Abstract
Malignant pleural mesothelioma (MPM) is a fatal disease with a median survival of less than 14 months. For the first time, a genetically engineered vaccinia virus is shown to produce efficient infection, replication, and oncolytic effect against MPM. GLV-1h68 is a replication-competent engineered vaccinia virus carrying transgenes encoding Renilla luciferase, green fluorescent protein (both inserted at the F14.5L locus), β-galactosidase (inserted at the J2R locus, which encodes thymidine kinase), and β-glucuronidase (at the A56R locus, which encodes hemagglutinin). This virus was tested in six human MPM cell lines (MSTO-211H, VAMT, JMN, H-2373, H-2452, and H-2052). GLV-1h68 successfully infected all cell lines. For the most sensitive line, MSTO-211H, expression of green fluorescent protein (GFP) started within 4 hr with increasing intensity over time until nearly 100% of cells expressed GFP at 24 hr. All cell lines were sensitive to killing by GLV-1h68, with the degree of sensitivity predictable by infectivity assay. Even the most resistant cell line exhibited 44 ± 3.8% cell survival by day 7 when infected at a multiplicity of infection of 1.0. Viral proliferation assays demonstrated 2-to 4-fold logarithmic replication of GLV-1h68 in the cell lines tested. In an orthotopic model, GLV-1h68 effectively prevented development of cachexia and tumor-related morbidity, reduced tumor burden, and cured MPM in both early and late treatment groups. GLV-1h68 was successfully used to treat MPM in vitro and in an orthotopic model (in vivo). These promising results warrant clinical investigation of GLV-1h68 as a novel agent in the treatment of MPM.
Introduction
Malignant pleural mesothelioma (MPM) is a highly aggressive disease with essentially no effective therapies available. Median survival with current standard treatment regimens ranges from 10 to 16 months (Borasio et al., 2008; Schipper et al., 2008). Most patients with MPM are found to have unresectable disease at the time of diagnosis and must resort to chemotherapy for treatment. The newest chemotherapeutic agent showing promise for treatment of MPM is pemetrexed, but even in combination with other first-line agents, median survival is 14 months (Castagneto et al., 2008). Because of the etiology of this disease from asbestos exposure and the latency period of approximately 40 years to development of disease after the exposure event (Bianchi and Bianchi, 2007), it is estimated that the annual incidence will continue to increase through the next decade (Walker et al., 1983; Connelly et al., 1987).
Given the dismal prognosis with current standard therapies, several gene therapy strategies are under investigation for MPM and have gone as far as phase I clinical trials. Al-belda and colleagues investigated the use of a suicide gene therapy strategy employing a replication-deficient recombinant adenovirus expressing the herpes simplex thymidine kinase gene (TK) in combination with ganciclovir (Sterman et al., 2005). Evidence of transgene expression was found in 11 of 25 patients who received treatment. No definitive antitumor responses were observed, however. A recombinant vaccinia virus expressing interleukin (IL)-2 was used in an attempt to incite an antitumor immune response by a group in Western Australia (Mukherjee et al., 2000; Blattman et al., 2003; van der Most et al., 2006). This study, which involved six MPM patients, reported an excellent safety profile for IL-2-expressing vaccinia virus, but no clinical responses.
The modality presented here involves oncolytic viral therapy with a vaccinia virus, which aims to directly lyse tumor cells by means of viruses preferentially designed to infect and kill tumors. Vaccinia virus has many characteristics that make it an excellent candidate for recombinant viral therapy. This virus demonstrates natural tumor tropism (Yu et al., 2004). Its large genome allows for insertion of multiple foreign genes without detrimental effects on replicative ability (McFadden, 2005; Thorne et al., 2005). In addition, it is naturally highly immunogenic and is able to incite strong host immune responses against virus-infected cells (Thorne et al., 2005; Woo et al., 2006). Last, vaccinia virus has already demonstrated an acceptable safety profile in humans as it has been administered to millions in the smallpox vaccine.
The virus used in the current study was GLV-1h68. This is a vaccinia virus recombinant expressing transgenes for Renilla luciferase, green fluorescent protein (GFP), and β-galactosidase. It has been shown to be effective against anaplastic thyroid carcinoma in vitro (Lin et al., 2007) and breast carcinoma in vitro and in vivo (Zhang et al., 2007). We sought to determine whether this virus could selectively target and kill a panel of human MPM cell lines in vitro and in an orthotopic animal model, and to explore the potential application of this virus as a novel, clinically relevant therapeutic agent for MPM.
Materials and Methods
Cell lines
Six human malignant mesothelioma cancer cell lines of various histologic subtypes, including epithelioid (H-2452), sarcomatoid (H-2052, H-2373, and VAMT), and biphasic (MSTO-211H and JMN), were studied. The MSTO-211H cell line and African green monkey kidney fibroblasts (CV-1) were obtained from the American Type Culture Collection (ATCC, Manassas, VA). JMN and VAMT cell lines were a kind gift from F. Sirotnik (Memorial Sloan-Kettering Cancer Center, New York, NY). H-2052, H-2452, and H-2373 cell lines were a kind donation from H.I. Pass (Karmanos Cancer Institute, Wayne State University, Detroit, MI). In addition, the murine mesothelioma cell line AC29 was obtained as a gift from S.M. Albelda (University of Pennsylvania, Philadelphia, PA). All cells were grown in appropriate media and were maintained at 37°C in a humidified incubator supplied with 5% CO2.
Viral strains
GLV-1h68 is a replication-competent, recombinant vaccinia virus derived from the vaccinia virus LIVP strain (Lister strain from the Institute for Research on Virus Preparations, Moscow, Russia), the construction of which was previously described (Zhang et al., 2007). GLV-1h68 carries three gene cassettes inserted into the viral genome: a Renilla luciferase-GFP (RUC-GFP) fusion cassette at the F14.5L locus, a reverse inserted human transferrin receptor and β-galactosidase cassette at the J2R locus (which encodes TK), and a β-glucuronidase cassette at the A56R locus (encoding hemagglutinin).
GFP expression
Cells were plated at 2 × 104 per well in 12-well plates in 1 ml of medium per well. After 6 hr of incubation, GLV-1h68 in 100 μl of medium (Dulbecco's modified Eagle's medium [DMEM] with 2% fetal calf serum [FCS]) was added to each well at multiplicities of infection (MOIs; number of viral particles per cancer cell) of 1.00, 0.10, and 0.01. All variations were performed in triplicate. At time intervals of 6, 12, 24, and 48 hr postinfection, cells were examined with an inverted fluorescence microscope (Nikon Eclipse TE300; Nikon, Tokyo, Japan) for GFP expression and photographs were taken.
5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside cytochemistry
Cells were plated at 2 × 104 per well in 24-well plates with 1 ml of medium per well. After incubation for 6 hr, cells were infected with GLV-1h68 at an MOI of 1.00. At various intervals, cells were fixed with 1% glutaraldehyde for 5 min. Cells were stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; 1 mg/ml) in an iron solution of 5 mM K4Fe(CN)6, 5 mM K3Fe(CN)6, and 2 mM MgCl2 for 4 hr. Cells were then washed with phosphate-buffered saline (PBS) and examined by light microscopy.
β-Galactosidase assays
Cells were plated at 1 × 104 per well in 96-well plates with 100 μl of medium per well. After incubation for 6 hr, cells were infected with GLV-1h68 at an MOI of 1.00 in 50 μl of medium per well. At various intervals, cells were lysed and β-galactosidase activity was measured with an enhanced β-galactosidase assay kit (Gene Therapy Systems, San Diego, CA), using a spectrophotometer (EL321e; Bio-Tek Instruments, Winooski, VT) at 570 nm. All samples were measured in triplicate.
Cytotoxicity assay
Cells were plated at 2 × 104 per well in 12-well plates in 1 ml of medium per well. After incubation for 6 hr, cells were infected with GLV-1h68 at MOIs of 1.00, 0.10, 0.01, and 0 (control wells). Viral cytotoxicity was measured daily for 7 days. Cells were washed with PBS and lysed in 1.5% Triton × (200 μl per well; Sigma-Aldrich, St. Louis, MO) to release intracellular lactate dehydrogenase, which was quantified with a CytoTox 96 kit (Promega, Madison, WI) and a spectrophotometer (EL321e; Bio-Tek Instruments) at 490 nm. Results are expressed as the percentage of surviving cells. This percentage was determined by comparing the measured lactate dehydrogenase of each infected sample with that in uninfected, control cells. All samples were analyzed in triplicate.
Viral titration
Cells were plated at 2 × 104 per well in 12-well plates. After 6 hr, cells were infected with GLV-1h68 at an MOI of 0.10. Culture well supernatants were collected daily for 7 days and immediately frozen at −80°C for storage. After thawing, serial dilutions of the supernatant samples were made and standard plaque assays performed on 6-well plates of confluent CV-1 cells. All samples were measured in triplicate.
Establishment of an animal model of malignant pleural mesothelioma
Athymic female mice were purchased from the National Cancer Institute (Bethesda, MD) and were provided with food and water ad libitum. All animals received humane care in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and the animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Memorial Sloan-Kettering Cancer Center. Anesthesia was induced with a mixture of isoflurane (2 liters/min) and oxygen (4 liters/min) in an induction chamber and was maintained with a nasal cone. Mice were placed in the left lateral position. The right chest was prepared with 10% povidone–iodine solution. An incision of 3 to 5 mm was made over the fourth to fifth intercostal space. Sharp dissection was carried out, exposing but not breaching the parietal pleura. The underlying inflating and deflating lung was thereby easily visualized through the thin membrane. Slowly, 100 μl of MSTO-211H malignant mesothelioma cellular suspension (5 × 106 cells) was injected through the pleura with a 27-gauge needle. Puncture of the lung was easily avoided because the needle tip was clearly observed to be superficial to the lung surface during injection through the transparent parietal pleura. After the injection the skin was closed with surgical staples. Recovery was observed for 15 min before mice were returned to their cages.
Treatment of malignant pleural mesothelioma
Intrapleural treatment with virus was performed in a fashion similar to that described above 5 days after tumor cell instillation into the pleural cavity for the early treatment group, and 10 days afterward for the late treatment group. GLV-1h68 (1 × 107 plaque-forming units [PFU]) was administered in 100 μl of PBS and animals were gently rotated from side to side to help distribute the virus throughout the pleural cavity. Animals were regularly assessed for weight loss and tachypnea throughout the experimental period. Mice were killed on day 21 and weighed, and all visible tumor in the chest was collected and weighed. In treated animals with gross disease, tissue from the chest cavity was harvested for immunohistochemistry.
Survival analysis
Efficacy of GLV-1h68 in prolonging survival was assessed in two groups of animals, with early and advanced disease. In the first experiment, the ability of GLV-1h68 to treat microscopic disease and prolong survival was assessed. Sixteen mice (n = 8 per group) underwent intrapleural injection of MSTO-211H (1 × 107 cells). Five days later, mice were treated with intrapleural injections of either PBS or GLV-1h68 (1 × 107 PFU). In a second experiment, the ability of GLV-1h68 to treat macroscopic disease and prolong survival was assessed. Two groups of mice (n = 8 per group) were implanted with MSTO-211H (1 × 107 cells) and treated 10 days later with PBS or GLV-1h68 (1 × 107 PFU). Two additional mice were injected with tumor cells and were killed at 10 days to verify the presence of macroscopic disease. Kaplan–Meier statistics were used for analysis of survival data.
Viral colonization of tumors
Samples of tissue emitting GFP under stereomicroscopy from the chest cavity were frozen in Tissue-Tek embedding medium (Sakura Finetek, Torrance, CA) and sectioned by cryotome for histologic examination. Slides were fixed with paraformaldehyde and stained with hematoxylin and eosin (H&E) to determine whether GFP expression localized to foci of cancer. To confirm that GFP expression was localizing virus, serial sections that expressed GFP were stained with X-Gal (1 mg/ml) in an iron solution of 5 mM K4Fe(CN)6, 5 mM K3Fe(CN)6, and 2 mM MgCl2 to identify virally mediated lacZ expression.
Evaluation of viral biodistribution and toxicity
After the establishment of macroscopic intrapleural disease, animals were injected intrapleurally with 1 × 107 PFU of GLV-1h68. Tissue from normal organs (lung, heart, liver, spleen, kidney, brain, bowel, uterus, and ovaries), as well as from tumor, was harvested at serial time points, weighed, and snap-frozen in liquid nitrogen. At the time of thawing, frozen tissue samples were suspended in 1 ml of tissue protein extraction reagent (T-PER, Pierce Protein Research Products/Thermo Fisher Scientific, Rockford, IL) and homogenized for 20 sec at a speed of 6500. After homogenization, samples were subject to three freeze–thaw cycles. Samples were then centrifuged for 10 min at 1000 × g at 4°C. Supernatants were collected and serial dilutions were made. Standard plaque assays were performed on 6-well plates of confluent CV-1 cells. All samples were assessed in triplicate.
Results
GFP microscopy
GFP expression was assessed 12, 24, and 48 hr after viral infection at an MOI of 1.0 by fluorescence microscopy. In all cell lines tested, cells were found to express GFP by 12 hr after infection. More green cells were seen at 24 hr and more still at 48 hr. MSTO-211H and H-2052 cells demonstrated near 100% infectivity (all cells green) at 24 hr (Fig. 1A and B). H-2452 and JMN displayed moderate GFP expression by this time, and VAMT and H-2373 displayed the least amount of GFP expression.
FIG. 1.
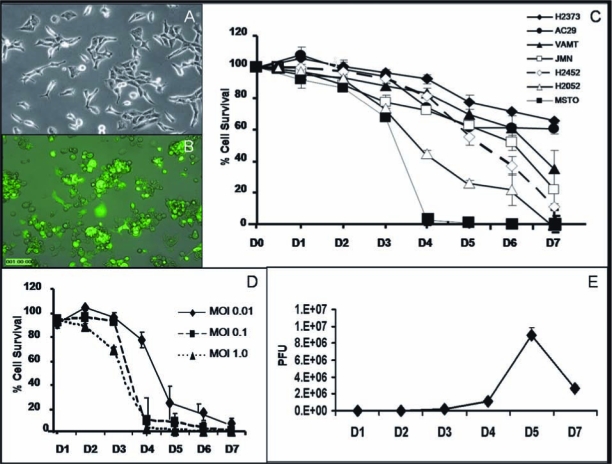
GLV-1h68 infects, replicates in, and has varying and dose-dependent cytotoxicity in a panel of malignant mesothelioma cell lines in vitro. (A) Overlay image showing no GFP expression in MSTO-211H cells immediately after infection with GLV-1h68. (B) Abundant GFP expression in MSTO-211H cells 24 hr after infection with virus. On close examination, all the infected cells have lost their normal morphology and have detached from the bottom of the plate, and some of them seem to be undergoing oncolysis. (C) The percentage cell survival of all the human (epithelioid, mixed, and sarcomatoid) and murine mesothelioma cell lines tested at an MOI of 1.0 demonstrates the cytotoxic effect of GLV-1h68 over time. Oncolytic vaccinia virus killed 100% of MSTO-211H and H-2052 cells by days 4 and 7, respectively. (D) LDH cytotoxicity assay on MSTO-211H cells performed daily after viral treatment shows effective oncolytic activity of GLV-1h68 at all doses tested (MOIs of 1.0, 0.1, and 0.01); activity occurred in a dose-dependent manner with 100% cell killing by days 4, 5, and 7, respectively. (E) Viral proliferation assay of GLV-1h68-treated MSTO-211H cells shows a 4-log increase in plaque-forming units of GLV-1h68 by day 7, demonstrating that the oncolytic vaccinia virus is able to replicate within the mesothelioma cells in vitro.
X-Gal cytochemistry
X-Gal cytochemistry was assessed 6, 12, and 24 hr after viral infection at an MOI of 1.0 as a marker of viral entry. X-Gal staining demonstrated the same results as GFP fluorescence in terms of relative amounts of lacZ-expressing cells in the various cell lines tested. In any given cell line, more blue cells were seen at progressively later time points. Again, MSTO-211H and H-2052 exhibited the strongest lacZ expression, followed by H-2452 and JMN, and VAMT and H-2373 showed the least expression even at the latest time point of 48 hr.
β-Galactosidase assays
β-Galactosidase activity was quantified 6, 12, and 24 hr after viral infection at an MOI of 1.0. As expected, amounts of β-galactosidase correlated with qualitative visual findings by X-Gal cytochemistry. At 24 hr, MSTO-211H displayed the highest expression (8.735 mU), followed by H-2052 (5.715 mU), JMN (5.065 mU), H-2452 (4.981 mU), H-2373 (1.927 mU), and VAMT (1.680 mU).
Cytotoxicity assays
All of the cell lines tested demonstrated significant sensitivity to cytotoxicity by GLV-1h68. MSTO-211H, the most sensitive cell line tested, showed nearly complete cell kill (<4% cell viability, or >96% cell kill) by day 4 after viral infection. H-2052 and H-2452 showed 89 and 78% cell kill by day 7, respectively. The remaining three human cell lines also demonstrated sensitivity to virus, with significant cell kill by day 7 after infection with an MOI of 1.0: JMN (75%), VAMT (60%), and H-2373 (34%). In addition, the murine mesothelioma cell line AC29 demonstrated 45% cell kill by day 7 after infection at an MOI of 1.0. In order of decreasing sensitivity to viral oncolysis, the tested cell lines were as follows: MSTO-211H > H-2052 > H-2452 > JMN > VAMT > AC29 > H-2373 (Fig. 1C). GLV-1h68 demonstrated a dose-dependent killing effect in all cell lines tested. This is shown for MSTO-211H cells (Fig. 1D), where even at an MOI of 0.01, 70% cell kill was achieved by day 5.
Viral proliferation
Viral proliferation was assessed after infection with GLV-1h68 at an MOI of 0.1. Assays demonstrated 2- to 3-fold logarithmic replication of GLV-1h68 in all cell lines tested. Proliferation data for MSTO-211H cells showed a 4-log increase in viral titer by day 5 (Fig. 1E).
Cytotoxicity in relation to viral entry
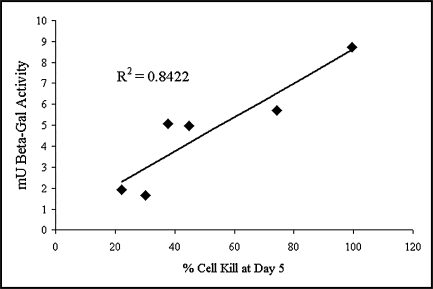
Although all tested MPM cell lines demonstrated sensitivity to oncolysis by GLV-1h68, there was marked variation in degree of sensitivity from 100% cell kill on day 7 in MSTO-211H cells versus 34% in H-2373 cells. Among these cell lines, rapidity of GLV-1h68 entry, as measured by quantitated β-galactosidase activity, was found to be directly proportional to viral cytotoxic efficacy (R2 = 0.8422) (Fig. 2).
FIG. 2.
Entry of GLV-1h8 into malignant pleural mesothelioma cells is directly proportional to the oncolytic effect of the virus. In a panel of MPM cell lines, viral entry as assayed by quantitated β-galactosidase activity 24 hr after infection correlates with viral cytotoxic efficacy as measured by percentage cell kill 5 days after infection.
Optical imaging of mouse tumors
After administration of GLV-1h68 in vivo, GFP expression was easily visualized by fluorescence microscopy in an orthotopic pleural tumor model (Fig. 3A–D). GFP expression was localized to tumor deposits, sparing normal tissues. Because the pleural cavities are connected in mice, even a single dose of GLV-1h68 was able to spread, infect, and replicate in tumor tissue, propagating to neighboring deposits. GFP-expressing tissue and tissue not expressing the protein were biopsied to confirm malignant status. All biopsied GFP-positive tissues proved to be MPM on H&E staining (Fig. 3E), whereas biopsied areas not expressing GFP were confirmed to be normal tissue. In addition, all biopsied GFP-expressing areas were also positive when stained for β-galactosidase activity, confirming that the green fluorescence was not autofluorescence but was actual GLV-1h68 transgene expression (Fig. 3F).
FIG. 3.
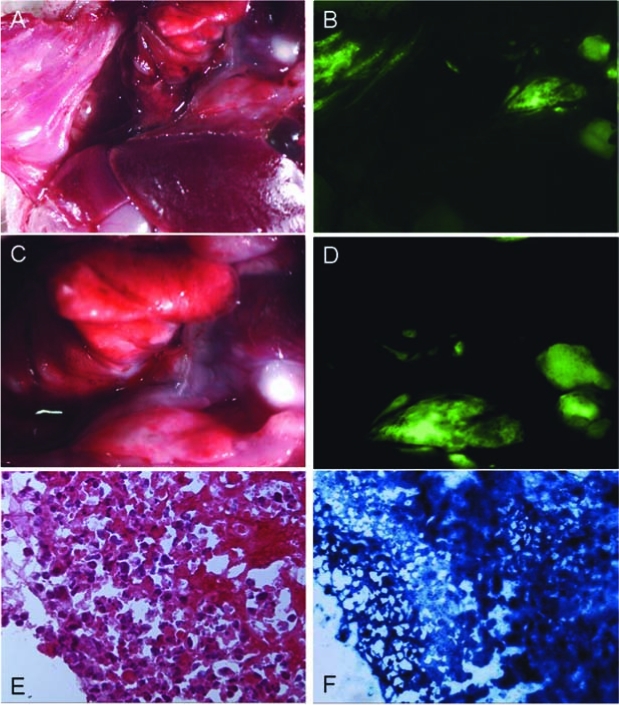
Intraoperative fluorescence imaging within the pleural cavity demonstrates the ability of GLV-1h68 to localize tumor nodules after regional delivery. (A and C) Bright-field images taken within the pleural cavities of virus-treated animals with mesothelioma. (B and D) Corresponding fluorescence images where virally mediated fluorescence allows for localization of tumor deposits. (E) Biopsied GFP-expressing areas were confirmed to be cancerous by H&E staining. (F) Biopsied GFP-positive areas were also β-galactosidase positive, confirming that the green signal was in fact due to viral transgene expression and not autofluorescence.
Intrapleural injection of GLV-1h68 is effective in reducing pleural mesothelioma tumor burden
In these experiments, GLV-1h68-treated animals continued to exhibit normal activity, feeding, and grooming, and were able to maintain their body weights. PBS-treated animals, however, demonstrated a 23% weight loss by day 20 after tumor inoculation (Fig. 4). Average tumor weights of control, early-treated (5 days after tumor inoculation), and late-treated (10 days after tumor inoculation) animals were 404, 16, and 7 mg, respectively (Fig. 5A). By day 28 after treatment, only 4 of 9 virus-treated animals had macroscopic pleural disease versus 10 of 10 PBS-treated control animals (Fig. 5B and C).
FIG. 4.
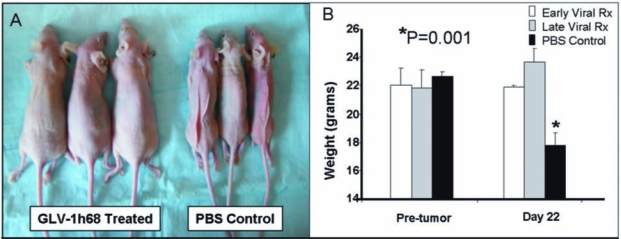
(A and B) Virus-treated animals maintained eating and grooming habits and normal weights whereas PBS-treated animals became cachectic. Before tumor inoculation the average weights of the three groups of animals were the same (early treatment, 22.0 ± 1.2 g; late treatment, 21.8 ± 1.3 g; control, 22.6 ± 0.36 g). After 22 days, the control PBS-treated group lost a significant amount of weight compared with animals in both GLV-1h68-treated groups (early treatment, 21.9 ± 0.2 g; late treatment, 23.7 ± 1.0 g; PBS, 17.8 ± 0.9 g) (Student t test).
FIG. 5.
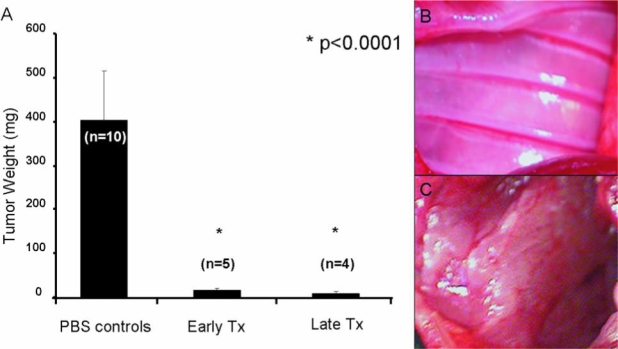
GLV-1h68 effectively clears animals of pleural mesothelioma whereas PBS-treated animals are overwhelmed by pleural disease burden. (A) The average tumor burden of PBS-treated animals is significantly greater compared with early and late treatment animals. Tumor weights of the PBS-treated control group ranged between 209.5 and 560.1 mg, with an average weight of 404.4 mg. The early treatment group had minimal tumor burden, 16.1 ± 2.5 mg, whereas the late treatment group had an even lesser tumor burden of 7.0 ± 7.1 mg. The differences were statistically significant, with both p values less than 0.0001 (Student t test). The chest cavities of all animals were examined on day 22. (B) The chest wall of an animal after regional treatment with GLV-1h68 (1 × 107 PFU) on day 10. Three of five late treatment and two of five early treatment animals were completely cured of their disease as seen on gross examination; whereas the other virally treated animals had minimal mediastinal disease. (C) Extensive tumor covering the chest wall of an animal within 22 days of tumor inoculation followed by PBS treatment.
GLV-1h68 is effective in achieving cures and improving survival of mice with MPM
In mice with established MPM (MSTO-211H), the median survival for PBS-treated control animals was 22 days. Animals from early and late treatment groups monitored for survival did well for >60 days, at which time they were killed as an end point for the experiment (Fig. 6). None of the GLV-1h68-treated animals suffered from any clinically apparent side effects attributable to administration of virus. A postmortem examination was performed after killing the mice in both groups. In the PBS-treated control group, gross disease was confirmed in all mice, with mediastinal encasement by tumor in several animals. In the GLV-1h68-treated group, in mice that were killed after prolonged survival, minimal intrathoracic disease was noted in two of eight animals. In addition, no evidence of gross metastatic disease was found.
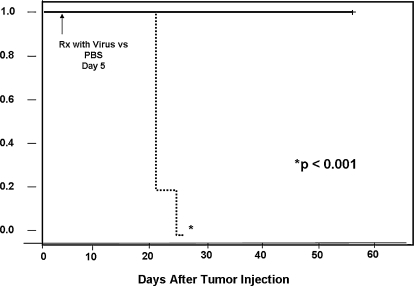
FIG. 6.
Treatment with GLV-1h68 provides a striking survival advantage to animals with malignant pleural mesothelioma as seen by Kaplan–Meier curve analysis. The animals were treated on day 5 after tumor inoculation and monitored daily for morbidity and mortality until day 38. All animals in the GLV-1h68-treated group were alive on day 38 and found to be disease free on autopsy, whereas no PBS-treated animals survived (log-rank analysis).
Selective viral colonization of tumors
GLV-1h68 particles were recovered from tumor tissues of virus-treated animals 24 hr, 48 hr, and 4 weeks after intrapleural administration of virus on the order of 2.5 to 5 × 104 PFU per gram of tissue. Trace amounts of virus were detected in brain, liver, and ovaries 24 hr after administration. These values decreased by 48 hr. By 4 weeks after administration of virus, no viral particles were recovered from any normal tissues whereas the virus continued to replicate in tumor (Table 1).
Table 1.
Biodistribution of GLV-1h68a
| Brain | Lung | Tumor | Heart | Liver | Spleen | Bowel | Kidney | Uterus | Ovary | |
|---|---|---|---|---|---|---|---|---|---|---|
| 24 hr (n = 2) | 0.3 | 0 | 5.5 × 104 | 0 | 36.1 | 0 | 0 | 0 | 0.8 | 10.8 |
| 48 hr (n = 2) | 0 | 0 | 2.5 × 104 | 0 | 0 | 0 | 0 | 0 | 0 | 4.8 |
| 4 weeks (n = 2) | 0 | 0 | 3.4 × 104 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Expressed as plaque-forming units per gram tissue. Biodistribution data after intrapleural administration of VLR-1h68 demonstrate that the virus is highly specific for infection of tumor for a time period ranging from 24 hr to 4 weeks after administration. Trace amounts of virus are detected in the brain, liver, and ovaries at early time points after administration. By 4 weeks after administration of virus, no viral particles are detected in any normal tissues, while the virus is still replicating within tumor.
Discussion
Many replication-competent oncolytic viruses are being designed for targeted killing of cancer. Examples include herpes simplex virus, Newcastle disease virus, adenovirus, vesicular stomatitis virus, myxoma virus, lentivirus, reovirus, and vaccinia virus. These viruses have deletions of specific virulence genes without which they cannot replicate in normal cells. Cancer cells, however, often have upregulated homologs of these same deleted genes. The attenuated viruses can therefore still achieve productive infection and replication in malignant cells. In addition, many oncolytic viruses carry transgenes inserted to allow for assaying or tracking of the virus. The insertion of transgenes into the viral genome can further impede the ability of oncolytic viruses to productively replicate in normal cells.
One characteristic of vaccinia that makes it a particularly good candidate as an oncolytic vector is its large genome, which is able to accommodate multiple transgenes without any effect on the replicative ability of the virus. Thorne and colleagues have published encouraging results based on the use of a vaccinia virus recombinant derived from the western reserve (WR) strain, JX-963, for tumor targeting. This virus has deletions of viral thymidine kinase (TK) and vaccinia growth factor (VGF) and expresses human GM-CSF. They have shown this virus to be effective against a variety of cancer types (including breast, lung, pancreas, colon, prostate, and ovarian cancer cell lines). They have demonstrated antitumor efficacy with this virus in both xenograft and immunocompetent animal models with both intratumoral and systemic delivery (Thorne et al., 2007).
In addition to therapeutic genes, marker genes can be inserted into oncolytic vectors. Viruses carrying marker genes, which are not expressed in uninfected cells, can be localized and the course of viral therapy monitored. Here we describe the use of a vaccinia virus construct, GLV-1h68, specifically targeted for treatment of cancer by deletion of virulence genes for hemagglutinin and vaccinia TK, and carrying marker genes encoding GFP, LacZ, and Renilla luciferase (Zhang et al., 2007), for treatment of MPM in vitro and in vivo.
GLV-1h68 is able to productively infect, replicate in, and lyse a variety of human malignant mesothelioma cell lines, including epithelioid, sarcomatoid, and biphasic subtypes. Of the cell lines tested, GLV-1h68 displays the greatest cytotoxic efficacy and replicative ability in MSTO-211H cells. This oncolytic effect was found to be dose dependent. Among the various cell lines tested, viral entry (as measured by quantitated expression of the inserted lacZ transgene) correlates with rapidity of cell kill. This is not surprising as more rapid entry of the virus into the host cell allows for more rapid transcription of viral proteins, assembly of new virions, and lysis of the host cell. We have shown that for human malignant mesothelioma cell lines, early viral entry can be used as a predictor of successful cell kill. The practical implication of this finding is that an ex vivo assay for infectivity may be worked out for prediction of successful response to viral therapy in vivo.
GLV-1h68 was able to maintain the successful oncolytic effect achieved against MSTO-211H cells in the in vitro setting in an orthotopic animal model of MPM (in vivo). GLV-1h68 successfully localized, infected, and displayed detectable transgene expression in tumor tissues after intrapleural administration. Animals in both early and late treatment groups maintained normal weight and food intake whereas control animals developed marked cachexia over a period of 3 weeks after tumor inoculation. GLV-1h68 demonstrated effective cure and marked reduction of tumor burden in settings of microscopic and macroscopic pleural disease. In addition, intrapleural therapy with GLV-1h68 resulted in increased survival in both microscopic and macroscopic disease settings.
Importantly, although GLV-1h68 displayed successful treatment of pleural disease, it did not result in any signs of toxicity or significant infection of normal host tissues over a varied time period, from 24 hr to 4 weeks after administration of virus. Trace amounts of virus were found in the liver and ovaries 24 hr after administration, but these values decreased by 48 hr. Four weeks after administration of virus, viral particles were detected only in tumor tissue. GLV-1h68 therapy was well tolerated by athymic mice and resulted in essentially no signs of toxicity and displayed no replicative ability in normal tissues. A limitation of this model is the fact that these mice are immunocompromised. We are confident that GLV-1h68 would be nontoxic to humans as vaccinia virus of the same strain has been safely administered to millions of people via the smallpox vaccine. We do not know, however, what effect the human immune system might have on viral efficacy.
Malignant pleural mesothelioma is a deadly disease with a great need for novel therapeutic options. Gene therapy strategies have been investigated in phase I clinical studies, but have not demonstrated therapeutic benefits as of yet. In our laboratory, we have done preclinical studies showing the efficacy of oncolytic herpes simplex viruses in the detection and treatment of MPM (Adusumilli et al., 2006a,b). We have also shown that oncolytic therapy with herpesvirus can be synergistic with radiation therapy in mesothelioma (Adusumilli et al., 2007). Here we show for the first time that an oncolytic vaccinia virus construct, GLV-1h68, is effective in killing MPM in vitro and in vivo. In conclusion, GLV-1h68 has excellent tumor specificity, replicative ability, and cytotoxic efficacy in MPM and warrants further investigation as a clinical diagnostic and therapeutic agent against this uniformly fatal disease.
Acknowledgments
The authors thank Sen Li for excellent technical assistance and Meryl Greenblatt for outstanding editorial assistance. This work was supported by the Flight Attendant Medical Research Institute (FAMRI), the National Institutes of Health (NIH) (R01 CA 75416), and with grant support by the Research and Development Division of Genelux Corporation (A.A. Szalay).
Author Disclosure Statement
Yong A. Yu, Nanhai Chen, and Aladar Szalay, are affiliated with Genelux Corporation. No competing financial interests exist for Kaitlyn Kelly, Yanghee Woo, Peter Brader, Christopher Riedl, Shu-Fu Lin, Zhenkun Yu, Valerie Rusch, and Yuman Fong.
References
- Adusumilli P.S. Eisenberg D.P. Stiles B.M. Chung S. Chan M.K. Rusch V.W. Fong Y. Intraoperative localization of lymph node metastases with a replication-competent herpes simplex virus. J. Thorac. Cardiovasc. Surg. 2006a;132:1179–1188. doi: 10.1016/j.jtcvs.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Adusumilli P.S. Stiles B.M. Chan M.K. Mullerad M. Eisenberg D.P. Ben-Porat L. Huq R. Rusch V.W. Fong Y. Imaging and therapy of malignant pleural mesothelioma using replication-competent herpes simplex viruses. J. Gene Med. 2006b;8:603–615. doi: 10.1002/jgm.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adusumilli P.S. Chan M.K. Hezel M. Yu Z. Stiles B.M. Chou T.C. Rusch V.W. Fong Y. Radiation-induced cellular DNA damage repair response enhances viral gene therapy efficacy in the treatment of malignant pleural mesothelioma. Ann. Surg. Oncol. 2007;14:258–269. doi: 10.1245/s10434-006-9127-4. [DOI] [PubMed] [Google Scholar]
- Bianchi C. Bianchi T. Malignant mesothelioma: Global incidence and relationship with asbestos. Ind. Health. 2007;45:379–387. doi: 10.2486/indhealth.45.379. [DOI] [PubMed] [Google Scholar]
- Blattman J.N. Grayson J.M. Wherry E.J. Kaech S.M. Smith K.A. Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat. Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- Borasio P. Berruti A. Bille A. Lausi P. Levra M.G. Giardino R. Ardissone F. Malignant pleural mesothelioma: Clinicopathologic and survival characteristics in a consecutive series of 394 patients. Eur. J. Cardiothorac. Surg. 2008;33:307–313. doi: 10.1016/j.ejcts.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Castagneto B. Botta M. Aitini E. Spigno F. Degiovanni D. Alabiso O. Serra M. Muzio A. Carbone R. Buosi R. Galbusera V. Piccolini E. Giaretto L. Rebella L. Mencoboni M. Phase II study of pemetrexed in combination with carboplatin in patients with malignant pleural mesothelioma (MPM) Ann. Oncol. 2008;19:370–373. doi: 10.1093/annonc/mdm501. [DOI] [PubMed] [Google Scholar]
- Connelly R.R. Spirtas R. Myers M.H. Percy C.L. Fraumeni J.F., Jr. Demographic patterns for mesothelioma in the United States. J. Natl. Cancer Inst. 1987;78:1053–1060. [PubMed] [Google Scholar]
- National Research Council, Institute of Laboratory Animal Resources, Commission on Life Sciences. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- Lin S.F. Yu Z. Riedl C. Woo Y. Zhang Q. Yu Y.A. Timiryasova T. Chen N. Shah J.P. Szalay A.A. Fong Y. Wong R.J. Treatment of anaplastic thyroid carcinoma in vitro with a mutant vaccinia virus. Surgery. 2007;142:976–983. doi: 10.1016/j.surg.2007.09.017. discussion 976–983. [DOI] [PubMed] [Google Scholar]
- McFadden G. Poxvirus tropism. Nat. Rev. Microbiol. 2005;3:201–213. doi: 10.1038/nrmicro1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S. Haenel T. Himbeck R. Scott B. Ramshaw I. Lake R.A. Harnett G. Phillips P. Morey S. Smith D. Davidson J.A. Musk A.W. Robinson B. Replication-restricted vaccinia as a cytokine gene therapy vector in cancer: Persistent transgene expression despite antibody generation. Cancer Gene Ther. 2000;7:663–670. doi: 10.1038/sj.cgt.7700133. [DOI] [PubMed] [Google Scholar]
- Schipper P.H. Nichols F.C. Thomse K.M. Deschamps C. Cassivi S.D. Allen M.S. Pairolero P.C. Malignant pleural mesothelioma: Surgical management in 285 patients. Ann. Thorac. Surg. 2008;85:257–264. doi: 10.1016/j.athoracsur.2007.06.066. discussion 264. [DOI] [PubMed] [Google Scholar]
- Sterman D.H. Recio A. Vachani A. Sun J. Cheung L. Delong P. Amin K.M. Litzky L.A. Wilson J.M. Kaiser L.R. Albelda S.M. Long-term follow-up of patients with malignant pleural mesothelioma receiving high-dose adenovirus herpes simplex thymidine kinase/ganciclovir suicide gene therapy. Clin. Cancer Res. 2005;11:7444–7453. doi: 10.1158/1078-0432.CCR-05-0405. [DOI] [PubMed] [Google Scholar]
- Thorne S.H. Bartlett D.L. Kirn D.H. The use of oncolytic vaccinia viruses in the treatment of cancer: A new role for an old ally? Curr. Gene Ther. 2005;5:429–443. doi: 10.2174/1566523054546215. [DOI] [PubMed] [Google Scholar]
- Thorne S.H. Hwang T.H. O'Gorman W.E. Bartlett D.L. Sei S. Kanji F. Brown C. Werier J. Cho J.H. Lee D.E. Wang Y. Bell J. Kirn D.H. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J. Clin. Invest. 2007;117:3350–3358. doi: 10.1172/JCI32727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Most R.G. Robinson B.W. Nelson D.J. Gene therapy for malignant mesothelioma: Beyond the infant years. Cancer Gene Ther. 2006;13:897–904. doi: 10.1038/sj.cgt.7700935. [DOI] [PubMed] [Google Scholar]
- Walker A.M. Loughlin J.E. Friedlander E.R. Rothman K.J. Dreyer N.A. Projections of asbestos-related disease 1980–2009. J. Occup. Med. 1983;25:409–425. [PubMed] [Google Scholar]
- Woo Y. Adusumilli P.S. Fong Y. Advances in oncolytic viral therapy. Curr. Opin. Investig. Drugs. 2006;7:549–559. [PubMed] [Google Scholar]
- Yu Y.A. Shabahang S. Timiryasova T.M. Zhang Q. Beltz R. Gentschev I. Goebel W. Szalay A.A. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat. Biotechnol. 2004;22:313–320. doi: 10.1038/nbt937. [DOI] [PubMed] [Google Scholar]
- Zhang Q. Yu Y.A. Wang E. Chen N. Danner R.L. Munson P.J. Marincola F.M. Szalay A.A. Eradication of solid human breast tumors in nude mice with an intravenously injected light-emitting oncolytic vaccinia virus. Cancer Res. 2007;67:10038–10046. doi: 10.1158/0008-5472.CAN-07-0146. [DOI] [PubMed] [Google Scholar]