Abstract
Glioblastoma patients are immunosuppressed, yet glioblastomas are highly infiltrated by monocytes/macrophages. Myeloid-derived suppressor cells (MDSC; immunosuppressive myeloid cells including monocytes) have been identified in other cancers and correlate with tumor burden. We hypothesized that glioblastoma exposure causes normal monocytes to assume an MDSC-like phenotype and that MDSC are increased in glioblastoma patients. Healthy donor human CD14+ monocytes were cultured with human glioblastoma cell lines. Controls were cultured alone or with normal human astrocytes. After 48 hours, glioblastoma-conditioned monocytes (GCM) were purified using magnetic beads. GCM cytokine and costimulatory molecular expression, phagocytic ability, and ability to induce apoptosis in activated lymphocytes were assessed. The frequency of MDSC was assessed by flow cytometry in glioma patients' blood and in GCM in vitro. As predicted, GCM have immunosuppressive, MDSC-like features, including reduced CD14 (but not CD11b) expression, increased immunosuppressive interleukin-10, transforming growth factor-β, and B7-H1 expression, decreased phagocytic ability, and increased ability to induce apoptosis in activated lymphocytes. Direct contact between monocytes and glioblastoma cells is necessary for complete induction of these effects. In keeping with our hypothesis, glioblastoma patients have increased circulating MDSC compared with normal donors and MDSC are increased in glioma-conditioned monocytes in vitro. To our knowledge, this has not been reported previously. Although further study is needed to directly characterize their origin and function in glioblastoma patients, these results suggest that MDSC may be an important contributor to systemic immunosuppression and can be modeled in vitro by GCM.
Keywords: B7-H1, immunosuppression, malignant glioma, monocyte, myeloid-derived suppressor cells
Gliomas, the most common primary central nervous system (CNS) neoplasms, are thought to originate from transformed glial cells such as astrocytes, oliogodendrocytes, and ependymal cells or their precursors.1 Glioblastomas (grade 4 astrocytomas) are the most common glioma with an annual incidence of 3 per 100 000.2 Despite aggressive therapy, they recur relentlessly within the brain, and the average survival after diagnosis remains just over 1 year. Effective new therapies are urgently needed.
Immunotherapy is one such promising strategy. Immunotherapies like dendritic cell and immunogene therapy vaccines have produced dramatic results in preclinical glioma studies.3–5 However, these have not been translated into effective clinical therapies.6–8 Although increased circulating anti-glioma lymphocytes have been documented following vaccination, few objective clinical responses have been reported to date. While there are many potential reasons for this, glioma-mediated immunosuppression may be a critical factor. Glioma patients are systemically immunosuppressed, with decreased T-cell responsiveness9,10 and increased circulating immunosuppressive CD4+/CD25+/FoxP3+ regulatory T cells (Treg).11,12 Furthermore, gliomas are potently immunosuppressive locally where they express and/or secrete multiple immunosuppressive factors including transforming growth factor-β (TGF-β), PGE2, and B7-H1.13,14
Human glioblastomas are heavily infiltrated by monocytes/microglia, but have relatively few tumor-infiltrating lymphocytes.15–17 The role of glioma-infiltrating monocytes/microglia in glioma immunology is enigmatic. They have reduced antigen-presenting potential and their surface marker profile is compatible with immature antigen-presenting cells, suggesting that they are not part of an effective anti-glioma immune response.12,18,19 Furthermore, several lines of evidence suggest that they actively suppress immune responses. For example, they secrete immunosuppressive interleukin-10 (IL-10), secrete Fas-ligand, and induce apoptosis in activated lymphocytes in murine models.20,21 We have previously demonstrated that human glioma-infiltrating monocytes are predominantly CD45bright/CD11b+.17 This surface marker pattern is consistent with infiltrating systemic macrophages, as opposed to resident microglia that are CD45dim/CD11b+. Taken together, these findings suggest that systemic monocytes/macrophages infiltrating gliomas might assume an immunosuppressive phenotype. Interestingly, glioma-infiltrating monocytes appear to have reduced CD14 expression.17 This is a relatively unusual phenomenon as CD14 expression on monocytes/microglia is increased in almost all other CNS pathologies.22
There is a precedent for immunosuppressive monocytic cells with reduced CD14 expression in other cancers. Myeloid-derived suppressor cells (MDSC), a heterogeneous population of CD14− myeloid progenitors at earlier stages of differentiation,23 increase peripherally in patients with head and neck squamous cell carcinoma, nonsmall cell lung cancer, and breast cancer.24 MDSC (also termed immature myeloid cells) do not express CD15 (a granulocytic marker) and approximately one-third express CD115 (M-CSF receptor; specific for mature monocytes/macrophages).24 They have multiple passive and active immunosuppressive effects including impaired antigen presentation to T cells, ability to induce apoptosis in activated T cells, and ability to stimulate regulatory T-cell proliferation.23–28 They have recently been shown to alter antigen recognition by nitrating CD8+ T-cell receptors, leading to tolerance.29 Functionally, MDSC exert their immunosuppressive effects at least partly in response to interferon-γ (IFN-γ) secreted by activated T cells, which induces secretion of IL-10 and TGF-β.30 Indeed, others have shown that the suppressive activity of MDSC is contingent on IFN-γ expression, as MDSC from IFN-γ−/− mice lose this.31 In practical terms, human MDSC can be identified by flow cytometry as cells within the monocyte/granulocyte gate (based on forward/side scatter profile) that express CD33 but do not express class II MHC or a panel of mature myeloid and lymphoid lineage markers (CD33+/HLA-DR−/ Lin−).24 The lineage cocktail (Lin) consists of antibodies directed against mature T, B, and NK cell and monocyte markers (CD3, CD19, CD57, and CD14, respectively). We hypothesized that normal human monocytes exposed to glioblastoma cells would assume an immunosuppressive phenotype similar to MDSC and that MDSC would be increased in glioblastoma patients. Here we present evidence supporting this hypothesis.
Materials and Methods
Cell Lines and Specimens
Cell lines were obtained from American Type Culture Collection. Normal human astrocytes (NHA) (>95% pure) were cultured as previously described from human temporal lobe epilepsy specimens.32 Peripheral blood mononuclear cells (PBMC) were obtained from normal donors or glioma patients. Fresh tumor specimens and blood were obtained at surgery from patients undergoing craniotomy. All samples were obtained with written, informed consent, and all protocols were reviewed and approved by the Conjoint Health Research Ethics Board at the University of Calgary or the Institutional Review Board at the Mayo Clinic College of Medicine.
Cell Sorting and Coculture
PBMC were isolated from whole blood using Ficoll-Paque Plus (GE Healthcare) density gradient centrifugation. The interphase containing PBMC was harvested and sorted using CD14-conjugated magnetic assisted cell sorting (MACS) microbeads (Miltenyi-Biotec) as per the manufacturer's instructions. Briefly, PBMs from 100 mL blood were resuspended in 100 µL of degassed buffer (phosphate-buffered saline + 2 mM EDTA + 0.5% bovine serum albumin) to which 20 µL of FcR blocking reagent (Milenyi-Biotec) was added. CD14-positive cells were isolated using LS MACS columns and a quadroMACS separator (Miltenyi-Biotec). In 12-well plates, 2 × 105 CD14+ monocytes were incubated with 4 × 104 U251, U87, or NHA for 48 hours at 37°C in AIM V serum-free media (GibcoBRL). Cells were harvested by scraping, glioma-conditioned monocytes (GCM) were sorted as described above except CD11b-conjugated MACS microbeads, as well as MS MACS columns and octoMACS separator were used (Miltenyi-Biotec). In experiments where cultured monocytes were physically separated from glioma cells (U251), monocytes were placed at the bottom of the wells while glioma cells were placed on well inserts with 1 µm pores (Millipore PIRP30R48). Glioblastoma-infiltrating monocytes were isolated from fresh tumor specimens by processing tissue with mechanical dissociation in Hanks' buffered saline solution (HBSS), trituration, and repeated low-speed centrifugation. Cells were then passed through a 30 µm filter (Miltenyi-Biotec) and sorted as above with CD11b-conjugated MACS microbeads.
Flow Cytometry
Cells were resuspended in 100 µL degassed buffer and 10 µL of the appropriate antibody or isotype control was added (FITC-CD14, FITC-ms-IgG1κ, PE-CD11b, PE-ms-IgG1κ; all from eBioscience; APC-CD15, APC-msIgM, Miltenyi Biotec). Fc receptors were blocked by preincubating cells with 20 µL of FcR blocker (Miltenyi Biotec). Cells were incubated at 4°C for 15 minutes, washed and read immediately using a BD FACScalibur flow cytometer and CellQuest software (Becton Dickinson). Cells were analyzed using FlowJo analysis software (Treestar). For intracellular cytokine FACS, naïve and GCM were cultured for 48 hours in AIMV followed by 6 hours in RPMI media (GibcoBRL) supplemented with 10% human AB serum (Sigma) and 100 U/mL IFN-γ (Calbiochem), as has previously been described for MDSC studies.30 Golgi Plug (1 µg/mL) (BD Biosciences) was added for the last 4 hours of culture, cells were harvested and then fixed in 4% para-formaldehyde solution for 20 minutes at room temperature (RT), washed, and resuspended in permeabilization buffer (eBioscience) for 10 minutes at RT. Cells were again washed, and resuspended in 50 µL permeabilization buffer with 10 µL of appropriate antibody or isotype control (PE-IL-10 [eBioscience], TGF-β1,2,3 [R and D systems], PE-IL-12p40/70 [eBioscience], FITC-IFN-γ [eBioscience]). Commercially prepared human intracellular cytokine control cells (BD Biosciences except eBioscience for IFN-γ) were used as positive (permeabilized) and negative (nonpermeabilized) controls for all cytokines except TGF-β. Permeabilized and nonpermeabilized U251 cells were used as controls for TGF-β. Cells were incubated with the antibody for 20 minutes at RT, washed, and read immediately on the flow cytometer. For unconjugated antibodies (anti-TGF-β1,2,3), secondary FITC-goat-anti-human was used (KPL) after washing the primary and incubating for 20 minutes at RT (1:5 dilution). For costimulatory molecule expression, cells were resuspended in PBS and blocked with FcR reagent as described above. PE-Cy5 antibodies were used at a 3:50 dilution (B7.1, CD40), and APC antibodies at a 1:25 dilution (B7.2, B7-H1). Anti-CD40 and B7.2 antibodies were obtained from BD Pharmingen and anti-B7.1 and B7-H1 antibodies were obtained from eBioscience.
T-cell Apoptosis
T cells from within the CD14− (lymphocyte enriched) PBMC population were activated by 48 hour culture in anti-CD3 (2 µg/mL)/anti-CD28 (4 µg/mL)-coated 48-well plates in AIM V containing 10 U/mL hrIL-2 (Chemicon, IL002). Activation was confirmed by blast formation in culture. Activated peripheral blood lymphocytes (aPBL) were cultured overnight alone or with autologous CD11b-purified conditioned monocytes (CM) at 5 × 104 cells/well in a 48-well plate (aPBL:CM = 1:1). Cultures were carried out in serum-free media (AIM V, GibcoBRL) at 37°C − 5% CO2. Cells were harvested, washed, and re-suspended in 100 µL FACS buffer. They were stained for 30 minutes at RT in the dark with anti-human CD3-PE or isotype-matched control antibody (7 µL; eBioscience) and Annexin V-FITC (1.5 µL, Calbiochem). They were washed once and re-suspended in 100 µL of FACS buffer. They were incubated for a further 10 minutes at RT in the dark after adding 3 µL of 7-amino-actinomycin D (7AAD, final concentration = 1 µg/mL; Calbiochem). The final volume was adjusted to 500 µL with FACS buffer and cells were read immediately on the flow cytometer.
Phagocytosis Assays
Glioma cell lines were labeled with 200 nM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes) for 10 minutes at 37°C in HBSS. After quenching excess CFSE with FBS, cells were washed once in complete media and CD11b-sorted GCM were added at phagocyte:target ratio 10:1. After overnight incubation at 37°C, cells were harvested by scraping and stained with PE-CD11b or isotype control (PE-ms-IgG1) (eBioscience) for 10 minutes at 4°C, washed, and analyzed immediately by flow cytometry for CFSE-uptake in CD11b+ cells.
For the E. coli cell wall particle phagocytosis, a flow cytometry assay was adapted from a commercially available kit (Molecular Probes, V6694). Briefly, NM, NM plus E. coli lipopolysaccaride (E. coli 055:B5 LPS; 1 µg/mL final concentration), or CD11b-purified conditioned-monocytes (preincubated with U87, U251, or NHA) in Hanks' buffered saline solution (HBSS) were incubated for 2–3 hours in 37°C, 5% CO2 incubator in the dark with briefly sonicated FITC-E. coli (K-12) bio-particles. Cells were harvested and subjected to very brief (no more than 2 minutes) quenching of external cellular fluorescence using trypan blue (0.25 mg/mL) at low pH (citrate-balanced salt solution, pH 4.4). After washing and centrifugation, cells were re-suspended in normal FACS buffer (Dulbecco's phosphate-buffered saline, DPBS, 0.1% NaN3) before analysis by flow cytometry. FITC-uptake was determined in monocytes (identified by forward and side scatter profile). Phagocytic uptake in NM plus LPS was taken as the maximum attainable.
For the confocal microscopy experiments, naïve and U251-CM were cultured as described above. CM were separated from U251 using CD11b-microbeads (Miltenyi 130-049-601). Both unconditioned and CM in AIM V were attached to glass bottom wells of 96-well glass bottom plates (MatTek P96G-1.5-5-F) before phagocytosis assay was performed. In different wells, pHrodo-E. coli particles (Invitrogen P35361) were added alone, or in conjunction with LPS (Calbiochem) as positive effector or cytochalasin D (CCD) (Sigma C2618) as inhibitor of phagocytosis. Cells were incubated for 3–4 hours before counterstaining with FITC-CD11b (Ebioscience 11-0118-73) for 20 minutes at 4°C. Microscopic analysis was done with Carl Zeiss LSM 510 Inverted Laser Scanning Confocal Microscope. Rodo exited at 568 nm laser line and FITC at 488 nm. Rodo and FITC data were collected at 585 nm and 505–550, respectively. Fluorescent photomicrographs were obtained. pHrodo-E.coli particles are nonfluorescent outside the cell, but fluoresce brightly red in phagosomes. Phagocytically active cells were defined as those with significant red or yellow staining (because of a combination of red pHrodo-E.coli particle and green CD11b-FITC staining). Total cell number and phagocytic cell number were determined visually for two high-power fields per experiment.
MDSC Assays
PBMC were isolated from peripheral blood and stained with a cocktail of PE-conjugated monoclonal antibodies directed at mature myeloid and lymphoid markers consisting of 1 µL of each of anti-CD3, CD14, CD19, and CD56 (eBioscience except CD56 from BD Pharmingen), 3 µL of anti-HLA-DR PE –Cy5 (BD Pharmingen), and 4 µL of anti-CD33-FITC (eBioscience) in 50 µL of PBS for 30 minutes on ice. Cells were re-suspended in PBS and analyzed immediately on a FACScalibur flow cytometer (Becton Dickinson). Initial acquisition was performed using Cell Quest software (Becton Dickinson) and subsequent analysis was performed using FlowJo software (Treestar). We employed the following strategy to identify MDSC. First, a generous forward and side scatter gate was used to identify monocytic and granulocytic cells (excluding lymphocytes). Lineage negative cells from this population were then analyzed for CD33 and HLA-DR expression. MDSC were defined as CD33+/Lin−/HLA-DR− cells. MDSC among GCM were identified using the same staining technique after sorting with CD11b-microbeads as described above. In a second set of experiments, MDSC levels in PBMC from a new set of normal donors and glioblastoma patients was determined in a similar manner except that a commercially available lineage cocktail (Linage Cocktail 1; BD Pharmingen) including antibodies directed against CD3, CD14, CD16, CD19, CD20, and CD56 was used.
Enzyme-linked Immunosorbent Assay
Serum from normal donors and glioma patients was collected and stored at −80°C, and later analyzed using commercially available ELISA kits IL-6, IL-10 (eBioscience), TGF-β2, PGE2, and VEGF (R&D Systems) following the manufacturer's instructions.
Statistics
t-Tests were performed using Sigma plot statistical software where two conditions were compared. One-way ANOVA (Dunnett's) was used for analysis of multiple groups (>2) using Graph Prism software. Pearson coefficient was calculated to determine the correlation between two data sets.
Results
Human Monocytes Decrease CD14 but Maintain CD11b Expression after Coculture with Human Glioma Cell Lines
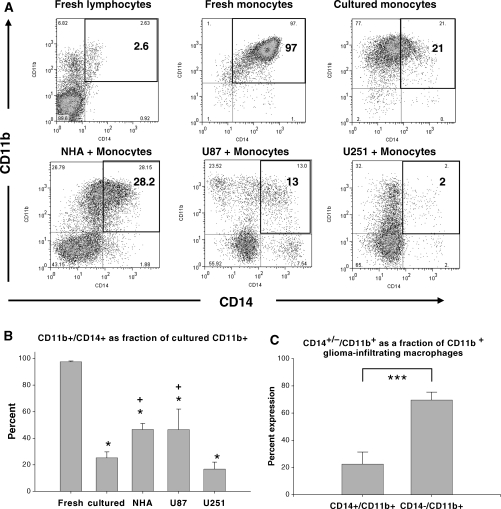
Fresh monocytes strongly express both CD11b and CD14 while cultured monocytes maintain CD11b, but have reduced CD14 expression (Fig. 1A and B). This reduction in CD14 expression may reflect relative inactivation when cultured in serum-free media in the absence of any other stimulus. CD14+ monocytes cocultured with NHA or (less consistently) U87 have a relative increase in CD14 expression compared with monocytes cultured alone, suggesting some degree of activation. In contrast, monocytes cultured with U251 glioma had a trend to decreased CD14 expression compared with monocytes cultured alone. As a correlate to our in vitro system, we tested CD14 expression on CD11b+ glioma-infiltrating monocytes from fresh tumor specimens. In keeping with our previous findings,17 we found that the majority of glioma-infiltrating monocytes do not express CD14 (Fig. 1C).
Fig. 1.
Decreased CD14 expression in GCM and glioblastoma-infiltrating monocytes. (A) Representative flow cytometry dot plots of CD14 and CD11b expression for fresh lymphocytes and monocytes, cultured monocytes, and bulk (unsorted) monocyte and astrocyte (NHA) or glioma (U87, U251) cocultures. Double negatives indicate glioma cells or astrocytes, CD14+/−/CD11b+ indicate monocytes. The number of CD14+/CD11b+ monocytes as percentage of total cell number is indicated in bold. Note that the autofluorescence for cultured monocytes was slightly greater than for fresh monocytes or lymphocytes (Supplementary Material, Fig. S1A), resulting in slightly different quadrant placement. (B) Bar graph showing the average number of double positive (CD14+/CD11b+) cells among fresh monocytes, cultured monocytes, and monocyte/glioma cocultures expressed as a percentage of total monocytes (CD11b+ cells) in 4 separate experiments. *P < .05 compared with fresh monocytes. +P < .05 compared with cultured monocytes alone. (C) Bar graph showing the average number of CD14+ and CD14− cells expressed as a percentage of CD11b+ glioblastoma-infiltrating monocytes from 6 different operative specimens. A significant majority of glioblastoma-infiltrating monocytes were CD14− (***P < .001). Graphs are mean ± SEM.
CM Can Be Purified Using CD11b Microbeads
Because of the low CD14 expression in GCM (particularly with U251), we chose to purify GCM from cocultures based on CD11b expression. CD11b+ cells were sorted with an average of greater than 90% purity and viability (by flow cytometry and trypan blue exclusion, respectively, data not shown).
GCM Have an Immunosuppressive Molecular Profile
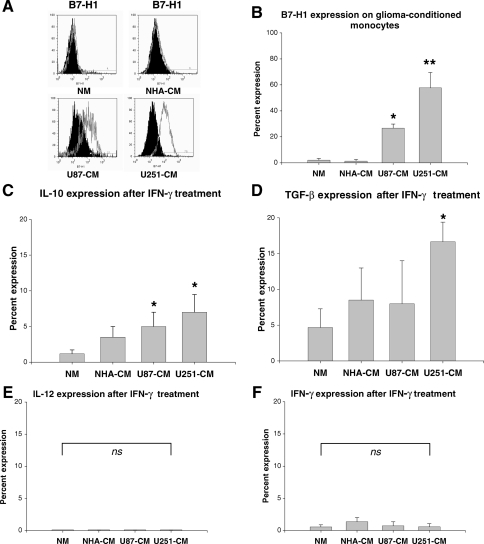
We first determined costimulatory molecule expression (B7.1, B7.2, CD40, and B7-H1) in GCM and compared this to naïve and NHA-CM. B7-1, B7-2, and CD40 were all present to varying degrees, but did not differ significantly between naïve and NHA-conditioned or GCM (data not shown). In contrast, the immunosuppressive T-cell costimulatory molecular homologue B7-H1 was essentially absent in naïve and NHA-CM, but strongly present in GCM (Fig. 2A and B). We next determined immunosuppressive (IL-10, TGF-β) and pro-inflammatory (IL-12, IFN-γ) cytokine expression in GCM using intracellular flow cytometry (Fig. 2C–F). U87 and U251-CM contained increased IL-10+ cells and U251-CM contained increased TGF-β+ cells compared with naïve or NHA-CM. This appeared to be specific for immunosuppressive cytokines as no corresponding increase in proinflammatory cytokines (IL-12, IFN-γ) was seen. Representative dot plots for these cytokine expression experiments (including positive and negative control cells) are shown in Supplementary Material, Fig. S1.
Fig. 2.
In vitro GCM have an immunosuppressive molecular profile. (A) Representative histograms showing B7-H1 expression in GCM, but not naïve or astrocyte-conditioned monocytes. NM, naïve monocytes; NHA-CM, normal human astrocytes-conditioned monocytes; U87-CM, U87-conditioned monocytes; U251-CM, U251-conditioned monocytes. (B) Graph showing percentage of cells expressing B7-H1 among NM and CM. U87-CM and U251-CM expressed significantly more B7-H1 than NM or NHA-CM. (C) Graph showing percentage of cells expressing IL-10 among NM and CM. U87-CM and U251-CM express significantly more IL-10 than NM. (D) Graph showing percentage of cells expressing TGF-β among NM and CM. U251-CM expressed significantly more TGF-β than NM. (E) Graph showing percentage of cells expressing IL-12 among NM and CM. IL-12 expression is minimal under all conditions and does not vary significantly. (F) Graph showing percentage of cells expressing IFN-γ among NM and CM. IFN-γ expression is minimal under all conditions and does not vary significantly. The scale in (C)–(F) have been kept uniform in order to allow easier relative comparisons between immunosuppressive (IL-10, TGF-β) and proinflammatory (IL-12, IFN-γ) cytokines. All graphs show mean ± SEM of three separate experiments using different PBMC donors. *P < .05; **P < .01; ns,not significant.
GCM Are Functionally Immunosuppressive
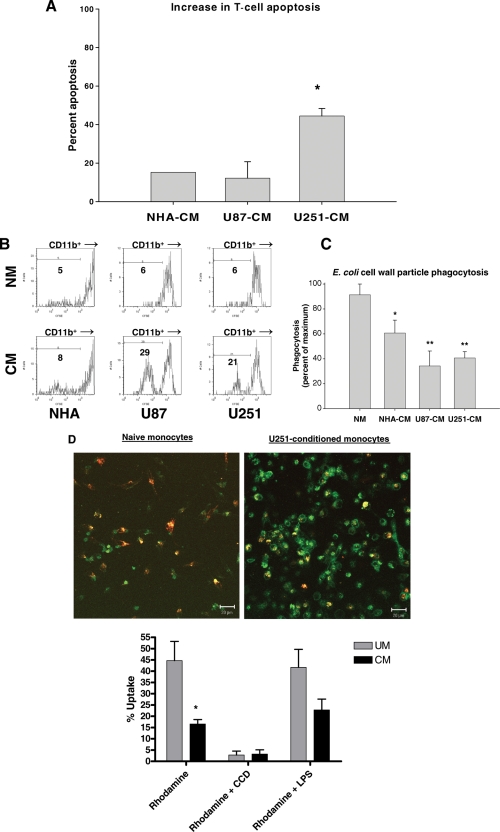
Activated autologous T cells exposed to either astrocytes or GCM undergo increased apoptosis compared with T cells exposed to NM based on flow cytometry staining for CD3+/Annexin-V+/7-AAD+ cells (Fig. 3A). This increase is relatively modest for NHA-CM or U87-CM, but marked for U251-CM.
Fig. 3.
In vitro GCM are functionally immunosuppressive. (A) Bar graph summarizing experiments where CM induced increased activated T-cell apoptosis over background with NM by flow cytometry for CD3+/Annexin V+/7AAD+ cells. This increase is modest for NHA-CM and U87-CM, but marked for U251-CM. (B) Representative histograms showing decreased CFSE uptake in CD11b+ U87-CM and U251-CM compared with NHA-CM or matched (same donor) NM after coculture with CFSE-labeled glioma cells. This indicates decreased tumor cell phagocytosis in GCM. (C) Bar graph summarizing experiments comparing uptake of FITC-labeled E. coli particles by NM and CM measured by flow cytometry. Results are expressed as a percentage of maximum uptake (uptake by LPS-stimulated NM; data not shown). GCM (U251-CM, U87-CM) had significantly decreased phagocytosis of FITC-labeled E. coli particles compared with NM. NHA-CM also demonstrated a modest reduction in phagocytosis. (D) Representative confocal photomicrographs of NM and U251-CM after culture with pHrodo-E. coli particles (red) and counter stained with CD11b-FITC (green). Cells with significant red or yellow staining are defined as phagocytically active. Bar graphs show percent uptake (number of phagocytically active cells/total number of cells). U251-CM have decreased phagocytic activity. This can be decreased further by adding the phagocyotsis inhibitor CCD or increased by adding bacterial LPS. Graphs are mean ± SEM of 3 separate experiments using different PBMC donors. *P < .05; **P < .01.
Phagocytic ability is reduced for GCM compared with both astrocyte-CM and NM. The number of CFSE-negative monocytes present after coculture with CFSE-labeled glioma cells is substantially increased if the monocytes are preconditioned with U87 or U251 (Fig. 3B). Similarly, fluorescently-labeled E. coli cell wall particle uptake is diminished in U87 or U251-CM compared with naïve or NHA-CM by flow cytometry (Fig. 3C). These findings were confirmed morphologically using pHrodo-E.coli particles and confocal microscropy (Fig. 3D).
Immunosuppressive Phenotype in U251-CM Is Dependent on Direct Cell Contact with Gliomas
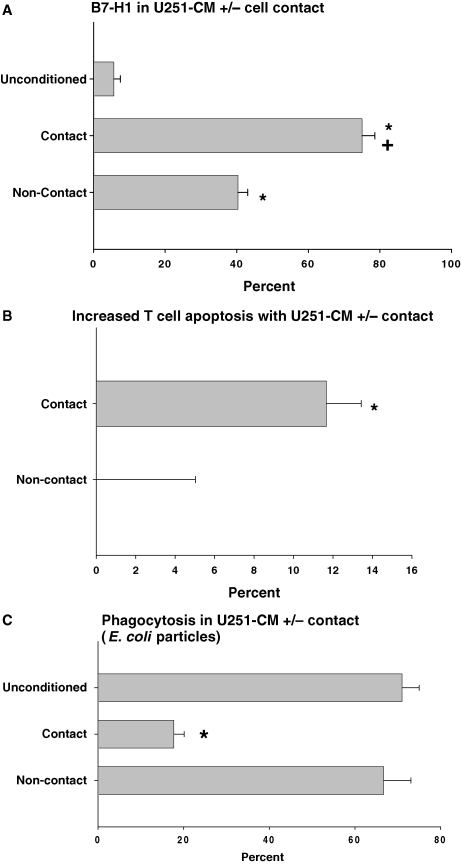
B7-H1 expression was minimal in NM, but markedly increased in U251-CM with direct cell contact compared with NM (Fig. 4A). It was also moderately increased in U251-CM without direct cell contact compared with NM), but this was significantly less than the increase seen with direct cell contact. Similarly, increased late apoptosis in activated T cells (CD3+/Annexin-V+/7-AAD+) over background after exposure to NM was seen for U251-CM with direct cell contact, but not U251-CM without direct cell contact (Fig. 4B). Finally, decreased phagocytosis of E. coli cell wall particles was seen for U251-CM with direct cell contact compared with NM, but not for U251-CM without direct cell contact (Fig. 4C). Thus, direct cell contact between monocytes and glioma cells appears to be necessary for monocytes to develop the full immunosuppressive phenotype.
Fig. 4.
Developing an immunosuppressive phenotype in U251-conditioned monocytes in vitro depends on direct monocyte/glioma cell contact. (A) B7-H1 expression by flow cytometry in naïve (unconditioned) monocytes, monocytes cultured directly with U251 (contact), and monocytes cultured with U251, but separated by a membrane with 0.1 µm pores (noncontact). Both contact and noncontact CM had increased B7-H1 expression compared with unconditioned monocytes (*P < .05), but contact CM had significantly more B7-H1 expression than noncontact-CM (+, *, P < .001). (B) Increased T-cell apoptosis by flow cytometry (CD3+/AnV+/7AAD+) over background (not shown) in activated T cells cultured with U251-CM (contact or noncontact). Only U251-contact CM significantly increase activated T-cell apoptosis over background (*P < .05). (C) E. coli cell wall particle phagocytosis in naïve (unconditioned), contact-U251-conditioned, and noncontact-U251-CM. Contact-U251-conditioned phagocytosis is significantly decreased (*P < .05) compared with unconditioned or noncontact-U251 CM. Graphs are mean ± SEM of three separate experiments using different PBMC donors.
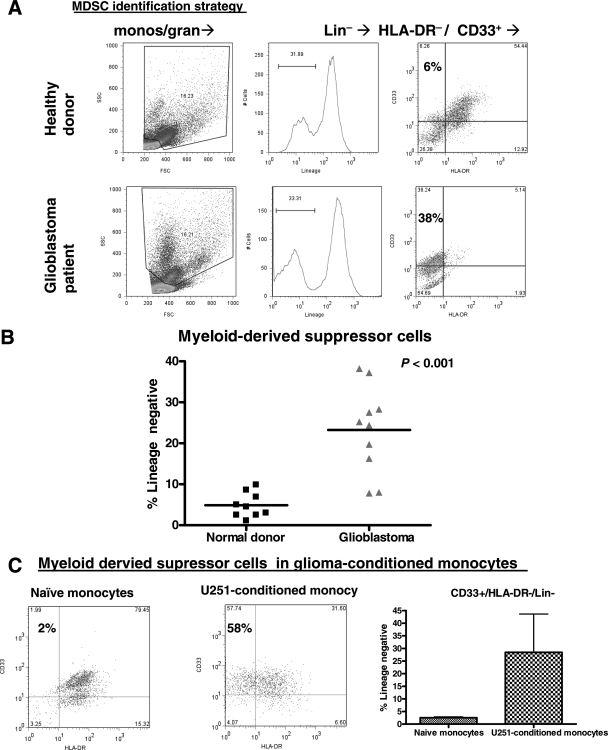
MDSC Are Increased in Glioblastoma Patients' Blood and GCM In Vitro
The immunosuppressive properties described above for GCM in vitro are similar to those described for MDSC. We therefore screened glioblastoma patients' peripheral blood for MDSC to see if there was an in situ correlatation to our in vitro findings. MDSC were defined as monocytic/granulocytic cells that were CD33+/Lin−/HLA-DR− (Fig. 5A). MDSC frequency expressed as the percentage of lineage marker-negative monocytes/granulocytes that are CD33+/HLA-DR− is shown for normal donors and glioblastoma patients in Fig. 5B. Glioblastoma patients had significantly increased peripheral MDSC compared with healthy donors. There were no significant differences in either monocyte/granulocyte frequency (as a percentage of PBMC) or lineage-negative frequency (as a percentage of monocytes/granulocytes) between glioblastoma patients and normal donors (data not shown).
Fig. 5.
MDSC are increased in glioma patients' blood and GCM in vitro. (A) Representative dot plots and histograms showing strategy for determining MDSC frequency in peripheral blood. After gating on monocytes/granulocytes by forward and side scatter, lineage negative/HLA-DR−/CD33+ MDSC were identified in patients and normal donors. (B) Glioma patients had increased MDSC (expressed as a percentage of Lin− cells) compared with normal donors. (C) Representative dot plots demonstrating CD33 and HLA-DR expression patterns in lineage-negative monocytic/granulocytic cells from among NM and U251-CM. The glioma (U251)-CM have consistently increased CD33+/HLA-DR−/Lin− MDSC (n = 3).
We then applied this detection strategy to GCM (specifically U251-conditioned) in vitro to see if these also contained increased MDSC using this definition. MDSC (CD33+/HLA-DR−/Lin−) were increased among U251-CM (Fig. 5C). This further emphasizes the correlation between our in vitro findings and glioblastoma patients.
MDSC Express B7-H1
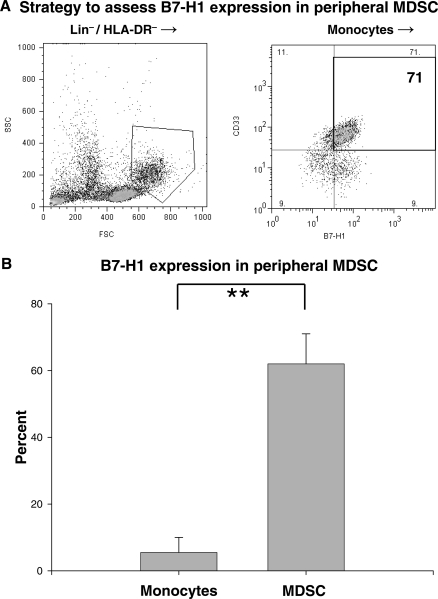
Since GCM in vitro expressed the immunosuppressive T-cell costimulatory molecular homologue B7-H1 and had many other established characteristics of MDSC, we sought to determine if peripheral MDSC also expressed B7-H1. Mature lineage marker-negative and class II MHC-negative cells were purified from normal donor PBMC by negative selection for CD3, CD14, CD19, CD56, and HLA-DR using magnetic beads. These cells were then stained for CD33 and B7-H1 expression. MDSC were identified from among these cells as monocytes (determined by forward and side scatter profile) expressing CD33. Representative dot plots demonstrating this strategy are shown in Fig. 6A. B7-H1 expression was significantly elevated in MDSC compared with peripheral blood CD14+ monocytes (Fig. 6B). These experiments required relatively large volumes of blood to complete (100 mL) that were not readily available from sick glioblastoma patients, making them impossible to repeat for glioblastoma patients using this strategy. Work is ongoing in our laboratory to develop techniques to identify B7-H1 expression on glioblastoma patients' MDSC using smaller blood volumes. Nevertheless, to our knowledge, this is the first report of B7-H1 expression by MDSC and further establishes the link between our in vitro findings and circulating MDSC.
Fig. 6.
MDSC express increased immunosuppressive B7-H1 compared with monocytes. (A) Representative dot plots demonstrating B7-H1 expression on peripheral MDSC from a healthy donor. Lin−/HLA-DR− cells were purified from PBMC using negative selection with magnetic beads. After gating on monocytes/granulocytes by forward/side scatter, CD33+ cells (MDSC) were identified. These cells were predominantly B7-H1+. (B) Bar graph of costimulatory molecule expression on peripheral blood MDSC (isolated as above) and CD14+ monocytes isolated from normal donors (n = 3). B7-H1 expression was significantly increased in MDSC compared with circulating monocytes. Graphs are mean ± SEM. **P < .01.
Granulocytes Do Not Contribute to GCM and MDSC
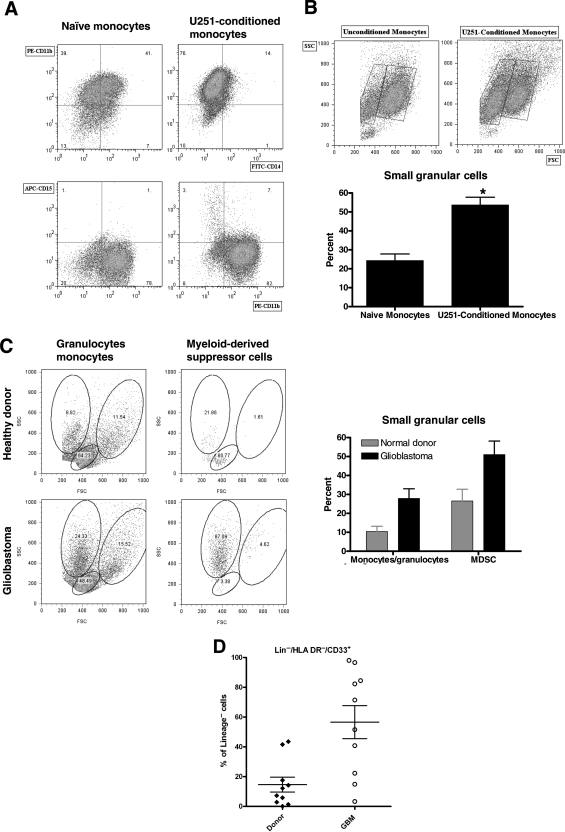
Although the monocytes included in our coculture are highly pure (>95% CD11b+/CD14+; see “Fresh Monocytes” in Fig. 1A), they do contain a small number of other cells that could include granulocytes. If these granulocytes selectively proliferated in response to glioma coculture, they could represent a significant proportion of our glioma-conditioned “monocytes.” To rule this out, we performed three-color flow cytometry for CD11b, CD14, and CD15 in naïve and U251-CM. As expected, CD11b expression was maintained and CD14 expression was reduced (particularly in U251-CM; Fig. 7A). CD15 expression was absent in all of these cells, essentially ruling out a contribution of granulocytes to our GCM. Despite this, we did find a reproducible increase in a population of small, relatively granular cells by forward scatter and side scatter analysis in GCM (Fig. 7B). These cells are not granulocytes based both on their lack of CD15 expression and on their relatively low size (less forward scatter than other monocytes).
Fig. 7.
True granulocytes do not contribute to GCM or MDSC although both are enriched for small granular cells. (A) Representative dot plots from 1 of 3 separate experiments performing 3 color flow cytometry for NM and U251-CM. CD11b expression is constant while CD14 expression is reduced (particularly for U251-CM). CD15 expression is absent, ruling out a contribution of granulocytes to our GCM. (B) U251-CM are enriched for small, relatively granular cells (low-forward scatter, relatively high-side scatter). Representative dot plots and pooled data from 3 experiments are shown. (C) MDSC from normal donors and (particularly) glioblastoma patients are also enriched for relatively small, granular cells (low-forward scatter, high-side scatter). Representative dot plots (corresponding to the normal donor and patient shown in Fig. 5A) and pooled data from all patients and donors shown in Fig. 5B. (D) Scatter plot showing increased MDSC in glioblastoma patients compared with normal donors using a lineage cocktail (CD3, CD14, CD16, CD19, CD20, CD56) that excludes granulocytes (CD16+) in the lineage negative fraction. This rules out a contribution of granulocytes to the MDSC we have identified.
Intriguingly, we noticed that both normal donors' and glioblastoma patients' MDSC also seemed to be enriched for a population of relatively small, granular cells based on forward and side scatter analysis (Fig. 7C). This effect was most notable in glioblastoma patients. These cells were purified by density gradient centrifugation with Ficoll-Paque prior to analysis, which should have eliminated most granulocytes. Nevertheless, it was possible that some granulocytes of slightly lower density persisted and could account for the CD33+/HLA-DR−/Lin− MDSC we were seeing, particularly as the lineage cocktail we used initially (CD3/CD14/CD19/CD56) did not include anything that would specifically exclude granulocytes. Therefore, we repeated our analysis using a commercially available lineage cocktail (CD3/CD14/CD16/CD19/CD20/CD56) that included a granulocyte marker (CD16) in 10 new normal donors and 10 new glioblastoma patients (Fig. 7D). This effectively eliminated granulocytes (CD16+) as contributors to our MDSC. Once again, glioblastoma patients had a marked increase in MDSC compared with healthy donors. The overall percentage of MDSC (expressed as a percentage of lineage negative cells) was slightly higher for both normal donors and glioblastoma patients compared with our earlier data (Fig. 5B), likely reflecting the more stringent lineage cocktail (ie, the number of MDSC remained constant but the number of lineage negative cells decreased).
Serum Immunomodulatory/Immunoinhibitory Cytokine Levels
The source of peripheral MDSC in glioma patients is not certain. Potentially, these changes could be induced in normal circulating monocytes by humoral factors produced by the glioma. Gliomas are known to secrete IL-6, IL-10, VEGF, PGE2, and TGF-β2,33 all of which have been linked with stimulating MDSC proliferation.23,26 Therefore, we analyzed serum from 17 glioblastoma patients and 8 normal donors for expression levels of these cytokines using a series of ELISAs. There were no significant increases in serum levels for IL-6, IL-10, PGE2, or TGF-β2 in glioblastoma patients compared with normal donors (data not shown). VEGF was the one exception to this pattern and was increased in glioblastoma patients' serum compared with normal donors (45.2 ± 5.5 vs 15.4 ± 6.6 pg/mL, P < .01). However, there was no association between VEGF and MDSC levels when they were plotted against one another (Pearson coefficient r = −.16, P = .66). This suggests that, while glioblastomas may secrete these factors locally, most do not enter the circulation in significant amounts and serum levels of those that do (ie, VEGF) do not correlate with MDSC levels. Systemic secretion of these factors is unlikely to be a significant factor in stimulating MDSC proliferation in glioblastoma patients.
Discussion
The experiments described in this study grew out of several earlier observations. First, the relative failure of clinical glioma immunotherapy trials to yield objective clinical responses led us to re-evaluate the nature of glioma-mediated immunosuppression as a potential explanation. In previous studies, we explored monocytic cell infiltrates in human malignant gliomas as a source of local immunosuppression. We were struck both by their relative frequency compared with lymphocytic infiltrates and by their macrophage-like (as opposed to microglial-like) surface marker profile.17 Our preliminary findings also suggested a surprising lack of CD14 expression by glioma-infiltrating macrophages. This is unusual because monocytes/microglia within the CNS up-regulate CD14 expression in response to almost all other pathologies.22 A literature search for a precedent for CD14−, immunosuppressive monocytic cells led us to MDSC, an immunosuppressive population of myelomonocytic CD14− cells that are increased in a number of other cancers.23–27 Increased MDSC have been reported in rodent glioma models34,35 and some have speculated that they might be increased in glioma patients,36 but this has not been demonstrated directly before, to our knowledge. Putting all of this together, we hypothesized that normal human monocytes exposed to malignant glioma cells would adopt an MDSC-like phenotype and that MDSC would be increased in glioblastoma patients.
The data presented here support this hypothesis. Normal human monocytes exposed to malignant glioma cells do adopt an MDSC-like phenotype where they maintain CD11b expression, but have low CD14 expression. Like MDSC, they have an immunosuppressive molecular profile (express B7-H1, IL-10, TGF-β) and are functionally immunosuppressive (reduced phagocytic/antigen presenting ability, increased ability to induce apoptosis in activated T cells). Based on surface marker analysis, MDSC (CD33+/HLA-DR−/Lin−) are consistently increased in GCM in vitro. Finally, MDSC are significantly increased in glioblastoma patients' peripheral blood compared with healthy donors.
This study highlights an increasingly important concept in glioma-mediated immunosuppression: immunosuppression does not result exclusively from glioma-derived factors. While glioblastoma cells express multiple immunosuppressive factors including TGF-β2, PGE2, IL-6, and B7-H1,13,14 the effects of these factors are likely to be local and cannot explain many of the systemic immune defects seen in glioblastoma patients. Local and/or systemic immunosuppressive effects of glioma-infiltrating macrophages/microglia and circulating regulatory T cells have already been described.11,12,20,21 To this list, we may also now add MDSC. Thus, multiple, immunologically active cell types are present in malignant glioma patients that may all contribute to immunosuppression.
The origin of increased MDSC in glioblastoma patients is unknown. In other cancers, it has been hypothesized that MDSC proliferation occurs systemically in response to circulating tumor-derived factors such as IL-6, IL-10, PGE2, TGF-β2, and VEGF.23, 26 This is relatively easy to conceive in the context of widely disseminated systemic cancers secreting large amounts of these factors. However, even a large glioblastoma represents a relatively small tumor burden by comparison. In keeping with this, we found that serum levels of glioblastoma-secreted factors known to stimulate MDSC proliferation were mostly similar to normal donors or did not correlate with MDSC levels. Furthermore, our in vitro data suggest that direct monocyte/glioma cell contact is necessary to acquire the full immunosuppressive phenotype. Together, these findings suggest an alternative hypothesis: increased circulating MDSC in glioblastoma patients arise from tumor-infiltrating macrophages that have undergone immunosuppressive “education” in the tumor itself. This remains conjecture and it is still possible that other as yet untested humoral factors produced by glioblastomas are responsible for MDSC proliferation. Rigorous evaluation will likely require specific manipulation of appropriate model systems in vivo to determine what, if any, relationship exists between glioma-infiltrating monocytes and MDSC.
Similarly, it is also unknown why regulatory T cells are increased in glioblastoma patients. Our results would also suggest that systemic Treg proliferation in response to glioblastoma-secreted cytokines is unlikely as these factors do not appear to be increased in glioblastoma patients' serum compared with normal donors. It has been proposed that Treg proliferation in glioblastoma patients occurs as a result of direct intra-tumoral glioma cell/T-cell interactions,11,12 much as we are suggesting for MDSC. However, glioma-infiltrating lymphocytes are relatively rare in our experience.15,17 Furthermore, T-cell proliferation usually requires stimulation by a professional antigen presenting cell. MDSC are reported to stimulate regulatory T-cell proliferation in other cancers.28,30 Therefore, an alternative hypothesis is that increased Treg in glioblastoma patients occurs in response to stimulation of naïve T cells by MDSC. This also remains conjecture and awaits rigorous evaluation in vivo.
Finally, we were struck by the fact that many of the immunosuppressive properties ascribed to MDSC (ability to induce apoptosis in activated T cells, stimulate Treg proliferation from among naïve T cells) are similar to the immunosuppressive properties attributed to cells expressing the immunosuppressive T-cell costimulatory molecular homologue B7-H1.37–39 Supporting this potential connection, we show that B7-H1 expression is increased in GCM in vitro and in circulating MDSC from normal donors. To our knowledge, this is the first time that B7-H1 expression has been documented in MDSC. Because the amount of blood we are able to obtain from glioblastoma patients is relatively limited (10 mL per patient), we have not yet been able to carry out the analysis of B7-H1 expression in glioblastoma patients' MDSC. Work is ongoing in our laboratory assessing the functional importance of B7-H1 in both MDSC- and GCM-mediated immunosuppression.
There are clear limitations to the present study. For example, all glioma patients in this study received high-dose dexamethasone therapy to reduce cerebral edema. Dexamethasone's many immunosuppressive effects could potentially be responsible for the alterations in MDSC in these patients.40,41 However, MDSC are increased in other malignancies where patients do not routinely receive corticosteroids23,24 and we have shown that MDSC precursors and (occasionally) true MDSC are increased in GCM in vitro prepared in the absence of dexamethasone. Nevertheless, longitudinal studies in glioblastoma patients correlating MDSC levels with tumor burden and dexamethasone dose will be required to definitively address this question. Similar prospective studies will be required to define the impact of treatment (surgery, radiation, chemotherapy) and tumor status (complete response, partial response, stable disease, progression) on MDSC levels. Furthermore, although in vitro GCM are clearly immunosuppressive and there is compelling evidence that MDSC in other cancers are immunosuppressive, direct evidence that glioblastoma patients' MDSC are immunosuppressive and the exact roles of MDSC-derived B7-H1, IL-10, and TGF-β in this immunosuppression remains to be seen. Studies are ongoing in our laboratory to address these issues.
Summary
Normal human monocytes acquire MDSC-like properties when exposed to glioma cells in vitro. Circulating MDSC are increased in malignant glioma patients. We have demonstrated that GCM in vitro express multiple immunosuppressive factors including IL-10, TGF-β, and B7-H1. Others have reported MDSC expression of IL-10 and TGF-β and we have shown for the first time that MDSC express B7-H1. The relative contributions of these factors to MDSC-mediated immunosuppression remain to be determined. However, it is clear that multiple immunosuppressive cells are present in glioma patients including glioma cells, glioma-infiltrating monocytes, MDSC, and regulatory T cells. Understanding the roles of these cells may be imperative to reversing glioma-mediated immunosuppression.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was supported primarily by the Alberta Heritage Foundation for Medical Research (AHFMR), the Canadian Institutes of Health Research (CIHR), the Alberta Cancer Board, the Department of Clinical Neurosciences at the University of Calgary. Additional support was obtained from the National Institutes of Health (Mayo Clinic Brain SPORE, CA108961-05CDA), and the Department of Neurologic Surgery at the Mayo Clinic. Ms Rodrigues was supported by an Alberta Cancer Board / Canadian Institutes for Health Research Translational Research Training in Cancer fellowship.
Supplementary Material
Acknowledgment
Dr Parney has been an AHFMR Clinical Investigator and a CIHR New Investigator.
Conflict of interest statement. None declared.
References
- 1.Holland EC. Progenitor cells and glioma formation. Curr Opin Neurol. 2001;14:683–688. doi: 10.1097/00019052-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol (Berl). 2005;109:93–108. doi: 10.1007/s00401-005-0991-y. [DOI] [PubMed] [Google Scholar]
- 3.Liau LM, Black KL, Prins RM, et al. Treatment of intracranial gliomas with bone marrow-derived dendritic cells pulsed with tumor antigens. J Neurosurg. 1999;90:1115–1124. doi: 10.3171/jns.1999.90.6.1115. [DOI] [PubMed] [Google Scholar]
- 4.Parney IF, Petruk KC, Zhang C, et al. GM-CSF and B7-2 combination immunogene therapy in an allogeneic hu-PBL-SCID/beige mouse—human glioblastoma multiforme model. Hum Gene Ther. 1997;8:1073–1085. doi: 10.1089/hum.1997.8.9-1073. [DOI] [PubMed] [Google Scholar]
- 5.Sampson JH, Archer GE, Ashley DM, et al. Subcutaneous vaccination with irradiated, cytokine-producing tumor cells stimulates CD8+ cell-mediated immunity against tumors located in the "immunologically privileged" central nervous system. Proc Natl Acad Sci USA. 1996;93:10399–10404. doi: 10.1073/pnas.93.19.10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 7.Parney IF, Chang LJ, Farr-Jones MA, et al. Technical hurdles in a pilot clinical trial of combined B7-2 and GM-CSF immunogene therapy for glioblastomas and melanomas. J Neurooncol. 2006;78:71–80. doi: 10.1007/s11060-005-9058-0. [DOI] [PubMed] [Google Scholar]
- 8.Yu JS, Liu G, Ying H, et al. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 9.Morford LA, Elliott LH, Carlson SL, et al. T cell receptor-mediated signalling is defective in T cells obtained from patients with primary intracranial tumors. J Immunol. 1997;159:4415–4425. [PubMed] [Google Scholar]
- 10.Dix AR, Brooks WH, Roszman TL, et al. Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol. 1999;100:216–232. doi: 10.1016/s0165-5728(99)00203-9. [DOI] [PubMed] [Google Scholar]
- 11.Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 12.Hussain SF, Yang D, Suki D, et al. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro-oncology. 2006;8:261–279. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parney IF, Farr-Jones MA, Chang L-J, et al. Human glioma immunobiology in vitro: implications for immunogene therapy. Neurosurgery. 2000;46:1169–1178. doi: 10.1097/00006123-200005000-00030. [DOI] [PubMed] [Google Scholar]
- 14.Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 15.Hao C, Parney IF, Roa WH, et al. Cytokine and cytokine receptor mRNA expression in human glioblastomas: evidence of Th1, Th2, and Th3 dysregulation. Acta Neuropathol (Berl) 2002;103:171–178. doi: 10.1007/s004010100448. [DOI] [PubMed] [Google Scholar]
- 16.Morimura T, Neuchrist C, Kitz K, et al. Monocyte subpopulations in human gliomas: expression of Fc and complement receptors and correlation with tumor proliferation. Acta Neuropathol (Berl) 1990;80:287–294. doi: 10.1007/BF00294647. [DOI] [PubMed] [Google Scholar]
- 17.Parney IF, Waldron JT, Parsa AT. Flow cytometry and in vitro analysis of human glioma-associated macrophages. J Neurosurg. 2009;110:572–582. doi: 10.3171/2008.7.JNS08475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flugel A, Labeur MS, Grasbon-Frodl E-M, et al. Microglia only weakly present glioma antigen to cytotoxic T cells. Int J Devl Neurosci. 1999;17:547–556. doi: 10.1016/s0736-5748(99)00020-9. [DOI] [PubMed] [Google Scholar]
- 19.Taniguchi Y, Ono K, Yoshida S, et al. Antigen-presenting capability of glial cells under glioma-harboring conditions and the effect of glioma-derived factors on antigen presentation. J Neuroimmunol. 2000;111:177–185. doi: 10.1016/s0165-5728(00)00361-1. [DOI] [PubMed] [Google Scholar]
- 20.Wagner S, Czub S, Greif M, et al. Microglial/macrophage expression of interleukin 10 in human glioblastomas. Int J Cancer. 1999;82:12–16. doi: 10.1002/(sici)1097-0215(19990702)82:1<12::aid-ijc3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Badie B, Schartner J, Prabakaran S, et al. Expression of Fas ligand by microglia: possible role in glioma immune evasion. J Neuroimmunol. 2001;120:19–24. doi: 10.1016/s0165-5728(01)00361-7. [DOI] [PubMed] [Google Scholar]
- 22.Rivest S. Molecular insights on the cerebral innate immune system. Brain Behav Immun. 2003;17:13–19. doi: 10.1016/s0889-1591(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 23.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 25.Bronte V, Apolloni E, Cabrelle A, et al. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- 26.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 27.Mazzoni A, Bronte V, Visintin A, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–695. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald KP, Rowe V, Clouston AD, et al. Cytokine expanded myeloid precursors function as regulatory antigen-presenting cells and promote tolerance through IL-10-producing regulatory T cells. J Immunol. 2005;174:1841–1850. doi: 10.4049/jimmunol.174.4.1841. [DOI] [PubMed] [Google Scholar]
- 29.Nagaraj S, Gupta K, Pisarev V, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang B, Pan PY, Li Q, et al. Gr-1 + CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 31.Gallina G, Dolcetti L, Serafini P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vecil GG, Larsen PH, Corley SM, et al. Interleukin-1 is a key regulator of matrix metalloproteinase-9 expression in human neurons in culture and following mouse brain trauma in vivo. J Neurosci Res. 2000;61:212–224. doi: 10.1002/1097-4547(20000715)61:2<212::AID-JNR12>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 33.Parney IF, Hao C, Petruk KC. Glioma immunology and immunotherapy: a review. Neurosurgery. 2000;46:778–792. doi: 10.1097/00006123-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Graf MR, Sauer JT, Merchant RE. Tumor infiltration by myeloid suppressor cells in response to T cell activation in rat gliomas. J Neurooncol. 2005;73:29–36. doi: 10.1007/s11060-007-9442-z. [DOI] [PubMed] [Google Scholar]
- 35.Prins RM, Scott GP, Merchant RE, et al. Irradiated tumor cell vaccine for treatment of an established glioma. II. Expansion of myeloid suppressor cells that promote tumor progression. Cancer Immunol Immunother. 2002;51:190–199. doi: 10.1007/s00262-002-0270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogden AT, Horgan D, Waziri A, et al. Defective receptor expression and dendritic cell differentiation of monocytes in glioblastomas. Neurosurgery. 2006;59:902–909. doi: 10.1227/01.NEU.0000233907.03070.7B. discussion 909–910. [DOI] [PubMed] [Google Scholar]
- 37.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 38.Cao Y, Zhou H, Tao J, et al. Keratinocytes induce local tolerance to skin graft by activating interleukin-10-secreting T cells in the context of costimulation molecule B7-H1. Transplantation. 2003;75:1390–1396. doi: 10.1097/01.TP.0000061599.24682.EC. [DOI] [PubMed] [Google Scholar]
- 39.Gray CP, Arosio P, Hersey P. Heavy chain ferritin activates regulatory T cells by induction of changes in dendritic cells. Blood. 2002;99:3326–3334. doi: 10.1182/blood.v99.9.3326. [DOI] [PubMed] [Google Scholar]
- 40.Leussink VI, Jung S, Merschdorf U, et al. High-dose methylprednisolone therapy in multiple sclerosis induces apoptosis in peripheral blood leukocytes. Arch Neurol. 2001;58:91–97. doi: 10.1001/archneur.58.1.91. [DOI] [PubMed] [Google Scholar]
- 41.Wilkinson JR, Lane SJ, Lee TH. Effects of corticosteroids on cytokine generation and expression of activation antigens by monocytes in bronchial asthma. Int Arch Allergy Appl Immunol. 1991;94:220–221. doi: 10.1159/000235365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.