expressing cells. Our findings are concordant with the general perception that mitochondrial depolarization per se may be insufficient to elicit apoptotic cell death22,51,52 in the absence of other causative factors that may include a p53-regulated mediator of mitochondrial apoptosis such as bax-1.22
Although the mechanisms underlying the antitumoral effects of chloroquine are only beginning to emerge, it has now become clear that chloroquine has antineoplastic effects in different types of cancers including gliomas. The ability to induce apoptosis and inhibit glioma growth as well as the long history of safe clinical use suggest chloroquine as a candidate drug in the treatment of malignant gliomas.
Conflict of interest statement. None declared.
Funding
This study was supported by grants from University of Schleswig-Holstein (A.G., E.K.), Fond der Chemischen Industrie (W.D.), EC FP6 funding (W.D.), and by a Cure for Life Foundation™ (D.S. and E.K.). D.S. is a Cancer Institute NSW Fellow.
References
- 1.Reardon DA, Wen PY. Therapeutic advances in the treatment of glioblastoma: rationale and potential role of targeted agents. Oncologist. 2006;11:152–164. doi: 10.1634/theoncologist.11-2-152. [DOI] [PubMed] [Google Scholar]
- 2.Lonardi S, Tosoni A, Brandes AA. Adjuvant chemotherapy in the treatment of high grade gliomas. Cancer Treat Rev. 2005;31:79–89. doi: 10.1016/j.ctrv.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Reardon DA, Rich JN, Friedman HS, Bigner DD. Recent advances in the treatment of malignant astrocytoma. J Clin Oncol. 2006;24:1253–1265. doi: 10.1200/JCO.2005.04.5302. [DOI] [PubMed] [Google Scholar]
- 4.Salhia B, Tran NL, Symons M, Winkles JA, Rutka JT, Berens ME. Molecular pathways triggering glioma cell invasion. Expert Rev Mol Diagn. 2006;6:613–626. doi: 10.1586/14737159.6.4.613. [DOI] [PubMed] [Google Scholar]
- 5.Terzis AJ, Niclou SP, Rajcevic U, Danzeisen C, Bjerkvig R. Cell therapies for glioblastoma. Expert Opin Biol Ther. 2006;6:739–749. doi: 10.1517/14712598.6.8.739. [DOI] [PubMed] [Google Scholar]
- 6.Naumann U, Kügler S, Wolburg H, et al. Chimeric tumor suppressor 1, a p53-derived chimeric tumor suppressor gene, kills p53 mutant and p53 wild-type glioma cells in synergy with irradiation and CD95 ligand. Cancer Res. 2001;61:5833–5842. [PubMed] [Google Scholar]
- 7.Vecil GG, Lang FF. Clinical trials of adenoviruses in brain tumors: a review of Ad-p53 and oncolytic adenoviruses. J Neurooncol. 2003;65:237–246. doi: 10.1023/b:neon.0000003653.45635.32. [DOI] [PubMed] [Google Scholar]
- 8.Cerrato JA, Khan T, Koul D, et al. Differential activation of the Fas/CD95 pathway by Ad-p53 in human gliomas. Int J Oncol. 2004;24:409–417. [PubMed] [Google Scholar]
- 9.Nakamizo A, Amano T, Zhang W, et al. Phosphorylation of Thr18 and Ser20 of p53 in Ad-p53-induced apoptosis. Neuro Oncol. 2008;10:275–291. doi: 10.1215/15228517-2008-015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang FF, Bruner JM, Fuller GN, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003;21:2508–2518. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- 11.Vousden KH. Outcomes of p53 activation—spoilt for choice. J Cell Sci. 2006;119:5015–5020. doi: 10.1242/jcs.03293. [DOI] [PubMed] [Google Scholar]
- 12.Vousden KH, Prives C. P53 and prognosis: new insights and further complexity. Cell. 2005;120:7–10. doi: 10.1016/j.cell.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 13.Bossi G, Sacchi A. Restoration of wild-type p53 function in human cancer: relevance for tumor therapy. Head Neck. 2007;29:272–284. doi: 10.1002/hed.20529. [DOI] [PubMed] [Google Scholar]
- 14.Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 15.Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinbach JP, Weller M. Apoptosis in gliomas: molecular mechanisms and therapeutic implications. J Neurooncol. 2004;70:247–256. doi: 10.1007/s11060-004-2753-4. [DOI] [PubMed] [Google Scholar]
- 17.Horn HF, Vousden KH. Coping with stress: multiple ways to activate p53. Oncogene. 2007;26:1306–1316. doi: 10.1038/sj.onc.1210263. [DOI] [PubMed] [Google Scholar]
- 18.Paul M, Gafter-Gvili A, Fraser A, Leibovici L. The anti-cancer effects of quinolone antibiotics? Eur J Clin Microbiol Infect Dis. 2007;26:825–831. doi: 10.1007/s10096-007-0375-4. [DOI] [PubMed] [Google Scholar]
- 19.Radl S. From chloroquine to antineoplastic drugs? The story of antibacterial quinolones. Arch Pharm (Weinheim). 1996;329:115–119. doi: 10.1002/ardp.19963290302. [DOI] [PubMed] [Google Scholar]
- 20.Savarino A, Lucia MB, Giordano F, Cauda R. Risks and benefits of chloroquine use in anticancer strategies. Lancet Oncol. 2006;7:792–793. doi: 10.1016/S1470-2045(06)70875-0. [DOI] [PubMed] [Google Scholar]
- 21.Mitscher LA. Bacterial topoisomerase inhibitors: quinolone and pyridone antibacterial agents. Chem Rev. 2005;105:559–592. doi: 10.1021/cr030101q. [DOI] [PubMed] [Google Scholar]
- 22.Boya P, Gonzalez-Polo RA, Poncet D, et al. Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene. 2003;22:3927–3936. doi: 10.1038/sj.onc.1206622. [DOI] [PubMed] [Google Scholar]
- 23.Boya P, González-Polo RA, Casares N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amaravadi RK, Yu D, Lum JJ, et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117:326–336. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shacka JJ, Klocke BJ, Shibata M, et al. 1 inhibits chloroquine-induced death of cerebellar granule neurons. Mol Pharmacol. 2006;69:1125–1136. doi: 10.1124/mol.105.018408. [DOI] [PubMed] [Google Scholar]
- 26.Maclean KH, Dorsey FC, Cleveland JL, Kastan MB. Targeting lysosomal degradation induces p53-dependent cell death and prevents cancer in mouse models of lymphomagenesis. J Clin Invest. 2008;118:79–88. doi: 10.1172/JCI33700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan C, Wang W, Zhao B, Zhang S, Miao J. Chloroquine inhibits cell growth and induces cell death in A549 lung cancer cells. Bioorg Med Chem. 2006;14:3218–3222. doi: 10.1016/j.bmc.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 28.Gurova KV, Hill JE, Guo C, et al. Small molecules that reactivate p53 in renal cell carcinoma reveal a NF-kappaB-dependent mechanism of p53 suppression in tumors. Proc Natl Acad Sci USA. 2005;102:17448–17453. doi: 10.1073/pnas.0508888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Briceno E, Reyes S, Sotelo J. Therapy of glioblastoma multiforme improved by the antimutagenic chloroquine. Neurosurg Focus. 2003;14:e3. doi: 10.3171/foc.2003.14.2.4. [DOI] [PubMed] [Google Scholar]
- 30.Briceno E, Calderon A, Sotelo J. Institutional experience with chloroquine as an adjuvant to the therapy for glioblastoma multiforme. Surg Neurol. 2007;67:388–391. doi: 10.1016/j.surneu.2006.08.080. [DOI] [PubMed] [Google Scholar]
- 31.Kim EL, Yoshizato K, Kluwe L, et al. Comparative assessment of the functional p53 status in glioma cells. Anticancer Res. 2005;25:213–224. [PubMed] [Google Scholar]
- 32.Cossarizza A, Baccarani-Contri M, Kalashnikova G, Franceschi C. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1) Biochem Biophys Res Commun. 1993;197:40–45. doi: 10.1006/bbrc.1993.2438. [DOI] [PubMed] [Google Scholar]
- 33.Brockmann MA, Westphal M, Lamszus K. Improved method for the intracerebral engraftment of tumour cells and intratumoural treatment using a guide screw system in mice. Acta Neurochir (Wien). 2003;145:777–781. doi: 10.1007/s00701-003-0091-5. discussion 781. [DOI] [PubMed] [Google Scholar]
- 34.Zaidi AU, McDonough JS, Klocke BJ, et al. Chloroquine-induced neuronal cell death is p53 and Bcl-2 family-dependent but caspase-independent. J Neuropathol Exp Neurol. 2001;60:937–945. doi: 10.1093/jnen/60.10.937. [DOI] [PubMed] [Google Scholar]
- 35.Amaravadi RK, Thompson CB. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin Cancer Res. 2007;13:7271–7279. doi: 10.1158/1078-0432.CCR-07-1595. [DOI] [PubMed] [Google Scholar]
- 36.Xu Y. Regulation of p53 responses by post-translational modifications. Cell Death Differ. 2003;10:400–403. doi: 10.1038/sj.cdd.4401182. [DOI] [PubMed] [Google Scholar]
- 37.Brooks CL, Gu W. Ubiquitination, phosphorylation and acetylation: the molecular basis for p53 regulation. Curr Opin Cell Biol. 2003;15:164–171. doi: 10.1016/s0955-0674(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 38.Loehberg CR, Thompson T, Kastan MB, et al. Ataxia telangiectasia-mutated and p53 are potential mediators of chloroquine-induced resistance to mammary carcinogenesis. Cancer Res. 2007;67:12026–12033. doi: 10.1158/0008-5472.CAN-07-3058. [DOI] [PubMed] [Google Scholar]
- 39.Appella E, Anderson CW. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem. 2001;268:2764–2772. doi: 10.1046/j.1432-1327.2001.02225.x. [DOI] [PubMed] [Google Scholar]
- 40.Bunz F, Hwang PM, Torrance C, et al. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999;104:263–269. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finkelstein SD, Black P, Nowak TP, Hand CM, Christensen S, Finch PW. Histological characteristics and expression of acidic and basic fibroblast growth factor genes in intracerebral xenogeneic transplants of human glioma cells. Neurosurgery. 1994;34:136–143. [PubMed] [Google Scholar]
- 42.Moll UM, Wolff S, Speidel D, Deppert W. Transcription-independent pro-apoptotic functions of p53. Curr Opin Cell Biol. 2005;17:631–636. doi: 10.1016/j.ceb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 44.Batista LF, Roos WP, Christmann M, Menck CF, Kaina B. Differential sensitivity of malignant glioma cells to methylating and chloroethylating anticancer drugs: p53 determines the switch by regulating xpc, ddb2, and DNA double-strand breaks. Cancer Res. 2007;67:11886–11895. doi: 10.1158/0008-5472.CAN-07-2964. [DOI] [PubMed] [Google Scholar]
- 45.Xu GW, Mymryk JS, Cairncross JG. Pharmaceutical-mediated inactivation of p53 sensitizes U87MG glioma cells to BCNU and temozolomide. Int J Cancer. 2005;116:187–192. doi: 10.1002/ijc.21071. [DOI] [PubMed] [Google Scholar]
- 46.Xu GW, Nutt CL, Zlatescu MC, Keeney M, Chin-Yee I, Cairncross JG. Inactivation of p53 sensitizes U87MG glioma cells to 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer Res. 2001;61:4155–4159. [PubMed] [Google Scholar]
- 47.Xu GW, Mymryk JS, Cairncross JG. Inactivation of p53 sensitizes astrocytic glioma cells to BCNU and temozolomide, but not cisplatin. J Neurooncol. 2005;74:141–149. doi: 10.1007/s11060-004-6601-3. [DOI] [PubMed] [Google Scholar]
- 48.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 49.Kim E, Deppert W. The versatile interactions of p53 with DNA: when flexibility serves specificity. Cell Death Differ. 2006;13:885–889. doi: 10.1038/sj.cdd.4401909. [DOI] [PubMed] [Google Scholar]
- 50.Cheng J, Zeidan R, Mishra S, et al. Structure-function correlation of chloroquine and analogues as transgene expression enhancers in nonviral gene delivery. J Med Chem. 2006;49:6522–6531. doi: 10.1021/jm060736s. [DOI] [PubMed] [Google Scholar]
- 51.Levraut J, Iwase H, Shao ZH, Vanden Hoek TL, Schumacker PT. Cell death during ischemia: relationship to mitochondrial depolarization and ROS generation. Am J Physiol Heart Circ Physiol. 2003;284:H549–H558. doi: 10.1152/ajpheart.00708.2002. [DOI] [PubMed] [Google Scholar]
- 52.Krohn AJ, Wahlbrink T, Prehn JH. Mitochondrial depolarization is not required for neuronal apoptosis. J Neurosci. 1999;19:7394–7404. doi: 10.1523/JNEUROSCI.19-17-07394.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]