Abstract
Immunostimulating oligodeoxynucleotides containing CpG motifs (CpG-ODN) have shown promising efficacy in cancer models when injected locally. In a phase I clinical trial, intratumoral infusions of CpG-ODN in glioblastoma (GBM) patients were well tolerated at doses up to 20 mg. This phase II trial was designed to study the efficacy of a local treatment by CpG-ODN in patients with recurrent GBMs. Patients with recurrent GBM occurring at least 3 months after radiotherapy, and previously treated with 1 or 2 regimens of chemotherapy received 20 mg of CpG-ODN (CpG-28) by convection-enhanced delivery. The primary endpoint was the percentage of patients without tumor progression 6 months after inclusion. Secondary endpoints were tolerance, survival, and radiological response. Thirty-four patients were enrolled in two centers between November 2004 and March 2006. Thirty-one patients received CpG-ODN treatment. The progression-free survival (PFS) at 6 months was 19%. One partial response and 3 minor responses were observed. The median overall survival was 28 weeks. Eight patients (24%) were alive 1 year after inclusion and 5 patients (15%) were alive after 2 years. Treatment was usually well tolerated. As reported previously, the most common toxicities were lymphopenia, mild fever, seizures, and transient neurological worsening. Despite a few cases showing a radiological response, CpG-28 showed modest activity on the 6-month PFS in this patient population. The molecular or clinical characteristics of a subgroup of patients that could potentially benefit from such an approach remain to be defined.
Keywords: CpG-ODN, convection-enhanced delivery, glioblastoma, phase II, TLR9
Glioblastoma (GBM) is the most frequent primary brain tumor in adults. Despite surgical resection and radiotherapy associated with or not associated with chemotherapy, the prognosis in GBM patients remains poor, with a median time to relapse of around 7 months.1 At recurrence, the efficacy of chemotherapy is limited, with a progression-free survival (PFS) at 6 months of 15%–20% and a median survival of around 6 months,2,3 underscoring the need for new therapeutic approaches such as immunotherapy.
Oligodeoxynucleotides containing unmethylated cytosine-guanosine motifs (CpG-ODNs) display potent immunomodulatory effects. CpG-ODNs exert their effects through activation of the toll-like receptor 9 (TLR9).4 In humans, TLR9 is mainly expressed by plasmacytoid dendritic cells and B cells.5 TLR9 is also expressed in human microglial cells,6 and we have recently shown TLR9 expression in human GBM samples.7
The identification of tumor antigens is a limiting step for the design of cancer vaccines. To overcome this problem, CpG-ODNs alone can be directly injected into the tumor, with the expectation that the immune system will select the most relevant antigens by itself. The validity of such an approach was shown in various cancer models, including malignant glioma, with both activation of innate immunity and triggering of tumor-specific T-lymphocytes.8–11 In addition, regulatory T-lymphocytes (Tregs) play a key role in immune tolerance, and intratumoral Treg depletion was recently reported after treatment with a TLR9 agonist in a murine glioma model, a biological effect which partly explained tumor rejection.12
A recent phase I clinical trial using local administration of CpG-ODN into the tumor mass of 24 patients with recurrent GBM showed a good safety profile and a minor response in 2 patients.11
This multicenter phase II trial was designed to study the efficacy and tolerance of a local treatment by CpG-ODN in patients with recurrent GBM. The patients received CpG-ODN by catheters implanted in the tumor mass. The primary objective was efficacy, based on PFS. Secondary outcomes were tolerance, survival, and radiological response.
Patients and Methods
Patient Eligibility Criteria
Eligibility criteria were defined as follows: age >18 years; histologically proven GBM (WHO criteria) with or without an oligodendroglial component; radiological progression occurring at least 3 months after surgery and at least 3 months after completion of radiotherapy; measurable contrast enhancement (1–6 cm) on MRI; Karnofsky performance status score of 60 or higher; life expectancy >3 months; patients previously treated by a minimum of 1 and a maximum of 2 regimens of chemotherapy; 4-week interval from last chemotherapy (6-week interval for nitrosourea); and adequate bone marrow and hepatic functions.
Ineligibility criteria were pregnancy and breast-feeding, past history of autoimmune disease, refractory epilepsy, MRI contraindication, and increase in the steroid dosage within 7 days of inclusion MRI. Before being included in the study, patients signed an informed consent form, which was approved by the institutional review board.
Treatment and Drug Administration
CpG-28 (sequence 5′-TAAACGTTATAACGTTATGACGTCAT-3′)11 synthesized with a wholly phosphorothioate backbone was supplied by Oligovax (Paris, France) in vials containing 10 mg of CpG-28 in 1 mL. The total drug volume injected was 2 mL per patient (1 mL per implanted catheter).
Two catheters (Seldiflex and Plastimed) were inserted intracerebrally in patients under local anesthesia using stereotactic guidance through small twist-drill holes. No surgical resections of the tumors were performed. The tips of the catheters targeted the contrast-enhanced areas, and they had to be at least 2 cm deep within the brain. After surgery, a CT scan was performed to check the positions of the catheters and the absence of hemorrhage. The infusion began a minimum of 1 hour and a maximum of 24 hours after implantation and used an infusion rate of 3.3 µm/hour (DPS Orchestra electric syringe; Fresenius Vial, France) for 6 hours (1 hour for the dead volume of the catheter and 5 hours for drug infusion).
In addition to their usual treatment, patients received clonazepam (1 mg intravenous [iv] at the start of CpG-28 administration and 1 mg at the end) to prevent seizures and 2 g of oxacilline to prevent infections. The catheters were removed 1 hour after completion of CpG-28 administration. Steroids and anticonvulsant treatment were allowed. Treatment with any other approved or investigational chemotherapeutic agents was not permitted.
Patient Evaluations
Patients were assessed monthly for 6 months after CpG-28 administration. At each follow-up visit, neurological and general examinations, complete blood count, serum biochemistry, and liver function tests were performed. Antinuclear antibody titers were measured at baseline and day 30. Toxicity was graded according to the NCI expanded common toxicity criteria (NCI CTC 3.0). Radiographic response was evaluated using the Macdonald criteria.13 MRI scans were performed 4 and 8 weeks after treatment, then every other month. Because of possible modifications of radiological images after local infusion of an immunostimulating agent, the earliest time point for progression was considered as 8 weeks. MRI images were centrally reviewed in a nonsequential order by two investigators (F.L.-D.; A.F.C.). The patients in the intent-to-treat (ITT) group who did not receive the study drug were not included in the safety analysis.
Statistical Considerations
The primary objective was to assess the percentage of patients without tumor progression at 6 months after treatment (6-month PFS). A sequential Bayesian strategy was chosen to allow continuous monitoring of outcomes throughout the trial. The prior distribution of the success rate for the 6-month PFS was centered on 30%. As the patient outcomes in the trial were recorded, the subsequent distribution of the outcome probability under experimental treatment was computed by applying Bayes' theorem, which yielded a mean estimated success rate with a 95% credibility interval (measure of Bayesian precision). On the basis of this subsequent distribution, early stopping criteria were calculated to allow early termination of the trial: (i) for inefficacy if the estimated success rate was <15% or (ii) for futility if the expected gain from further inclusions in the reliability of the estimated success rate was low (0.05 or less). The qualitative and quantitative results were expressed as percentage and median (range), respectively, and event-free survival and survival curves were calculated by the Kaplan–Meier method.
MGMT Methylation Status
Where paraffin blocks of the initial tumor at the time of diagnosis were available, genomic DNA was extracted (DNA purification kit, Promega, Lyon, France). DNA was then converted by the Epitect bisulfite kit (Qiagen, Courtaboeuf, France). Methylation analysis was performed with the methylight technique14 using theLightCycler 480 (Roche, Meylan, France). Bisulfate converted genomic DNA was amplified using a set of primers and a fluorescent dye–labeled oligonucleotide probe, resulting in a semiquantitative methylation analysis. For the CpG islands of the 15 investigated gene regions (GENE), the primers and probes were designed specifically for methylated DNA. The differences in amounts of input genomic DNA were normalized by the Col2A1 gene. SssI-treated genomic DNA was used as a standard. The percentage of methylated reference (PMR) was calculated as follows: the methylated MGMT/COL2A1 ratio for each sample was divided by the GENE/COL2A1 ratio of 100% SssI-treated genomic DNA, and the values were multiplied by 100 to give the PMR. Tumors were considered methylated if the calculated PMR was above zero.
Pharmacokinetic Studies
Pharmacokinetics (PKs) of CpG-28 in the blood was studied in 6 patients. Blood samples (2 mL serum per sample) were drawn at baseline, 3 hours after the start of the infusion, at the end of infusion, and then 1, 2, 4, 6, and 15 hours after the end of the perfusion. The terminal elimination phase of the PK profile was identified (where possible) based on the line of best fit (R2) using at least the final 3 observed concentration values. The slope of the terminal elimination phase was calculated using a log-linear regression using the unweighted concentration data.
Assessment of Treg
The number of lymphocytes and the CD4 + CD25high FoxP3+ Treg cell subsets in the circulating blood at the start and 1 month after treatment with CpG-28 was determined in 4 patients. The peripheral blood mononuclear cell (PBMC) were purified on Ficoll (Eurobio, Les Ulis, France) gradients and kept frozen in liquid nitrogen. Mononuclear cells were stained with anti-hCD4 (-FITC from Becton Dickinson, San Diego, California) and anti-hCD25 (-PC7 from Becton Dickinson). Intracellular detection of FoxP3 with anti-hFoxP3 (-PE, clone 236E/A7, e-Bioscience, San Diego, California) was performed on fixed and permeabilized cells using Cytofix/cytoperm (e-Bioscience). Flow cytometry was performed on LSRII (Becton Dickinson), and data were analyzed with DIVA.
Results
Patient Characteristics
Thirty-four GBM patients with recurrent tumors were enrolled in two centers between November 2004 and March 2006. Their characteristics are summarized in Table 1. Three patients were included but did not receive the experimental drug and therefore were not evaluated for response to CpG-ODN treatment or safety analysis (1 patient had a major tumor progression between inclusion and planned treatment; 2 patients had wrongly placed catheters).
Table 1.
Patient characteristics
| Characteristic | Number of patients (n = 34) | Percent |
|---|---|---|
| Male/female | 18/16 | |
| Median age (range, years) | 57 (31–71) | |
| KPS (%) | ||
| 80–100 | 15 | 44 |
| 70 | 14 | 41 |
| 60 | 5 | 15 |
| Median (range; %) | 70 (60–100) | |
| Time from first diagnosis to treatment (mo) | ||
| Median (range) | 11.3 (5.2–43.0) | |
| Prior therapy | ||
| Radiotherapy only | 0 | 0 |
| Radiotherapy combined with TMZ (concomitant) | 10 | 29 |
| Radiotherapy followed by 1 adjuvant regimen of chemotherapy | 10 | 30 |
| Radiotherapy followed by 2 regimens of chemotherapy (inclusion in this trial at time of second recurrence) | 14 | 41 |
| Corticoids treatment at the time of inclusion (mg/d) | ||
| >60 | 4 | 12 |
| 30–60 | 15 | 44 |
| 5–30 | 11 | 32 |
| 0 | 4 | 12 |
Abbreviations: KPS, Karnofsky performance status; TMZ, temozolomide.
Primary Endpoint, PFS, and Radiological Response
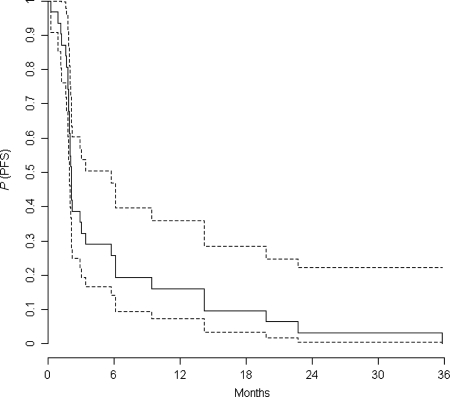
The 3 patients in the ITT population who did not receive the study drug were included in this analysis. In the treated population (31 patients), the 6-month PFS was 19% and the median PFS was 9.1 weeks (Fig. 1). As the predictive probability of observing 3 or more successes in the next 5 patients was 3% according to the Bayesian analysis, the inclusions were stopped based on no predictive significant gain (<0.05) from the next 5 inclusions.
Fig. 1.
PFS in the treated population (with 95% confidence interval in dashed lines).
One partial response (−78%) and 3 minor responses (−33%, −26%, and −25%) were observed. The patient with a partial response relapsed after 19 months and was still alive at the time of last follow-up. Patients with minor responses had recurrences at 6.1, 6.1, and 5.7 months after inclusion, respectively. Three additional patients achieved stable disease over 6 months. Two of these patients subsequently developed progressive disease 9 and 22 months after study enrolment. One patient remains alive without active disease 36 months after study enrolment without any further treatment.
Survival and Patient Follow-Up
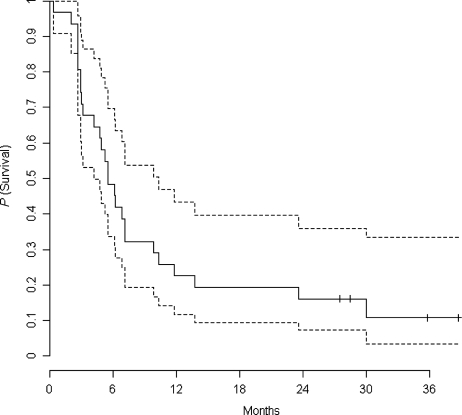
Overall survival (OS) in the ITT population is presented as a Kaplan–Meier curve in Fig. 2. The 6-month OS is 59%. The median OS is 28 weeks. Eight patients (24%) were alive after 1 year and 5 patients (15%) were alive at 2 years after inclusion in the protocol.
Fig. 2.
OS in the intent-to-treat population (with 95% confidence interval in dashed lines).
Postdiscontinuation systemic therapy was received by 84% patients. Therapies administered included BCNU, carboplatin, temozolomide (TMZ), CCNU, or VP16. Only 2 patients were treated with bevacizumab–irinotecan at relapse, 22 and 27 months after inclusion in the protocol and therefore, did not impact survival at earlier time points.
At the time of last follow-up, 4 patients are still alive (28, 29, 36, and 39 months). One of these patients remains alive without active disease 36 months from study enrolment without any further treatment, and 1 has no evidence of recurrence after the completion of one chemotherapy course with nitrosourea.
Sufficient genomic DNA for MGMT methylation status determination was obtained in 8 patients. Three patients were MGMT-methylated, and 5 were not. The median survival was 7.1 months for methylated patients and 5.7 for unmethylated patients. Among these 8 patients, 2 patients survived more than 1 year. Both were MGMT-unmethylated.
Toxicity
Adverse events (≥grade 2) related to the procedure and/or studied drug according to the investigator in charge of the patients are summarized in Table 2. As previously reported, the most common toxicities were lymphopenia, mild fever, seizures, and neurological worsening.
Table 2.
Reported adverse events (≥grade 2)
| Adverse event | Certainly related |
Probably related |
Possibly related |
|||
|---|---|---|---|---|---|---|
| Procedure | Treatment | Procedure | Treatment | Procedure | Treatment | |
| Clinical AEs | ||||||
| Partial seizures | 0 | 3 | 0 | 0 | 3 | 7 |
| General seizures | 0 | 3 (SAE) | 0 | 2 | 0 | 0 |
| Fever (grade 2) | 0 | 0 | 0 | 1 | 0 | 0 |
| Worsening of previous neurological condition | 0 | 0 | 3 | 4 | 7 | 6 |
| Fatigue (grade 2) | 0 | 0 | 0 | 0 | 0 | 2 |
| Hemorrhage leading to death 8 days after treatment | 1 (SAE) | |||||
| Other clinical symptoms (grade ≥2) | 2 (SAE) | |||||
| Biological AEs ≥grade 2a | ||||||
| Lymphopenia (grade 2) | 22 | |||||
| Lymphopenia (grade 3) | 15 | |||||
| ALT | 3 | |||||
| AST | 0 | |||||
| Other biological alterations | 0 | |||||
aCounted several times if they lasted several follow-up visits.
Of the 31 patients treated with CpG-28, 15 experienced grade 3 lymphopenia (<500/mm3) and 4 of these already had a grade 2 lymphopenia at the time of inclusion. None of them experienced an opportunist infection. All these patients had chemotherapy between 1 and 4 months before study enrolment and a relationship with the studied drug is possible.
Body temperature was monitored in 25 patients. A temperature greater than 38°C was noted in 9 patients, which was well tolerated and disappeared within 5 days without antibiotics. A relationship with the studied drug is probable.
Within the month following administration of CpG-28, 10 patients had short partial seizures. In 3 patients, a relationship with the treatment is probable because the seizure occurred just after administration of CpG-28.
Transient worsening of baseline neurological symptoms or fatigue was seen in 22 patients. This worsening was moderate and resolved within 4 weeks. When a neurological worsening did occur, although no increase in peritumoral edema was seen on MRI, the steroid dosage was increased, and this was usually sufficient for the patients to recover.
In addition, there were 6 serious adverse events (SAEs) considered as possibly or probably related to the protocol. Three SAEs were considered as related to the procedure (local infection regressive in 2 weeks with surgical drainage and antibiotics, postprocedure pneumoencephaly regressive within a month, and one hemorrhage leading to death 8 days after catheter implantation and treatment). The 3 other SAEs considered to be related to the infusion of CpG-28 were general seizures occurring within 24 hours of infusion. In 2 cases, these seizures occurred after protocol violations regarding the infusion rate of CpG-28 or in clonazepam administration. It should be noted that all seizures were resolved without recurrence following adjustments to the anticonvulsant medication.
PKs and Monitoring of Circulating Treg
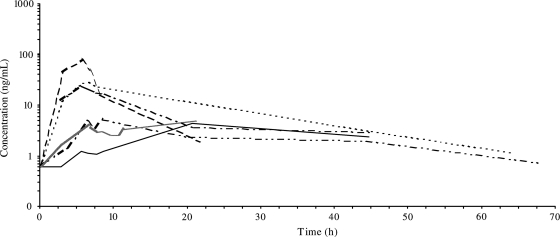
Among the 6 patients studied, the PK profile of CpG-28 in the blood was heterogeneous (Fig. 3). This was considered to result from the inherent variability in tumor location and the site of CpG-28 administration. CpG-28 plasma concentrations were shown to reach up to 79 ng/mL at the end of infusion and to be eliminated or reach baseline concentration at around 70 hours after the start of infusion. Half-life estimates were variable ranging from 3 to 24 hours. The mean area under the plasma concentration-time curve (AUC) ranged from 0.07 to 0.65 µg h/mL.
Fig. 3.
PKs in 6 patients after intracerebral infusion of CpG-28 in logarithmic scale (each line represents 1 patient).
The number of lymphocytes and the CD4 + CD25high FoxP3+ Treg cell subsets in the circulating blood of the 4 patients studied were not significantly modified by CpG-28 treatment (Table 3).
Table 3.
Circulating lymphocytes and Treg
| Patient | Date | Lymphocytes count/mm3 | CD4/T-lymphocytes (%) | CD4 + CD25hight/CD4 (%) | CD4 + Foxp3/CD4 (%) |
|---|---|---|---|---|---|
| 1 | Day 0 | ND | 28 | 0.9 | 0.9 |
| Day 30 | 738 | 29 | 1.5 | 1.6 | |
| 2 | Day 0 | 1394 | 46 | 0.9 | 0.7 |
| Day 30 | 1654 | 51 | 0.6 | 0.5 | |
| 3 | Day 0 | 630 | 6 | 2.1 | 2.4 |
| Day 30 | 816 | 8 | 2 | 1.9 | |
| 4 | Day 0 | 2760 | 22 | 1.0 | 0.9 |
| Day 30 | 438 | 20 | 1 | 1.5 |
Discussion
CpG-ODNs are powerful new immunostimulating agents currently in clinical trials in cancer patients, either as single agents or combined with targeted agents or chemotherapy. In the present study, we report a phase II trial with local administration by convection-enhanced delivery of a CpG-ODN in patients with recurrent GBM.
Efficacy was the primary endpoint of this trial. One radiological response was seen, the median survival was 6.4 months, and the PFS at 6 months was 19%, similar to most phase II studies with new therapeutic agents2,3,15,16 (except bevacizumab–iriniotecan, which gives a higher response rate17). Different interpretations can be drawn from our data. On the one hand, the less number of radiological responses and the standard PFS6 could suggest that the treatment is poorly effective. On the other hand, one can be surprised by the number of long-term survivors. In the literature, the 1-year survival is usually less than 15%,2,15–16 contrasting with the 1-year survival of 24% and a 2-year survival of 15% in our study, although we acknowledge that this could result from a selection bias in an uncontrolled study. The MGMT methylation status of the initial tumor was not correlated with the long-term survival in the few patients in whom material was still available. Using the recursive partitioning analysis (RPA) classification,18 most of our patients (23 of 31) were RPA class 7; the median OS for this group in our study was 6.0 months compared with the 4.9 months reported in the RPA classification (Appendix). A randomized design for this trial would have been more appropriate to clarify this point; unfortunately, this study was designed without a control group, due to the limited data on efficacy available at the time.
If we consider that treatment with local CpG-ODN can be of benefit in some patients, efficacy could depend on 2 factors: the success of catheter implantation and subsequent exposure to the drug, and/or a variable immune response triggered by CpG-ODN among patients, related to variable TLR9 expression within the tumors. Poor implantation of the catheters in some patients, hence a poor exposure to the drug, cannot be ruled out, although great care was made for correct implantations by the surgeon. Defining the fraction of drug that was not retained in the brain and leaked into the bloodstream during convection-enhanced delivery is not directly possible but can be estimated. The concentration steady state (Css) of 2 different phosphorothioate ODNs after a continuous intravenous perfusion (2 mg/kg/24 hour) were shown to be around 1400 ng/mL.19 The rate of infusion in our study was 4 mg/hour, corresponding to approximately 1.4 mg/kg/24 hour. We would therefore expect a Css of around 1000 ng/mL if our ODN had been fully injected into the bloodstream and not into the brain due to poor catheter implantation. The plasma concentration after a 5-hour infusion can be a good estimation of what the Css would have been if the infusion had been totally intravenous because the half-life of intravenous phosphorothioate ODN is known to be less than 1 hour.20 As the theoretical Css of 1000 ng/mL is far above the maximum of 79 ng/mL observed in one patient at the end of perfusion, we can estimate that less than 10% of CpG-28 leaked directly into the bloodstream. We acknowledge that this estimation is subject to criticism.
Regarding a variable immune response triggered by CpG-ODN among patients, this individual susceptibility has already been reported in clinical trials with CpG-ODN,21,22 although the underlying mechanism is unclear. Major polymorphisms of the TRL9 gene have not been found.23 One possible explanation could be variable TLR9 expression within tumors due to the degree of tumor infiltration by immune cells, as we have previously reported in patients with newly resected GBM.7 Translational research for TLR9 expression in our series is on-going but has been limited so far by poor antibody labeling on paraffin blocks. Unfortunately, no immunological surrogate markers such as tumor lymphocyte cytotoxicity were available in those patients who did not have surgery following relapse. In a murine model, CpG-induced depletion of Treg has also been suggested as a mechanism for tumor rejection.12 We did not find such depletion in the PBMC of the few patients with available frozen lymphocytes. This discrepancy might be explained in our patients by the treatment with steroids that can impact Treg levels24 and by the fact that Tregs were studied in the blood and not within the tumor as in the murine model.
Intracranial administration of CpG-28 was generally well tolerated and the toxicity profile was very similar to the phase I data. Transient neurological worsening or fatigue was the most significant toxicity observed, an adverse event that was not related to increased edema. Lymphopenia was frequent but well tolerated. It remains unclear whether this lymphopenia is related to the studied drug or to previous treatments with chemotherapy. Perhaps the most potentially SAE was seizures. Within the month following administration of CpG-28, 10 patients had short partial seizures. In 3 patients, a relationship with the treatment is probable because the seizure occurred just after administration of CpG-28. All seizures were resolved without recurrence following anticonvulsant treatment. One severe hemorrhage occurred during this study, leading to death 8 days after treatment. According to the scientific committee, this hemorrhage was more likely related to the procedure than to CpG infusion. Indeed, symptomatic hemorrhages are well known complications of stereotactic surgery procedures. The frequency of such events in deep-brain stimulation, a procedure very close to ours, has been estimated at 0.6%–2.0% per lead implant.25–27 Intracerebral hemorrhage after catheter placement was also reported in other trials using convection-enhanced delivery with other targeted therapeutics.28 If we combine results from the previous phase I trial11 and this phase II trial, out of 95 catheter implantations in 55 patients, 2 symptomatic hemorrhages occurred. This gives an incidence of 2% of symptomatic hemorrhage per implanted catheter in our protocol, which fits with the risk reported by other studies.
In conclusion, this phase II trial evaluating the efficacy of CpG-28 did not meet the targeted PFS benefit in our patients with recurrent GBM. However, the occurrence of a few long-term survivors could suggest that some GBM patients might benefit from this treatment. Translational studies are on-going to clarify the criteria for selection of such a subgroup of patients, if any. In addition, a randomized phase II trial is currently ongoing in newly diagnosed GBM in combination with surgical resection, radiotherapy, and concomitant TMZ.
Funding
This work was supported by the Fondation pour la Recherche Médicale (FRM), a grant from the Agence Nationale de la Recherche (ANR RIB 2006), the Association Oligocyte, the Association pour la Recherche sur les Tumeurs Cérébrales (ARTC), Oligovax (supply of CpG-ODN), and the Assistance Publique-Hôpitaux de Paris (AP/HP).
Acknowledgments
Members of the independent scientific committee were Jean-Sebastien Guillamo, Caen; Eric Raymond, Paris; François Lafitte, Paris; Michel Kalamarides, Clichy, and Bernard Asselain, Paris. Co-investigators were: Jean-Yves Delattre (Paris), Khe Hoang-Xuan (Paris), Sophie Taillibert (Paris), Antony Behin (Paris), Remy van Effenterre (Paris), Philippe Cornu (Paris), Laurent Capelle (Paris), Hugues Duffau (Paris), Damien Bresson (Paris), Bernard George (Paris), Selma Elouahdi, (Paris), François Grisoli (Marseille). Sylvie Chevret was involved in the statistical analysis of the trial. L'houcine Oauafik and Catherine Carpentier made the MGMT analysis.
Conflict of interest statement. The Assistance Publique – Hôpitaux de Paris (sponsor of the trial) and AF Carpentier hold a patent position on CpG in cancers. AF Carpentier has shares in Oligovax. Margaretha Richard is an employee of Oligovax.
Appendix: Survival in Our Trial, According To RPA Classes16
| RPA class | Number of patients | Median OS in the trial (mo) | Expected median OS (mo) |
|---|---|---|---|
| 4 (GBM, <50 y, KPS 90-100) | 1 | 13.7 | 10.4 |
| 5 (GBM, <50 y, KPS 60-80) | 6 | 5.6 | 5.6 |
| 6 (GBM, > 50 y, no steroids) | 4 | 6.1 | 6.4 |
| 7 (GBM, > 50 y, taking steroids) | 23 | 6.0 | 4.9 |
Abbreviations: KPS, Karnofsky performance status; GBM, glioblastoma; RPA, recursive partitioning analysis; OS, overall survival.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamborn KR, Yung WK, Chang SM, et al. North American Brain Tumor Consortium. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro-Oncology. 2008;10:162–170. doi: 10.1215/15228517-2007-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krieg AM. Antitumor applications of stimulating toll-like receptor 9 with CpG oligodeoxynucleotides. Curr Oncol Rep. 2004;6:88–95. doi: 10.1007/s11912-004-0019-0. [DOI] [PubMed] [Google Scholar]
- 5.Takeshita F, Leifer CA, Gursel I, et al. Cutting edge: role of Toll-like receptor 9 in CpG DNA-induced activation of human cells. J Immunol. 2001;167:3555–3558. doi: 10.4049/jimmunol.167.7.3555. [DOI] [PubMed] [Google Scholar]
- 6.Jack CS, Arbour N, Manusow J, et al. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- 7.Meng Y, Kujas M, Marie Y, et al. Expression of TLR9 within human glioblastoma. J Neurooncol. 2008;88:19–25. doi: 10.1007/s11060-008-9536-2. [DOI] [PubMed] [Google Scholar]
- 8.Carpentier AF, Chen L, Maltonti F, Delattre JY. Oligodeoxynucleotides containing CpG motifs can induce rejection of a neuroblastoma in mice. Cancer Res. 1999;59:5429–5432. [PubMed] [Google Scholar]
- 9.Carpentier AF, Xie J, Mokhtari K, Delattre JY. Successful treatment of intracranial gliomas in rat by oligodeoxynucleotides containing CpG motifs. Clin Cancer Res. 2000;6:2469–2473. [PubMed] [Google Scholar]
- 10.Carpentier AF, Auf G, Delattre JY. CpG-oligonucleotides for cancer immunotherapy: review of the literature and potential applications in malignant glioma. Front Biosci. 2003;8:115–127. doi: 10.2741/934. [DOI] [PubMed] [Google Scholar]
- 11.Carpentier A, Laigle-Donadey F, Zohar S, et al. Phase 1 trial of a CpG oligodeoxynucleotide for patients with recurrent glioblastoma. Neuro-Oncology. 2006;8:60–66. doi: 10.1215/S1522851705000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Andaloussi A, Sonabend AM, Han Y, Lesniak MS. Stimulation of TLR9 with CpG ODN enhances apoptosis of glioma and prolongs the survival of mice with experimental brain tumors. Glia. 2006;54:526–535. doi: 10.1002/glia.20401. [DOI] [PubMed] [Google Scholar]
- 13.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 14.Eads CA, Danenberg KD, Kawakami K, et al. MethyLight: a high-throughput assay to measure DNA methylation. Nucleic Acids Res. 2000;28 doi: 10.1093/nar/28.8.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine HA, Kim L, Royce C, et al. Results from phase II trial of enzastaurin (LY317615) in patients with recurrent high grade gliomas [abstract] J Clin Oncol. 2005;23(suppl 16) A-1504, 115s. [Google Scholar]
- 16.Van den Bent MJ, Brandes A, Rampling R, et al. Randomized phase II trial of erlotinib (E) versus TMZ or BCNU in recurrent glioblastoma multiforme (GBM): EORTC 26034 [Meeting Abstracts] J Clin Oncol. 2007;25 2005. [Google Scholar]
- 17.Vredenburgh JJ, Desjardins A, Herndon JE, II, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 18.Carson AK, Grossman SA, Fisher JD, Shaw EG. Prognostic Factors for Survival in adult with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS Consortium phase I and II clinical trials. J Clin Oncol. 2007;25:2601–2606. doi: 10.1200/JCO.2006.08.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tolcher AW, Reyno L, Venner PM, et al. A randomized phase II and pharmacokinetic study of the antisense oligonucleotides ISIS 3521 and ISIS 5132 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2000;8:2530–2535. [PubMed] [Google Scholar]
- 20.Yuen AR, Halsey J, Fisher GA, et al. Phase I study of an antisense oligonucleotide to protein kinase C-( ( ISIS 3521/CPG 64128A) in patients with cancer. Clin Cancer Res. 1999;5:3357–3363. [PubMed] [Google Scholar]
- 21.Cooper CL, Davis HL, Morris ML, et al. Safety and immunogenicity of CPG 7909 injection as an adjuvant to fluarix influenza vaccine. Vaccine. 2004;22:3136–3143. doi: 10.1016/j.vaccine.2004.01.058. [DOI] [PubMed] [Google Scholar]
- 22.Krieg AM. Therapeutic potential of toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 23.Krieg AM, Efler SM, Wittpoth M, Al Adhami MJ, Davis HL. Induction of systemic TH1-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-class CpG oligodeoxynucleotide TLR9 agonist. J Immunother. 2004;27:460–471. doi: 10.1097/00002371-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Braitch M, Harikrishnan S, Robins RA, et al. Glucocorticoids increase CD4CD25 cell percentage and Foxp3 expression in patients with multiple sclerosis. Acta Neurol Scand. 2009;119:239–245. doi: 10.1111/j.1600-0404.2008.01090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyons KE, Wilkinson SB, Overman J, Pahwa R. Surgical and hardware related complications of subtalamic stimulation: a series of 160 procedures. Neurology. 2004;63:612–616. doi: 10.1212/01.wnl.0000134650.91974.1a. [DOI] [PubMed] [Google Scholar]
- 26.Gorgulho A, De Salles AA, Frighetto L, Behnke E. Incidence of hemorrhage associated with electrophysiological studies performed using macroelectrodes and microelectrodes in functional neurosurgery. J Neurosurg. 2005;105:888–896. doi: 10.3171/jns.2005.102.5.0888. [DOI] [PubMed] [Google Scholar]
- 27.Sansur CA, Frysinger RC, Pouratian N, et al. Incidence of symptomatic hemorrhage after stereotactic electrode placement. J Neurosurg. 2007;107:998–1003. doi: 10.3171/JNS-07/11/0998. [DOI] [PubMed] [Google Scholar]
- 28.Sampson JH, Akabani G, Archer GE, et al. Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J Neurooncol. 2003;65:27–35. doi: 10.1023/a:1026290315809. [DOI] [PubMed] [Google Scholar]