Abstract
The frequency of meningeal dissemination (MD) in primary CNS lymphoma (PCNSL), its prognostic impact, and optimal management have not been defined thus far. In 69 of 92 (75%) immunocompetent patients, primarily diagnosed with PCNSL at our institution between January 1994 and February 2007, cerebrospinal fluid was analyzed for MD. MD was found by cytomorphology in 7/63 (11%), by immunophenotyping in 1/32 (3%), and by PCR of the IgH CDR III region in 6/37 (16%). Neuroradiologic examination revealed MD in 3 of 69 patients (4%). Median event-free survival (EFS) of patients with MD diagnosed by any of the methods was 26 months, of those without MD 34.1 months (P = .24); median overall survival (OAS) of these two patients' groups was 45.5 and 42.5 months, respectively (P = .34). Patients with cytomorphologic proof of MD had a median EFS of 15.4 months and OAS of 18.5 months, those without MD 34.3 and 45 months (P = .018 and .017, respectively). We found a low frequency of MD despite the use of putatively sensitive diagnostic methods. No impact on outcome was seen for MD, diagnosed by any of the methods used; however, patients with cytomorphologic proof of MD had a significantly shorter median EFS and OAS.
Keywords: cerebrospinal fluid, CNS lymphoma, meningeal dissemination, prognosis
The incidence of meningeal dissemination (MD) in primary CNS lymphoma (PCNSL) is not exactly known. In 7%–42% of PCNSL patients, MD has been reported to be diagnosed by morphologic cerebrospinal fluid (CSF) assessment,1–3 which is considered the gold standard in detecting MD in malignant disease.4 In malignant lymphoma, however, a high rate of both false-negative and false-positive results has been reported.5,6 False-negative results can be attributed to the paucity of tumor cells, frequently due to an upfront use of corticosteroids, and might be reduced by larger sample volumes, repeated sampling, and avoiding corticosteroids until definite diagnosis when possible.4,7 The misinterpretation of reactive CSF lymphocytes as lymphoma cells, leading to false-positive results, represents another obstacle for the cytomorphlogic detection of lymphoma cells in the CSF.8–10
The optimal therapy of MD in PCNSL has not been established due to the rarity of the disease and the lack of adequately designed prospective trials. Moreover, the evaluation of the prognostic impact of MD has yielded conflicting results. In one study an inferior outcome was observed after deleting intrathecal chemotherapy from a combined systemic/intrathecal chemotherapy regimen.11 These data raise the possibility of occult meningeal spread in many PCNSL patients. Addressing the meningeal compartment by intrathecal chemotherapy might improve the outcome of these patients. However, intrathecal chemotherapy bears the inconvenience of repeated lumbar punctures, the risk of an Ommaya reservoir infection,12 and the increased risk of neurotoxicity.13
An optimal diagnostic CSF work-up including putatively sensitive methods such as immunophenotyping and PCR of the CDR III region of the rearranged IgH genes could help improve diagnostic accuracy for MD detection in PCNSL to ensure optimal management while omitting unnecessary treatment. Thus, we retrospectively evaluated all our immunocompetent PCNSL patients for incidence of MD diagnosed with different methods and assessed the therapeutic management and the impact of MD on survival.
Methods
Patients and Treatment
Records of all 92 consecutive immunocompetent patients diagnosed with PCNSL or its subtype primary intraocular lymphoma (PIOL) from January 1994 to February 2007 at our institution were reviewed, and 69 patients (75%) in whom CSF analysis by at least one method capable of detecting lymphoma cells (cytomorphology, immunophenotyping, or PCR of the rearranged IgH gene CDR III region) was performed at first diagnosis were identified (in two further patients CSF was analyzed for cell count and protein only). The diagnosis of PCNSL/PIOL had to be confirmed histologically by tumor biopsy and/or cytologically from CSF or aqueous humor (in case of leptomeningeal involvement or ocular involvement, respectively). In rare cases where biopsy was not feasible or inconclusive, a strong suspicion of PCNSL based on radiomorphological and clinical features after exclusion of other conditions was accepted if treatment was started accordingly. Before treatment contrast-enhanced cerebral computed tomography (CT) scans or magnetic resonance imaging (MRI) of the brain with gadolinium, CSF analysis by a single lumbar puncture, CT of the chest/abdomen/pelvis, bone marrow biopsy, creatinine clearance, and HIV testing were performed in all patients. Slit lamp examination of the eye was performed only in patients with ocular symptoms.
Patients were intended to be treated with high-dose methotrexate (HDMTX), depending on their performance status and organ function. Three regimens were used: from January 1994 to April 2000 HDMTX 1.5 g/m2 i.v. over 24 hours on day 2, carmustine 80 mg/m2 i.v. on day 1 and procarbazine 100 mg/m2 p.o. on days 1–8, repeated every 4 weeks (BMPD [carmustine, methotrexate, procarbazine, dexamethasone] protocol);14 from May 2000 to November 2006 HDMTX 4 g/m2 i.v. over 4 hours on day 1 as monotherapy, repeated every 2 weeks for maximal 6 courses; and from December 2006 to February 2007 HDMTX 4 g/m2 i.v. over 4 hours on day 1 and ifosfamide 1.5 g/m2 i.v. over 3 hours on days 3–5. All patients additionally received dexamethasone 3×8 mg/day in the first course, and thereafter only if clinically indicated. HDMTX dose was adjusted to patients' creatinine clearance.3 Intrathecal treatment was given only to patients on the BMPD protocol: 15 mg MTX on day 1 of each course to patients with cytomorphologic proof of lymphoma in the CSF and in course 1 only to all other patients. Treatment response was evaluated by MRI or CT after the first and third BMPD treatment course and in case of HDMTX monotherapy or HDMTX/ifosfamide combination treatment after the third and the sixth course. Sequential CSF evaluations during the course of treatment were not performed routinely, regardless of initial presence of MD. During the follow-up all patients were followed longitudinally with surveillance brain MRI or CT scans and neurologic examinations every 3 months in the first year, every 4 months in the second year and every 6 months thereafter. Additional neuroradiologic evaluation was performed upon clinical suspicion.
Survival data of 49 of the patients have been previously reported.15
CSF Analysis
CSF was obtained by lumbar puncture at first diagnosis of PCNSL prior to any chemo- or radiotherapy (excluding corticosteroids) and immediately processed for cell count, cytomorphology, and protein concentration. The CSF evaluation for MD included: cytomorphology in 63 patients, immunophenotyping in 32 patients (immunocytology in 4 patients and flow cytometry with fluorescent-amplified cell-sorting [FACS] analysis in 28 patients), and PCR of the rearranged IgH gene CDR III region in 37 patients. Additionally, CSF was evaluated for cell count in 64 patients and for protein in 55 patients.
Cytomorphology was interpreted by at least two experienced hematopathologists/neurologists, and was termed positive for conclusive detection of lymphoma cells and suspicious when atypical lymphatic cells not clearly diagnostic of malignancy were detectable; the remaining cases were termed negative.
For immunophenotyping, CSF was either centrifuged within 2 hours after asservation and spotted on poly-l-lysin-coated glass slides with subsequent antibody staining for CD19, CD3, lambda, and kappa chains16 or measured by flow cytometry with 2- or 4-color staining of the same antigens. Immnunophenotyping was regarded positive when a monoclonal B-cell population (exclusive expression of lambda or kappa immunoglobulin light chains) was detected.
For molecular genetic analysis, the PCR of the rearranged IgH genes was performed as described recently until March 2006.17 Briefly, a seminested PCR of the IgH chain CDR III region was performed using the primers LJH in the first, VLJH in the second, and FR3A in both PCR reactions. All samples were tested with a human growth hormone PCR to check for amplifiable DNA. All specimens were amplified at least twice in independent PCR runs to avoid false monoclonal interpretation of pseudomonoclonal rearrangement patterns.18 From April 2006 on, samples (n = 5) were subjected to a PCR using 3 sets of family-specific IgH primers according to the BIOMED-2 protocol, which was modified to apply 50 instead of 35 cycles for increased sensitivity. Three different framework region primer sets (FR1, FR2, and FR3) were applied separately to all samples in conjunction with an IgH joining segment (JH) consensus primer (JH22).19 In each PCR, positive (DNA from a B-cell line) and negative controls (sterile water) were included. A monoclonal pattern was defined as the detection of a single or dominating amplicon of identical size in repetitive experiments. Multiple peaks characterized polyclonality.
Elevated cell count was defined as ≥5/μl, and elevated protein as >450 mg/l.
Neuroimaging
Brain MRIs were obtained before treatment in 58 patients and CT in 10 patients; in 1 patient imaging modality is unknown. All scans available (n = 63) were reviewed by an experienced neuroradiologist. All examinations included T1- and T2-weighted sequences as well as contrast-enhanced studies. Cerebral imaging was performed on various MR scanners with field strengths of 1.0–1.5 T. For the T1-weighted scans, all patients received 0.1-mmol gadolinium–DTPA per kg body weight. CT scans were performed as contrast-enhanced cerebral CTs. MD on neuroimaging was defined as contrast-enhancement of the leptomeninges.
Definition of MD
Patients were regarded having MD if at least one of the following conditions was fulfilled: conclusive cytomorphological detection of lymphoma cells, light-chain restricted B-cell population detected by immunocytology or flow cytometry, presence of a dominant amplicon in PCR analysis, or evidence of MD on MRI.
Statistics
For statistical analysis, patient characteristics were grouped according to prognostic factors previously published: age ≤50 and >50 years, age ≤60 and >60 years and age as a continuous variable, Karnofsky performance score ≥70 and <70, the MSKCC prognostic score,20 and superficial and deep lesion location. Event-free survival (EFS) was defined as the time from histologic diagnosis to first documentation of relapse (on imaging or in CSF) or death from any cause in patients responding (complete response and partial response) to first therapy since the survival of non-responders and patients treated with steroids only is usually very short in PCNSL. Overall survival (OAS) was defined as the time from beginning of treatment to death from any cause, according to the standardized response criteria for non-Hodgkin's lymphoma.21
EFS and OAS were estimated by the Kaplan–Meier method. Group comparisons were made using the log-rank test. Distribution of patient characteristics to different groups was analyzed by the chi-square test. MD status and pleocytosis or elevated CSF protein concentration were compared by Fisher's exact test. Mean values of independent groups were compared with Student's t-test. The level of significance was .05 (2-sided). Commercially available statistical software was used (SPSS for Windows, release 14.0).
Results
Patient Characteristics
Of 69 patients with CSF analysis for MD parenchymal brain involvement was present in 66 (96%), isolated meningeal lymphoma (diagnosed by CSF cytomorphology) in 2 patients (3%) and isolated intraocular lymphoma in 1 patient.
In 6 patients diagnosis could not be established by conclusive histology or positive CSF/vitreous cytology, in two of these patients biopsy was not feasible due to lesion location, and in one patient clinical condition was too critical for biopsy. In another three patients biopsy was inconclusive showing non-specific reactive tissue. A probable diagnosis was then made on clinical (rapid onset of symptoms and response to corticosteroids) and radiologic features (proximity to the subarachnoid space, strong and homogeneous contrast enhancement, moderate edema, and absence of necrosis) and after exclusion of infectious diseases by serology and CSF analysis (e.g. herpes virus, cytomegaly virus, Epstein–Barr virus, HIV, JC virus, toxoplasmosis, mycobacteria, and cryptococci).
Concomitant ocular involvement was detected by slit lamp examination in 1 of 19 (5%) patients examined. Data on steroid exposure before MD evaluation was available for 23 (33%) patients; of those 13 (57%) were on steroids.
Characteristics of all patients and the comparison between patients with MD vs those without MD are given in Table 1. No statistically significant difference was observed between both groups for any parameter. Moreover, no significant difference was found for the MSKCC score.
Table 1.
Patient characteristics
| Total, n = 69 | Percentage | With MD, n = 11 | Without MD, n = 58 | |
|---|---|---|---|---|
| Age (years) | ||||
| Median | 62 | 61 | ||
| Range | 16–87 | 45–78 | ||
| Sex | ||||
| Male | 32 | 46 | 7 | 25 |
| Female | 37 | 54 | 4 | 33 |
| Karnofsky index (%) | ||||
| ≥ 70 | 45 | 65 | 8 | 37 |
| < 70 | 21 | 30 | 2 | 19 |
| Unknown | 3 | 4 | 1 | 2 |
| MSKCC score | ||||
| 1 | 11 | 16 | 0 | 11 |
| 2 | 36 | 52 | 8 | 28 |
| 3 | 19 | 28 | 2 | 17 |
| Unknown | 3 | 4 | 1 | 2 |
| Intracerebral lesion location | ||||
| No intracerebral lesion | 3 | 4 | 2 | 1 |
| Superficial lesion | 36 | 52 | 6 | 30 |
| Deep lesion | 29 | 42 | 2 | 27 |
| Unknown | 1 | 1 | 1 | 0 |
| Pathologic diagnosis confirmation | ||||
| Stereotactic biopsy | 32 | 46 | 4 | 28 |
| Partial tumor resection | 19 | 28 | 3 | 16 |
| Total tumor resection | 6 | 9 | 0 | 6 |
| Surgical procedure unknown | 1 | 1 | 0 | 1 |
| CSF only | 4 | 6 | 4 | 0 |
| Vitrectomy only | 1 | 1 | 0 | 1 |
| None | 6 | 9 | 0 | 6 |
| Histology | ||||
| No histology | 8 | 12 | 4 | 4 |
| DLCBL | 52 | 75 | 5 | 47 |
| T-NHL | 1 | 1 | 1 | 0 |
| Low-grade B-NHL | 4 | 6 | 1 | 3 |
| Inconclusive | 3 | 4 | 0 | 3 |
| Unknown | 1 | 1 | 0 | 1 |
Abbreviations: MD, meningeal dissemination; MSKCC, Memorial Sloan-Kettering Cancer Center; MRI, magnetic resonance imaging; DLCBL, diffuse large-cell B-cell lymphoma; T-NHL, T-cell non-Hodgkin lymphoma; B-NHL, B-cell non-Hodgkin lymphoma.
Cerebrospinal Fluid
Lymphoma cells were found by cytomorphologic examination in 7 of 63 (11%) samples and suspicious lymphocytes in 4 samples (6%). Of the 32 samples evaluated by immunophenotyping, 1 (3%) was positive and 31 negative. Of the 37 samples with PCR analysis, 6 (16%) showed a monoclonal pattern, 24 (65%) a polyclonal pattern, and in 7 (19%) no DNA was amplifiable.
Based on the results of all methods together, 11 patients (16%) were regarded as having MD (Table 2).
Table 2.
Results of different methods for MD detection
| Cytomorphology |
||||
|---|---|---|---|---|
| Negative (n = 52) | Positive (n = 7) | Suspicious (n = 4) | Not done (n = 6) | |
| Immunocytology | ||||
| Positive | 0 | 1 | 0 | 0 |
| Negative | 24 | 3 | 1 | 3 |
| Not done | 28 | 3 | 3 | 3 |
| PCR | ||||
| Monoclonal | 3 | 2 | 1 | 0 |
| Polyclonal | 17 | 1 | 2 | 4 |
| DNA not amplifiable | 6 | 0 | 0 | 1 |
| Not done | 26 | 4 | 1 | 1 |
| Neuroimaging | ||||
| Positive | 0 | 3 | 0 | 0 |
| Negative | 52 | 4 | 4 | 6 |
While steroid medication was part of the initial treatment in all patients, information on concomitant steroid medication at the time of CSF sampling was available for 23 patients. Of those, 13 (57%) were on corticosteroids (2 of 5 with MD and 11 of 18 patients without MD).
Median CSF cell count was 5/μl (range, 0–237; n = 64) and median protein concentration of 760 mg/l (range, 93–4117; n = 55). An elevated cell count (5/μl) was found in 26 (41%) patients, an elevated protein level in 44 patients (80%). No significant correlation was found between proof of MD and CSF pleocytosis or elevated protein (Table 3).
Table 3.
CSF pleocytosis and protein concentration
| Number of patients | Number of patients with MD | OR (95% CI) | P value | |
|---|---|---|---|---|
| CSF pleocytosis | ||||
| No | 38 | 5 | 1.98 (0.53–7.34) | 0.33 |
| Yes | 26 | 6 | ||
| Missing | 5 | – | ||
| CSF protein | ||||
| Normal | 11 | 1 | 1.58 (0.17–14.66) | 1.0 |
| Elevated | 44 | 6 | ||
| Missing | 14 | 4 | ||
Abbreviation: OR, odds ratio.
Initial Treatment
Sixty-one patients (88%) received the intended HDMTX-based chemotherapy: 44 as monotherapy and 17 in combination with other cytostatics (ifosfamide, high-dose cytarabine, BCNU, and procarbazine). Eight patients did not receive HDMTX-based chemotherapy due to poor physical condition or renal insufficiency: 2 received topotecan monotherapy, 3 steroids only, and 2 were treated with whole-brain irradiation (WBI). One patient with PIOL had vitrectomy and local irradiation only. Twelve patients treated with BMPD additionally received intrathecal treatment with methotrexate: 10 patients without cytomorphologic proof of MD in course 1 only and 2 patients with lymphoma cells in CSF in each course. In patients receiving HDMTX-based chemotherapy, 10 additionally had WBI as part of their initial treatment.
Nine of the 11 patients with MD received HDMTX-based therapy (2 of them with consecutive WBI), 1 patient had WBI only, and 1 patient received steroids only.
Outcome
The median follow-up was 42.8 months (95% CI: 23.9–61.7). The median OAS of all patients with CSF analysis for MD was 42.5 months (95% CI: 33.4–51.6), and of those without MD was 23.8 months (95% CI: 0–65.8) (P = .3).
Of the 11 patients with MD, 7 responded to primary treatment, and had a median EFS of 26 months (95% CI: 4.3–47.7), and 4 progressed. Four of the 7 responders relapsed: 3 in the brain and 1 in the skin. Of patients without MD, 34 responded to primary treatment with a median EFS of 34.1 months (95% CI: 26.2–42): 14 relapsed within the brain parenchyma and 1 patient each in the CSF, in the lung/mediastinal lymph nodes and in the testes. The difference in EFS of patients with MD vs those without was not significant (P = .24).
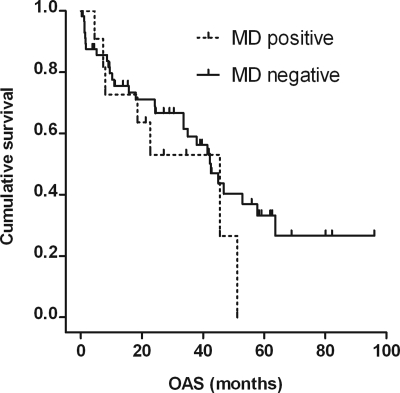
Median OAS of the 11 patients with MD (diagnosed by either of the methods) was 45.5 months (95% CI: 16.6–74.4) as compared with 42.5 months (95% CI: 33.8–51.2) in the 58 patients without MD (P = .34; Fig. 1).
Fig. 1.
Kaplan–Meier estimate of OAS for MD-positive (n = 11) and MD-negative patients (n = 58). Median OAS was 45.5 (95% CI: 16.6–74.4) months (7 events, 4 patients censored) and 42.5 (95% CI: 33.8–51.2) months (29 events, 29 patients censored), respectively. P = .34 (log-rank test).
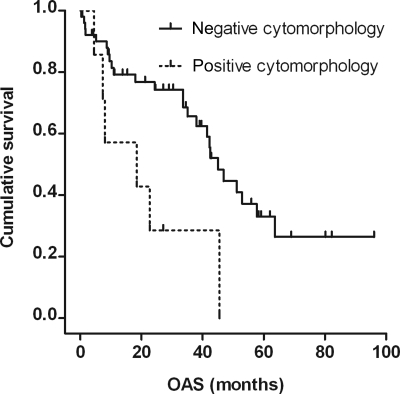
Patients with cytomorphologic proof of MD (n = 7; Table 4) had a median OAS of 18.5 months (95% CI: 0–45.4), those with a negative or suspicious CSF cytomorphology (n = 56) of 45 months (95% CI: 38.7–51.3); this difference was significant (P = .017; Fig. 2). A significant difference between these groups was also found for median EFS with 15.4 months (95% CI: 0–36.5) vs 34.3 months (95% CI: 28.2–40.4 months), respectively (P = .018). No significant difference was found for MSKCC score between these groups.
Table 4.
Characteristics of patients with cytomorphologic proof of MD in CSF
| Age (sex) | Pathologic diagnosis | Disease localization on MRI | Primary treatment/response | Relapse (TTR, months)/salvage treatment/response | OAS (months)/cause of death |
|---|---|---|---|---|---|
| 54 (f) | CSF cytology only | Brain | WBI/CR | Brain and CSF (2)/2× BMPD/CR | 27.1 + /lost to follow-up |
| 83 (m) | DLBCL | Meninges and brain | 4× HDMT × /SD | Brain (4)/WBI/PD | 18.5/lymphoma |
| 70 (f) | DLBCL | Brain and meninges | 3× BMPD + WBI/CR | – | 4.5/neurotoxicity |
| 74 (f) | T-NHL | Brain | Steroids only/PD | – | 7.3/lymphoma |
| 64 (m) | CSF cytology only | Meninges | 2× BMPD + 2× HDMTX/CR | Cutaneous (28)/6× CHOP/CR | 45.5/sepsis on therapy |
| Second relapse lung and tonsils/1× ICE/unknown | |||||
| 62 (f) | DLBCL | Brain | 2× HDMTX/PD, 1× HD-AraC/PD | – | 8.0/lymphoma |
| WBI/PD | |||||
| 76 (m) | CSF cytology only | Brain | 6× HDMTX + 2× HD-AraC/CR | Brain and CSF (16)/4× HDMTX + Ifosfamide/PR | 22.7/lymphoma |
Abbreviations: TTR, time to relapse; OAS, overall survival; f, female; m, male; CSF cerebrospinal fluid; DLBCL, diffuse large B-cell lymphoma; T-NHL, T cell Non-Hodgkin's lymphoma; HDMTX, high-dose methotrexate; WBI, whole-brain irradiation; BMPD, carmustine, methotrexate, procarbazine, dexamethasone and 1× 15 mg MTX intrathecally in each course; CHOP, cyclophosphamide, adriamycin, vincristin, prednisolone; HD-AraC, high-dose cytarabine; ICE, ifosfamide, carboplatin, etoposide; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Fig. 2.
Kaplan–Meier estimate of overall survival for patients with positive cytomorphology (n = 7) and patients with negative or suspicious cytomorphology (n = 56). Median OAS was 18.5 (95% CI: 0–45.4) months (6 events, 1 patient censored), and 45 (95% CI: 38.7–51.3) months (27 events, 29 patients censored), respectively. P = .017 (log-rank test).
With a median OAS of 52.9 months (95% CI: 40.5–65.3), patients with an elevated cell count in the CSF (n = 26) had a trend towards longer survival compared with patients with a normal cell count (n = 8) with a median OAS of 37.9 months (95% CI: 27.2–48.6; P = .095). No significant OAS difference was found for patients with elevated vs normal CSF protein concentration.
Discussion
Our study population might have been selected by the referral bias, since patients in smaller community hospitals might not have received biopsy or not have been referred to our institution due to poor physical condition, and a selection bias, because patients in poor condition might not have been subjected to lumbar puncture. This hypothesis is supported by a considerably poorer OAS of patients without CSF analysis for MD, although the difference is not statistically significant, probably because patient numbers were too small.
In this study, an extended diagnostic work-up was used, and MD was defined by a positive result of any of the methods used, although, in the absence of a reliable diagnostic “gold standard”, their equivalence for MD detection in lymphoma can only be assumed. The frequency of MD found is in the lower range of frequencies reported in studies usually using the cytomorphologic examination only, ranging from 7%–42% (Table 5). Underestimation due to a singular puncture and a prior use of steroids in more than half of all documented patients can be assumed in our cohort; however, these limitations also apply to the majority of other studies. MD was detected with an equal frequency in patients with and without prior steroid use in our study, but the number of patients might have been too low to detect a significant difference.
Table 5.
Studies (more than 40 patients) reporting frequencies of MD in PCNSL
| Author | Patients with CSF/all patients | % MD positive | Diagnostic methods (% positive) | Prior steroid use | Prognostic impact on OAS |
|---|---|---|---|---|---|
| Abrey et al.20 | 279/338 | 17 | Cytomorphologic examination | Not reported | No impact |
| Ferreri et al.2 | 241/378 | 18 | Cytomorphologic examination | Not reported | No impact for cytomorphologic proof |
| Negative impact for CSF protein >450 mg/l | |||||
| Blay et al.32 | 157/226 | 16 | Cytomorphologic examination | Not reported | Negative impact for CSF protein >600 mg/l and positive cytomorphology (univariate) |
| Fischer et al.33 | 116/145 | 18 | Cytomorphologic examination | 71/91 with available data | Not evaluated |
| Balmaceda et al.7 | 86/96 | 42 | Cytomorphologic examination (27%), imaging, meningeal biopsy | 56/69 with available data | No impact |
| DeAngelis et al.34 | 81/98 | 21 | Cytomorphologic examination | Not reported | Not evaluated |
| Gleissner et al.17 | 76/76 | 16 | Cytomorphologic examination (8%), CDR III PCR (11%) | 60/68 | Not evaluated |
| Present study | 69/92 | 16 | Cytomorphologic examination (11%), immunophenotyping (3%), CDR III PCR (16%) | 13/23 with available data | Negative impact for positive cytomorphology only |
| Pels et al.12 | 58/65 | 12 | Cytomorphologic examination | Not reported | Not evaluated |
| Gavrilovic et al.35 | 57/57 | 18 | Cytomorphologic examination | Not reported | Not evaluated |
| Abrey et al.36 | 52/52 | 21 | Cytomorphologic examination | Not reported | Not evaluated |
| Hoang-Xuan et al.37 | 50/50 | 18 | Not reported | Not reported | Not evaluated |
| Korfel et al.14 | 45/60 | 18 | Cytomorphologic examination | Not reported | No impact |
| Poortmans et al.38 | 43/52 | 16 | Not reported | Not reported | Not evaluated |
| O'Brien et al.39 | 42/46 | 7 | Cytomorphologic examination, immunophenotyping | Not reported | Not evaluated |
Abbreviations: OAS, overall survival; CSF, cerebrospinal fluid.
The very low frequency of MD detection by immunophenotyping in this study is remarkable. A monoclonal B-cell population was found only in 1 of the 4 cytomorphologically positive samples evaluated by immunophenotyping. Small cell counts and rapid cell decay in a delayed analysis are major limitations of this method. However, a higher sensitivity of immunocytology as compared with cytomorphology for MD detection in hematologic malignancies primarily localized outside the CNS is suggested in the literature. In 3 small studies comparing flow cytometry with conventional cytomorphology, a much higher frequency of MD found by immunophenotyping was reported.22–24 In the largest study investigating 51 newly diagnosed aggressive B-cell lymphomas with risk for CNS involvement, MD was detected more than twice as frequently using flow cytometry compared with conventional cytomorphology.25
The high rate of discordant cytomorphologic and PCR results is another striking finding in this study. Of 3 cytomorphologically positive samples evaluated by PCR, only in 1 a monoclonal PCR product was found. Conversely, only 1 of 6 samples with a monoclonal PCR product was positive on cytomorphologic examination, and 1 was regarded suspicious. False negatives in PCR can be explained by a high mutational frequency of PCNSL as compared with nodal diffuse-large B-cell lymphomas due to the introduction of further point mutations after immunoglobulin gene rearrangement, which can prevent annealing of PCR primers.26,27 On the other hand, a misinterpretation of a peak caused by a single B-cell as a monoclonal population (“pseudomonoclonality”) may result in a false-positive PCR. This can be minimized to some extent by repeated PCR in independent runs. However, the presence of false-positive cytology results cannot be finally ruled out. A high rate of discordant cytomorphologic and PCR results has also been reported in two other studies.17,28 In a smaller study by Ekstein et al.,29 9 of 15 PCNSL patients with active disease had positive PCR results (60%); however, multiple samples from single patients during the course of disease, including relapse, were included in the evaluation.
The rate of MD detection by neuroradiologic evaluation was very low in our study. All these patients also had positive CSF findings. This corresponds to the data published by others who found the sensitivity of radiological methods for detection of MD in lymphoma lower than in solid tumors.30,31 It cannot be excluded, however, that a higher detection frequency would have been found with an additional spinal imaging.
The prognostic impact of MD in PCNSL has not been defined yet. Blay et al.32 found a trend towards worse survival in patients with a cytomorphologic proof of MD as compared with those without, whereas no impact of MD on survival was found by others.2,7,14,20 The outcome of our patients with MD (diagnosed by either of the methods) was comparable to that of patients without MD with no differences in the relapse pattern between the two groups, but the power of our study was clearly limited by the relatively small number of patients. In the framework of our study, we could have detected a difference in median OAS of more than 3 years with 80% power. However, we saw a difference of only 3 months, which at least indicates that MD as defined in this study is not an important predictor of survival in PCNSL when treated with HDMTX. Remarkably, patients with cytomorphologic evidence of lymphoma cells had a significantly poorer outcome than those without. This may indicate a stronger prognostic impact of conventional CSF diagnostics, possibly detecting a higher tumor burden as compared with the subclinical MD detection by putatively more sensitive methods.
In some studies, CSF protein concentration has been suggested to be an independent prognostic factor,2,32 whereas no prognostic role has been reported by others.20 In our study, no influence of CSF protein concentration on patients' outcome has been found.
Within limitations mentioned above, the diagnostic yield for MD detection in newly diagnosed PCNSL seems not improved by CSF immunophenotyping and MRI as compared with CSF cytomorphology. The value of a positive PCR result remains unclear since only a positive cytomorphologic result had an impact on outcome. Thus, considering cytomorphology a diagnostic gold standard for diagnosing MD in PCNSL seems still justified. These findings need to be confirmed by a prospective analysis of a larger patient cohort.
Conflict of interest statement. None declared.
Funding
This study was supported by the “Kompetenznetz Maligne Lymphome” and the “Deutsches Zentrum für Luft- und Raumfahrt (DLR)”.
References
- 1.Batchelor T, Carson K, O'Neill A, et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96-07. J Clin Oncol. 2003;21:1044–1049. doi: 10.1200/JCO.2003.03.036. [DOI] [PubMed] [Google Scholar]
- 2.Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21:266–272. doi: 10.1200/JCO.2003.09.139. [DOI] [PubMed] [Google Scholar]
- 3.Jahnke K, Korfel A, Martus P, et al. on the behalf of the German Primary Central Nervous System Lymphoma Study Group (G-PCNSL-SG) High-dose methotrexate toxicity in elderly patients with primary central nervous system lymphoma. Ann Oncol. 2005;16:445–449. doi: 10.1093/annonc/mdi075. [DOI] [PubMed] [Google Scholar]
- 4.Glantz MJ, Cole BF, Glantz LK, et al. Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer. 1998;82:733–739. doi: 10.1002/(sici)1097-0142(19980215)82:4<733::aid-cncr17>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Freilich RJ, Krol G, DeAngelis LM. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol. 1995;38:51–57. doi: 10.1002/ana.410380111. [DOI] [PubMed] [Google Scholar]
- 6.Glass JP, Melamed M, Chernik NL, Posner JB. Malignant cells in cerebrospinal fluid (CSF): the meaning of a positive CSF cytology. Neurology. 1979;29:1369–1375. doi: 10.1212/wnl.29.10.1369. [DOI] [PubMed] [Google Scholar]
- 7.Balmaceda C, Gaynor JJ, Sun M, Gluck JT, DeAngelis LM. Leptomeningeal tumor in primary central nervous system lymphoma: recognition, significance, and implications. Ann Neurol. 1995;38:202–209. doi: 10.1002/ana.410380212. [DOI] [PubMed] [Google Scholar]
- 8.Weller M. Glucocorticoid treatment of primary CNS lymphoma. J Neurooncol. 1999;43:237–239. doi: 10.1023/a:1006254518848. [DOI] [PubMed] [Google Scholar]
- 9.Cartmill M, Allibone R, Bessell EM, Byrne PO. Primary cerebral non-Hodgkin's lymphoma: problems with diagnosis and development of a protocol for management. Br J Neurosurg. 2000;14:313–315. doi: 10.1080/026886900417270. [DOI] [PubMed] [Google Scholar]
- 10.Haldorsen IS, Espeland A, Larsen JL, Mella O. Diagnostic delay in primary central nervous system lymphoma. Acta Oncol. 2005;44:728–734. doi: 10.1080/02841860500256272. [DOI] [PubMed] [Google Scholar]
- 11.Pels H, Juergens A, Glasmacher A, et al. Early relapses in primary CNS lymphoma after response to polychemotherapy without intraventricular treatment: results of a phase II study. J Neurooncol. 2009;91:299–305. doi: 10.1007/s11060-008-9712-4. [DOI] [PubMed] [Google Scholar]
- 12.Pels H, Schmidt-Wolf IGH, Glasmacher A, et al. Primary central nervous system lymphoma: results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol. 2003;21:4489–4495. doi: 10.1200/JCO.2003.04.056. [DOI] [PubMed] [Google Scholar]
- 13.Sandor V, Stark-Vancs V, Pearson D, et al. Phase II trial of chemotherapy alone for primary CNS and intraocular lymphoma. J Clin Oncol. 1998;16:3000–3006. doi: 10.1200/JCO.1998.16.9.3000. [DOI] [PubMed] [Google Scholar]
- 14.Korfel A, Martus P, Nowrousian MR, et al. German primary central nervous system lymphoma Study Group (G-PCNSL-SG) Response to chemotherapy and treating institution predict survival in primary central nervous system lymphoma. Br J Haematol. 2005;128:177–183. doi: 10.1111/j.1365-2141.2004.05284.x. [DOI] [PubMed] [Google Scholar]
- 15.Kiewe P, Fischer L, Martus P, Thiel E, Korfel A. Primary central nervous system lymphoma: monocenter, long-term, intent-to-treat analysis. Cancer. 2008;112:1812–1820. doi: 10.1002/cncr.23377. [DOI] [PubMed] [Google Scholar]
- 16.Kranz BR, Thiel E, Thierfelder S. Immunocytochemical identification of meningeal leukemia and lymphoma: poly-L-lysine-coated slides permit multimarker analysis even with minute cerebrospinal fluid cell specimens. Blood. 1989;73:1942–1950. [PubMed] [Google Scholar]
- 17.Gleissner B, Siehl J, Korfel A, Reinhardt R, Thiel E. CSF evaluation in primary CNS lymphoma patients by PCR of the CDR III IgH genes. Neurology. 2002;58:390–396. doi: 10.1212/wnl.58.3.390. [DOI] [PubMed] [Google Scholar]
- 18.Galoin S, Daste G, Apoil PA, et al. Polymerase chain reaction on cerebrospinal fluid cells in the detection of leptomeningeal involvement by B-cell lymphoma and leukaemia: a novel strategy and its implications. Br J Haematol. 1997;99:122–130. doi: 10.1046/j.1365-2141.1997.3423153.x. [DOI] [PubMed] [Google Scholar]
- 19.van Dongen JJ, Langerak AW, Brüggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 20.Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24:5711–5715. doi: 10.1200/JCO.2006.08.2941. [DOI] [PubMed] [Google Scholar]
- 21.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. (Erratum in: J Clin Oncol 2000;18:2351) [DOI] [PubMed] [Google Scholar]
- 22.Finn WG, Peterson LC, James C, Goolsby CL. Enhanced detection of malignant lymphoma in cerebrospinal fluid by multiparameter flow cytometry. Am J Clin Pathol. 1998;110:341–346. doi: 10.1093/ajcp/110.3.341. [DOI] [PubMed] [Google Scholar]
- 23.French CA, Dorfman DM, Shaheen G, Cibas ES. Diagnosing lymphoproliferative disorders involving the cerebrospinal fluid: increased sensitivity using flow cytometric analysis. Diagn Cytopathol. 2000;23:369–374. doi: 10.1002/1097-0339(200012)23:6<369::aid-dc1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Bromberg JE, Breems DA, Kraan J, et al. CSF flow cytometry greatly improves diagnostic accuracy in CNS hematologic malignancies. Neurology. 2007;68:1674–1679. doi: 10.1212/01.wnl.0000261909.28915.83. [DOI] [PubMed] [Google Scholar]
- 25.Hegde U, Filie A, Little RF, et al. High incidence of occult leptomeningeal disease detected by flow cytometry in newly diagnosed aggressive B-cell lymphomas at risk for central nervous system involvement: the role of flow cytometry versus cytology. Blood. 2005;105:496–502. doi: 10.1182/blood-2004-05-1982. [DOI] [PubMed] [Google Scholar]
- 26.Montesinos-Rongen M, Küppers R, Schlüter D, et al. Primary central nervous system lymphomas are derived from germinal-center B cells and show a preferential usage of the V4-34 gene segment. Am J Pathol. 1999;155:2077–2086. doi: 10.1016/S0002-9440(10)65526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompsett AR, Ellison DW, Stevenson FK, Zhu D. V(H) gene sequences from primary central nervous system lymphomas indicate derivation from highly mutated germinal center B cells with ongoing mutational activity. Blood. 1999;94:1738–1746. [PubMed] [Google Scholar]
- 28.Fischer L, Martus P, Weller M, et al. Meningeal dissemination in primary CNS lymphoma: prospective evaluation of 282 patients. Neurology. 2008;71:1102–1108. doi: 10.1212/01.wnl.0000326958.52546.f5. [DOI] [PubMed] [Google Scholar]
- 29.Ekstein D, Ben Yehuda D, Slyusarevsky E, Lossos A, Linetsky E, Siegal T. CSF analysis of IgH gene rearrangement in CNS lymphoma: relationship to the disease course. J Neurol Sci. 2006;247:39–46. doi: 10.1016/j.jns.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Zeiser R, Burger JA, Bley TA, Windfuhr-Blum M, Schulte-Mönting J, Behringer DM. Clinical follow-up indicates differential accuracy of magnetic resonance imaging and immunocytology of the cerebral spinal fluid for the diagnosis of neoplastic meningitis—a single centre experience. Br J Haematol. 2004;124:762–768. doi: 10.1111/j.1365-2141.2004.04853.x. [DOI] [PubMed] [Google Scholar]
- 31.Kuker W, Nagele T, Korfel A, et al. Primary central nervous system lymphomas (PCNSL): MRI features at presentation in 100 patients. J Neurooncol. 2005;72:169–177. doi: 10.1007/s11060-004-3390-7. [DOI] [PubMed] [Google Scholar]
- 32.Blay JY, Conroy T, Chevreau C, et al. High-dose methotrexate for the treatment of primary cerebral lymphomas: analysis of survival and late neurologic toxicity in a retrospective series. J Clin Oncol. 1998;16:864–871. doi: 10.1200/JCO.1998.16.3.864. [DOI] [PubMed] [Google Scholar]
- 33.Fischer L, Jahnke K, Martus P, Weller M, Thiel E, Korfel A. The diagnostic value of cerebrospinal fluid pleocytosis and protein in the detection of lymphomatous meningitis in primary central nervous system lymphomas. Haematologica. 2006;91:429–430. [PubMed] [Google Scholar]
- 34.DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ Radiation Therapy Oncology Group Study 93-10. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol. 2002;20:4643–4648. doi: 10.1200/JCO.2002.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Gavrilovic IT, Hormigo A, Yahalom J, DeAngelis LM, Abrey LE. Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2006;24:4570–4574. doi: 10.1200/JCO.2006.06.6910. [DOI] [PubMed] [Google Scholar]
- 36.Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: the next step. J Clin Oncol. 2000;18:3144–3150. doi: 10.1200/JCO.2000.18.17.3144. [DOI] [PubMed] [Google Scholar]
- 37.Hoang-Xuan K, Taillandier L, Chinot O, et al. Chemotherapy alone as initial treatment for primary CNS lymphoma in patients older than 60 years: a multicenter phase II study (26952) of the European Organization for Research and Treatment of Cancer Brain Tumor Group. J Clin Oncol. 2003;21:2726–2731. doi: 10.1200/JCO.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 38.Poortmans PM, Kluin-Nelemans HC, Haaxma-Reiche H, et al. European Organization for Research and Treatment of Cancer Lymphoma Group. High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962. J Clin Oncol. 2003;21:4483–4488. doi: 10.1200/JCO.2003.03.108. [DOI] [PubMed] [Google Scholar]
- 39.O'Brien P, Roos D, Pratt G, et al. Phase II multicenter study of brief single-agent methotrexate followed by irradiation in primary CNS lymphoma. J Clin Oncol. 2000;18:519–526. doi: 10.1200/JCO.2000.18.3.519. [DOI] [PubMed] [Google Scholar]